Abstract
Preterm birth (PTB, <37 completed weeks’ gestation) is one of the leading obstetrical problems in the United States, affecting approximately one of every nine births. Even more concerning are the persistent racial disparities in PTB, with particularly high rates among African Americans. There are several recognized pathophysiologic pathways to PTB, including infection and/or exaggerated systemic or local inflammation. Intrauterine infection is a causal factor linked to PTB thought to result most commonly from inflammatory processes triggered by microbial invasion of bacteria ascending from the vaginal microbiome. Trials to treat various infections have shown limited efficacy in reducing PTB risk, suggesting that other complex mechanisms, including those associated with inflammation, may be involved in the relationship between microbes, infection, and PTB. The complement system, a key mediator of the inflammatory response, is an innate defense mechanism involved in both normal physiologic processes that occur during pregnancy implantation and processes that promote the elimination of pathogenic microbes. Recent research has demonstrated an association between this system and PTB. The purpose of this article is to present a mechanistic model of inflammation-associated PTB, which hypothesizes a relationship between the microbiome and dysregulation of the complement system. Exploring the relationships between the microbial environment and complement biomarkers may elucidate a potentially modifiable biological pathway to PTB.
Keywords: preterm birth, microbiome, inflammation in pregnancy, complement system
Preterm birth (PTB), defined as birth at <37 completed weeks’ gestation, is one of the leading obstetrical problems in the United States, affecting nearly 500,000—or 1 of every 9—infants born annually (Centers for Disease Control and Prevention, 2014). PTB is the greatest risk factor for infant death, contributing to 35% of all infant deaths and health-care costs in excess of US $26 billion annually (March of Dimes, 2015). Although PTB rates have declined in the general U.S. population since 2007, the overall rate remains around 12%, which exceeds the March of Dimes Prematurity Campaign 2020 PTB rate goal of 8.1% (Centers for Disease Control and Prevention, 2014; March of Dimes, 2016; J. A. Martin, Hamilton, Osterman, Curtin, & Matthews, 2015). Additionally, a higher prevalence of PTB persists among ethnic and racial subgroups (Culhane & Goldenberg, 2011). African American (AA) women, one of the groups at highest risk, are 1.5 times as likely to experience PTB and nearly 2 times as likely to have an early PTB (<32 weeks’ gestation) compared to Caucasian women (Culhane & Goldenberg, 2011; Kramer, Hogue, Dunlop, & Menon, 2011; March of Dimes, 2014). Authors have reported that identified risk factors associated with PTB such as maternal age, history of infection, and substance use explain less than half of the disparity in rate of PTB between AA and Caucasian women (Goldenberg, Culhane, & Iams, 2008; McGrady, Sung, Rowley, & Hogue, 1992). Identification of novel biobehavioral pathways to PTB may ultimately lead to the development of interventions to reduce the risk of PTB, particularly among groups with the greatest risk.
Although many mammalian species exhibit slight variations in gestational length, PTB appears to be primarily a human phenomenon (Phillips, Abbot, & Rokas, 2015). Identification of the underlying biological mechanisms of PTB is difficult as the complication is syndromic in nature and includes biological (e.g., infection, hypothalamic–pituitary–adrenal [HPA] axis activation, uterine overdistention), behavioral (e.g., stress, substance abuse, anxiety), and epidemiologic (e.g., demographic, social, economic) contributing factors (Goldenberg et al., 2008; Kramer & Hogue, 2009). Of the many biological processes implicated in the onset of PTB, intrauterine infection and/or inflammation are strongly associated with an increased risk for PTB (Goldenberg, Hauth, & Andrews, 2000; Goncalves, Chaiworapongsa, & Romero, 2002; Romero et al., 2006). In this pathway to PTB, microbial invasion triggers inflammation, which is driven by the release of pro-inflammatory cytokines, prostaglandins, and matrix metalloproteinases (MMP) that promote cervical ripening and weakening of the amniotic membranes. This process suggests that mechanisms that alter inflammatory events at the maternal–fetal interface may be predictive of PTB (Holst, Mattsby-Baltzer, Wennerholm, Hagberg, & Jacobsson, 2005; Vogel, Thorsen, Curry, Sandager, & Uldbjerg, 2005). Despite this relationship between infection and PTB, randomized controlled trials directly targeting known contributors of intrauterine infection—including the elimination of bacterial vaginosis (BV), sexually transmitted infections, and periodontal disease during pregnancy—have shown limited efficacy in reducing the risk of PTB (Lynch, Wagner, Deterding, et al., 2016; Lynch, Wagner, Giclas, et al., 2016). These findings suggest that there may be other more complex mechanisms involved in the infection-associated pathway to PTB.
The inflammatory response to infection is mediated by the activities of several immune-cell populations, including a group of blood proteins known as complement. The complement system is an innate immune mechanism composed of more than 30 blood proteins that initiate a series of enzymatic reactions that drive the initiation of the inflammatory response that ultimately results in elimination of a pathogen (Markiewski & Lambris, 2007; Sjoberg, Trouw, & Blom, 2009; Walport, 2001). Complement dysregulation, defined as excess activation or poor regulation, may promote an exaggerated inflammatory response and damage to self-tissues in the process (Girardi, Bulla, Salmon, & Tedesco, 2006). The complement pathway has recently been associated with cervical remodeling, preterm premature rupture of membranes (PPROM), and PTB (Gonzalez, Franzke, Yang, Romero, & Girardi, 2011; Lynch et al., 2011), suggesting that complement dysregulation in the intrauterine tissues and maternal vasculature may be predictive of PTB.
Although not previously linked together in the literature in regard to PTB, complement and the microbiome are linked in oral (Astafurov et al., 2014; Boackle, 1991; Hajishengallis & Lambris, 2012; Hajishengallis et al., 2011; Hajishengallis, Maekawa, Abe, Hajishengallis, & Lambris, 2015), gut (Lu, Knutson, Wishnok, Fox, & Tannenbaum, 2012; Yoshiya et al., 2011), skin (Chehoud et al., 2013), and nasopharynx (Domenech, Ramos-Sevillano, Garcia, Moscoso, & Yuste, 2013) health. Given the ability of the microbiome to influence the expression of complement in other situations and the suspected associations of vaginal and even oral microbial dysbiosis as a risk for PTB, in the present article, we summarize the current knowledge regarding the role of infection and inflammation in the context of PTB with a specific focus on the role of the microbiome and the complement system. We conclude with a presentation of a mechanistic model for inflammation-associated PTB, which hypothesizes a relationship between the microbiome and dysregulation of the complement system. Identification of biological pathways of inflammation, particularly in high-risk groups, may identify potential targets for intervention to eliminate this stubbornly persistent adverse pregnancy outcome and health disparity.
Mechanisms of PTB: Infection and Inflammation
The study of PTB has involved the investigation of a variety of anatomical, endocrinological, immunological, and clinical events that affect both the mother and fetus. While many organ systems are involved in the labor process, only changes within the intrauterine environment are directly involved in the labor and birth process. The accepted framework is that preterm parturition results from the physiologic activation of pathways that result in increased uterine contractility, cervical ripening, amniotic membrane weakening, and decidual activation (Romero et al., 2006; Romero, Gotsch, Pineles, & Kusanovic, 2007). The mechanisms involved in initiating the process remain unclear, but one of the major focal areas of obstetrical research is the study of pathogenic processes highly associated with PTB, including infection and inflammation.
Infection and PTB
Evidence suggesting a relationship between infection and preterm parturition is readily available: (1) Extrauterine infections such as pyelonephritis (Kaul et al., 1999; Wren, 1969), pneumonia (Madinger, Greenspoon, & Ellrodt, 1989; Munn, Groome, Atterbury, Baker, & Hoff, 1999), and periodontal disease (Goepfert et al., 2004; Jeffcoat, Geurs, Reddy, Goldenberg, & Hauth, 2001; Offenbacher, 2004) are associated with PTB; (2) several PTB studies have identified pathogenic microbes from the chorioamnion and amniotic fluid of women with PTB (DiGiulio, 2012; Marconi, de Andrade Ramos, Peracoli, Donders, & da Silva, 2011); (3) subclinical intrauterine infection is associated with PTB (Gomez et al., 1995); and (4) animal studies show that intrauterine infection or systemic microbes stimulate preterm labor (PTL) and birth (Gonzalez et al., 2011; Romero et al., 1988). In fact, intrauterine infection caused by the invasion of bacteria into the uterine cavity is the leading cause of infection-associated PTB (Goldenberg et al., 2008; Romero et al., 2006; Vogel et al., 2005). The pathway to microbial invasion of the amniotic cavity (MIAC) is thought to result primarily from ascending microbes from the vagina and cervix; however, authors have proposed other pathways including transplacental infection, retrograde exposure from the peritoneal cavity through the fallopian tube, or introduction during invasive procedures such as amniocentesis (Romero et al., 2001). Recent evidence supports hematogenous transfer of oral microbes to the placenta as well (Aagaard et al., 2014).
