Abstract
Among protozoa, Tetrahymena pyriformis is the most commonly ciliated model used for laboratory research. All living organisms need to adapt to ever changing adverse conditions in order to survive. This article focuses on the phenomenon that exposure to toxic doses of the toxicants protects against a normally harmful dose of the same stressor. This first encounter by toxicant provokes the phenomenon of epigenetical imprinting, by which the reaction of the cell is quantitatively modified. This modification is transmitted to the progeny generations. The experiments demonstrate the possibility of epigenetic effects at a unicellular level and call attention to the possibility that the character of unicellular organisms has changed through to the present day due to an enormous amount of non-physiological imprinter substances in their environment. The results point to the validity of epigenetic imprinting effects throughout the animal world. Imprinting in Tetrahymena was likely the first epigenetic phenomenon which was justified at cellular level. It is very useful for the unicellular organisms, as it helps to avoid dangerous molecules more easily or to find useful ones and by this contributes to the permanence of the population’s life.
Keywords: epigenetic imprinting, cadmium, dichlorvos, ochratoxin, Tetrahymena
Introduction
Tetrahymena pyriformis is a ubiquitous, free-living freshwater ciliate whose physiology and biochemistry has been extensively studied [1]. Its cultivation in the laboratory is very simple and inexpensive. At pH between 6.5 and 7.0 and at temperature 25°C, its reproductive cycle is very short [2, 3]. Under these conditions Tetrahymena create up to 8 generations per day, 56 in a week, 240 in a month, and almost 3000 in a year and therefore is an appropriate model for epigenetic studies allowing the simultaneous study of several subjects and their progeny during many generations [4, 5]. In studies of hormone-induced imprinting (term imprinting is not exact here, it is used with respect to predecessing works. Probably better equivalent should be methylation-pattern change with followed activation or suppression of genes and imprinting is used here in this way) in relation to receptors they were commonly followed up to 1000 generations [6, 7].
In addition to these advantages, members of the genus Tetrahymena have other advantages, e.g. their cultures are highly sensitive to growth conditions, various chemical substances in their environment and also to different external physical factors [8, 9].
The sensitivity of T.pyriformis to different external factors and their high reproduction rate means that they are a reliable bio-assay model to determine the harmful effects of hazardous chemicals in the environment [10, 11], to monitor industrial and agricultural contaminants, to determine the presence of toxic substances of biological origin in the natural resources, etc. [12].
In addition, Tetrahymena as a unicellular animal model has enormous potential of application for a wide range of pharmacological, molecular biological, genetic, immunotoxicological and other experimental studies. They can replace experiments on higher animals for the study of different functions as cell growth arrest, inhibition of respiration and metabolism, synthesis and degradation of specific molecules, etc. [13, 14]. Tetrahymena plays an important role in a large number of impressive new molecular genetic technologies and research in the field of functional genomics and provides valuable insights into how these findings are extrapolated to the human genomics [6, 15–17]. Tetrahymena was used also in two Nobel-prize winning experimental series; namely in studies of self-splicing character of RNA [18, 19], in discovering the function of telomers and telomerase [20] and contributed to more pioneer studies as (description of lysosome and peroxisome; first isolation of motor protein dynein; (iii) description of somatic genome rearrangement; (iv) introduction of artificial synchronization of cell cycle; (v) discovery of self-splicing RNA; (vi) discovery of the function of histone acetylation, and many other.
In our previous study [21], we proved that T.pyriformis W is a suitable model for the study of environmental and industrial pollutants and biological toxins. In this study, we found that cells surviving exposure to LC50 of various toxic substances became more resistant to them. The aim of this study was to determine the number of cell generations after exposure to some toxic substances, which are able to keep this “cell memory” of higher resistance.
Results
In Table 1 we present the results of cytotoxic effects of the tested toxic substances expressed as LC50/2 h.
