Abstract
Consumption of polluted fish may lead to high levels of persistent organic pollutants (POPs) in humans, potentially causing adverse health effects. Altered DNA methylation has been suggested as a possible contributor to a variety of adverse health effects. The aim of this study was to evaluate the relationship between serum POP levels (dioxins, polychlorobiphenyls, and perfluoroctane sulphonate) and DNA methylation. We recruited a total of 80 Dutch men who regularly consumed eel from either low- or high-polluted areas, and subsequently had normal or elevated POP levels. Clinical parameters related to e.g. hormone levels and liver enzymes were measured as biomarkers for adverse health effects. The Infinium 450K BeadChip was used to assess DNA methylation in a representative subset of 34 men. We identified multiple genes with differentially methylated regions (DMRs; false discovery rate <0.05) related to POP levels. Several of these genes are involved in carcinogenesis (e.g. BRCA1, MAGEE2, HOXA5), the immune system (e.g. RNF39, HLA-DQB1), retinol homeostasis (DHRS4L2), or in metabolism (CYP1A1). The DMRs in these genes show mean methylation differences up to 7.4% when comparing low- and high-exposed men, with a mean difference up to 14.4% for single positions within a DMR. Clinical parameters were not significantly associated with serum POP levels. This is the first explorative study investigating extensive DNA methylation in relation to serum POP levels among men. We observed that elevated POP levels are associated with aberrant DNA methylation profiles in adult men who consumed high-polluted eel. These preliminary findings warrant further confirmation in other populations.
Keywords: DNA methylation, Illumina 450K BeadChip, persistent organic pollutants, dioxins, PCBs, PFOS
Introduction
Persistent organic pollutants (POPs) are widely present in the environment and humans are mainly exposed to POPs through the consumption of food, especially fish and seafood [1]. In Dutch men, serum POP levels were shown to be elevated in consumers of high-polluted eel, coming from rivers with a high degree of urbanization and industrialization, compared with men consuming eel from relatively low-polluted areas or aquaculture [2]. Especially, polychlorobiphenyls (PCBs) (median increases 4–10 times), perfluoroctane sulphonate (PFOS) (median increase 2 times), and the total amount of dioxins and dioxin-like compounds (median increase 2 times) were found to be elevated. In 2011, a ban on eel fishing in high-polluted areas was implemented since risk assessors had concluded that the consumption of one portion eel per month would lead to unsafe levels of especially dioxins and dioxin-like compounds [3]. The safe level is derived from animal reproduction studies [4], and there is a lack of information regarding the exact safe levels in humans.
In adults, POPs can cause various adverse health effects of which the majority is related to endocrine disruption, neurological disorders, immune function and cancer [5]. More specifically, hydroxylated (OH–) PCBs might affect thyroid and retinol regulation [6], while dioxins and PFOS might affect testosterone levels [7, 8], liver enzymes [9, 10], hematocrit (Ht) and hemoglobin (Hb) levels [11, 12]. Furthermore, POP mixtures were shown to interfere with glucose and high-density lipoprotein (HDL) cholesterol levels, and possibly attribute to an increased risk of diabetes mellitus type II [13]. Although some mechanisms explaining adverse health effects of POPs are clear, like dioxins binding to the aryl hydrocarbon receptor [14], OH-PCBs binding to thyroid transport proteins [6], and PFOS resembling fatty acids [15], not all adverse health effects can be explained by these mechanisms of action. Epigenetic phenomena, such as DNA methylation, have been proposed as a possible molecular mechanism underlying adverse health effects of pollutants [16]. Observational studies have indeed shown that POPs can affect global DNA methylation, although both hypo- (decrease) and hyper- (increase) methylation have been found [17–20]. Furthermore, animal studies showed that dioxin [21], PCBs [22], and PFOS [23] might cause adverse health effects due to gene-specific methylation changes. Also in vitro experiments showed that POPs can affect gene-specific DNA methylation [24, 25].
Therefore, we hypothesized that men with elevated POP levels (i.e. dioxins, PCBs, and PFOS) might have aberrant DNA methylation profiles which may possibly explain the suggested adverse health effects. In this study, we assessed DNA methylation in blood collected from male eel consumers, as well as some clinical parameters, and associated these outcomes with serum POP levels. This is the first study to explore the association between extensive DNA methylation and serum POP-levels in humans.
Material and Methods
Study Population
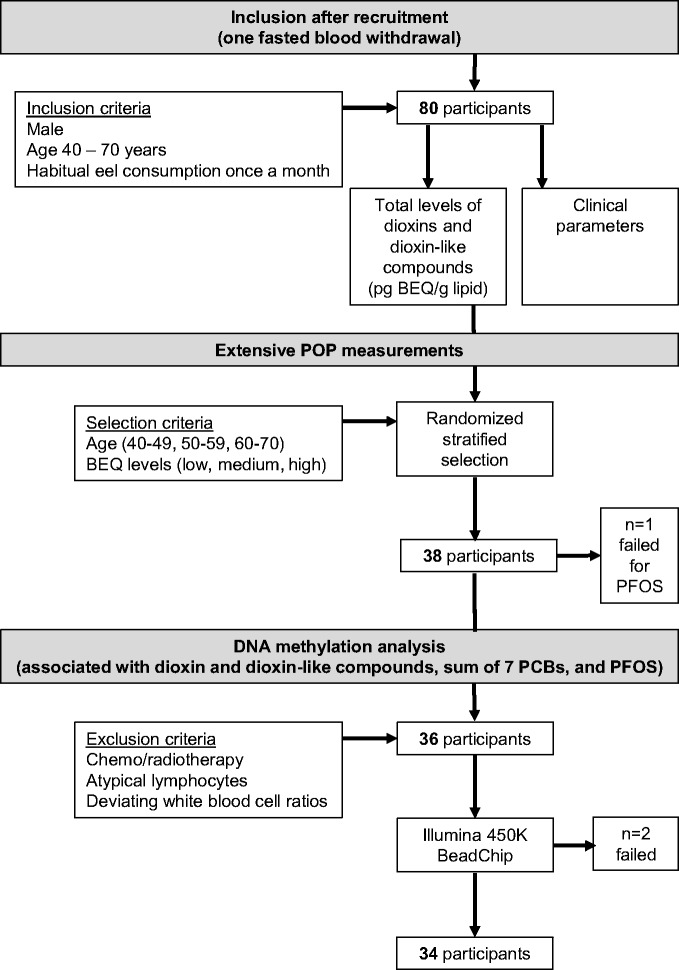
The population consisted of 80 Dutch men aged 40–70 years, with a habitual eel consumption of at least one portion a month [2]. Men were invited to participate through professional and recreational fishermen associations and through advertisements in local newspapers and webpages. Participants filled out a questionnaire at home about fish consumption habits (including the origin of the eel), smoking habits and alcohol consumption. Blood samples were obtained between February and June 2015 by venipuncture after overnight fasting. Furthermore, height and weight were measured using standard methods in light clothes. Waist circumference was measured midway between the iliac crest and the costal margin at the end of gentle expiration and hip circumference was measured over the great trochanters. This study was approved by the Medical Ethical Committee of Wageningen University, and written informed consent was obtained from all participants before inclusion in the study. The design of the study can be found in Fig. 1.