Intrauterine infection accounts for 25–40% of PTBs (Goldenberg et al., 2000; Goncalves et al., 2002). The isolation of microorganisms from the amniotic cavity is considered an abnormal finding as the uterus is traditionally thought to be sterile. However, research has demonstrated MIAC in women with PTL (regular uterine contractions with cervical change at <37 weeks’ gestation), PTB (delivery at <37 weeks’ gestation), and PPROM (rupture of membranes at <37 weeks’ gestation). Studies investigating the relationship between MIAC and PTL in women with and without intact membranes suggest that there are differences in the prevalence of MIAC based on the patient clinical presentation, with greater microbial invasion being associated with actual preterm delivery and PPROM. The mean rate of positive amniotic fluid cultures in women with PTL and intact membranes is 12.8%, whereas that rate in women with PTL and delivery with intact membranes it is 22% and in women with PPROM is 32.4% (Goncalves et al., 2002). MIAC may occur in 51% of women who present with cervical shortening (Romero et al., 1992); a shortened cervix, as diagnosed by sonography (cervical length <25 mm), is a significant predictor of preterm delivery (Iams et al., 1996; Moroz & Simhan, 2014). Common microorganisms isolated from women with intrauterine infection and PTB include Mycoplasma, Streptococcus, Ureaplasma, Bacteroides, and Prevotella species (Hill, 1998). These findings suggest that microbial invasion and inflammation in the maternal reproductive tissues may be predictive of PTB.
Inflammation and PTB
Review of the inflammatory response to infection
The immune system protects the body from infection and invasion by microorganisms via a highly complex and organized system of various cell-based populations that interact via various effector mechanisms to eliminate pathogens (Mogensen, 2009). Macrophages are one of the first effector cells to be activated in the innate immune response, as they are resident in the soft tissues. Macrophages and other cellular populations use a variety of pattern-recognition receptors such as complement receptors (e.g., CR1, CR3, CR4), mannose, and toll-like receptors (TLR) to recognize molecular structures found on the surface of pathogens (Hargreaves & Medzhitov, 2005; Mogensen, 2009; Taylor et al., 2005). Upon exposure of these various cellular populations to bacterial antigens, a signal transduction cascade is triggered, resulting in the activation of NF-kappaB (NF-κβ) and other transcription factors that regulate the genes involved in the production of inflammatory cytokines. These cytokines then attract other cellular populations to the site of infection (Lawrence, 2009). There are many different types of cytokines, but interleukin 1 beta (IL-1β), IL-6, IL-8, and IL-12, in particular, are heavily involved in the recruitment of different cellular populations during the early stages of infection (Janeway & Medzhitov, 2002). The cytokine tumor necrosis factor-alpha (TNF-α), in turn, drives the vasodilation of blood vessels and is heavily involved in endothelial leukocyte cellular activity (Bradley, 2008), which allows for the movement of fluid, plasma proteins, and white blood cells into the tissue, resulting in inflammation.
Role of exaggerated inflammation in PTB
There is strong evidence supporting the role of various inflammatory mediators in the etiology of PTB (Gibbs, Romero, Hillier, Eschenbach, & Sweet, 1992; Gomez et al., 1995; Keelan et al., 2003). Researchers have posited that the release of various inflammatory cytokines, such as IL-8, IL-1β, and TNF-α, along with microbial endotoxins stimulates the production of other inflammatory mediators including prostaglandins and MMPs (Goldenberg et al., 2000; Keelan et al., 2003; Romero et al., 2007). IL-1β, in fact, was one of the first cytokines implicated in the onset of spontaneous PTL (Romero, Durum, et al., 1989), and the human decidua produces IL-1β in response to exposure to bacteria (Romero, Wu, et al., 1989). IL-1β concentrations are increased in the amniotic fluid of women with PTL and infection, and it is thought to stimulate the production of prostaglandins and initiate intrauterine contractions (Marconi et al., 2011; Nadeau-Vallee et al., 2016). Similarly, TNF-α can stimulate prostaglandin production in the intrauterine tissues and also in response to bacterial components. Researchers have found elevated TNF-α concentrations in women with PPROM and intrauterine infection and have identified increased TNF-α as a mechanism for bacteria-induced PTB (Hillier et al., 1993; Romero et al., 2007). Other cytokines, including IL-6, IL-10, and IL-18, have also been implicated in the biologic pathway to PTB. Authors have suggested that early and prolonged activation of these inflammatory processes break down collagen, stimulate uterine contractions, facilitate cervical ripening, and promote decidual activation, resulting in incompetent cervix and weakening of the amniotic membrane (Goldenberg et al., 2000; Romero et al., 2007), both key risk factors in the development of PTB (Holst et al., 2005; Romero et al., 2007).
The Microbiome as a Mechanism for PTB
The study of the human microbiome refers to the evaluation of the composition and metabolic potential of the ecologic community of microbes residing within the human body (Peterson et al., 2009). Newer culture-independent DNA sequencing strategies using polymerase chain reaction (PCR) methods have made evaluation of a variety of microbes found in different body sites possible, which, in turn, has allowed for the identification of a significantly greater number and diversity of intrauterine microbes not typically found with culture-dependent methods (Shendure & Ji, 2008). PCR analysis of amniotic fluid of women with PTB indicates a 30–50% higher prevalence of intrauterine infection and identifies a 1.5–3.5 times greater number of bacterial taxa as compared to studies using culture-based methods (DiGiulio, 2012; Han, Shen, Chung, Buhimschi, & Buhimschi, 2009; Marconi et al., 2011). Specifically, 16S ribosomal RNA (rRNA) gene-based sequencing is used to characterize and compare bacterial communities, thereby allowing for a more detailed evaluation of the microbiota present (Eckburg et al., 2005; Lane et al., 1985). Using these newer methods to explore routes associated with intrauterine infection, such as the bacterial flora of the vagina, may be beneficial, particularly in women at risk for PTB, and may reveal a pathway to PTB in women who present without overt signs of clinical infection.
Although there are many proposed routes to intrauterine infection including hematogenous spread of microbes from the oral (Han et al., 2006; Solt, 2015), gut (Cani, Osto, Geurts, & Everard, 2012), and respiratory (Sandu, Folescu, Pop, & Motoc, 2013) communities, the primary pathway to intrauterine infection is thought to involve the ascension of pathogenic microbes from the vagina and cervix into the uterus (Bastek, Gomez, & Elovitz, 2011; Romero et al., 2001). The microbes present in the vagina form a complex ecosystem, collectively known as the vaginal microbiome, which plays a role in both normal physiologic function and infection. Protective bacterial species, such as those in the Lactobacillus genus (e.g., L. crispatus, L. iners, L. jensenii, L. gasseri), produce lactic acid and other bacteriostatic compounds that prevent the overgrowth of other, more pathogenic microbiota (Boskey, Cone, Whaley, & Moench, 2001; Kaewsrichan, Peeyananjarassri, & Kongprasertkit, 2006). When the vaginal equilibrium of lactobacilli are disturbed, either via replacement or due to the overgrowth of select anaerobes, vaginal infections such as BV are more likely to occur (Hummelen et al., 2010; McMillan et al., 2015).
The microbes that colonize epithelial surfaces of the vagina communicate with a wide variety of pattern-recognition receptors in the uterus, cervix, and amniotic membranes (Bastek et al., 2011; Takeuchi & Akira, 2010) directly or through the release of products such as lipids, carbohydrates, proteins, or nucleic acids (Chu & Mazmanian, 2013). This communication facilitates the activation of various pro- and anti-inflammatory mechanisms to prevent the elimination of commensal bacteria and stimulate an aggressive inflammatory response to eliminate pathogenic bacteria, thereby maintaining a healthy vaginal microenvironment. A healthy vaginal microbiome plays a role in the prevention of several reproductive tract infections associated with PTB including BV, sexually transmitted infections, and urinary tract infections (Donders et al., 2000; Gupta et al., 1998; H. L. Martin et al., 1999; Wiesenfeld, Hillier, Krohn, Landers, & Sweet, 2003).
