Abstract
We developed a simple, noninvasive artificial insemination technique to study epigenetic germline inheritance in mice. This technique avoids interfering factors introduced by superovulation, surgery, in vitro culture or mating that can confound the transmission of acquired epigenetic information through the germline. Using a stress model, we demonstrate that our method is suited to test the causal involvement of the male germline in transmitting acquired information from father to offspring.
Keywords: epigenetic inheritance, artificial insemination, germline-dependence, mice, transgenerational, sperm
Introduction
Defying the dogma of classic genetic inheritance, evidence is mounting that parental experiences can have heritable effects on subsequent generations [ 1 , 2 ]. However, it remains challenging to provide causal proof for this concept and demonstrate that acquired traits can be passed to the offspring via the germline. Breeding males to generate patrilines is often used to show transmission through germ cells. However, the interaction between male and female during mating, and the paternal influence on the intrauterine environment or on postnatal maternal care can be important confounding factors [ 1 , 3 ]. A way to avoid these confounds is to employ assisted reproductive techniques (ARTs) to eliminate interactions between male and female. In vitro fertilization [ 4 ] and sperm RNA injection into fertilized oocytes [ 5 ] have thus been used to demonstrate the germline-dependence of environmentally induced effects in rodents. However, these methods are laborious and require technical expertise and setups, which limit their routine use in many labs. Further, the required in vitro culture conditions and ovarian stimulation can alter the epigenome of the developing embryo and induce long-term biological alterations [ 6 ]. Thus, the results obtained from animals derived from these methods can be difficult to interpret. When the transgenerational phenotype under study is not reproduced, it remains unclear if the effect is germline-independent or if technical confounding factors have interfered with transmission [ 4 ].
To circumvent these shortcomings, we developed a simple and minimally invasive artificial insemination protocol in mice ideally suited to study nongenetic germline inheritance. Artificial insemination is rarely used in mice, and the available protocols are labor intense and involve similar confounding factors as other ARTs, introduced by superovulation, surgery or restraint during insemination, or breeding with vasectomized males [ 7–9 ]. Here, we present an improved method for artificial insemination in mice that avoids these confounds and simplifies the procedure. Ovulation is induced naturally by exposing group-housed females to male odors. Insemination is conducted rapidly in nonrestrained females using a nonsurgical injection procedure that delivers sperm through the cervix into the uterine horns. A balloon catheter and cotton tampon mimicking the male ejaculatory response and mating plug, respectively, are used to replace the need of mating to a vasectomized male following insemination. The method is efficient and reliable, and we demonstrate that it gives rise to healthy offspring with no obvious developmental or behavioral alterations. Using an established model of paternal stress that induces metabolic changes in the offspring [ 10 ], we demonstrate that the method allows to causally test the involvement of the germline in models of transgenerational epigenetic inheritance.
Results
Sperm Collection
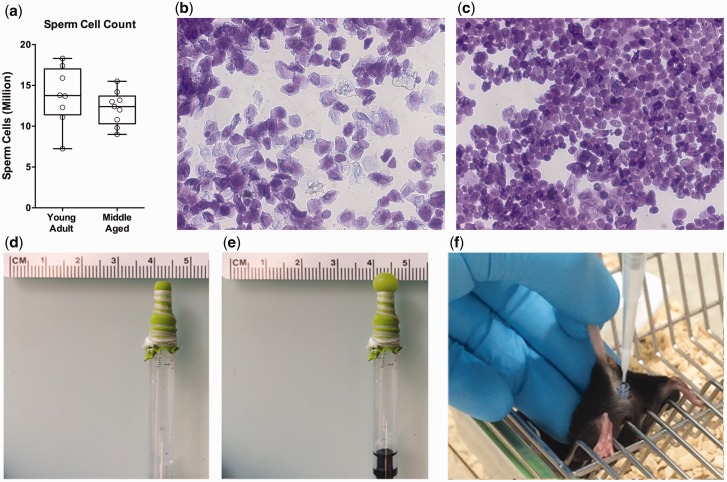
Sperm from adult male mice was collected from the cauda epididymis in 150 µl of 9.5% milk [ 8 ] and maintained at 35°C using a heating pad. On average, 13.7 ± 1.3 million sperm cells were harvested from young adult males ( n = 8), and 12.2 ± 0.7 million cells from middle-aged (12 months) males ( n = 9) ( Fig. 1 a). This high cellular yield allows artificial insemination of multiple females (up to 6) with the sperm of one male, and males of a broad age-range can be used for the generation of offspring.
Figure 1.
Sperm collection and natural induction of ovulation. Large amounts of sperm cells can be harvested from young adult ( n = 8) and middle-aged ( n = 9) males ( a ), sufficient to inseminate up to six females with the sperm of one male. Seventy-two hours after adding dirty male bedding to group-housed females, most females (10/12) had vaginal cytology characterized by cornified epithelial cells typical of estrous ( b ), whereas only a single female had round nucleated and cornified cells ( c ), indicative of late proestrus. A simple balloon catheter ( d ), which can be inflated ( e ), was used to induce pseudopregnancy following noninvasive artificial insemination (AI) ( f )
Induction of Ovulation
In a pilot experiment, we established artificial insemination following hormonal induction of ovulation. Mice maintained on a reverse 12/12 h light-dark cycle (light on at 08:30) received 5U pregnant mare serum gonadotropin (PMSG) (G4877, Sigma) by i.p. injection at 21:00 (light phase) followed by 5U human chorionic gonadotropin (HCG) (CG10-1VL, Sigma) 48 h later. The next morning, sperm was collected at 9:00 and females were inseminated between 10:00 and 11:00 (dark phase). This gave rise to offspring in 3 out of 8 inseminated females ( = 37.5% success rate). Litter size ranged from 8 to 12 pups, with a total of 17 males and 12 females.
To avoid undesired epigenetic confounds induced by superovulation [ 6 ], we established a protocol for natural induction of ovulation. We took advantage of the fact that group-housed female mice suppress each other’ estrous cycle [ 11 ]. Synchronous estrous can be induced in such acyclic females through exposure to male pheromones present in male urine [ 12 , 13 ]. For this, we added dirty male bedding to a cage of virgin females group-housed (4 per cage) since weaning and not previously exposed to any male odor. In a pilot study, we collected vaginal smears to assess the stage of estrous cycle 72 h after exposure to dirty male bedding. Vaginal cytology showed that 10 out of 12 females were in estrus 3 days after adding male bedding, as indicated by vaginal smears dominated by cornified epithelial cells typical of estrous in mice ( Fig. 1 b) [ 14 ]. For one mouse, insufficient cells were collected to determine the cycle stage, and one mouse was in late proestrus/early estrus (high number of round, well-formed nucleated cells as well as cornified cells, Fig. 1 c). These results indicate that timed estrous can be naturally induced using exposure of group-housed females to male bedding.