Figure 1.
flow diagram for the recruitment and selection of the participants
Serum POP Levels
Full details regarding blood withdrawal and POP measurements are described previously [2]. In short, the total dioxin-like toxic potency of compounds in serum was measured for all 80 participants with the DR CALUX bioassay at RIKILT Institute for Food Safety [26, 27] and expressed in bioanalytical equivalent (BEQ) per gram lipid. Extensive analyses for individual POPs were performed, due to financial limitations, in a subset of 38 participants with an age distribution that represents the whole study population. First, all participants were categorized in three age groups (40–49, 50–59, 60–70 years), and subsequently by the measured BEQ levels with equal numbers of participants for low, medium or high levels (see online supplementary material for Table S1). The 38 participants were randomly selected from each of the categories. Stratification for age is important because POP levels are related to age [28] as is DNA methylation [29, 30].
The extensive POP measurements included 48 individual compounds and were performed at the Laboratory of Environmental Toxicology at the Norwegian University of Life Sciences (NMBU), according to the requirements of the ISO/IEC 17025 accreditation (TEST 137). Of the 48 POPs, 30 compounds reached levels above the detection limit in at least 60% of the participants, and were therefore used in the statistical analyses (see online supplementary material for Table S1). The seven indicator PCBs (sum of PCB-28, 52, 101, 118, 138, and 153), recommended by the European Union Community Bureau of Reference based on their relatively high concentrations in technical mixtures and their wide chlorination range, were measured with gas chromatography followed by electron-capture detection (GC-ECD) after liquid–liquid extraction and expressed per gram lipid [31]. PFOS was measured with liquid chromatography-mass spectrometry (LC-MS) after methanol extraction and expressed per gram wet weight (ww) [32].
Clinical Parameters
Liver parameters (aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transpeptidase (GGT) levels), HDL cholesterol, and thyroid hormones (free thyroxine (T4) and thyroid-stimulating hormone (TSH) levels) were determined in venous blood collected in lithium heparin vacutainers. Blood collected in a sodium fluoride/potassium oxalate vacutainer was analyzed for glucose, while testosterone and retinol were quantified in serum. Whole blood collected in an EDTA vacutainer was used to measure hemoglobin (Hb), hematocrit (Ht), and white blood cell counts (WBC). All samples were transferred to the clinical chemistry laboratory at the Hospital Gelderse Vallei (Ede, the Netherlands) within 3 h after blood sampling for analysis.
Associations between total BEQ levels as well as all levels of individual POPs and the clinical parameters were investigated using multivariable linear regression analyses. The association between log-transformed POP levels and log-transformed parameters was assessed while adjusting for age and waist-to-hip ratio, because these covariates had statistically significant effects on some of the clinical parameters measured. Waist-to-hip ratio was not significantly related to the measured POP levels. Some of the POPs were significantly related to age, but adjustment is still important to correctly interpret results as both POPs [28] and DNA methylation [29, 30] are related to age. The standardized β coefficients are reported for the linear regression analyses and were used to interpret the influence of the POP levels on the clinical parameters. Participants with known or expected diabetes mellitus (due to high glucose levels) were excluded from the analyses on glucose and POP levels (6 out of 80).
DNA Isolation
Two participants were excluded for DNA methylation analysis since their WBC showed deviating ratios (percentage of either monocytes, lymphocytes, or granulocytes outside two times the standard deviation of the population). Therefore, DNA methylation was assessed for 36 participants with extensive POP measurements. Genomic DNA was isolated from the buffy coat using the DNeasy blood and tissue kit according to the protocol from the manufacturer (Qiagen, Venlo, The Netherlands). DNA concentration and quality (ratio 260/280 close to 1.8 and 260/230 above 1.8) were determined using a NanoDrop ND-1000 UV–vis spectrophotometer.
DNA Methylation Measurements
DNA methylation was analyzed using the Illumina Infinium HumanMethylation450 BeadChip (Infinium Inc., San Diego, CA), which addresses 485 512 cytosine positions. For the 36 participants, a total of 500 ng DNA was sent to the Human Genotyping Facility of the Erasmus University Medical Centre (Rotterdam, The Netherlands) who performed the bisulfite conversion (EZ DNA Methylation Kit, Zymo Research, Freiburg, Germany) and processed the BeadChip. Two samples had to be excluded due to technical failure during processing of the BeadChip, resulting in a total of 34 remaining samples (Fig 1).
Data analysis was conducted as described previously [33]. In short, the minfi package (v1.16.1) [34] was used to process the intensity iDAT files in R-based open software (R 3.2.4). Positions with a detection P value of >0.01 in at least one sample were filtered, as these positions did not surpass the background signal. Filtered data were normalized using the SWAN normalization procedure. Autosomes and the X and Y chromosomes were included in the analysis, but positions containing a SNP (based on the dbSNP version 137 [35]) at the CpG interrogation and/or the single nucleotide extension with a frequency of at least 1% were excluded. Finally, there were 467 974 positions included in the analyses and annotated based on the ilmn12.hg19 annotation [36]. Differentially methylated positions were identified as a function of log-transformed BEQ levels, sum of seven indicator PCBs, and PFOS levels (continuous variables), using the dmpFinder function for continuous phenotypes with a linear regression analyses adjusted for age and leukocyte counts (percentage of monocytes, lymphocytes, and granulocytes). Other possible confounders, such as smoking, were tested but did not significantly change the results and were not taken into account to prevent decreasing the statistical power. Statistical analyses were adjusted for multiple testing with the Benjamini–Hochberg (BH) method [37]. A false discovery rate (FDR) value of 0.05 was used as the threshold to define statistical significance. Furthermore, we considered positions with a non-adjusted P value below 1E−04. For descriptive purposes, we also focused on a selection of the 1000 positions with the lowest P-values for each of the three individual POP groups. M values (log2 intensity ratios) were used for statistical testing [38], while β-values were used for visualization purposes. These β-values range from 0% (completely unmethylated) to 100% (completely methylated). All β-values as well as individual iDAT files and other relevant information are available through the Gene Expression Omnibus (GEO) repository with accession number GSE79329.
Differentially Methylated Regions
Identification of differentially methylated regions (DMRs) was performed with the DMRcate package (v1.6.1) from Bioconductor [39]. This method identifies DMRs across the genome based on tunable kernel smoothing. This analysis was again performed for BEQ levels, sum of seven indicator PCBs, and PFOS levels as these POPs showed the largest elevation upon consumption of eel from high-polluted areas. For this study, DMRs were defined as regions of at least 5 positions with a distance of less than 200 nucleotides between the consecutive positions. The default settings of DMRcate were used for the bandwidth scaling factor (C = 2, resulting in a kernel size of 200/2 = 100 base pairs). Statistical analyses were adjusted for multiple testing with the BH method and a FDR value of 0.05 was used as the threshold to define statistically significant DMRs.