The vaginal tract is home to more than 50 nonpathogenic species of commensal flora (Cribby, Taylor, & Reid, 2008; Oakley, Fiedler, Marrazzo, & Fredricks, 2008). These flora vary widely among women due to host and environmental factors (Costello et al., 2009; Ravel et al., 2011). A recent study of the vaginal microbiomes of 396 asymptomatic nonpregnant women from various ethnic groups identified five clusters of microbial flora, four of which were dominated by lactobacillus species; these clusters varied significantly based on ethnicity (Ravel et al., 2011). Another study found that AA women were more likely than Caucasian women to have vaginal microbiota that were not dominated by lactobacilli (Zhou et al., 2007). Pathogenic vaginal microbes known to be highly associated with PTB, such as Gardnerella vaginalis, Bacteroides, Mobiluncus, and Prevotella, disturb protective microbial species (e.g., Lactobacillus) resulting in vaginal infections such as BV, the most common vaginal infection affecting women aged 15–44 years in the United States (Centers for Disease Control and Prevention, 2016; Culhane & Goldenberg, 2011; Denney & Culhane, 2009). The differences in the vaginal microenvironment among women of various ethnicities are likely due to differences in host and environmental factors such as diet or cultural practices. These findings suggest that these differences in the vaginal microenvironment may have implications for infection/inflammation-associated pregnancy outcomes, such as PTB.
Recent studies of the vaginal microbiome in pregnancy suggest that there is a reduction in taxonomic diversity of microorganisms present as pregnancy progresses (Aagaard et al., 2012). However, the factors that influence the structure and dynamics of the vaginal microbiome in pregnancy are relatively unknown. A study of 27 pregnant women found an association between oral intake of probiotics and changes in the vaginal microbiome that favored a decrease in pro-inflammatory vaginal cytokines (Vitali et al., 2012). A recent case control study of 49 pregnant women found that the bacterial taxonomic composition of the vaginal microenvironment remained stable throughout pregnancy and that prevalence of Lactobacillus-deficient vaginal communities was inversely associated with the gestational age of delivery. Additionally, the risk for PTB was higher in women with a greater prevalence of Lactobacillus-deficient communities combined with elevated levels of Gardnerella and Ureaplasma species (DiGiulio et al., 2015).
The Complement System as a Mechanism for PTB
Overview of the Complement System
The complement pathway is a component of the innate immune response and is so named for its primary function, which is to “complement” the activities of antibodies in the destruction of pathogens. The pathway is composed of more than 30 soluble and membrane-bound proteins that initiate a series of enzymatic reactions that facilitate the destruction of pathogens, eliminate immune complexes, and facilitate removal of cellular debris following tissue injury (Markiewski & Lambris, 2007; Sjoberg et al., 2009; Walport, 2001). Complement drives the initiation of inflammation and promotes opsonization and macrophage activation. The complement system functions at a low-level steady state until its activities are amplified via activation of various pathways.
The complement activation pathway actually involves three pathways, as Lynch and colleagues (2011) described: the classical, lectin, and alternative pathways. The classical pathway is triggered by the presence of bound antigen–antibody complexes; the alternative pathway is continuously activated due to the presence of foreign invaders as well as the presence of damaged self-tissues; and the lectin pathway is activated via the binding of mannose-binding lectin to carbohydrate or glycoprotein groups present on the surface of microorganisms (Denny, Woodruff, Taylor, & Callaway, 2013; Lynch et al., 2011; Regal, Gilbert, & Burwick, 2015).
Regardless of the particular initiating component, all three pathways lead to enzymatic cleavage of complement component C3 by a pathway-specific C3 convertase (enzyme) into fragments C3a and C3b. C3a is a cleavage product that functions as an anaphylatoxin, which recruits and activates inflammatory cells, amplifies local inflammation, and affects vascular permeability and smooth muscle contractility (Sjoberg et al., 2009; Walport, 2001). C3b opsonizes pathogens and/or nonself cells for destruction by phagocytic cells and also initiates the activation of downstream complement proteins that form the membrane attack complex (C5–C9), leading to pathogen-cell lysis and death (Denny et al., 2013; Lynch et al., 2011; Markiewski & Lambris, 2007). Factors B, D, and properdin lead to the activation of C3 via the alternative pathway; Bb, a derivative of Factor B, is an activation product that assists in the additional cleavage of component C3 (Lynch et al., 2008). Given that complement activation products have the potential to initiate damage against self-tissues, the products are quickly removed by plasma carboxypeptides such as C3a des-Arg; factors such as C3b and C4b are quickly deactivated and cleaved into fragments by serine proteases. Ineffective clearance may result in deposits of complement products into host tissues, thereby promoting host tissue damage (Sarma & Ward, 2011).
The Complement System in Pregnancy
The state of pregnancy is associated with increased complement activation both as a mechanism of host defense and as a necessary component in normal fetal/placental development (Baines, Millar, & Mills, 1974; Richani et al., 2005). In both normal and complicated pregnancies, researchers have found complement products in placental tissues (Faulk, Jarret, Keane, Johnson, & Boackle, 1980; Richani et al., 2005; Weir, 1981), and these products likely serve as a protective mechanism against potential infection for both the mother and fetus. Complement is involved in both fetal and placental development, as activated C3 is involved in phagocytic activities of the mouse trophoblastic invasion of the uterine vasculature (Albieri, Kipnis, & Bevilacqua, 1999). Researchers have also noted the participation of complement component C1q in normal physiologic trophoblastic invasion of human uteroplacental tissues (Bulla et al., 2008). Components of the complement system are important in embryonic and fetal development as well, as the most abundant embryotrophic factor in humans, ETF-3, contains C3, C3b, and iC3b, suggesting that C3 is important in fetal development prior to development of the placenta (Lee, Cheong, Chow, Lee, & Yeung, 2009).
Dysregulation of complement, which results from excess activation or poor regulation of the complement pathways, promotes a heightened inflammatory state (Lynch et al., 2011). The uteroplacental tissues have complement regulatory proteins, such as decay-accelerating factor, membrane cofactor protein, and CD59, to prevent excessive complement activation (Holmes et al., 1990; Hsi, Hunt, & Atkinson, 1991; Liszewski, Farries, Lublin, Rooney, & Atkinson, 1996; Nishikori, Noma, Hirakawa, Amano, & Kudo, 1993). At the same time, however, deficiencies in complement components, such as C1q, C2, and C4, predispose individuals to increased risk for infection by encapsulated bacteria (Botto et al., 2009; Regal et al., 2015) as well as collagen vascular disorders (Aggarwal et al., 2010) and abnormal placentation and pregnancy complications (Singh, Ahmed, & Girardi, 2011). During early pregnancy, the classical, lectin, and/or alternative complement pathways may be activated in women who have one or more additional triggers, including foreign substances or damaged tissue. Excessive complement activation in response to infection or other triggers may overwhelm regulatory systems, thereby increasing the risk for perinatal complications. Investigators have found deposits of complement activation products on various reproductive tissues including the placenta, cervix, and decidual spiral arteries (Girardi et al., 2006; Gonzalez et al., 2011).
The dysregulation of complement has been implicated in a variety of adverse pregnancy outcomes including hypertensive diseases of pregnancy (Lynch et al., 2012; Lynch et al., 2008; Lynch, Wagner, Giclas, et al., 2016), antiphospholipid antibody syndrome–associated fetal loss (Breen et al., 2012), and recurrent fetal loss and PTB (Lynch et al., 2012; Lynch et al., 2011; Lynch, Wagner, Deterding, et al., 2016). In studies on hypertensive diseases of pregnancy, antiphospholipid syndrome, and PTB, researchers determined the occurrence of complement dysregulation via the discovery of elevated circulating plasma levels of complement activation fragments (e.g., C3a/Bb) in the presence of the select health complication. In the studies of recurrent fetal loss and miscarriage, researchers determined the occurrence of complement dysregulation via both elevated complement plasma levels and genetic deficiencies in complement regulatory genes and complement regulators (e.g., CD55) that resulted in increased complement activation (Mohlin et al., 2013). Complement products, such as C3a and C5a, are typically cleared quickly from the system as a result of innate regulatory mechanisms, as previously described; this process is essential given the effects of these products on inflammatory responses, such as induction of chemoattraction, vasodilation, smooth muscle contraction, histamine release, and cytokine production (Sarma & Ward, 2011). As such, the identification of elevated plasma levels of select complement activation fragments in the presence of disease likely indicates some degree of dysregulation in the system.