Artificial Insemination
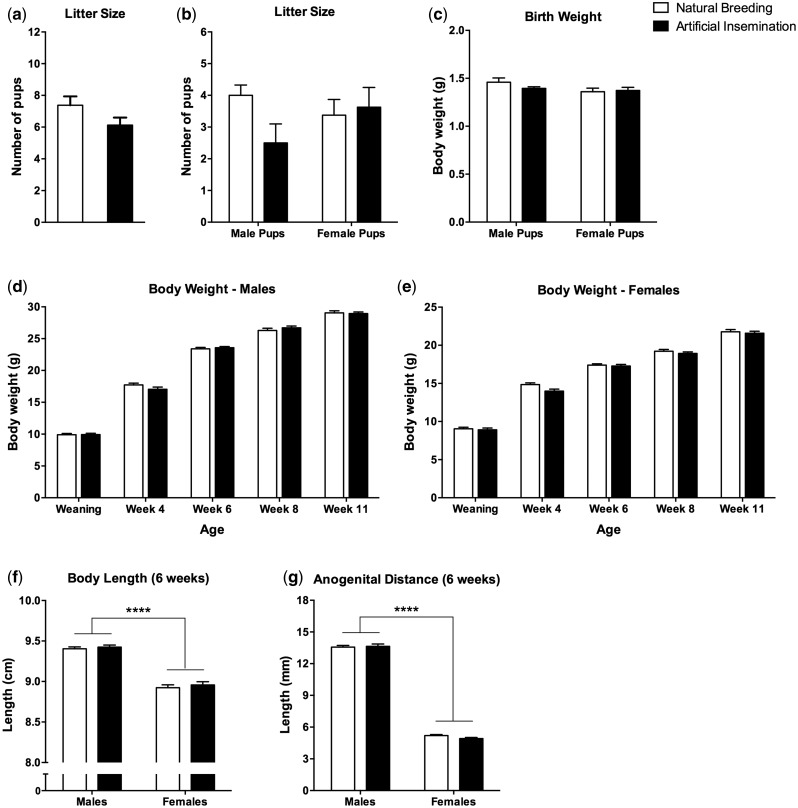
We then tested whether artificial insemination can be successful following natural induction of ovulation, and whether the resulting offspring is physiologically and behaviorally comparable to naturally conceived offspring. We focused our analyses on features known to be sensitive to paternal environmental exposures [ 1 ]. After natural induction of ovulation, females were inseminated (3–4 females with the sperm of a single male) and treated with a balloon catheter ( Fig. 1 d and e) and cotton tampon (to substitute for mating with vasectomized males) as described in the Methods section. Artificial insemination was carried out without restraint using a rapid, noninvasive technique ( Fig. 1 f, see Methods for details). Of 22 inseminated female mice, eight had litters (36.4% success rate). This was repeated in a separate batch of animals with similar success rate (5/15 females successfully inseminated). In parallel, 19 females were naturally mated by pairing with males for 24 h after natural induction of ovulation. Of these, eight had litters (42% success rate). Litter size after artificial insemination or natural breeding was similar ( Fig. 2 a), with a slight but not statistically significant reduction in number of male pups after artificial insemination [ F (1,26) = 2.78, P = 0.11; Fig. 2 b]. Each litter was culled to 6–8 pups on the first postnatal day (PND1) to equalize the number of pups per litter, and body weight was regularly measured until early adulthood. Birth weight was similar between groups in males and females ( Fig. 2 c), and there was neither difference in body weight during development and adulthood ( Fig. 2 d and e), nor in body size or anogenital distance ( Fig. 2 f and g), parameters known to be sensitive to paternal environmental challenges [ 10 , 15–17 ].
Figure 2.
Artificial insemination results in healthy offspring which develops normally. Litter size ( a, b ) and birth weight ( c ) of pups is similar after AI ( n = 8 dams) and natural breeding ( n = 8 dams). There is no difference between groups in body weight ( d, e ), body length ( f ) and AD ( g ) during development or in adulthood ( n = 20–31 mice per sex per group). All values are mean ± SEM. ANOVAs were used for all analyses. **** P < 0.0001
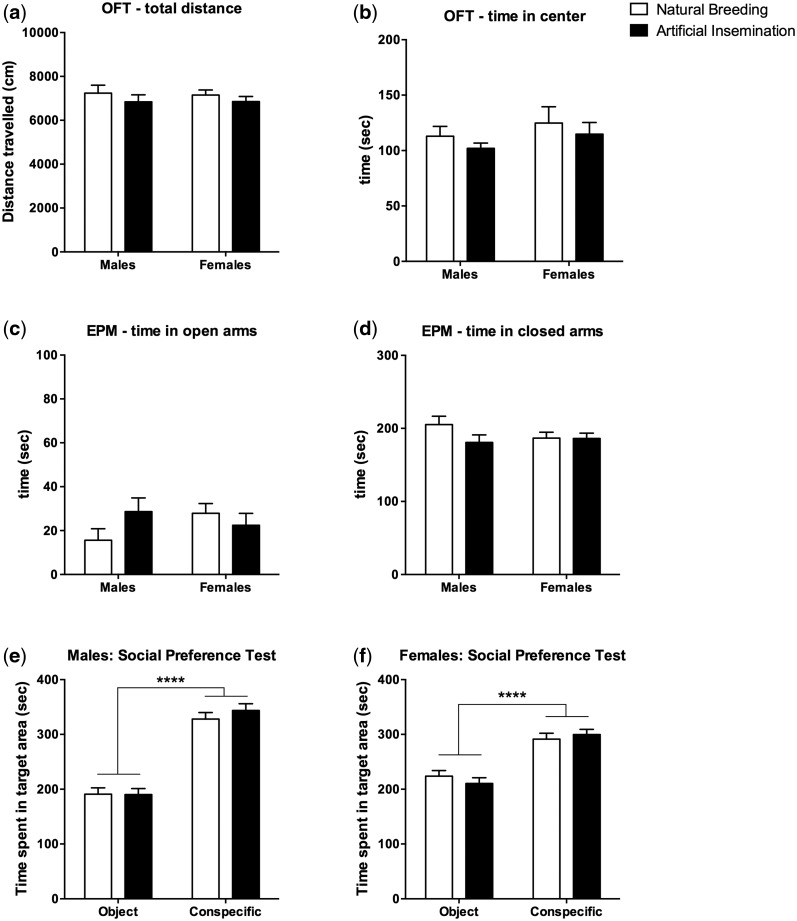
We also tested the behavior of the offspring resulting from artificial insemination or natural breeding. Since anxiety and social behaviors are known to be altered transgenerationally by adverse environmental conditions [ 4 , 5 , 18 , 19 ], we first tested the animals on an open field test (OFT) and an elevated plus maze (EPM), which assess behavioral responses to adversity. On OFT, mice from both groups had similar overall activity ( Fig. 3 a) and spent a similar amount of time in the aversive center of the open field ( Fig. 3 b). On EPM, mice from both groups had a strong preference for the closed arms, and spent a similar amount of time on the more aversive open arms ( Fig. 3 c and d). We then tested social behaviors using a three-chamber social preference task. Mice from both groups preferred a conspecific partner over a novel object, and exploration of the conspecific/object was similar between groups ( Fig. 3 e and f). Overall, these results suggest that animals generated by artificial insemination are healthy, develop normally, have normal general activity in an open field, and behave normally in novel/aversive environments and in social contexts.