Results
The 80 men included in this study showed high variability in their serum levels of dioxins and dioxin-like compounds (range from 4.9 to 145 pg BEQ/g lipid). Demographic characteristics can be found in Table 1. Men with ‘low’ or ‘high’ POP-levels (based on the median-splits of the different POPs) did not differ regarding their demographic characteristics as confirmed by the absence of statistical significance (data not shown).
Table 1.
characteristics of the study participants. Values are given as median (25–75 percentile) or number (percentage) of participants
| All participants | Participants with DNA methylation analyses | ||
|---|---|---|---|
| Number of participants (n) | 80 | 34 | |
| Age (years) | 58.6 (49.0–63.9) | 57.3 (49.2–65.5) | |
| Body Mass Index (kg/m2) | 29.2 (26.5–31.0) | 29.4 (26.7–32.3) | |
| Waist-to-hip ratio | 0.98 (0.93–1.02) | 0.98 (0.94–1.00) | |
| Smoking habits | Current | 11 (14) | 5 (15) |
| Former | 48 (60) | 21 (62) | |
| Never | 21 (26) | 8 (24) | |
| Alcohol consumption (minimal once per week) | Yes | 62 (78) | 28 (82) |
| No | 18 (23) | 6 (18) | |
| Serum levels of persistent organic pollutants (POPs) | |||
| Dioxins and dioxin-like compounds (pg BEQ/g lipid) | 22 (16–32) | 26 (17–47) | |
| Sum of 7 indicator PCBs (ng/g lipid) | 841 (249–1551) | ||
| PFOS levels (ng/g wet weight) | 40 (15–93) |
We first analyzed the BEQ levels for all 80 men and the selected panel of clinical parameters previously shown in the literature to be affected by POP exposure (ALT, AST, GGT, glucose, HDL cholesterol, Hb, Ht, testosterone, TSH, free T4, and retinol) and detected no statistically significant associations (see online supplementary material for Table S2). For the 38 participants for whom extensive POP analyses were performed (see flow diagram Fig. 1), we analyzed an extensive panel of 30 POPs in relation to the same clinical parameters. Again, for most POPs no statistically significant associations were detected. Statistically significant associations were only found for two organochlorine pesticides, namely HCB levels which were inversely associated with HDL cholesterol and trans-nonachlor which was inversely associated to both Ht and Hb levels (see online supplementary material for Table S2). The biological effects, however, were moderate with standardized β coefficients ranging from −0.30 to −0.50 in the multivariate regression analyses.
We continued with comprehensive DNA methylation analyses to determine whether BEQ, PCBs, and PFOS levels were associated with differentially methylated positions or regions (as these three POP groups showed the largest elevation in blood levels upon consumption of high-polluted eel). No statistically significant positions were detected after correction for multiple testing (FDR < 0.05). However, we found 22, 17, and 29 positions with a P value below 1E−04 that were associated with BEQ levels, PCBs, and PFOS, respectively (see online supplementary material for a color version of Figs S1–S3).
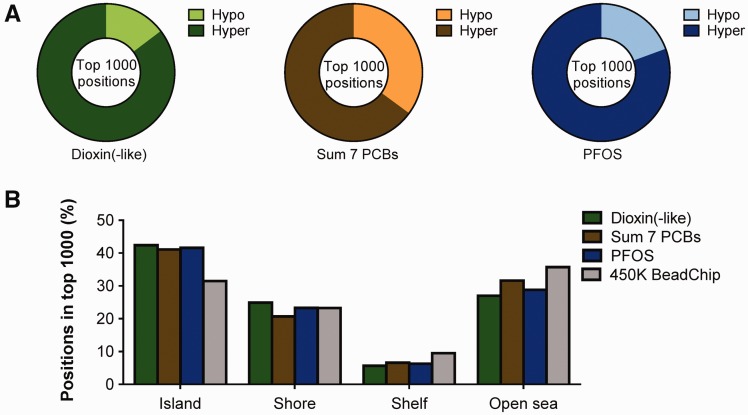
We further explored the DNA methylation differences based on the top 1000 positions with the lowest P value (ranging from 8.8E−07 to 5.5E−03) in more detail. Elevated levels of all three POP groups were associated with hypermethylation of most of the positions (Fig. 2A). Approximately 42% of the top 1000 positions for each of the respective POP groups (BEQ levels 42.4%, PCBs 41.1%, and PFOS 41.6%) were located in CpG islands (CGIs), compared with 31% of the positions on the 450K BeadChip (Fig. 2B). With higher POP-levels, slightly fewer positions were detected in the open sea and shelf as compared with all available positions on the 450K BeadChip. Most positions in the top 1000 were unique between the different POP groups, with only 82 positions identified in all three exposure groups.
Figure 2.
General features of extensive DNA methylation associations with higher POP levels. (A) The fraction of hypo- (inverse association with POP levels) and hypermethylated (positively associated with POP levels) positions, based on the top 1000 most statistically significant positions, determined with a continuous analyses for serum levels of dioxins and dioxin-like compounds (P values between 8.8E−07 and 3.5E−03), the sum of seven indicator PCBs (P values between 1.8E−05 and 5.5E−03), and PFOS (P values between 2.4E−06 and 3.0E−03). (B) Percentages of positions expressed in relationship to CpG density, for the top 1000 positions with the highest statistical significance, for the three POP groups (colored bars) as well as all considered positions on the Illumina 450K BeadChip (grey bars). ‘Islands’ indicate the CpG islands (CGI), ‘Shore’ the CGI shores (0-2 kb from CGI), ‘Shelf’ the CGI shelves (2–4 kb from CGI) and ‘Open sea’ the single positions outside CGIs
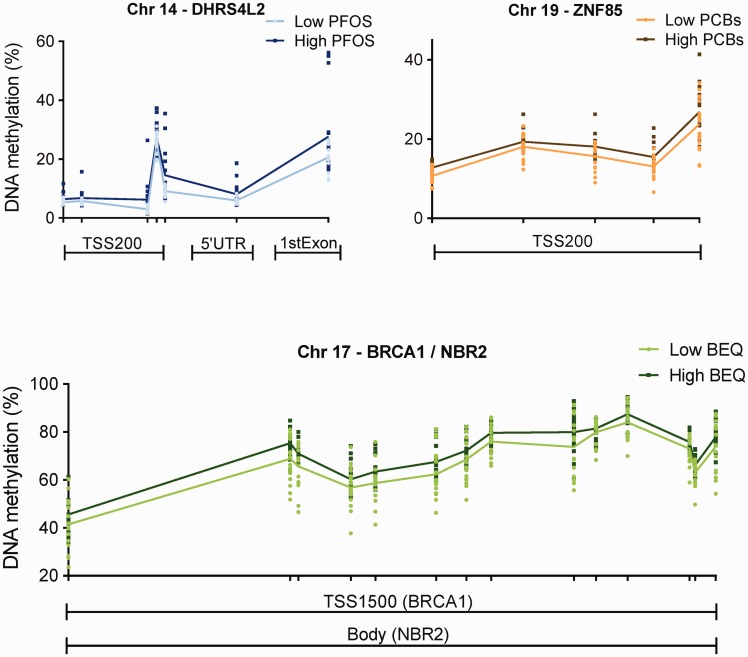
We continued our analysis by searching for regions, rather than single positions, that were differentially methylated upon higher POP levels. In total, we detected 13, 8, and, 38 DMRs for BEQ levels, PCBs, and PFOS, respectively (see online supplementary material for Tables S3–S5). Interestingly, three regions were detected for all three POP groups, namely DHRS4L2, ZNF85, and BRCA1/NBR2 (Fig. 3). DHRS4L2 encodes retinol oxidoreductase protein and ZNF85 encodes a zinc finger (ZNF) protein. BRCA1 is a tumor suppressor gene, while NBR2 (the neighbor of BRCA1) does not encode a protein.