The Complement System in PTB: Animal Studies
Both human and animal models have demonstrated that increased levels of complement markers are associated with PTB. Gonzalez, Franzke, Yang, Romero, and Girardi (2011) found that the vaginal administration of lipopolysaccharide (LPS) in pregnant mice resulted in cervical C3b deposits, collagen degradation, increased MMP-9 activity, and PTB. Mice lacking a C5a receptor (C5aR-deficient) that were exposed to LPS did not show cervical remodeling or increased incidence of PTB, but the C5aR-positive wild-type mice did. These researchers also found that complement activation via C5a/C5aR interactions promoted macrophage infiltration and MMP activation, thereby promoting cervical ripening and PTB. The mechanism of cervical ripening is different in term deliveries, in which it results from activities of cervical fibroblasts and epithelial cells (Gonzalez et al., 2011; Regal et al., 2015). Similarly, in a later study, Gonzalez, Pedroni, and Girardi (2014) found that complement activation may play a role in myometrial contractility, as they found elevated levels of C5a in the myometrium of PTL mice administered vaginal LPS; these mice additionally exhibited increased expression of a contraction-associated protein, connexin 43.
In contrast, in a similar study investigating the rates of PTB and miscarriage among C5a receptor (C5aR1)-deficient knockout (KO) mice exposed to intraperitoneal LPS, researchers found that genetic KO of C5aR1 was associated with miscarriage and did not prevent the occurrence of PTB (Denny et al., 2015). In that study, investigators administered LPS into the intraperitoneal space, which may more accurately mimic a systemic maternal infection than the methods in the Gonzalez study, which explored the effects of vaginally administered LPS. Although the results of these two studies differ in regard to the role of the C5a receptor, they both support the theory that microbes are key factors in the etiology of PTB. The differences in study findings suggest that localized infections may work more through complement-associated changes in cervical remodeling, whereas systemic exposure to LPS is likely more complex and not entirely complement dependent.
The Complement System in PTB: Human Studies
An elevated level of C3a in the first trimester of pregnancy may be an independent predictive factor for adverse pregnancy outcomes including PTB and PPROM (Lynch et al., 2011). Specifically, Lynch et al. found that women in the upper quartile of C3a level were 3 times more likely to have an adverse outcome later in pregnancy after controlling for parity and prepregnancy Body mass index (BMI) compared to women in lower quartiles. In a more recent biomarker discovery study, Lynch, Wagner, Deterding, et al. (2016) also found that the complement factors B and H along with coagulation factors IX and IXab were the highest ranking proteins found in cases of PTB compared to term controls, suggesting that the leading pathways to PTB include the complement, immune, and clotting systems. Similarly, Lynch et al. (2008) found that women with elevated levels of the complement factor Bb in early pregnancy were nearly 4 times as likely to have PTB after controlling for known PTB-related risk factors than women whose levels were not elevated. Researchers have also identified elevated levels of complement factors C3a, C4a, C5a, and Bb in the amniotic fluid of women with PTL with microbial invasion of the amniotic cavity (Soto et al., 2009; Vaisbuch et al., 2009).
The Microbiome and Complement Activation: A Mechanistic Model for PTB
Intrauterine infection and inflammation have been identified as definitive risk factors in the etiology of PTB; however, interventions directly targeting the elimination of infection have not resulted in reducing the risk of PTB. We hypothesize that the risk may not be reduced because there may be additional pathogenic bacterial species present that stimulate inflammatory pathways, some of which may not be detectable with traditional culture-based methods, sensitive to standard therapies, or associated with overt clinical infection. The analysis of samples collected from various sites using 16S DNA sequencing could allow for the identification of a greater number of potentially virulent microbial species. Evaluation of the composition and metabolic potential of the human microbiome along with complement markers may provide a more comprehensive evaluation of the community of microorganisms present in various locations of the human body and may elucidate their relationship to the onset of inflammation and the increased risk for PTB.
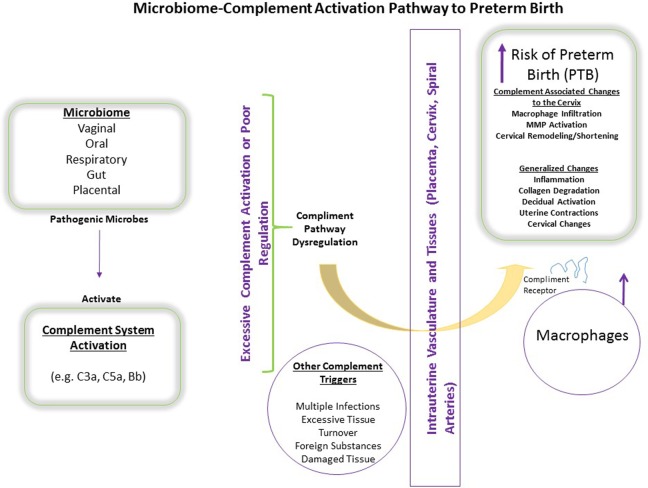
In Figure 1, we present a model of a hypothesized microbiome–complement activation pathway to PTB. We posit that pathogenic microbes from various locations (i.e., vaginal, oral, respiratory, gut, placental) influence the activation of complement pathways (i.e., C3a and Bb) in the maternal vasculature and reproductive tissues. Although complement proteins are found throughout the body without adverse effects, during infection the level of complement activation is increased to assist in the osponization (tagging) and elimination of pathogens via phagocytes (e.g., macrophages). We hypothesize that the composition of the microbiome primarily in the vagina, but elsewhere as well, influences the degree to which the complement system is activated. The resulting inflammatory response involves the production of various inflammatory mediators, which, if the response is dysregulated, may break down collagen, stimulate uterine contractions, facilitate cervical ripening, and promote decidual activation, thereby increasing the risk for PTB.
Figure 1.
Microbiome and complement dysregulation pathway to preterm birth. The composition of the microbiome may influence the activation/dysregulation of complement pathways in the maternal vasculature and reproductive tissues. We hypothesize that complement dysregulation in the intrauterine environment enhances inflammation and the production of inflammatory mediators, which in turn promote direct changes to the cervix, collagen degradation, activation of the uterine decidua, and uterine contractility, thereby increasing the risk for preterm birth.
Although regulatory mechanisms are present to prevent uncontrolled complement activation, the system may become overwhelmed depending upon the number of factors present. Complement pathway dysregulation (defined as excess activation or poor regulation) not only promotes destruction of pathogens but may also cause injury to self-tissues (Girardi et al., 2006), as evidenced by findings of deposits of complement activation products on the placenta, cervix, and decidual spiral arteries (Gonzalez et al., 2011). If complement attaches to these reproductive tissues, then macrophages can likewise attach via their complement receptors and begin to degrade the tissue, which may be another pathway to PTB (Figure 1). We propose that women who have one or more triggers for complement activation, including foreign substances, damaged tissue, and/or infection, may be prone to complement dysregulation, leading to overwhelmed regulatory systems and increasing the risk for perinatal complications via direct injury from the effects of complement dysregulation in the reproductive tissues or via effects of the resulting inflammatory response, as previously described.
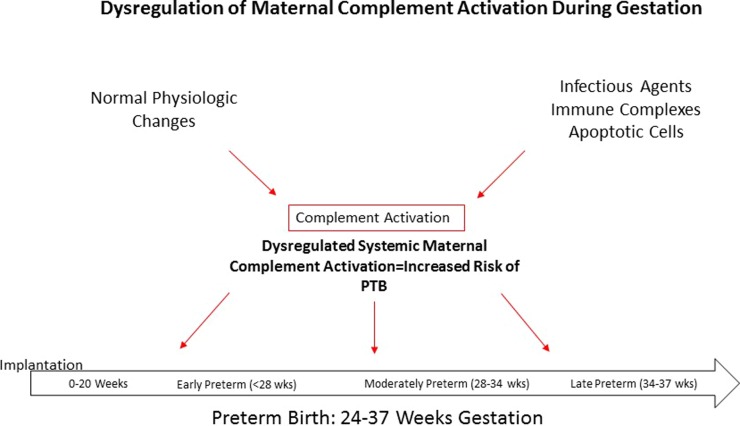
We propose that the dysregulation of maternal complement activation at any point across gestation (implantation to 37 weeks’ gestation) may increase the risk of PTB, as shown in Figure 2. During early pregnancy, there is normal physiologic activation of complement to prevent infection at the maternal–fetal interface and to promote the clearance of tissue debris resulting from implantation and placental development. This last task, clearing of tissue debris, is essential for successful trophoblast invasion. Coupled with early pregnancy infection and/or shifts in the microbiome composition, however, there may be excess activation of complement. Dysregulation of complement during placental development or early pregnancy may promote an altered inflammatory profile more favorable for the development of inflammation-related adverse pregancy outcomes such as PTB. This hypothesis is supported by studies that have shown that elevated levels of first-trimester complement factors C3a and Bb are associated with PTB and PPROM (Lynch et al., 2008, 2011). Similarly, we propose that dysregulation of maternal complement could also occur later during gestation as a result of similar factors including infection, shifts in the microbiome composition, or normal physiologic activation.