Figure 3.
Offspring generated by AI is behaviorally normal. Offspring of both groups show similar overall activity ( a ) and anxiety level ( b ) on OFT. Both groups prefer the protected closed arms on an EPM over the exposed open arms, with no difference between groups ( c, d ). Offspring of both groups similarly prefer a social conspecific over an innate object in the three-chamber social preference test ( e, f ). For behavior tests, 10–13 mice per sex per group were used. All values are mean ± SEM. ANOVAs were used for all analyses. **** P < 0.0001
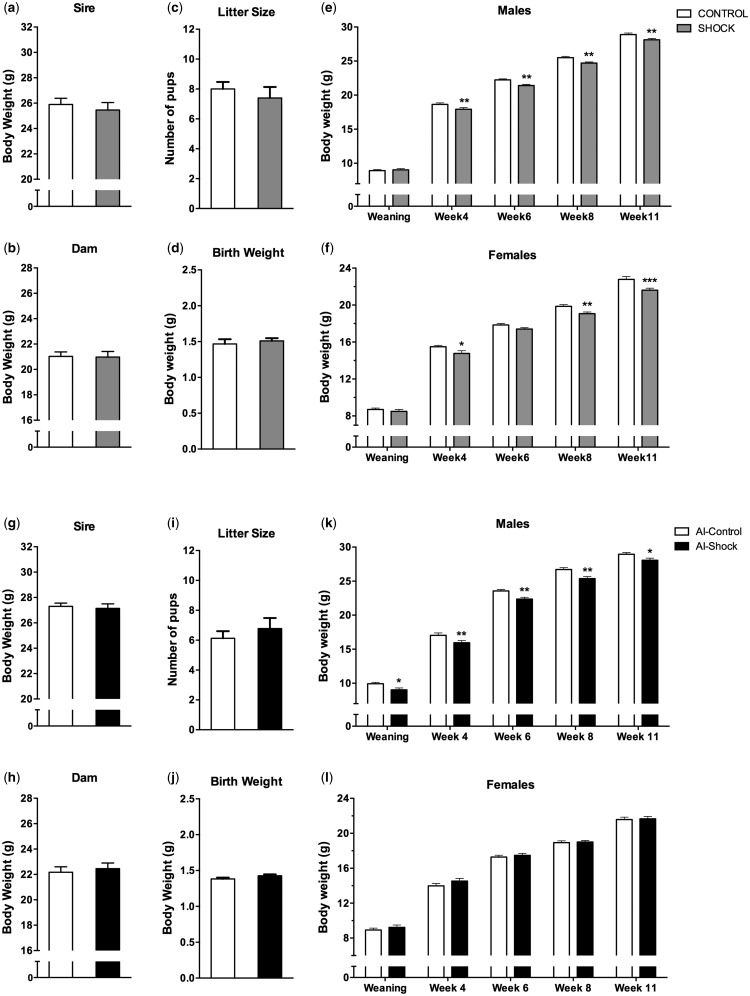
We next tested the potential of our method for probing and confirming the germline-dependence of an environmentally induced phenotype. We used a mouse model of adult stress in which exposure of an adult male to a single stressful event a few weeks before breeding, alters the offspring’s body weight [ 10 ]. We first established this model in our lab by exposing 8-week-old male mice to a foot-shock, and after 2 weeks of recovery, pairing each male with a naïve, age-matched female for 7 days ( n = 10/group). Breeding pairs were then separated and the resulting offspring was weighed 1 day after birth, at weaning, and every 2 weeks until adulthood. Body weight of sires or dams was similar between groups ( Fig. 4 a and b) and there was no difference in litter size and body weight at birth ( Fig. 4 c and d). However, body weight was significantly lower in both males and females after weaning, and remained lower until adulthood [males: F (1,77) = 7.22, P = 0.009, females: F (1,65) = 9.34, P = 0.003, Fig. 4 e and f)]. To test whether this effect is transmitted through the germline, independent of any interaction between male and female during mating, we repeated the stress paradigm in an independent group of males but generated offspring using artificial insemination with the sperm of stress-exposed or control fathers, instead of natural breeding. Body weight of sires or dams before breeding was similar between groups ( Fig. 4 g and h), and dams of both groups gave birth to litters of similar size and body weight ( Fig. 4 i and j). However, the male offspring generated with the sperm of stress-exposed males had a significantly lower body weight than the offspring generated with the sperm of control males at weaning and when adult [ F (1,42) = 10.01, P = 0.003, Fig. 4 k]. The female offspring had no difference in body weight [ F (1,56) = 0.64, P = 0.43, Fig. 4 l]. These results demonstrate that sperm from stressed males is sufficient to reproduce a reduced body weight in the male offspring, which confirms that the effect of stress exposure is transmitted to the offspring by the germline. At the same time, it suggests that in the female offspring, transmission of the effect is germline-independent, suggesting the involvement of in utero effects or maternal care. Therefore, artificial insemination is an efficient method to causally test germline-dependence of environmentally-induced heritable traits.