Figure 3.
representative differentially methylated regions detected in leukocytes in relation to PFOS (DHRS4L2), the sum of seven indicator PCBs (ZNF85) and to dioxins and dioxin-like compounds expressed in BEQ (BRCA1/NBR2), although similar DMRs were detected in relation to the other POPs (see online supplementary material for Tables S3–S5). Each dot represents methylation rate at the indicated position of individual samples and the lines show the average methylation levels. Analyses were based on continuous levels of the POPs; however, participants were categorized into two groups for visualization purposes. The light color represents the low-exposed group, while the dark color represents the high-exposed group based on the serum levels (median-split). Median (25–75 percentile) levels for the low- and the high-exposed group are 14.3 (6.9–33.6) and 96.9 (71.8–114) ng/g wet weight for PFOS, 246 (115–371) and 1635 (1095–2484) ng/g lipid for PCBs, and 17.1 (12.2–19.3), and 49.6 (35.2–70.7) for BEQ levels. 5’UTR, five prime untranslated region; Chr, chromosome; TSS200, 200 base pairs from the transcription start site; TSS1500, 1500 base pairs from the transcription start site
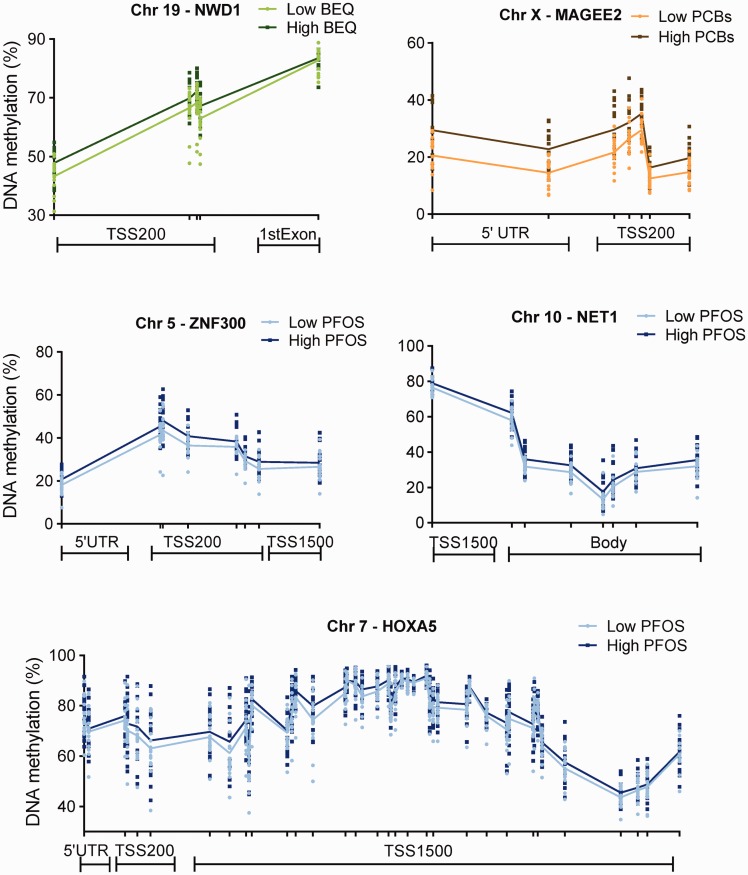
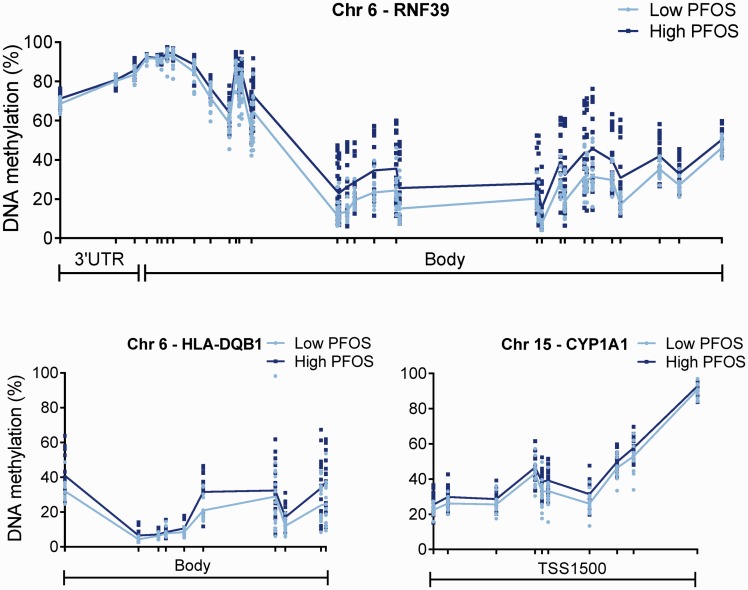
Most of the detected DMRs are annotated to known genes involved in different biological functions. Nevertheless, there were multiple cancer-related genes containing a DMR apart from BRCA1, including MAGEE2, NWD1, ZNF300, HOXA5, NET1, RHOBTB1, EDNRB, and SFRP2 (summary in Fig. 4 and see online supplementary material for Tables S3–S5). Furthermore, DMRs were found in genes with an immune-related function (such as RNF39, HLA-DQB1, (PUS7L)/IRAK, MPZL2, TFCP2, LY6G5C, HCG9, and ZNF416; specific exposures associated with these DMRs are mentioned in online supplementary Tables S3–5). Especially RNF39 and HLA-DQB1 (related to PFOS levels) showed a long stretch of DNA that was differentially methylated (Fig. 5). Also, a DMR associated with PFOS was found in CYP1A1, which plays an important role in the metabolism of many chemicals (Fig. 5 and see online supplementary material for Table S5). In addition, some genes characteristic for germ-line cells were differentially methylated like ESX1 (with PCBs) and MPZL2 (with PFOS), as well as genes associated with neurodevelopment, such as PRRT1 and SLC6A11 in relation to PFOS (see online supplementary material for Tables S3–S5).