Figure 2.
Dysregulation of maternal complement activation during gestation. The dysregulation of maternal complement activation at any point across gestation (implantation to 37 weeks’ gestation) may increase the risk of preterm birth.
Conclusions
The relationships between infection and PTB and PPROM suggest a major role for both the microbiome and complement dysregulation in the biological pathway to PTB. The presence of pathogenic microbes in low abundance in the absence of clinical symptoms may promote the activation of complement and increase the risk for PTB (Ravel et al., 2011). We have presented a hypothesis, therefore, that the infection-associated pathway to PTB does not involve only the presence and activities of pathogenic microbiota; rather, it is more complex and may involve the combination of pathogenic microbes present, the ratio of pathogens to one another, the number of “protective” species present in the environment, and the individual woman’s inflammatory—and complement—response to these factors.
In summary, intrauterine infection and the activation of pro-inflammatory processes in the intrauterine environment are definitive risk factors for PTB. In addition, the immunological response to infection may be influenced by immunogenetic factors, such as gene polymorphisms or gene–environment interactions (Moura et al., 2009), which may make some exposed women more susceptible to inflammation-associated PTB. Failure to identify the most susceptible women may help to explain why the treatment of infection has not resulted in decreased PTB risk. Also, some women may be infected with previously unidentifiable microbes. Newer culture-independent PCR methods have allowed for the identification of an even greater number of microbes not typically found with culture-dependent methods via identification of the 16S rRNA gene, thereby allowing for a more detailed DNA-based evaluation of the microbiota present (Aagaard et al., 2012; Eckburg et al., 2005; Lane et al., 1985). Using these newer methods to explore routes associated with intrauterine infection, such as the vaginal, oral, gut, or even skin microbiome, in conjunction with measures of complement activation may elucidate a potentially modifiable biobehavioral pathway of inflammation-associated PTB.
Footnotes
Authors’ Note: The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contribution: A. Dunn contributed to conception and design, acquisition, analysis, and interpretation; drafted the manuscript; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. A. Dunlop contributed to design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. C. Hogue contributed to design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. A. Miller contributed to design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy. E. Corwin contributed to design, acquisition, analysis, and interpretation; critically revised the manuscript; gave final approval; and agrees to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The theoretical framework presented is currently under investigation with funds awarded by a fellowship grant to Alexis B. Dunn (1F31NR015400-01A1) from the National Institutes of Health, National Institute of Nursing Research (NINR), the American College of Nurse-Midwives Foundation Fellowship for Graduate Education Award, and the Southern Nursing Research Society Dissertation Award.
References
- Aagaard K., Ma J., Antony K. M., Ganu R., Petrosino J., Versalovic J. (2014). The placenta harbors a unique microbiome. Science Translational Medicine, 6, 237ra265 doi:10.1126/scitranslmed.3008599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aagaard K., Riehle K., Ma J., Segata N., Mistretta T. A., Coarfa C.…Versalovic J. (2012). A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One, 7, e36466 doi:10.1371/journal.pone.0036466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggarwal R., Sestak A. L., D’Sousa A., Dillon S. P., Namjou B., Scofield R. H. (2010). Complete complement deficiency in a large cohort of familial systemic lupus erythematosus. Lupus, 19, 52–57. doi:10.1177/0961203309346508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albieri A., Kipnis T., Bevilacqua E. (1999). A possible role for activated complement component 3 in phagocytic activity exhibited by the mouse trophoblast. American Journal of Reproductive Immunology, 41, 343–352. [DOI] [PubMed] [Google Scholar]
- Astafurov K., Elhawy E., Ren L., Dong C. Q., Igboin C., Hyman L.…Danias J. (2014). Oral microbiome link to neurodegeneration in glaucoma. PLoS One, 9, e104416 doi:10.1371/journal.pone.0104416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines M. G., Millar K. G., Mills P. (1974). Studies of complement levels in normal human pregnancy. Obstetrics & Gynecology, 43, 806–810. [PubMed] [Google Scholar]
- Bastek J. A., Gomez L. M., Elovitz M. A. (2011). The role of inflammation and infection in preterm birth. Clinics in Perinatology, 38, 385–406. doi:10.1016/j.clp.2011.06.003 [DOI] [PubMed] [Google Scholar]
- Boackle R. J. (1991). The interaction of salivary secretions with the human complement system—A model for the study of host defense systems on inflamed mucosal surfaces. Critical Reviews in Oral Biology & Medicine, 2, 355–367. [DOI] [PubMed] [Google Scholar]
- Boskey E. R., Cone R. A., Whaley K. J., Moench T. R. (2001). Origins of vaginal acidity: High D/L lactate ratio is consistent with bacteria being the primary source. Human Reproduction, 16, 1809–1813. [DOI] [PubMed] [Google Scholar]
- Botto M., Kirschfink M., Macor P., Pickering M. C., Wurzner R., Tedesco F. (2009). Complement in human diseases: Lessons from complement deficiencies. Molecular Immunology, 46, 2774–2783. doi:10.1016/j.molimm.2009.04.029 [DOI] [PubMed] [Google Scholar]
- Bradley J. R. (2008). TNF-mediated inflammatory disease. Journal of Pathology, 214, 149–160. doi:10.1002/path.2287 [DOI] [PubMed] [Google Scholar]
- Breen K. A., Seed P., Parmar K., Moore G. W., Stuart-Smith S. E., Hunt B. J. (2012). Complement activation in patients with isolated antiphospholipid antibodies or primary antiphospholipid syndrome. Thrombosis and Haemostasis, 107, 423–429. doi:10.1160/th11-08-0554 [DOI] [PubMed] [Google Scholar]
- Bulla R., Agostinis C., Bossi F., Rizzi L., Debeus A., Tripodo C.…Tedesco F. (2008). Decidual endothelial cells express surface-bound C1q as a molecular bridge between endovascular trophoblast and decidual endothelium. Molecular Immunology, 45, 2629–2640. doi:10.1016/j.molimm.2007.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani P. D., Osto M., Geurts L., Everard A. (2012). Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes, 3, 279–288. doi:10.4161/gmic.19625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2014). Preterm birth. Retrieved from http://www.cdc.gov/reproductivehealth/MaternalInfantHealth/PretermBirth.htm
- Centers for Disease Control and Prevention. (2016). Bacterial vaginosis—CDC fact sheet. Retrieved from http://www.cdc.gov/std/bv/stdfact-bacterial-vaginosis.htm
- Chehoud C., Rafail S., Tyldsley A. S., Seykora J. T., Lambris J. D., Grice E. A. (2013). Complement modulates the cutaneous microbiome and inflammatory milieu. Proceedings of the National Academy of Sciences of the USA, 110, 15061–15066. doi:10.1073/pnas.1307855110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H., Mazmanian S. K. (2013). Innate immune recognition of the microbiota promotes host-microbial symbiosis. Nature Immunology, 14, 668–675. doi:10.1038/ni.2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello E. K., Lauber C. L., Hamady M., Fierer N., Gordon J. I., Knight R. (2009). Bacterial community variation in human body habitats across space and time. Science, 326, 1694–1697. doi:10.1126/science.1177486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cribby S., Taylor M., Reid G. (2008). Vaginal microbiota and the use of probiotics. Interdisciplinary Perspectives on Infectious Disease, 2008, 256490 doi:10.1155/2008/256490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culhane J. F., Goldenberg R. L. (2011). Racial disparities in preterm birth. Seminars in Perinatology, 35, 234–239. doi:10.1053/j.semperi.2011.02.020 [DOI] [PubMed] [Google Scholar]