Table 1:
LC50/2 h expressed as % viable T. pyriformis W cells
| Characteristic | C | OA | DVPP | Cd |
|---|---|---|---|---|
| Mean | 99.84 | 49.85 | 49.57 | 49.29 |
| S.D. | 1.17 | 4.47 | 3.44 | 2.67 |
| Maximum | 102.00 | 56.00 | 54.30 | 53.30 |
| Upper quartile | 100.55 | 54.48 | 52.50 | 51.20 |
| Lower quartile | 98.88 | 44.98 | 45.98 | 46.98 |
| Minimum | 98.00 | 44.00 | 45.00 | 44.00 |
| Range | 4.00 | 12.00 | 9.30 | 8.30 |
| Variation coeff. | 0.01 | 0.09 | 0.07 | 0.05 |
| P against C | <0.001 | <0.001 | <0.001 |
LC50/2 h = 50% lethal concentration after 2 h exposure; C = untreated control; OA = exposed to ochratoxin A (3.2 mg/l); DVPP = exposed to dichlorvos (9.6 mg/l); Cd = exposed to cadmium (24 mg/l); n = 10 in all groups. Mean values are inferred from regression lines.
Based on these data we confirmed the values of LC50/2 h for the protozoan T.pyriformis W for the applied toxic substances found in our previous experiments [21]. The largest variation coefficient was detected for exposure to ochratoxin A.
After exposure to toxic substances there has been a significant increase in resistance of protozoa against the toxic substances. Values of LC50/2 h increased significantly already in the first generation (Table 2 and Fig. 1). LC50/2 h after exposure to ochratoxin A in the F1 generation increased 1.9 times, corresponding to a concentration of ochratoxin A 6.08 mg/l, after exposure to dichlorvos increased 1.6 times, which corresponds to a concentration of 15.36 mg/l, and after exposure to cadmium increased 2.7 times, corresponding to a concentration of cadmium 64.8 mg/l.
Table 2:
Increase in LC50/2 h for further generations of T. pyriformis W expressed as multiples of the LC50/2 h used in the first exposure to the same toxic substance as in the parental generation
| Characteristic | C | OA | DDVP | Cd |
|---|---|---|---|---|
| Mean | 1.02 | 1.79 | 1.56 | 2.69 |
| S.D. | 0.03 | 0.07 | 0.05 | 0.10 |
| Maximum | 1.10 | 1.92 | 1.67 | 2.84 |
| Upper quartile | 1.05 | 1.86 | 1.59 | 2.75 |
| Lower quartile | 1.00 | 1.74 | 1.52 | 2.66 |
| Minimum | 0.97 | 1.69 | 1.48 | 2.49 |
| Range | 0.13 | 0.23 | 0.19 | 0.35 |
| Variation coeff. | 0.03 | 0.04 | 0.03 | 0.04 |
| P against C | <0.001 | <0.001 | <0.001 | |
| P against OA | <0.001 | <0.001 | <0.001 | |
| P against DDVP | <0.001 | <0.001 | <0.001 |
LC50/2 h = 50% lethal concentration after 2 h exposure; C = untreated control; OA = exposed to ochratoxin A (3.2 mg/l); DVPP = exposed to dichlorvos (9.6 mg/l); Cd = exposed to cadmium (24 mg/l); n = 10 in all groups. Mean values are inferred from regression lines.