Figure 4.
differentially methylated regions (DMRs) detected in genes which have a relationship with cancer (see online supplementary material for Tables S3–S5). DMRs were found in relation to dioxins and dioxin-like compounds (green lines), sum of PCBs (beige lines), and PFOS (blue lines). Each dot represents methylation rate at the indicated position of individual samples and the lines show the average methylation levels. Analyses were based on continuous levels of the POPs; however, participants were categorized into two groups for visualization purposes The light color represents the low-exposed group, while the dark color represents the high-exposed group (median-split). Median (25–75 percentile) levels for the low- and the high-exposed group are 17.1 (12.2–19.3) and 49.6 (35.2–70.7) for BEQ levels, 246 (115–371) and 1635 (1095–2484) ng/g lipid for PCBs, and 14.3 (6.9–33.6) and 96.9 (71.8–114) ng/g wet weight for PFOS. 5’UTR, five prime untranslated region; Chr, chromosome; TSS200, 200 base pairs from the transcription start site; TSS1500, 1500 base pairs from the transcription start site
Figure 5.
Differentially methylated regions (DMRs) detected in genes related to the immune system (RNF39 and HLA-DQB1) and metabolism (CYP1A1) associated with PFOS levels (see online supplementary material for Table S5). Each dot represents methylation rate at the indicated position of individual samples and the lines show the average methylation levels. Analyses were based on continuous levels of the POPs; however, participants were categorized into two groups for visualization purposes The light color represents the low-exposed group, while the dark color represents the high-exposed group (median-split). Median (25–75 percentile) levels for the low- and the high-exposed group are 14.3 (6.9–33.6) and 96.9 (71.8–114) ng/g wet weight. 3’UTR, three prime untranslated region; Chr, chromosome; TSS1500, 1500 base pairs from the transcription start site
Discussion
The present study indicated that elevated levels of dioxins and dioxin-like compounds, sum of seven indicator PCBs, and PFOS were associated with differentially methylated DNA regions. These pollutants, however, did not affect the selected clinical parameters such as hormone levels and liver enzymes. Altered DNA methylation was mostly detected in genes that could be related to other potential adverse health effects, such as cancer and impaired immune responses.
No differences were observed in selected clinical parameters among those with high versus low POP levels. Although thyroid-related health effects from POP exposure are mostly observed in the early life stage, we expected adverse health effects with these, sometimes very high, POP levels based on animal studies [40] and a human study with slightly lower (OH-) PCB levels [41]. In our study, we only found moderate associations between HCB and HDL cholesterol, and trans-nonachlor and both Hb and Ht levels. More pronounced adverse health effects could be expected during sensitive stages such as early development. These parameters are under tight homeostatic regulation, and the men in this study were possibly able to compensate POP-induced effects. Furthermore, the small sample size of our study could have attributed to the lack of power to detect an association between clinical parameters and POP levels. We conclude that in this study POP levels, including very high PCB levels, were not associated with measurable effects in adult men on the clinical parameters studied.
We did observe aberrant DNA methylation among those with high versus low POP levels. While previous observational studies investigating global DNA methylation found both hypo- and hypermethylation [17–20], our study investigating gene-specific methylation found mostly hypermethylation within the affected regions. Aberrant DNA methylation was not investigated in relationship with POP levels before, but was shown in relation to e.g. arsenic [42] and cadmium exposure [43]. Most gene-specific DNA methylation differences were detected in CGIs, where inter-individual and inter-tissue variation is expected to be low compared to CpG-poor areas [44]. CGIs are regions in the DNA that are often associated with transcription start sites, which can result in gene silencing upon hypermethylation [45]. The low variation in these regions might ensure that relatively small DNA methylation differences, due to higher POP-exposure, were detected. On the other side, differences in regions with higher variation may need more statistical power, and consequently larger population studies.
We identified three DMRs with increased methylation levels associated with higher levels of all of the three POP groups. One of these DMRs was located in the promoter of DHRS4L2, relevant for retinol homeostasis, and transcriptionally controlled by DNA methylation [46]. In our study, retinol levels were not affected. In others, plasma retinol levels were also not correlated to dioxins or PCBs, but hepatic retinol levels were dramatically decreased with increasing pollutant levels [47]. Although DHRS4L2 is only one of many genes involved in retinol homeostasis, and we measured DNA methylation in leukocytes instead of a more relevant tissue such as the liver, hypermethylation of this region associated with elevated POP levels, could theoretically be related to a disturbed retinol balance.
Also, ZNF85 was hypermethylated in men with elevated POP levels. ZNF proteins have many key functions and are important as transcriptional regulators [48]. ZNF85 is especially expressed in testicular tissues, with the highest expression levels described in germ-cell tumors and fetal tissue [49]. This gene might have a developmental function, and due to its interaction with many nuclear receptors, differential methylation could potentially lead to a diverse range of adverse health effects.
The third encoding gene detected for all three POP groups was BRCA1. Previous research revealed that dioxin-exposed rat offspring shows hypermethylation and reduced expression of this gene [50]. Also in human breast cancer cells, hypermethylation of BRCA1 was detected after dioxin exposure in vitro [24]. However, while we detected the hypermethylation in the CGI, Papoutsis et al. [51] reported enhanced methylation in the shore. BRCA1 expression is frequently silenced in breast carcinomas and promoter hypermethylation of BRCA1 is associated with tumor progression. Furthermore, it was shown that BRCA1 hypermethylation in leukocytes was more common among breast cancer patients as compared to cancer-free controls [52].
Also HOXA5, known for its developmental role during embryogenesis and the regulation of the tumor suppressor p53, was hypermethylated with higher PFOS levels. Methylation of HOXA5 results in loss of expression of both HOXA5 and p53 and is also related to human breast cancer [53]. Our results may indicate that DNA methylation changes could be an underlying mechanism in breast cancer development due to POP exposure [54]. More research is, however, needed to verify this hypothesis.
In addition, we also detected DMRs in other genes possibly related to cancer development. NWD1 is highly expressed in male reproductive organs, related to androgen receptor levels, and might be involved in prostate cancer development [55]. Melanoma antigen gene family member E2 (MAGEE2) belongs to the larger family of cancer-germline antigens that are frequently overexpressed in cancer. ZNF300 hypermethylation is related to down-regulation of this gene during toxicant-induced malignant formation [56], and hypermethylation of NET1 might affect DNA repair. The association between POPs and DNA methylation of cancer-related genes might explain part of the carcinogenic mechanisms these compounds have without being genotoxic [57].
Interestingly, also a DMR in CYP1A1 was detected, which protein is involved in the metabolism of many pollutants. A relationship between the role of PFOS and CYP1A1 in breast cancer has been proposed [58]. DNA methylation of CYP1A1 has been described to influence expression levels, which subsequently could both affect the half-lives of pollutants and alter the amount of carcinogenic metabolites produced [59].