Figure 4.
Inheritance of stress-induced alterations depends on the germline. Before mating, body weight of stress-exposed and control sires ( a ) or of dams ( b ) was similar (control: n = 10; stress: n = 10). After natural mating, offspring litter size ( c ) and birth weight ( d ) are similar in mice sired by stress-exposed or control males. After weaning, male ( e ) and female ( f ) offspring of stress-exposed males gain significantly less weight than controls ( n = 32–41/group). Repeating the experiment with AI instead of natural breeding, body weight of parents was again similar ( g, h ; control: n = 8; stress: n = 9). Dams deliver similar size litters ( i ) with no difference in birth weight between groups ( j ). Male offspring of stress-exposed fathers gain less weight than controls ( k ) but females show normal weight gain ( l , n = 20–31 per sex per group). * P < 0.5, ** P < 0.01 following Fisher’s post hoc test after ANOVAs
Discussion
This study presents an improved method for artificial insemination in mice that can be easily used without prior expertise in reproductive biology and without requiring any specialized equipment. Methods for artificial insemination in the mouse are available but involve either surgical procedures under anesthesia [ 8 , 9 , 20 ] or highly stressful restraint in awake mice [ 7 ]. Our method builds on previously reported noninvasive technique for embryo transfer and artificial insemination [ 21 , 22 ] but provides several major improvements to these techniques. It replaces hormone-induced superovulation [ 7 , 21 ] by natural induction of ovulation [ 12 ], eliminates the need for mating with vasectomized males by using a balloon catheter and cotton tampon [ 7 , 23 ], and removes the need for expensive commercial tools [ 21 ]. Natural induction of ovulation circumvents epigenetic confounds associated with superovulation [ 6 , 24 , 25 ], and limits unnecessary stress of the animals due to repeated i.p. injections known to activate stress-pathways [ 26 ]. Replacing the need for vasectomized males by using a catheter and cotton tampon effectively reduces the number of mice needed and animal suffering induced by surgical vasectomy, fully in line with the 3Rs guidelines to replace, reduce, and refine the number of animals in research [ 27 , 28 ]. Additionally, the new method greatly simplifies experimental design because (i) the insemination procedure can be performed by a trained experimenter in <2 min, (ii) no vasectomy and recovery time are needed for males, and no extra days are required for mating and plug-checking, which considerably saves time, and (iii) the natural induction of ovulation avoids strenuous timing of hormone injections which are usually performed both early in the morning and late at night [ 29 ]. Hence, for ethical, experimental and financial considerations, the newly designed method for artificial insemination is superior to previously established methods in the mouse.
An important concern with ARTs is whether the method itself induces physiological or behavioral alterations in the offspring [ 24 , 30 , 31 ]. Recent data have raised the possibility that ARTs in human are associated with neurodevelopmental and neuropsychiatric disease risk [ 30 , 32 , 33 ]. Although the concern is more relevant for hormone treatment and in vitro conditions involved in vitro fertilization [ 6 ], it is particularly important in the context of epigenetic inheritance to ensure that artificial insemination does not induce confounding factors that impact the offspring’s development. To our knowledge, this study represents the first systematic assessment of neurobehavioral function in the offspring generated by artificial insemination in any species. Our data indicate no gross physiological or behavioral difference between offspring generated by artificial insemination or natural conception. Importantly, epigenetic alterations associated with superovulation and in vitro culture conditions, which are normally associated with ARTs, are circumvented by our method. This reduces the likelihood to introduce epigenetic confounds in studies of nongenetic transgenerational inheritance [ 1 , 6 ]. However, future studies should systematically assess the epigenetic consequences of ARTs, e.g. by profiling DNA methylation [ 34 ], to develop a better understanding of the epigenome-wide effects potentially associated with the different techniques.
A key challenge in studies of transgenerational epigenetic inheritance remains to provide a causal proof of germline-dependence, particularly to rule out alternative routes of transmission such as the interaction between male and female during mating [ 1 , 3 , 35 ]. We show that artificial insemination can be used to provide causal proof for germline-dependence. We reproduce previous findings that exposure to brief traumatic stress prior to mating can reduce body weight in the offspring when adult [ 10 ]. Using artificial insemination, we demonstrate that this effect is passed to the male but not female offspring through sperm, allowing for a dissociation of germline-dependent and germline-independent mechanisms in a sex-dependent manner. Complex differences between sexes have often been reported in studies of nongenetic transgenerational inheritance [ 36–40 ], although the cause for these differences is unclear. As the effects of acute stress exposure on germ cells remain poorly understood, the mechanisms involved in the observed transmission are also unknown. However, it has been shown that several days of injections with the glucocorticoid receptor agonist dexamethasone can induce global changes in DNA methylation in sperm cells [ 41 ], offering one potential route via which germ cells may be sensitive to the effects of stressful experiences. Chronic exposure to stressful conditions, such as paternal or maternal exposure to traumatic stress in early postnatal life, induces profound transgenerational effects in mice, affecting physiology and behavior across generations [ 42–45 ]. These effects are associated with alterations in DNA methylation in sperm [ 36 , 42 , 44 ], and recent work has identified sperm RNAs as causal vectors of transmission of stress-induced changes to the offspring [ 5 ]. Paternal exposure to early life trauma alters emotional behaviors and metabolic functions, in a way that can be recapitulated in the offspring by injection of sperm RNAs into fertilized oocytes. The inherited metabolic alterations include hypermetabolism, characterized by a reduction in body weight despite an increase in caloric intake [ 5 ]. Therefore, future work will need to assess whether the observed decrease in body weight in the current model is associated with changes in overall food-intake or energy expenditure [ 46 ], and whether sperm RNAs are involved in transmission.