- Denney J. M., Culhane J. F. (2009). Bacterial vaginosis: A problematic infection from both a perinatal and neonatal perspective. Seminars in Fetal and Neonatal Medicine, 14, 200–203. doi:10.1016/j.siny.2009.01.008 [DOI] [PubMed] [Google Scholar]
- Denny K. J., Coulthard L. G., Mantovani S., Simmons D., Taylor S. M., Woodruff T. M. (2015). The role of C5a receptor signaling in endotoxin-induced miscarriage and preterm birth. American Journal of Reproductive Immunology, 74, 148–155. doi:10.1111/aji.12386 [DOI] [PubMed] [Google Scholar]
- Denny K. J., Woodruff T. M., Taylor S. M., Callaway L. K. (2013). Complement in pregnancy: A delicate balance. American Journal of Reproductive Immunology, 69, 3–11. doi:10.1111/aji.12000 [DOI] [PubMed] [Google Scholar]
- DiGiulio D. B. (2012). Diversity of microbes in amniotic fluid. Seminars in Fetal and Neonatal Medicine, 17, 2–11. doi:10.1016/j.siny.2011.10.001 [DOI] [PubMed] [Google Scholar]
- DiGiulio D. B., Callahan B. J., McMurdie P. J., Costello E. K., Lyell D. J., Robaczewska A.…Relman D. A. (2015). Temporal and spatial variation of the human microbiota during pregnancy. Proceedings of the National Academy of Sciences U.S.A., 112, 11060–11065. doi:10.1073/pnas.1502875112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenech M., Ramos-Sevillano E., Garcia E., Moscoso M., Yuste J. (2013). Biofilm formation avoids complement immunity and phagocytosis of Streptococcus pneumoniae . Infection and Immunity, 81, 2606–2615. doi:10.1128/iai.00491-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donders G. G., Bosmans E., Dekeersmaecker A., Vereecken A., Van Bulck B., Spitz B. (2000). Pathogenesis of abnormal vaginal bacterial flora. American Journal of Obstetrics and Gynecology, 182, 872–878. [DOI] [PubMed] [Google Scholar]
- Eckburg P. B., Bik E. M., Bernstein C. N., Purdom E., Dethlefsen L., Sargent M.…Relman D. A. (2005). Diversity of the human intestinal microbial flora. Science, 308, 1635–1638. doi:10.1126/science.1110591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulk W. P., Jarret R., Keane M., Johnson P. M., Boackle R. J. (1980). Immunological studies of human placentae: Complement components in immature and mature chorionic villi. Clinical and Experimental Immunology, 40, 299–305. [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. S., Romero R., Hillier S. L., Eschenbach D. A., Sweet R. L. (1992). A review of premature birth and subclinical infection. American Journal of Obstetrics and Gynecology, 166, 1515–1528. [DOI] [PubMed] [Google Scholar]
- Girardi G., Bulla R., Salmon J. E., Tedesco F. (2006). The complement system in the pathophysiology of pregnancy. Molecular Immunology, 43, 68–77. doi:10.1016/j.molimm.2005.06.017 [DOI] [PubMed] [Google Scholar]
- Goepfert A. R., Jeffcoat M. K., Andrews W. W., Faye-Petersen O., Cliver S. P., Goldenberg R. L., Hauth J. C. (2004). Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstetrics & Gynecology, 104, 777–783. doi:10.1097/01.AOG.0000139836.47777.6d [DOI] [PubMed] [Google Scholar]
- Goldenberg R. L., Culhane J. F., Iams J. D. (2008). Epidemiology and causes of preterm birth. Lancet, 371, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg R. L., Hauth J. C., Andrews W. W. (2000). Intrauterine infection and preterm delivery. New England Journal of Medicine, 342, 1500–1507. doi:10.1056/nejm200005183422007 [DOI] [PubMed] [Google Scholar]
- Gomez R., Ghezzi F., Romero R., Munoz H., Tolosa J. E., Rojas I. (1995). Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clinical Perinatology, 22, 281–342. [PubMed] [Google Scholar]
- Goncalves L. F., Chaiworapongsa T., Romero R. (2002). Intrauterine infection and prematurity. Mental Retardation and Developmental Disabilities Research Reviews, 8, 3–13. doi:10.1002/mrdd.10008 [DOI] [PubMed] [Google Scholar]
- Gonzalez J. M., Franzke C. W., Yang F., Romero R., Girardi G. (2011). Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. American Journal of Pathology, 179, 838–849. doi:10.1016/j.ajpath.2011.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez J. M., Pedroni S. M., Girardi G. (2014). Statins prevent cervical remodeling, myometrial contractions and preterm labor through a mechanism that involves hemoxygenase-1 and complement inhibition. Molecular Human Reproduction, 20, 579–589. doi:10.1093/molehr/gau019 [DOI] [PubMed] [Google Scholar]
- Gupta K., Stapleton A. E., Hooton T. M., Roberts P. L., Fennell C. L., Stamm W. E. (1998). Inverse association of H2O2-producing lactobacilli and vaginal Escherichia coli colonization in women with recurrent urinary tract infections. Journal of Infectious Diseases, 178, 446–450. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G., Lambris J. D. (2012). Complement and dysbiosis in periodontal disease. Immunobiology, 217, 1111–1116. doi:10.1016/j.imbio.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G., Liang S., Payne M. A., Hashim A., Jotwani R., Eskan M. A.…Curtis M. A. (2011). Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe, 10, 497–506. doi:10.1016/j.chom.2011.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G., Maekawa T., Abe T., Hajishengallis E., Lambris J. D. (2015). Complement involvement in periodontitis: Molecular mechanisms and rational therapeutic approaches. Advances in Experimental Medicine and Biology, 865, 57–74. doi:10.1007/978-3-319-18603-0_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. W., Ikegami A., Bissada N. F., Herbst M., Redline R. W., Ashmead G. G. (2006). Transmission of an uncultivated Bergeyella strain from the oral cavity to amniotic fluid in a case of preterm birth. Journal of Clinical Microbiology, 44, 1475–1483. doi:10.1128/jcm.44.4.1475-1483.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. W., Shen T., Chung P., Buhimschi I. A., Buhimschi C. S. (2009). Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. Journal of Clinical Microbiology, 47, 38–47. doi:10.1128/jcm.01206-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves D. C., Medzhitov R. (2005). Innate sensors of microbial infection. Journal of Clinical Immunology, 25, 503–510. doi:10.1007/s10875-005-8065-4 [DOI] [PubMed] [Google Scholar]
- Hill G. B. (1998). Preterm birth: Associations with genital and possibly oral microflora. Annals of Periodontology, 3, 222–232. doi:10.1902/annals.1998.3.1.222 [DOI] [PubMed] [Google Scholar]
- Hillier S. L., Witkin S. S., Krohn M. A., Watts D. H., Kiviat N. B., Eschenbach D. A. (1993). The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstetrics & Gynecology, 81, 941–948. [PubMed] [Google Scholar]
- Holmes C. H., Simpson K. L., Wainwright S. D., Tate C. G., Houlihan J. M., Sawyer I. H.…Tanner M. J. (1990). Preferential expression of the complement regulatory protein decay accelerating factor at the fetomaternal interface during human pregnancy. Journal of Immunology, 144, 3099–3105. [PubMed] [Google Scholar]
- Holst R. M., Mattsby-Baltzer I., Wennerholm U. B., Hagberg H., Jacobsson B. (2005). Interleukin-6 and interleukin-8 in cervical fluid in a population of Swedish women in preterm labor: Relationship to microbial invasion of the amniotic fluid, intra-amniotic inflammation, and preterm delivery. Acta Obstetricia et Gynecologica Scandinavica, 84, 551–557. doi:10.1111/j.0001-6349.2005.00708.x [DOI] [PubMed] [Google Scholar]
- Hsi B. L., Hunt J. S., Atkinson J. P. (1991). Differential expression of complement regulatory proteins on subpopulations of human trophoblast cells. Journal of Reproductive Immunology, 19, 209–223. [DOI] [PubMed] [Google Scholar]