Furthermore, dioxins and PCBs are known to suppress immune function [60], and also for PFOS such indications exist [61]. A relatively large DMR with a distinct profile in the high-exposed men was detected for RNF39, a transcription factor in the major histocompatibility complex. For both RNF39 [62] and HLA-DQB1 [63], methylation status might be related to impaired immune responses.
This study has several limitations. The cross-sectional nature of our study makes it impossible to determine whether the DNA methylation profiles are caused by the elevated POP levels. Additionally, we must consider that the different POPs investigated are correlated with each other, and therefore the interpretation of the results with regard to individual POPs should be done with caution. Furthermore, we assessed DNA methylation in leukocytes, while most differentially methylated genes are not primarily relevant in leukocytes but in other tissues. Linking DNA methylation and clinical parameters to POP levels is difficult due to the latency between exposure and the manifestation of health consequences. A strength of our study, on the other hand, is the large variation in POP levels in our population, enabling us to investigate DNA methylation in low- to high-exposed men. POP levels are very persistent in the body, making current exposure levels a good estimate of past exposures. Furthermore, the DMRs give more insight into which genes have aberrant DNA methylation than single positions do. We and other studies investigating environmental exposures found little or no statistically significant positions [42, 43], but the analysis for DMRs revealed differential methylation. Although the magnitude of the effects may seem to be modest, previous studies have also found modest effects and suggest it might be biological relevant when longer stretches of DNA have small changes in DNA methylation [64]. Also, this is the first study that explored the hypothesis that POPs could affect gene-specific DNA methylation in humans. Preliminary findings of this study warrant further confirmation in larger and independent study populations and explorative hypotheses generated from the results of our research should be tested using in vitro or in vivo studies.
Conclusions
In our study, we have shown that persistent organic pollutants in environmentally exposed men were associated with (modest) aberrant DNA methylation. The identified genes containing differentially methylated regions are involved in various biological functions implicated in health and disease. These findings carefully imply that DNA methylation may be an underlying mechanism of POP-induced adverse health effects. Additional studies with larger sample sizes are needed to confirm this trend. Revealing the biological implications of aberrant DNA methylation will be an important aspect for future studies.
Ethical Standards
The human study described in this manuscript has been approved by the Medical Ethical Committee of Wageningen University and was performed in accordance with the ethical standards laid down in the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Supplementary Material
Acknowledgements
We are very thankful to the study participants who kindly donated blood and their time, and to Lucy Okma for the blood withdrawal. Furthermore, we are thankful to staff of RIKILT Wageningen UR for the DR CALUX measurements and to staff of the Laboratory for Environmental Toxicology, NMBU, for the extensive POP measurements (especially PCBs and PFOS). This research was supported by the graduate school VLAG (Advanced Studies in Food Technology, Agrobiotechnology, Nutrition and Health Sciences) of Wageningen UR. The support by DUPAN (Dutch sustainable eel foundation) enabling chemical analyses of PCBs and PFOS is highly appreciated.
Supplementary data
Supplementary data is available at EnvEpig online.
Conflict of interest statement. None declared.
Data Availability
Data is available in supplementary material and through the Gene Expression Omnibus (GEO) repository with accession number GSE79329.
Author Contributions
All authors contributed to the study design. M.D. collected all the samples and isolated the DNA. M.D., W.S., and D.K. performed the data analysis. All authors contributed to writing and have approved the manuscript.
References
- 1. Liem AK, Furst P, Rappe C. Exposure of populations to dioxins and related compounds. Food Addit Contam 2000;17:241–59. [DOI] [PubMed] [Google Scholar]
- 2. van den Dungen MW, Kok DE, Polder A, Hoogenboom RL, van Leeuwen SP, Steegenga WT, Kampman E, Murk AJ. Accumulation of persistent organic pollutants in consumers of eel from polluted rivers compared to marketable eel. Environ Pollut 2016;219:80–8. [DOI] [PubMed] [Google Scholar]
- 3. Hoogenboom LAP, Kotterman MJJ, Zeilmaker M, Hoek-van Nieuwenhuizen MK, Van der Lee W, Traag Dioxin and PCB Levels in Eel from Large Rivers in the Netherlands, the Impact of the 2006 TEF Values, and the Risk for the Consumer. Organohalogen Compounds, 2007. 69: p. 122–125. [Google Scholar]
- 4. EU-SCF, Opinion on the risk assessment of dioxins and dioxins-like PCBs in food, 30 May 2001.2001.
- 5. Li Q, Loganath A, Chong YS, Tan J, Obbard JP. Persistent organic pollutants and adverse health effects in humans. J Toxicol Environ Health a 2006;69:1987–2005. [DOI] [PubMed] [Google Scholar]
- 6. Marchesini GR, Meimaridou A, Haasnoot W, Meulenberg E, Albertus F, Mizuguchi M, Takeuchi M, Irth H, Murk AJ. Biosensor discovery of thyroxine transport disrupting chemicals. Toxicol Appl Pharmacol 2008;232:150–60. [DOI] [PubMed] [Google Scholar]
- 7. Egeland GM, Sweeney MH, Fingerhut MA, Wille KK, Schnorr TM, Halperin WE. Total serum testosterone and gonadotropins in workers exposed to dioxin. Am J Epidemiol 1994;139:272–81. [DOI] [PubMed] [Google Scholar]
- 8. Joensen UN, Veyrand B, Antignac JP, Blomberg Jensen M, Petersen JH, Marchand P, Skakkebsek NE, Andersson AM, Le Bizec B, Jorgensen N. PFOS (perfluorooctanesulfonate) in serum is negatively associated with testosterone levels, but not with semen quality, in healthy men. Hum Reprod 2013;28:599–608. [DOI] [PubMed] [Google Scholar]
- 9. Calvert GM, Hornung RW, Sweeney MH, Fingerhut MA, Halperin WE. Hepatic and gastrointestinal effects in an occupational cohort exposed to 2,3,7,8-tetrachlorodibenzo-para-dioxin. JAMA 1992;267:2209–14. [PubMed] [Google Scholar]