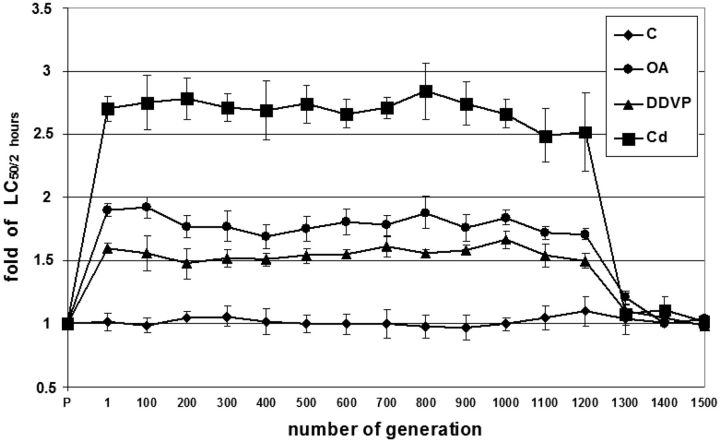
Figure 1:
The time course of LC50/2 h after exposure from parental generation to 1500th generation of T. pyriformis W. LC50/2 h = 50% lethal concentration after 2 h exposure; C = untreated control; OA = exposed to ochratoxin A; DVPP = exposed to dichlorvos; Cd = exposed to cadmium; n = 10 in all groups
As can be seen in Fig. 1, protozoan T.pyriformis W were able to resist higher concentrations of these toxic compounds during more than 1200 generations, and then their sensitivity was returned to the initial (original) level. Protozoa tolerated almost 3-fold dose for exposure to cadmium, almost nearly double for exposure to ochratoxin A, and 1.5-fold for exposure to dichlorvos.
Discussion
In this study, offspring of protozoa surviving exposure to LC50 of three different toxic substances increased their resistance to the appropriate toxic substance very quickly and in a rather uniform way. The increased resistance persisted for ∼1200–1300 generations and then suddenly returned to levels found at their first encounter of with the studied toxic substances. This loss can be explained only hypothetically due to possible rearrangement of epigenetic pattern (e.g. methylation) to the starting one, before encounter with toxic substance, as without further exposition, the gained pattern became redundant.
Recognition system of unicellular organisms is comprised by surface receptors recognizing the presence of harmful molecules. Subsequently, these signals through the signal-transduction pathway activate the defense systems, able to bind, neutralize and break down the toxic substance.
Furthermore, tested toxicants are from the point of view according to their structure complexity three different compounds. Cadmium is a basic metal element and also belongs to heavy metals, dichlorvos is a simple organophosphate and ochratoxin A is a common toxic product of food-contaminating mold.
In higher multicellular organisms’ different cells express different sets of receptors. On other hand, in unicellular organisms must be a complete repertoire of receptors expressed on a single cell. Enormous variety of molecules and stimuli can exist in natural aquatic environment of Tetrahymena and therefore it should be also a huge number of exprimed receptors on the surface of a single cell. It is not possible to express all of them simultaneously and any time but this paradox could be explained by the fluid mosaic theory of Koch et al. [22]. According to this theory, primitive organisms code only receptor subunits and these are continuously incorporated into the cytoplasmic membrane and subsequently recirculated into the cytoplasm. This leads to the fact that the surface of the cytoplasmic membrane structures recognizing foreign molecules is continuously changing. This dynamic system—if the theory is valid, and membrane turnover is sufficiently fast at a high speed and the organism is able to respond to almost unlimited number of molecules present in a changing environment. The fact that the increased resistance in cells surviving after encounter with toxic substance is higher in simple elements and compounds (such as cadmium chloride) and lower for complicated molecules requiring more complex receptor (organophosphate or mycotoxins) supports the validity of Koch’s hypothesis.
The higher resistance was transmitted by protozoa to the following generations is probably the consequence of imprinting, which in the case of hormone induced imprinting has been already described [5, 6]. There are only few data on fixation of imprinting. It is assumed that these are the earliest epigenetic changes, and it has been demonstrated that DNA methylation or rearrangement of histones in nucleosomes significantly affect expression of the genes and that these changes are inherited for following generations [23–26]. The fact that imprinting is an epigenetic process has been reliably demonstrated in the protozoan Tetrahymena [27]. Epigenetic modification is very durable and it can explain why is inherited during many generations. In our experiments, we found out that this imprinting persists over 1200 generations similar to hormonal imprinting, which is inherited by more than 1000 generations [27–31]. Imprinting is very beneficial for individual life of Tetrahymena offsprings population and helps to protect against dangerous molecules. Interaction of unicellular organism with damaging agents can be remembered, to form a memory of cell which activates defense mechanisms against the appropriate pollutant [17]. Creating of memory mechanisms is also based on epigenetic mechanisms. Though, cellular memory is insufficient itself for learning. Memory and learning are distinct advantages to organisms living in a changing, but recurrent environment [32] and some answers can be expected in these areas from the new results obtained in the study of epigenetics mechanisms. The concept of cellular memory is important in the study of cell biology and differentiation [33, 34]. This can be especially important for microorganisms that live in an environment that neither changes very quickly, nor very slowly.