- Hummelen R., Fernandes A. D., Macklaim J. M., Dickson R. J., Changalucha J., Gloor G. B., Reid G. (2010). Deep sequencing of the vaginal microbiota of women with HIV. PLoS One, 5, e12078 doi:10.1371/journal.pone.0012078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iams J. D., Goldenberg R. L., Meis P. J., Mercer B. M., Moawad A., Das A.…Roberts J. M. (1996). The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. New England Journal of Medicine, 334, 567–572. doi:10.1056/nejm199602293340904 [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Medzhitov R. (2002). Innate immune recognition. Annual Review of Immunology, 20, 197–216. doi:10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- Jeffcoat M. K., Geurs N. C., Reddy M. S., Goldenberg R. L., Hauth J. C. (2001). Current evidence regarding periodontal disease as a risk factor in preterm birth. Annals of Periodontology, 6, 183–188. doi:10.1902/annals.2001.6.1.183 [DOI] [PubMed] [Google Scholar]
- Kaewsrichan J., Peeyananjarassri K., Kongprasertkit J. (2006). Selection and identification of anaerobic lactobacilli producing inhibitory compounds against vaginal pathogens. FEMS Immunology & Medical Microbiology, 48, 75–83. doi:10.1111/j.1574-695X.2006.00124.x [DOI] [PubMed] [Google Scholar]
- Kaul A. K., Khan S., Martens M. G., Crosson J. T., Lupo V. R., Kaul R. (1999). Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infection and Immunity, 67, 5958–5966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keelan J. A., Blumenstein M., Helliwell R. J., Sato T. A., Marvin K. W., Mitchell M. D. (2003). Cytokines, prostaglandins and parturition—A review. Placenta, 24, S33–S46. [DOI] [PubMed] [Google Scholar]
- Kramer M. R., Hogue C. R. (2009). What causes racial disparities in very preterm birth? A biosocial perspective. Epidemiologic Reviews, 31, 84–98. doi:10.1093/ajerev/mxp003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer M. R., Hogue C. J., Dunlop A. L., Menon R. (2011). Preconceptional stress and racial disparities in preterm birth: An overview. Acta Obstetricia et Gynecologica Scandinavica, 90, 1307–1316. doi:10.1111/j.1600-0412.2011.01136.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane D. J., Pace B., Olsen G. J., Stahl D. A., Sogin M. L., Pace N. R. (1985). Rapid determination of 16S ribosomal RNA sequences for phylogenetic analyses. Proceedings of the National Academy of Sciences U.S.A., 82, 6955–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence T. (2009). The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harbor Perspectives in Biology, 1, a001651 doi:10.1101/cshperspect.a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. L., Cheong A. W., Chow W. N., Lee K. F., Yeung W. S. (2009). Regulation of complement-3 protein expression in human and mouse oviducts. Molecular Reproduction and Development, 76, 301–308. doi:10.1002/mrd.20955 [DOI] [PubMed] [Google Scholar]
- Liszewski M. K., Farries T. C., Lublin D. M., Rooney I. A., Atkinson J. P. (1996). Control of the complement system. Advances in Immunology, 61, 201–283. [DOI] [PubMed] [Google Scholar]
- Lu K., Knutson C. G., Wishnok J. S., Fox J. G., Tannenbaum S. R. (2012). Serum metabolomics in a Helicobacter hepaticus mouse model of inflammatory bowel disease reveal important changes in the microbiome, serum peptides, and intermediary metabolism. Journal of Proteome Research, 11, 4916–4926. doi:10.1021/pr300429x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A. M., Eckel R. H., Murphy J. R., Gibbs R. S., West N. A., Giclas P. C.…Holers V. M. (2012). Prepregnancy obesity and complement system activation in early pregnancy and the subsequent development of preeclampsia. American Journal of Obstetrics & Gynecology, 206, 428 e421–428. doi:10.1016/j.ajog.2012.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A. M., Gibbs R. S., Murphy J. R., Byers T., Neville M. C., Giclas P. C.…Holers V. M. (2008). Complement activation fragment Bb in early pregnancy and spontaneous preterm birth. American Journal of Obstetrics & Gynecology, 199, 354 e351–358. doi:10.1016/j.ajog.2008.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A. M., Gibbs R. S., Murphy J. R., Giclas P. C., Salmon J. E., Holers V. M. (2011). Early elevations of the complement activation fragment C3a and adverse pregnancy outcomes. Obstetrics & Gynecology, 117, 75–83. doi:10.1097/AOG.0b013e3181fc3afa [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A. M., Wagner B. D., Deterding R. R., Giclas P. C., Gibbs R. S., Janoff E. N.…Santoro N. F. (2016). The relationship of circulating proteins in early pregnancy with preterm birth. American Journal of Obstetrics & Gynecology, 214, 517 e511–518. doi:10.1016/j.ajog.2015.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch A. M., Wagner B. D., Giclas P. C., West N. A., Gibbs R. S., Holers V. M. (2016). The relationship of longitudinal levels of complement Bb during pregnancy with preeclampsia. American Journal of Reproductive Immunology, 75, 104–111. doi:10.1111/aji.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madinger N. E., Greenspoon J. S., Ellrodt A. G. (1989). Pneumonia during pregnancy: Has modern technology improved maternal and fetal outcome? American Journal of Obstetrics & Gynecology, 161, 657–662. [DOI] [PubMed] [Google Scholar]
- March of Dimes. (2014). Preterm by race: United States, 2010–2012 average. Retrieved from http://www.marchofdimes.org/Peristats/ViewSubtopic.aspx?reg=99&top=3&stop=62&lev=1&slev=1&obj=1
- March of Dimes. (2015). The impact of premature birth on society. Retrieved from http://www.marchofdimes.org/mission/the-economic-and-societal-costs.aspx
- March of Dimes. (2016). Premature birth report card. Retrieved from http://www.marchofdimes.org/materials/premature-birth-report-card-united-states.pdf
- Marconi C., de Andrade Ramos B. R., Peracoli J. C., Donders G. G., da Silva M. G. (2011). Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. American Journal of Reproductive Immunology, 65, 549–556. doi:10.1111/j.1600-0897.2010.00940.x [DOI] [PubMed] [Google Scholar]
- Markiewski M. M., Lambris J. D. (2007). The role of complement in inflammatory diseases from behind the scenes into the spotlight. American Journal of Pathology, 171, 715–727. doi:10.2353/ajpath.2007.070166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. L., Richardson B. A., Nyange P. M., Lavreys L., Hillier S. L., Chohan B.…Kreiss J. (1999). Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus Type 1 and sexually transmitted disease acquisition. Journal of Infectious Diseases, 180, 1863–1868. doi:10.1086/315127 [DOI] [PubMed] [Google Scholar]
- Martin J. A., Hamilton B. E., Osterman M. J., Curtin S. C., Matthews T. J. (2015). Births: Final data for 2013. National Vital Statistics Report, 64, 1–65. [PubMed] [Google Scholar]
- McGrady G. A., Sung J. F., Rowley D. L., Hogue C. J. (1992). Preterm delivery and low birth weight among first-born infants of black and white college graduates. American Journal of Epidemiology, 136, 266–276. [DOI] [PubMed] [Google Scholar]
- McMillan A., Rulisa S., Sumarah M., Macklaim J. M., Renaud J., Bisanz J. E.…Reid G. (2015). A multi-platform metabolomics approach identifies highly specific biomarkers of bacterial diversity in the vagina of pregnant and non-pregnant women. International Journal of Scientific Reports, 5, 14174 doi:10.1038/srep14174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogensen T. H. (2009). Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical Microbiology Reviews, 22, 240–273. doi:10.1128/cmr.00046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohlin F. C., Mercier E., Fremeaux-Bacchi V., Liszewski M. K., Atkinson J. P., Gris J. C., Blom A. M. (2013). Analysis of genes coding for CD46, CD55, and C4b-binding protein in patients with idiopathic, recurrent, spontaneous pregnancy loss. European Journal of Immunology, 43, 1617–1629. doi:10.1002/eji.201243196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz L. A., Simhan H. N. (2014). Rate of sonographic cervical shortening and biologic pathways of spontaneous preterm birth. American Journal of Obstetrics & Gynecology, 210, e551–e555. doi:10.1016/j.ajog.2013.12.037 [DOI] [PubMed] [Google Scholar]
- Moura E., Mattar R., de Souza E., Torloni M. R., Goncalves-Primo A., Daher S. (2009). Inflammatory cytokine gene polymorphisms and spontaneous preterm birth. Journal of Reproductive Immunology, 80, 115–121. doi:10.1016/j.jri.2008.11.007 [DOI] [PubMed] [Google Scholar]