It is generally thought that early life is a particularly sensitive period during which gametes may be more vulnerable to nongenetic (or epigenetic) changes induced by environmental factors [ 47 ]. However, recent evidence also shows that chronic stress in adolescence and adulthood can have heritable consequences [ 4 , 48 ], and the present results suggest that germ cells are even sensitive to brief environmental impacts in adulthood. Similar to our data, it was previously shown that a single exposure of sires to 24-h fasting several weeks before mating reduces offspring body weight and markedly disrupts glucose metabolism [ 49 ]. This raises several questions concerning the sensitivity of germ cells to environmental factors and the consequences for the offspring. First, the continuously renewing germ cell population is tightly protected by the blood-testis barrier and the blood-epididymis barrier in the adult [ 50 ], and the mechanisms that allow environmental factors to penetrate these barriers remain unknown. Promising candidate mechanisms include exosome signaling in the epididymis, Sertoli-cell mediated effects, or direct action of systemic signals through various receptors expressed on sperm cells [ 51–53 ] (see [ 1 ] for review). Second, it remains to be determined at which stage of spermatogenesis the traumatic experience can impact sperm cells. We chose a 2-week delay between stress exposure and mating, the time required for mature spermatogonia released into the lumen of the seminiferous tubes to travel through the epididymis and reach the cauda epididymis [ 54 , 55 ]. This suggests mature sperm cells stored in the cauda or spermatozoa traveling along the epididymis can be impacted by the stressful event. Previous work has demonstrated an effect of traumatic stress on offspring body weight after a 6-week delay between stress and mating but the effect was only observed in female offspring [ 10 ]. Notably, we show here that the effect on female offspring seems to be transmitted through a germline-independent mechanism, but through unknown mechanisms. Thus, it will be interesting to determine whether the germline-dependent effect on body weight of male offspring is transmitted also after a longer delay between stress exposure and mating, which would suggest that systemic, longer-lasting changes have occurred that also impact sperm cells that were at earlier developmental stages during paternal stress exposure. Finally, it will be necessary to determine whether the sensitivity of spermatogonia to stress depends on the modality of stress, or whether different stress paradigms can trigger similar heritable effects. If stress hormones or inflammatory signals are associated with the induction of epigenetic changes in germ cells, then different stressors and environmental factors could exert similar effects [ 56 , 57 ].
In summary, this study establishes a simple and rapid method of artificial insemination that gives rise to healthy offspring and can be applied to studies of epigenetic inheritance in mice. Reports of inter- and transgenerational effects induced by environmental factors have rapidly accumulated over the last couple of years and rigorous standards in study design are necessary to determine which of these effects are germline-dependent [ 1 ]. We believe that routine use of artificial insemination will help resolve this question by excluding germline-independent factors that currently limit the interpretation of many studies.
Methods
Mice
Male and female C57Bl/6J mice were purchased from Janvier (France) and used to set up an in-house breeding colony. For all experiments, we used adult males aged 2.5–5 months (unless otherwise stated), and virgin females aged 2.5–4 months. Artificial insemination in females younger than 2.5 months is more difficult, as the insertion of the larger speculum is problematic due to their smaller body size. However, if experimentally necessary, a smaller speculum can be used for insemination of younger mice (of reproductive age). All males and females were sexually naïve before natural breeding or sperm collection/artificial insemination. All procedures were carried out in accordance to Swiss cantonal regulations for animal experimentation under license numbers 55/2012 and 57/2015.
Sperm Isolation
A 9.5% milk solution was boiled for 10 min. After cooling to room temperature, 150 μl of boiled milk were pipetted into a single well of a 12-well cell culture dish and maintained at 35°C using a heating pad. After euthanasia (by cervical dislocation followed by decapitation), an incision was made in the abdomen of a male and testes were exposed by pulling the scrotal fat. Cauda epididymis was dissected from each testicle and transferred to the well containing the milk solution. Using 22G injection needles, several cuts were made in the cauda to release sperm cells into the solution. After at least 10 min of incubation, the leftover tissue was carefully removed from the well. Using a 1 ml pipette, the sperm solution was gently mixed by pipetting. This solution can be used immediately for insemination or kept for at least 1 h.
To determine how many sperm cells can be obtained per male, 10 μl of the sperm solution collected from individual adult males was diluted 1:100 in ddH 2 O before counting cells in a hemacytometer under ×20 magnification. We routinely use 2–3 million sperm cells in 25 μl of sperm solution to inseminate a single female, thus up to six females can be inseminated with the sperm of one male (collected in 150 μl solution).
Insemination
After sperm collection, 25 μl of the sperm solution are loaded into a western blot loading tip (Biorad, #223-9915) attached to a 200 μl pipette. A small cotton tampon is prepared, moistened in 0.9% saline solution and compressed between thumb and forefinger to reduce its size (10 mm length, 4 mm width). A female mouse is placed on an angled wire cage top and slightly pulled up by the tail with thumb and index finger, while the body of the female is gently pushed onto the wire grid using middle- and ring finger, as described previously [ 22 ]. Two different sizes of Teflon tubing are used as speculums and cut to different length (small speculum: 13 mm; large speculum: 10 mm). First, the small tubing/speculum (inner diameter, 3.28–3.58 mm, wall 0.38 mm, e.g. Angst+Pfister AG, Switzerland, Product number 0110350008) is gently inserted into the vaginal opening until slight resistance is felt (cervix). The speculum is removed and the larger speculum fully inserted [Teflon tubing (inner diameter, 2.06–2.31 mm, wall 0.38 mm), e.g. Angst+Pfister AG, Switzerland, Product number 0110350012]. Once in place, the plastic pipette containing the sperm solution is guided through the speculum, and when the cervical opening is found after gentle probing, the pipette tip is inserted for a total of 2 cm ( Fig. 1 f). The sperm solution is released and the pipette withdrawn. A small lamp (e.g. surgery light) can be used to visually locate the cervix through the speculum, although with little experience, this can easily be done without visual aid. Inseminations were routinely performed under dim red light during the active phase of the mice. It is noteworthy that females, also when never handled before and behaviorally naïve, do not show any signs of distress during this procedure and hold very still, particularly once the larger speculum has been inserted.