- 10. Gallo V, Leonardi G, Genser B, Lopez-Espinosa MJ, Frisbee SJ, Karlsson L, Ducatman AM, Fletcher T. Serum perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS) concentrations and liver function biomarkers in a population with elevated PFOA exposure. Environ Health Perspect 2012;120:655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim HA, Kim EM, Park YC, Yu JY, Hong SK, Jeon SH, Park KL, Hur SJ, Heo Y. Immunotoxicological effects of Agent Orange exposure to the Vietnam War Korean veterans. Ind Health 2003;41:158–66. [DOI] [PubMed] [Google Scholar]
- 12. Michalek JE, Akhtar FZ, Longnecker MP, Burton JE. Relation of serum 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) level to hematological examination results in veterans of Operation Ranch Hand. Arch Environ Health 2001;56:396–405. [DOI] [PubMed] [Google Scholar]
- 13. Lee DH, Porta M, Jacobs Dr, Jr, Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev 2014;35:557–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Noakes R. The aryl hydrocarbon receptor: a review of its role in the physiology and pathology of the integument and its relationship to the tryptophan metabolism. Int J Tryptophan Res 2015;8:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vanden Heuvel JP, Thompson JT, Frame SR, Gillies PJ. Differential activation of nuclear receptors by perfluorinated fatty acid analogs and natural fatty Acids: a comparison of human, mouse, and rat peroxisome proliferator-activated receptor-α, -β, and -γ, liver X receptor-β, and retinoid X receptor-α. Toxicol Sci 2006;92:476–89. [DOI] [PubMed] [Google Scholar]
- 16. Baccarelli A, Bollati V. Epigenetics and environmental chemicals. Curr Opin Pediatr 2009;21:243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Itoh H, Iwasaki M, Kasuga Y, Yokoyama S, Onuma H, Nishimura H, Kusama R, Yoshida T, Yokoyama K, Tsugane S. Association between serum organochlorines and global methylation level of leukocyte DNA among Japanese women: a cross-sectional study. Sci Total Environ 2014;490:603–9. [DOI] [PubMed] [Google Scholar]
- 18. Kim KY, Kim DS, Lee SK, Lee IK, Kang JH, Chang YS, Jacobs DR, Steffes M, Lee DH. Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environ Health Perspect 2010;118:370–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lind L, Penell J, Luttropp K, Nordfors L, Syvanen AC, Axelsson T, Salihovic S, van Bavel B, Fall T, Ingelsson E. et al. Global DNA hypermethylation is associated with high serum levels of persistent organic pollutants in an elderly population. Environ Int 2013;59:456–61. [DOI] [PubMed] [Google Scholar]
- 20. Rusiecki JA, Baccarelli A, Bollati V, Tarantini L, Moore LE, Bonefeld-Jorgensen EC. Global DNA hypomethylation is associated with high serum-persistent organic pollutants in Greenlandic Inuit. Environ Health Perspect 2008;116:1547–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Dioxin (TCDD) induces epigenetic transgenerational inheritance of adult onset disease and sperm epimutations. PLoS One 2012;7:e46249.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Desaulniers D, Xiao GH, Lian H, Feng YL, Zhu J, Nakai J, Bowers WJ. Effects of mixtures of polychlorinated biphenyls, methylmercury, and organochlorine pesticides on hepatic DNA methylation in prepubertal female Sprague-Dawley rats. Int J Toxicol 2009;28:294–307. [DOI] [PubMed] [Google Scholar]
- 23. Wan YJ, Li YY, Xia W, Chen J, Lv ZQ, Zeng HC, Zhang L, Yang WJ, Chen T, Lin Y. et al. Alterations in tumor biomarker GSTP gene methylation patterns induced by prenatal exposure to PFOS. Toxicology 2010;274:57–64. [DOI] [PubMed] [Google Scholar]
- 24. Papoutsis AJ, Borg JL, Selmin OI, Romagnolo DF. BRCA-1 promoter hypermethylation and silencing induced by the aromatic hydrocarbon receptor–ligand TCDD are prevented by resveratrol in MCF-7 cells. J Nutr Biochem 2012;23:1324–32. [DOI] [PubMed] [Google Scholar]
- 25. Tian M, Peng S, Martin FL, Zhang J, Liu L, Wang Z, Dong S, Shen H. Perfluorooctanoic acid induces gene promoter hypermethylation of glutathione-S-transferase Pi in human liver L02 cells. Toxicology 2012;296:48–55. [DOI] [PubMed] [Google Scholar]
- 26. Bovee TF, Hoogenboom LA, Hamers AR, Traag WA, Zuidema T, Aarts JM, Brouwer A, Kuiper HA. Validation and use of the CALUX-bioassay for the determination of dioxins and PCBs in bovine milk. Food Addit Contam 1998;15:863–75. [DOI] [PubMed] [Google Scholar]
- 27. Murk AJ, Leonards PEG, Bulder AS, Jonas AS, Rozemeijer MJC, Denison MS, Koeman JH, Brouwer A. The calux (chemical-activated luciferase expression) assay adapted and validated for measuring TCDD equivalents in blood plasma. Environ Toxicol Chem 1997;16:1583–9. [Google Scholar]
- 28. Hardell E, Carlberg M, Nordström M, van Bavel B. Time trends of persistent organic pollutants in Sweden during 1993–2007 and relation to age, gender, body mass index, breast-feeding and parity. Sci Total Environ 2010;408:4412–9. [DOI] [PubMed] [Google Scholar]
- 29. Steegenga WT, Boekschoten MY, Lute C, Hooiveld GJ, de Groot PJ, Morris TJ, Teschendorff AE, Butcher LM, Beck S, Müller M. Genome-wide age-related changes in DNA methylation and gene expression in human PBMCs. Age 2014;36:9648.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol 2013;14:R115.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Polder A, Gabrielsen GW, Odland JO, Savinova TN, Tkachev A, Loken KB, Skaare JU. Spatial and temporal changes of chlorinated pesticides, PCBs, dioxins (PCDDs/PCDFs) and brominated flame retardants in human breast milk from Northern Russia. Sci Total Environ 2008;391:41–54. [DOI] [PubMed] [Google Scholar]
- 32. Bytingsvik J, van Leeuwen SPJ, Hamers T, Swart K, Aars J, Lie E, Nilsen EME, Wiig Ø, Derocher AE, Jenssen BM. Perfluoroalkyl substances in polar bear mother–cub pairs: a comparative study based on plasma levels from 1998 and 2008. Environ Int 2012;49:92–9. [DOI] [PubMed] [Google Scholar]
- 33. Kok DE, Dhonukshe-Rutten R, Lute AC, Heil SG, Uitterlinden A, van der Velde GN, van Meurs JB, van Schoor NM, Hooiveld GJ, de Groot LC. et al. The effects of long-term daily folic acid and vitamin B12 supplementation on genome-wide DNA methylation in elderly subjects. Clin Epigenet 2015;7:121.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aryee MJ, Jaffe AE, Corrada-Bravo H, Ladd-Acosta C, Feinberg AP, Hansen KD, Irizarry RA. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics 2014;30:1363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sherry ST, Ward MH, Kholodov M, Baker J, Phan L, Smigielski EM, Sirotkin K. dbSNP: the NCBI database of genetic variation. Nucleic Acids Res 2001;29:308–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hansen K, IlluminaHumanMethylation450kanno.ilmn12.hg19: Annotation for Illumina's 450k methylation arrays. R package version 0.2.1.2015.