- Munn M. B., Groome L. J., Atterbury J. L., Baker S. L., Hoff C. (1999). Pneumonia as a complication of pregnancy. Journal of Maternal-Fetal & Neonatal Medicine, 8, 151–154. doi:10.1002/(sici)1520-6661(199907/08)8:4<151:: aid-mfm2>3.0.co;2-h [DOI] [PubMed] [Google Scholar]
- Nadeau-Vallee M., Obari D., Quiniou C., Lubell W. D., Olson D. M., Girard S., Chemtob S. (2016). A critical role of interleukin-1 in preterm labor. Cytokine & Growth Factor Reviews, 28, 37–51. doi:10.1016/j.cytogfr.2015.11.001 [DOI] [PubMed] [Google Scholar]
- Nishikori K., Noma J., Hirakawa S., Amano T., Kudo T. (1993). The change of membrane complement regulatory protein in chorion of early pregnancy. Clinical Immunology and Immunopathology, 69, 167–174. [DOI] [PubMed] [Google Scholar]
- Oakley B. B., Fiedler T. L., Marrazzo J. M., Fredricks D. N. (2008). Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Applied and Environmental Microbiology, 74, 4898–4909. doi:10.1128/aem.02884-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S. (2004). Maternal periodontal infections, prematurity, and growth restriction. Clinical Obstetrics and Gynecology, 47, 808–821. [DOI] [PubMed] [Google Scholar]
- Peterson J., Garges S., Giovanni M., McInnes P., Wang L., Schloss J. A.…Guyer M. (2009). The NIH Human Microbiome Project. Genome Research, 19, 2317–2323. doi:10.1101/gr.096651.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. B., Abbot P., Rokas A. (2015). Is preterm birth a human-specific syndrome? Evolution, Medicine, and Public Health, 2015, 136–148. doi:10.1093/emph/eov010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J., Gajer P., Abdo Z., Schneider G. M., Koenig S. S., McCulle S. L.…Forney L. J. (2011). Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences U.S.A., 108, 4680–4687. doi:10.1073/pnas.1002611107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regal J. F., Gilbert J. S., Burwick R. M. (2015). The complement system and adverse pregnancy outcomes. Molecular Immunology, 67, 56–70. doi:10.1016/j.molimm.2015.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richani K., Soto E., Romero R., Espinoza J., Chaiworapongsa T., Nien J. K.…Mazor M. (2005). Normal pregnancy is characterized by systemic activation of the complement system. Journal of Maternal-Fetal & Neonatal Medicine, 17, 239–245. doi:10.1080/14767050500072722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R., Durum S., Dinarello C. A., Oyarzun E., Hobbins J. C., Mitchell M. D. (1989). Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins, 37, 13–22. [DOI] [PubMed] [Google Scholar]
- Romero R., Espinoza J., Kusanovic J. P., Gotsch F., Hassan S., Erez O.…Mazor M. (2006). The preterm parturition syndrome. British Journal of Obstetrics and Gynaecology, 113, 17–42. doi:10.1111/j.1471-0528.2006.01120.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R., Gomez R., Chaiworapongsa T., Conoscenti G., Kim J. C., Kim Y. M. (2001). The role of infection in preterm labour and delivery. Paediatric and Perinatal Epidemiology, 15, 41–56. [DOI] [PubMed] [Google Scholar]
- Romero R., Gonzalez R., Sepulveda W., Brandt F., Ramirez M., Sorokin Y.…Cotton D. B. (1992). Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: Prevalence and clinical significance. American Journal of Obstetrics & Gynecology, 167, 1086–1091. [DOI] [PubMed] [Google Scholar]
- Romero R., Gotsch F., Pineles B., Kusanovic J. P. (2007). Inflammation in pregnancy: Its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutrition Reviews, 65, S194–S202. [DOI] [PubMed] [Google Scholar]
- Romero R., Mazor M., Wu Y. K., Sirtori M., Oyarzun E., Mitchell M. D., Hobbins J. C. (1988). Infection in the pathogenesis of preterm labor. Seminars in Perinatology, 12, 262–279. [PubMed] [Google Scholar]
- Romero R., Wu Y. K., Brody D. T., Oyarzun E., Duff G. W., Durum S. K. (1989). Human decidua: A source of interleukin-1. Obstetrics & Gynecology, 73, 31–34. [PubMed] [Google Scholar]
- Sandu C., Folescu R., Pop E., Motoc A. G. (2013). Hematogenous placental infection in acute respiratory infections. Romanian Journal of Morphology and Embryology, 54, 157–161. [PubMed] [Google Scholar]
- Sarma J. V., Ward P. A. (2011). The complement system. Cell and Tissue Research, 343, 227–235. doi:10.1007/s00441-010-1034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shendure J., Ji H. (2008). Next-generation DNA sequencing. Nature Biotechnology, 26, 1135–1145. doi:10.1038/nbt1486 [DOI] [PubMed] [Google Scholar]
- Singh J., Ahmed A., Girardi G. (2011). Role of complement component C1q in the onset of preeclampsia in mice. Hypertension, 58, 716–724. doi:10.1161/hypertensionaha.111.175919 [DOI] [PubMed] [Google Scholar]
- Sjoberg A. P., Trouw L. A., Blom A. M. (2009). Complement activation and inhibition: A delicate balance. Trends in Immunology, 30, 83–90. doi:10.1016/j.it.2008.11.003 [DOI] [PubMed] [Google Scholar]
- Solt I. (2015). The human microbiome and the great obstetrical syndromes: A new frontier in maternal–fetal medicine. Best Practice & Research: Clinical Obstetrics & Gynaecology, 29, 165–175. doi:10.1016/j.bpobgyn.2014.04.024 [DOI] [PubMed] [Google Scholar]
- Soto E., Romero R., Richani K., Yoon B. H., Chaiworapongsa T., Vaisbuch E.…Kusanovic J. P. (2009). Evidence for complement activation in the amniotic fluid of women with spontaneous preterm labor and intra-amniotic infection. Journal of Maternal-Fetal & Neonatal Medicine, 22, 983–992. doi:10.3109/14767050902994747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O., Akira S. (2010). Pattern recognition receptors and inflammation. Cell, 140, 805–820. doi:10.1016/j.cell.2010.01.022 [DOI] [PubMed] [Google Scholar]
- Taylor P. R., Martinez-Pomares L., Stacey M., Lin H. H., Brown G. D., Gordon S. (2005). Macrophage receptors and immune recognition. Annual Review of Immunology, 23, 901–944. doi:10.1146/annurev.immunol.23.021704.115816 [DOI] [PubMed] [Google Scholar]
- Vaisbuch E., Romero R., Erez O., Mazaki-Tovi S., Kusanovic J. P., Soto E.…Hassan S. S. (2009). Fragment Bb in amniotic fluid: Evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. Journal of Maternal-Fetal & Neonatal Medicine, 22, 905–916. doi:10.1080/14767050902994663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali B., Cruciani F., Baldassarre M. E., Capursi T., Spisni E., Valerii M. C.…Brigidi P. (2012). Dietary supplementation with probiotics during late pregnancy: Outcome on vaginal microbiota and cytokine secretion. BMC Microbiology, 12, 236 doi:10.1186/1471-2180-12-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel I., Thorsen P., Curry A., Sandager P., Uldbjerg N. (2005). Biomarkers for the prediction of preterm delivery. Acta Obstetricia et Gynecologica Scandinavica, 84, 516–525. doi:10.1111/j.0001-6349.2005.00771.x [DOI] [PubMed] [Google Scholar]
- Walport M. J. (2001). Complement. First of two parts. New England Journal of Medicine, 344, 1058–1066. doi:10.1056/nejm200104053441406 [DOI] [PubMed] [Google Scholar]
- Weir P. E. (1981). Immunofluorescent studies of the uteroplacental arteries in normal pregnancy. British Journal of Obstetrics and Gynaecology, 88, 301–307. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld H. C., Hillier S. L., Krohn M. A., Landers D. V., Sweet R. L. (2003). Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clinical Infectious Diseases, 36, 663–668. doi:10.1086/367658 [DOI] [PubMed] [Google Scholar]
- Wren B. G. (1969). Subclinical renal infection and prematurity. Medical Journal of Australia, 2, 596–600. [DOI] [PubMed] [Google Scholar]
- Yoshiya K., Lapchak P. H., Thai T. H., Kannan L., Rani P., Dalle Lucca J. J., Tsokos G. C. (2011). Depletion of gut commensal bacteria attenuates intestinal ischemia/reperfusion injury. American Journal of Physiology—Gastrointestinal and Liver Physiology, 301, G1020–G1030. doi:10.1152/ajpgi.00239.2011 [DOI] [PubMed] [Google Scholar]
- Zhou X., Brown C. J., Abdo Z., Davis C. C., Hansmann M. A., Joyce P.…Forney L. J. (2007). Differences in the composition of vaginal microbial communities found in healthy Caucasian and Black women. International Society for Microbial Ecology, 1, 121–133. doi:10.1038/ismej.2007.12 [DOI] [PubMed] [Google Scholar]