Balloon Catheter and Cotton Tampon to Mimic Copulation
We used a balloon catheter that can be inserted into the vagina and when inflated, applies gentle pressure on the vaginal walls similar to the male mouse penis during the ejaculatory response, stimulating pseudopregnancy as described previously [ 58 ]. It can be simply and inexpensively built in any lab, only with the use of a rubber balloon, a 1 ml syringe, silicone paste and dental floss [ 58 ], and is shown deflated in Fig. 1 d. Briefly, a sharp object is used (e.g. pliers) to carve a thin ridge into the plastic tip of a 1 ml plastic syringe (2–3 mm from the tip of the syringe). The syringe is filled with 500 μl glycerol (water can also be used but reduces the lifespan of the catheter) and air bubbles are removed. The rubber is stretched over the tip of the syringe and tied to the syringe using dental floss, so that the thread lies inside the carved-out ridge. Before use, the syringe is marked to indicate how far the plunger has to be depressed to inflate the rubber balloon to a diameter of 7–8 mm ( Fig. 1 e). After insemination, the speculum is removed, the balloon catheter is immediately inserted and gently inflated to the marked position. It is held in place for 10 s then removed. The moistened tampon is carefully inserted into the vagina using a wooden applicator stick or a blunt metal probe to mimic the mating plug that normally forms after copulation. To avoid injury, the cotton tampon was inserted with several gentle and brief pushes. Typically, mice remove the tampon within a few hours after insemination. The day after insemination, all mice should be inspected briefly to ensure the tampon was removed. In rare instances, the tampon is still inserted 24 h after insemination, in this case it can be gently removed using blunt-tip forceps.
Gestation
Pairs of gestating females were maintained in individual cages in a quiet facility. Since cannibalism is common in C57BL/6 dams, exposure of females to any noise or stress was avoided (disturbance only by cage change once a week). Single housing is not recommended since social isolation during gestation increases cannibalism.
Natural Induction of Ovulation and Vaginal Cytology
To induce ovulation, females were exposed to male odor by adding dirty bedding from males in a cage of group-housed females (4 per cage) at 14:00 on Friday, then females were inseminated on Monday between 10:00 and 13:00. Vaginal cytology was determined using a 100 μl pipette to gently flush the opening of the vaginal canal several times with autoclaved ddH 2 O, and collecting the liquid on a glass slide. After staining with 1% cresyl violet (Sigma-Aldrich, Product # C5042), vaginal cytology was assessed under a light microscope at 20-fold magnification. Vaginal cytology was determined as described previously [ 14 , 59 ].
Body Size and Anogenital Distance
Body size and anogenital distance (AD) were measured in 6-week-old mice under light isofluorane anesthesia. Body size was measured with a ruler, anogenital distance was measured using a caliper [ 60 ].
Open Field Test
The open field apparatus (70 × 70 × 30 cm) consists of gray Polyvinyl chloride (PVC) walls and a white Plexiglas floor. The room was dimly lit (around 1 lux in the center of the field) and the arena was lit from below with infrared light invisible to the mice. Each mouse was placed in a corner of the arena facing the wall at the start of the test. Using an infrared camera, the session was video-recorded and the animal automatically tracked (Viewpoint, France). The movement of the mouse and duration in the center square (25% area of the arena) were recorded [ 36 ].
Elevated Plus Maze
The maze consists of a platform with two open (no walls) and two closed (protected by walls) arms (dark gray PVC, 30 × 5 cm), elevated 60 cm above the floor [ 36 ]. Testing was performed in a dimly lit room with an opaque 25 W light bulb mounted directly above the maze (around 30 lux on the open and closed arms). Each mouse was placed in the center of the maze facing a closed arm and allowed to explore the maze for 5 min while being recorded via video camera. Total distance moved and time spent in open/closed arms were automatically recorded by a videotracking system (Viewpoint, France).
Three-Chamber Social Test
The testing apparatus was placed in a dimly lit room (light in arena ∼2 lux) and consisted of clear Plexiglas walls and a white Plexiglas floor. The floor was lit from below by infrared light invisible to the mice for animal tracking. The arena consists of two square partitions (30 × 30 × 30 cm) each connected to a center area (15 × 30 × 30 cm) through a 10 cm wide opening. A round Plexiglas container (10 cm diameter) is placed in the center of the two square areas to hold the target mouse or the innate object. Small airholes (10 mm diameter each) in the container allowed for olfactory and physical contact. Round metal weights were placed on top of cylinders to prevent movement. Same-sex target mice were habituated to the Plexiglas containers for 20 min to arena 1 day before testing. On test day, each mouse was habituated for 10 min to the arena including empty cylinders. After 10 min, the mouse was carefully led to the center and the entrance to both left/right compartments were blocked using gray PVC plastic dividers. A target mouse was then placed in one cylinder, and a round unfamiliar object inside the other cylinder. The dividers were removed and the mouse was allowed to explore the arena including target mouse and innate object for another 10 min. Time spent on each side of the arena was recorded using automated infrared video tracking software (Viewpoint, France).
Effect of Paternal Stress on Offspring Body Weight
Male mice, 7 weeks of age, were singly housed for 1 week. At 8 weeks of age, half of the mice were exposed to a single strong foot-shock (1.5 mA for 2 s) in a fear conditioning chamber (TSE, Germany). Simultaneously, control mice were placed into an identical chamber without receiving any foot-shock. After exposure, males were kept in single-housing for 2 weeks. Then, each male mouse was weighed and paired with a naïve, age-matched female for 7 days. After this time, males were separated from females and pregnant dams were left undisturbed during gestation (except for weekly cage changes). The resulting pups were weighed immediately after birth and at weaning on PND21. Weaned mice were ear clipped to allow tracking across age, and weight was measured every 2 weeks thereafter. The experiment was repeated in an independent cohort of mice with artificial insemination instead of natural breeding. Three days before artificial insemination, dirty bedding from male cages was added to the female cages and 3 days later (14 days after males had received the foot-shock or control treatment), male sperm was harvested and females were inseminated as described earlier. The females therefore never interacted with the males whose sperm was used for insemination.
Study Design and Statistical Analyses
Sample size was chosen based on our previous work and on literature [ 5 , 10 ]. Male mice were pseudorandomly assigned to stress or control groups. From cages of 4–5 group housed, males were randomly picked and assigned alternatingly to either control or stress group. Experimenters were blind to group assignment during all measurements. Variance was not significantly different between groups. Unpaired t-tests were used to compare two groups. Appropriate ANOVAs were used to compare four groups, significant main effects or interactions were followed up by Fisher’s posthoc test. A detailed list of all statistical tests used for each measure and statistical results are shown in Supplementary Table S1 .