- 37. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 38. Du P, Zhang X, Huang C, Jafari CN, Kibbe W, Hou AL, Lin SM. Comparison of Beta-value and M-value methods for quantifying methylation levels by microarray analysis. BMC Bioinformatics 2010;11:587.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Peters TJ, Buckley MJ, Statham A, Pidsley L, Samaras RK, Lord RV, Clark SJ, Molloy PL. De novo identification of differentially methylated regions in the human genome. Epigenet Chromatin 2015;8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Montano M, Gutleb AC, Murk AJ. Persistent toxic burdens of halogenated phenolic compounds in humans and wildlife. Environ Sci Technol 2013;47:6071–81. [DOI] [PubMed] [Google Scholar]
- 41. Dallaire R, Dewailly E, Pereg D, Dery S, Ayotte P. Thyroid function and plasma concentrations of polyhalogenated compounds in Inuit adults. Environ Health Perspect 2009;117:1380–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Argos M, Chen Jasmine L, Tong FL, Pierce B, Roy L, Paul-Brutus SR, Gamble MV, Harper K, Parvez NF. et al. ; Gene-specific differential DNA methylation and chronic arsenic exposure in an epigenome-wide association study of adults in Bangladesh. Environ Health Perspect 2014;123:64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kippler M, Engstrom K, Mlakar SJ, Bottai M, Ahmed S, Hossain MB, Raqib R, Vahter M, Broberg K. Sex-specific effects of early life cadmium exposure on DNA methylation and implications for birth weight. Epigenetics 2013;8:494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ziller MJ, Gu H, Muller F, Donaghey J, Tsai LTY, Kohlbacher O, De Jager PL, Rosen ED, Bennett DA, Bernstein BE. et al. Charting a dynamic DNA methylation landscape of the human genome. Nature 2013;500:477–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet 2012;13:484–92. [DOI] [PubMed] [Google Scholar]
- 46. Zhang Q, Li Y, Liu G, Xu X, Song X, Liang B, Li R, Xie J, Du M, Xiao L. et al. Alternative transcription initiation and splicing variants of the DHRS4 gene cluster. Biosci Rep 2009;29:47–56. [DOI] [PubMed] [Google Scholar]
- 47. Murk AJ, Leonards PE, van Hattum B, Luit R, van der Weiden ME, Smit M. Application of biomarkers for exposure and effect of polyhalogenated aromatic hydrocarbons in naturally exposed European otters (Lutra lutra). Environ Toxicol Pharmacol 1998;6:91–102. [DOI] [PubMed] [Google Scholar]
- 48. Berg JM, Shi Y. The galvanization of biology: a growing appreciation for the roles of zinc. Science 1996;271:1081–5. [DOI] [PubMed] [Google Scholar]
- 49. Poncelet DA, Bellefroid EJ, Bastiaens PV, Demoitie MA, Marine JC, Pendeville H, Alami Y, Devos N, Lecocq P, Ogawa T. et al. Functional analysis of ZNF85 KRAB zinc finger protein, a member of the highly homologous ZNF91 family. DNA Cell Biol 1998;17:931–43. [DOI] [PubMed] [Google Scholar]
- 50. Papoutsis AJ, Selmin OI, Borg JL, Romagnolo DF. Gestational exposure to the AhR agonist 2,3,7,8-tetrachlorodibenzo-p-dioxin induces BRCA-1 promoter hypermethylation and reduces BRCA-1 expression in mammary tissue of rat offspring: preventive effects of resveratrol. Mol Carcinog 2015;54:261–9. [DOI] [PubMed] [Google Scholar]
- 51. Alvarez S, Diaz-Uriarte R, Osorio A, Barroso A, Melchor L, Paz MF, Honrado E, Rodriguez R, Urioste M, Valle L. et al. A predictor based on the somatic genomic changes of the BRCA1/BRCA2 breast cancer tumors identifies the non-BRCA1/BRCA2 tumors with BRCA1 promoter hypermethylation. Clin Cancer Res 2005;11:1146–53. [PubMed] [Google Scholar]
- 52. Al-Moghrabi N, Nofel A, Al-Yousef N, Madkhali S, Bin Amer S, Alaiya A, Shinwari Z, Al-Tweigeri T, Karakas B, Tulbah A. et al. The molecular significance of methylated BRCA1 promoter in white blood cells of cancer-free females. BMC Cancer 2014;14:830.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Raman V, Martensen SA, Reisman D, Evron E, Odenwald WF, Jaffee E, Marks J, Sukumar S. Compromised HOXA5 function can limit p53 expression in human breast tumours. 2000;405:974–8. [DOI] [PubMed] [Google Scholar]
- 54. Brody JG, Moysich KB, Humblet O, Attfield KR, Beehler GP, Rudel RA. Environmental pollutants and breast cancer. Cancer 2007;109:2667–711. [DOI] [PubMed] [Google Scholar]
- 55. Correa RG, Krajewska M, Ware CF, Gerlic M, Reed JC. The NLR-related protein NWD1 is associated with prostate cancer and modulates androgen receptor signaling. Oncotarget 2014;5:1666–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Severson PL, Tokar EJ, Vrba L, Waalkes MP, Futscher BW. Coordinate H3K9 and DNA methylation silencing of ZNFs in toxicant-induced malignant transformation. Epigenetics 2013;8:1080–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. IARC, 2,3,7,8-TETRACHLORODIBENZOpara-DIOXIN, 2,3,4,7,8-PENTACHLORODIBENZOFURAN, AND 3,3′,4,4′,5-PENTACHLOROBIPHENYL.2012.
- 58. Ghisari M, Eiberg H, Long M, Bonefeld-Jørgensen EC. Polymorphisms in Phase I and Phase II genes and breast cancer risk and relations to persistent organic pollutant exposure: a case–control study in Inuit women. Environ Health 2014;13:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Fisel P, Schaeffeler E, Schwab M. DNA Methylation of ADME Genes. New York: Clinical Pharmacology & Therapeutics, 2016: p. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 60. Van Den Heuvel RL, Koppen G, Staessen JA, Hond ED, Verheyen G, Nawrot TS, Roels HA, Vlietinck R, Schoeters GE. Immunologic biomarkers in relation to exposure markers of PCBs and dioxins in Flemish adolescents (Belgium). Environ Health Perspect 2002;110:595–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Granum B, Haug LS, Namork E, Stølevik SB, Thomsen C, Aaberge IS, van Loveren H, Løvik M, Nygaard UC. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol 2013;10:�373–9. [DOI] [PubMed] [Google Scholar]
- 62. Lu Y, Cheng Y, Yan W, Nardini C. Exploring the molecular causes of hepatitis B virus vaccination response: an approach with epigenomic and transcriptomic data. BMC Med Genomics 2014;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Martino D, Joo JE, Sexton-Oates A, Dang T, Allen K, Saffery R, Prescott S. Epigenome-wide association study reveals longitudinally stable DNA methylation differences in CD4+ T cells from children with IgE-mediated food allergy. Epigenetics 2014;9:998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tobi EW, Slagboom PE, van Dongen J, Kremer D, Stein AD, Putter H, Heijmans BT, Lumey LH. Prenatal famine and genetic variation are independently and additively associated with DNA methylation at regulatory Loci within IGF2/H19. PLoS One 2012;7:e37933.. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is available in supplementary material and through the Gene Expression Omnibus (GEO) repository with accession number GSE79329.