The results of our experiments confirm that:
Toxic substances (ochratoxin A > dichlorvos > cadmium) are suitable ligands to develop epigenetic imprinting in Tetrahymena.
Imprinting developed by toxic substances may persist for more than 1200 generations.
The duration of the changed responsiveness is not affected by the nature of the imprinter toxic substance (possible reason is stated in the first paragraph of the discussion).
These results are preliminary and reliable explanation certainly requires further study.
Methods
Tetrahymena pyriformis S train and C ulture C onditions
Tetrahymena pyriformis strain W were axenically cultured in PPYS medium (pH 6.8–7.0) containing 0.75% (w/v) Proteose peptone (Difco, Detroit, MI, USA), 0.75% (w/v) Yeast extract (Difco) and inorganic salts [35] at a constant temperature of 28°C. Cultures of T. pyriformis were maintained by inoculation of 0.5 ml culture every 24 h (about eight generations) into a 100 ml freshly prepared PPYS medium in 500 ml Erlenmeyer bottles.
Toxic S ubstances
Ochratoxin A (OA), purity of 98% (Sigma-Aldrich, Calbiochem) at a final concentration in the culture medium 3.2 mg/l, which corresponds to a dose of LC50/2 h. Dichlorvos (DDVP; 2,2-dichlorovinyl dimethyl phosphate, efficiency 98%, Riedel-de Haën), at a final concentration in the culture medium 9.6 mg/l, which corresponds to a dose of about LC50/2 h. Cadmium chloride (pure, Sigma-Aldrich) at a final concentration of 24 mg Cd/l which corresponds to a dose of LC50/2 h. Tested compounds were added from stock solutions so that they have the required concentration in test cultures.
Exposure to T oxic S ubstances
Parental generation was exposed with a dose LC50/2 h of toxic substance in the logarithmic growth phase (10 parallel samples) for 2 h, in the second series of the similarly in 10 subsequent generations (10 parallel samples) for 2 h, respectively.
Determination of LC50/2 h in S urviving C ells
In subsequent generations after single and repeated exposure, we investigated the dose LC50/2 h in the logarithmic phase of growth in the 2nd generation and in approximately every 100th generation to the 1500th generation. Results were evaluated in cultures repeatedly exposed to different concentrations of the toxic substances (0.5, 1, 2 and 4 times the LC50/2 h) and the control groups (T. pyriformis culture with the addition of pure solvent which were used to dilution of tested toxic substances) after the appropriate number of generations of protozoa. Cell cultures were then incubated at 28°C, without shaking in darkness for 2 h. To determine the number of viable protozoa the samples were stained and fixed (by addition of equal volume of 0.4% trypane blue solution and subsequently 5.0% formalin) and calculated microscopically (Leica ICC50 HD) in hemocytometer (Fuchs-Rosenthal chamber). Toxicity of toxic substances was quantified as a percentage of viable cells (%) compared with the number of cells detected in the corresponding untreated control (100% survival), and determining of LC50/2 h, i.e. the concentration which causes 50% decrease in cell number, which means halved viability under toxic conditions, compared with the untreated control, respectively. For each tested compound and concentration were evaluated 10 parallel samples.
Statistical A nalyses
Data shown in figure represent mean ± SD. values. The 50% lethal concentration (LC50/2 h) were calculated by regression analysis with Microsoft Excel 2000 software [36]. A Student’s t-test was used for the statistical evaluation of the data. Significance levels were tested at the P < 0.05 level.
Conflict of interest statement. None declared.