Supplementary Material
Supplementary Data
Acknowledgements
The Mansuy laboratory is supported primarily by the University of Zürich, ETH Zürich and the Swiss National Science Foundation. We thank Roche for supporting JB. The authors thank Pawel Pelczar and Sarah Steinbacher for important suggestions and Yvonne Zipfel for animal care.
Supplementary data
Supplementary data are available at EnvEpig online.
Conflict of interest : None declared.
References
- 1. Bohacek J, Mansuy IM . Molecular insights into transgenerational non-genetic inheritance of acquired behaviours . Nat Rev Genet 2015. ; 16 : 641 – 52 . [DOI] [PubMed] [Google Scholar]
- 2. Nilsson EE, Skinner MK . Environmentally induced epigenetic transgenerational inheritance of disease susceptibility . Transl Res 2015. ; 165 : 12 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Curley JP, Mashoodh R, Champagne FA . Epigenetics and the origins of paternal effects . Horm Behav 2011. ; 59 : 306 – 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dietz DM, Laplant Q, Watts EL, et al. . Paternal transmission of stress-induced pathologies . Biol Psychiatry 2011. ; 70 : 408 – 14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gapp K, Jawaid A, Sarkies P, et al. . Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice . Nat Neurosci 2014. ; 17 : 667 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denomme MM, Mann MRW . Genomic imprints as a model for the analysis of epigenetic stability during assisted reproductive technologies . Reproduction 2012. ; 144 : 393 – 409 . [DOI] [PubMed] [Google Scholar]
- 7. Leckie PA, Watson JG, Chaykin S . An improved method for the artificial insemination of the mouse (Mus musculus) . Biol Reprod 1973. ; 9 : 420 – 5 . [DOI] [PubMed] [Google Scholar]
- 8. Wolfe HG . Artificial insemination of the laboratory mouse (Mus musculus) . Lab Anim Care 1967. ; 17 : 426 – 32 . [PubMed] [Google Scholar]
- 9. Snell GD, Hummel KP, Abelmann WH . A technique for the artificial insemination of mice . Anat Rec 1944. ; 90 : 243 – 53 . [Google Scholar]
- 10. Hoyer C, Richter H, Brandwein C, et al. . Preconceptional paternal exposure to a single traumatic event affects postnatal growth of female but not male offspring . Neuroreport 2013. ; 24 : 856 – 60 . [DOI] [PubMed] [Google Scholar]
- 11. Whitten WK . Occurrence of anoestrus in mice caged in groups . J Endocrinol 1959. ; 18 : 102 – 7 . [DOI] [PubMed] [Google Scholar]
- 12. Marsden HM, Bronson FH . Estrous synchrony in mice: alteration by exposure to male urine . Science 1964. ; 144 : 1469 . [DOI] [PubMed] [Google Scholar]
- 13. Whitten WK, Bronson FH, Greenstein JA . Estrus-inducing pheromone of male mice: transport by movement of air . Science 1968. ; 161 : 584 – 5 . [DOI] [PubMed] [Google Scholar]
- 14. McLean AC, Valenzuela N, Fai S, et al. . Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification . J Vis Exp 2012. ;( 67 ): e4389 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dunn GA, Bale TL . Maternal high-fat diet effects on third-generation female body size via the paternal lineage . Endocrinology 2011. ; 152 : 2228 – 36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fullston T, Ohlsson Teague E, Palmer N, et al. . Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content . FASEB J 2013. ; 27 : 4226 – 43 . [DOI] [PubMed] [Google Scholar]
- 17. Lephart ED, Fleming DE, Rhees RW . Fetal male masculinization in control and prenatally stressed rats . Dev Psychobiol 1989. ; 22 : 707 – 16 . [DOI] [PubMed] [Google Scholar]
- 18. Saavedra-Rodriguez L, Feig LA . Chronic social instability induces anxiety and defective social interactions across generations . Biol Psychiatry 2013. ; 73 : 44 – 53 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Crews D, Gillette R, Scarpino R, et al. . Epigenetic transgenerational inheritance of altered stress responses . Proc Natl Acad Sci 2012. ; 109 : 9143 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sato M, Kimura M . Intrabursal transfer of spermatozoa (ITS): a new route for artificial insemination of mice . Theriogenology 2001. ; 55 : 1881 – 90 . [DOI] [PubMed] [Google Scholar]
- 21. Stone BJ, Steele KH, Fath-Goodin A . A rapid and effective nonsurgical artificial insemination protocol using the NSET™ device for sperm transfer in mice without anesthesia . Transgenic Res 2015. ; 24 : 775 – 81 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Green M, Bass S, Spear B . A device for the simple and rapid transcervical transfer of mouse embryos eliminates the need for surgery and potential post-operative complications . Biotechniques 2009. ; 47 : 919 – 24 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McGill TE . Induction of luteal activity in female house mice . Horm Behav 1970. ; 1 : 211 – 22 . [Google Scholar]
- 24. van Montfoort AP, Hanssen L, de Sutter P, et al. . Assisted reproduction treatment and epigenetic inheritance . Hum Reprod Updat 2012. ; 18 : 171 – 97 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Market-Velker BA, Zhang L, Magri LS, et al. . Dual effects of superovulation: loss of maternal and paternal imprinted methylation in a dose-dependent manner . Hum Mol Genet 2009. ; 19 : 36 – 51 . [DOI] [PubMed] [Google Scholar]
- 26. Gray JD, Rubin TG, Hunter RG, et al. . Hippocampal gene expression changes underlying stress sensitization and recovery . Mol Psychiatry 2014. ; 19 : 1171 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Balls M . FRAME, animal experimentation and the Three Rs: past, present and future . Altern Lab Anim 2009. ; 37Suppl 2 : 1 – 6 . [DOI] [PubMed] [Google Scholar]
- 28. Russell WMS . The development of the three Rs concept . Altern Lab Anim 23 : 298 – 304 . [PubMed] [Google Scholar]
- 29. Luo C, Zuniga J, Edison E, et al. . Superovulation strategies for 6 commonly used mouse strains . J Am Assoc Lab Anim Sci 2011. ; 50 : 471 – 8 . [PMC free article] [PubMed] [Google Scholar]
- 30. Grace KS, Sinclair KD . Assisted reproductive technology, epigenetics, and long-term health: a developmental time bomb still ticking . Semin Reprod Med 2009. ; 27 : 409,416 . [DOI] [PubMed] [Google Scholar]