Acknowledgements
We thank editor and anonymous reviewers for helpful and valuable comments, which significantly contributed to the clarity of our work. Furthermore, we would like to express gratitude to Professor Csaba, who motivated us to use this mono-cellular model for toxicological studies.
References
- 1. Elliott A. Biology of Tetrahymena. Dowden, Hutchinson and Ross Inc, Stoudsburgh/Pennsylvania, 1973,1–508. [Google Scholar]
- 2. Scherbaum O. Studies on the mechanism of synchronous cell division in Tetrahymena pyriformis. Exp Cell Res 1957;13:11–23. [DOI] [PubMed] [Google Scholar]
- 3. Prescott D. Variations in the individual generation times of Tetrahymena geleii HS. Exp Cell Res 1959;16:279–84. [DOI] [PubMed] [Google Scholar]
- 4. Csaba G. The hormonal system of the unicellular Tetrahymena: a review with evolutionary aspects. Acta Microbiol Immunol Hung 2012;59:131–56. [DOI] [PubMed] [Google Scholar]
- 5. Csaba G. Hormonal imprinting in the unicellular Tetrahymena: the proto-model of epigenetics. Acta Microbiol Immunol Hung 2012;59:291–310. [DOI] [PubMed] [Google Scholar]
- 6. Kõhidai L, Lajkó E, Pállinger É. et al. Verification of epigenetic inheritance in an unicellular model system. Multigenerational effects of hormonal imprinting. Cell Biol Int 2012;36:951–9. [DOI] [PubMed] [Google Scholar]
- 7. Csaba G, Németh G, Vargha P. Development and persistence of receptor “memory” in a unicellular model system. Exp Cell Biol 1982;50:291–4. [DOI] [PubMed] [Google Scholar]
- 8. Ništiar F, Hrušovský J, Beneš J. Employment of the protozoon Tetrahymena pyriformis for testing biological material contamination by organophosphates and carbamates. Vet Med-Czech 1981;26:695–700. [PubMed] [Google Scholar]
- 9. Lukačínová A, Beňačka R, Ništiar F. Tetrahymena pyriformis—a model organism for the screening of mycotoxins. Physiol Res 2008;57:20P. [Google Scholar]
- 10. Sauvant M, Pepin D, Piccinni E. Tetrahymena pyriformis: a tool for toxicological studies. A review. Chemosphere 1999;38: 1631–69. [DOI] [PubMed] [Google Scholar]
- 11. Chen F, Leick V. The protozoan Tetrahymena as a bioindicator to screen bioactive substances. J Microbiol Methods 2004;59:233–41. [DOI] [PubMed] [Google Scholar]
- 12. Dayeh V, Lynn D, Bols N. Cytotoxicity of metals common in mining effluent to rainbow trout cell lines and to the ciliated protozoan, Tetrahymena thermophila. Toxicol in Vitro 2005;19:399–410. [DOI] [PubMed] [Google Scholar]
- 13. Gutierrez J, Martin-Gonzalez A, Diaz S. et al. Ciliates as a potential source of cellular and molecular biomarkers/biosensors for heavy metal pollution. Eur J Protistol 2003;39:461–7. [Google Scholar]
- 14. Ništiar F, Hrušovský J, Mojžiš J. The effect of dichlorvos and metathion on selected enzymes of the amoeba Tetrahymena pyriformis. Vet Med-Czech 1985;30:443–7. [PubMed] [Google Scholar]
- 15. Hamilton E, Bruns P, Lin C. et al. Genome-wide characterization of Tetrahymena thermophila chromosome breakage sites. I. Cloning and identification of functional sites. Genetics 2005;170:1611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yao M-C, Yao C-H, Halasz L. et al. Identification of novel chromatin-associated proteins, involved in programmed genome rearrangements in Tetrahymena. J Cell Sci 2007;120:1978–89. [DOI] [PubMed] [Google Scholar]
- 17. Ginsburg S, Jablonka E. Epigenetic learning in non-neural organisms. J Biosci 2009;34:633–46. [DOI] [PubMed] [Google Scholar]