- 31. Fernández-Gonzalez R, Ramirez MA, Bilbao A, et al. . Suboptimal in vitro culture conditions: an epigenetic origin of long-term health effects . Mol Reprod Dev 2007. ; 74 : 1149 – 56 . [DOI] [PubMed] [Google Scholar]
- 32. Abdel-Mannan O, Sutcliffe A . I was born following ART: how will I get on at school? Semin Fetal Neonatal Med 2014. ; 19 : 245 – 9 . [DOI] [PubMed] [Google Scholar]
- 33. Feuer SK, Camarano L, Rinaudo PF . ART and health: clinical outcomes and insights on molecular mechanisms from rodent studies . Mol Hum Reprod 2013. ; 19 : 189 – 204 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee HJ, Hore TA, Reik W . Reprogramming the methylome: erasing memory and creating diversity . Cell Stem Cell 2014. ; 14 : 710 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mashoodh R, Franks B, Curley JP, et al. . Paternal social enrichment effects on maternal behavior and offspring growth . Proc Natl Acad Sci U S A 2012. ; 109Suppl : 17232 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Weiss I, Franklin T, Vizi S, et al. . Inheritable Effect of Unpredictable Maternal Separation on Behavioral Responses in Mice . Frontiers in Behavioral Neuroscience 2011. ; 5 : 3 – 15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Byrnes JJ, Babb JA, Scanlan VF, et al. . Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring . Behav Brain Res 2011. ; 218 : 200 – 5 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolstenholme JT, Edwards M, Shetty S, et al. . Gestational exposure to bisphenol a produces transgenerational changes in behaviors and gene expression . Endocrinology 2012. ; 153 : 3828 – 38 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skinner MK, Anway MD, Savenkova MI, et al. . Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior . PLoS One 2008. ; 3 : e3745 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jimenez-Chillaron JC, Isganaitis M, Charalambous M, et al. . Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice . Diabetes 2009. ; 58 : 460 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Petropoulos S, Matthews SG, Szyf M . Adult glucocorticoid exposure leads to transcriptional and DNA methylation changes in nuclear steroid receptors in the hippocampus and kidney of mouse male offspring . Biol Reprod 2014. ; 90 : 43 . [DOI] [PubMed] [Google Scholar]
- 42. Franklin TB, Russig H, Weiss I, et al. . Epigenetic transmission of the impact of early stress across generations . Biol Psychiatry 2010. ; 68 : 408 – 15 . [DOI] [PubMed] [Google Scholar]
- 43. Franklin TB, Linder N, Russig H, et al. . Influence of early stress on social abilities and serotonergic functions across generations in mice . PLoS One 2011. ; 6 : e21842 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bohacek J, Farinelli M, Mirante O, et al. . Pathological brain plasticity and cognition in the offspring of males subjected to postnatal traumatic stress . Mol Psychiatry 2015. ; 20 : 621 – 31 . [DOI] [PubMed] [Google Scholar]
- 45. Gapp K, Soldado-Magraner S, Alvarez-Sánchez M, et al. . Early life stress in fathers improves behavioural flexibility in their offspring . Nat Commun 2014. ; 5 : 5466 . [DOI] [PubMed] [Google Scholar]
- 46. Langhans W, Geary N . Overview of the physiological control of eating . Forum Nutr 2010. ; 63 : 9 – 53 . [DOI] [PubMed] [Google Scholar]
- 47. Gapp K, von Ziegler L, Tweedie-Cullen RY, et al. . Early life epigenetic programming and transmission of stress-induced traits in mammals: how and when can environmental factors influence traits and their transgenerational inheritance? Bioessays 2014. ; 36 : 491 – 502 . [DOI] [PubMed] [Google Scholar]
- 48. Rodgers AB, Morgan CP, Bronson SL, et al. . Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation . J Neurosci 2013. ; 33 : 9003 – 12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anderson LM, Riffle L, Wilson R, et al. . Preconceptional fasting of fathers alters serum glucose in offspring of mice . Nutrition 2006. ; 22 : 327 – 31 . [DOI] [PubMed] [Google Scholar]
- 50. Mital P, Hinton BT, Dufour JM . The blood-testis and blood-epididymis barriers are more than just their tight junctions . Biol Reprod 2011. ; 84 : 851 – 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adeoya-Osiguwa SA, Gibbons R, Fraser LR . Identification of functional alpha2- and beta-adrenergic receptors in mammalian spermatozoa . Hum Reprod 2006. ; 21 : 1555 – 63 . [DOI] [PubMed] [Google Scholar]
- 52. Belleannée C, Calvo É, Caballero J, et al. . Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis . Biol Reprod 2013. ; 89 : 30 . [DOI] [PubMed] [Google Scholar]
- 53. Guerrero-Bosagna C, Savenkova M, Haque MM, et al. . Environmentally induced epigenetic transgenerational inheritance of altered Sertoli cell transcriptome and epigenome: molecular etiology of male infertility . PLoS One 2013. ; 8 : e59922 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. França LR, Avelar GF, Almeida FFL . Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs . Theriogenology 2005. ; 63 : 300 – 18 . [DOI] [PubMed] [Google Scholar]
- 55. Robaire B, Hinton B, Orgebin-Crist M . The epididymis. In: Knobil and Neill’s Physiology of Reproduction. Eds: Neill JD, Amsterdam-Boston Elsevier, 2006, 1071–148 . [Google Scholar]
- 56. Ulrich-Lai YM, Herman JP . Neural regulation of endocrine and autonomic stress responses . Nat Rev Neurosci 2009. ; 10 : 397 – 409 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sapolsky RM, Romero LM, Munck AU . How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions . Endocr Rev 2000. ; 21 : 55 – 89 . [DOI] [PubMed] [Google Scholar]
- 58. McGill TE . Induction of luteal activity in female house mice . Horm Behav 1970. ; 1 : 211 – 22 . [Google Scholar]
- 59. Bohacek J, Manuella F, Roszkowski M, et al. . Hippocampal gene expression induced by cold swim stress depends on sex and handling . Psychoneuroendocrinology 2015. ; 52 : 1 – 12 . [DOI] [PubMed] [Google Scholar]
- 60. Welsh M, Saunders P, Fisken M, et al. . Identification in rats of a programming window for reproductive tract masculinization, disruption of which leads to hypospadias and cryptorchidism . J Clin Invest 2008. ; 118 : 1479 – 90 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data