- 18. Cech T. Nobel lecture. Self-splicing and enzymatic activity of an intervening sequence RNA from Tetrahymena. Biosci Rep 1990;10:239–61. [DOI] [PubMed] [Google Scholar]
- 19. Cech T. Ribozymes, the first 20 years. Biochem Soc Trans 2002;30:1162–6. [DOI] [PubMed] [Google Scholar]
- 20. Blackburn E. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 2005;579:859–62. [DOI] [PubMed] [Google Scholar]
- 21. Ništiar F, Lukačínová A, Ništiarová A. Rapid bioassay that uses protozoan Tetrahymena pyriformis to asses the toxicity of various xenobiotics. Labor Diagnostika 2003;8:85–6. [Google Scholar]
- 22. Koch A, Nienhaus R, Lauitsh M. Metastable equilibrium with random local fluctuations: simulations of dynamic receptor pattern generation in a fluid mosaic membrane. Biol Cybern 1982;44:121–8. [DOI] [PubMed] [Google Scholar]
- 23. Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol 2010;28:1057–68. [DOI] [PubMed] [Google Scholar]
- 24. Daxinger L, Whitelaw E. Understanding transgenerational epigenetic inheritance via the gametes of mammals. Nat Rev Genet 2012;13:153–62. [DOI] [PubMed] [Google Scholar]
- 25. Golbabapour S, Abdulla M, Hajrezaei M. A concise review on epigenetic regulation: insight into molecular mechanisms. Int J Mol Sci 2011;12:8661–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hanson M, Skinner M. Developmental origins of epigenetic transgenerational inheritance. Environ Epigenet 2016;dvw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Csaba G, Kovács P. Impact of 5-azacytidine on insulin binding and insulin-induced receptor formation in Tetrahymena. Biochem Biophys Res Commun 1990;168:709–13. [DOI] [PubMed] [Google Scholar]
- 28. Csaba G. Phylogeny and ontogeny of hormone receptors: the selection theory of receptor formation and hormonal imprinting. Biol Rev 1980;55:47–63. [DOI] [PubMed] [Google Scholar]
- 29. Csaba G, Kovács P. Insulin treatment (hormonal imprinting) increases the insulin production of the unicellular Tetrahymena long term. Is there a simultaneous formation of hormone receptor and hormone? Cell Biol Int 1995;19:1011–4. [DOI] [PubMed] [Google Scholar]
- 30. Kőhidai L, Csaba G, László V. Persistence of receptor “memory” induced in Tetrahymena by insulin imprinting. Acta Microbiol Hung 1990;37:269–75. [PubMed] [Google Scholar]
- 31. Csaba G. Hormonal imprinting: phylogeny, ontogeny, diseases and possible role in present-day human evolution. Cell Biochem Funct 2008;26:1–10. [DOI] [PubMed] [Google Scholar]
- 32. Rando O, Verstrepen K. Timescales of genetic and epigenetic inheritance. Cell 2007;128:655–68. [DOI] [PubMed] [Google Scholar]
- 33. Holliday R. Epigenetics: an overview. Dev Genet 1994;15: 453–7. [DOI] [PubMed] [Google Scholar]
- 34. Jablonka E, Raz G. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity. Q Rev Biol 2009;84:131–76. [DOI] [PubMed] [Google Scholar]
- 35. Plesner P, Rasmussen L, Zeuthen E. Techniques used in the study of synchronous Tetrahymena In: Zeuthen E. (ed.), Synchrony in Cell Division and Growth. Interscience Publ, New York, 1964,543–63. [Google Scholar]
- 36. Bonnet J, Dusser M, Bohatier J. et al. Cytotoxicity assessment of three therapeutic agents, cyclosporine-A, cisplatin and doxorubicin, with the ciliated protozoan Tetrahymena pyriformis. Res Microbiol 2003;154:375–85. [DOI] [PubMed] [Google Scholar]