Abstract
Advanced testicular germ cells, expressing novel cell surface and intracellular proteins, appear after the establishment of central tolerance and thus are auto-immunogenic. However, due to testis immune privilege these germ cells normally do not evoke a detrimental immune response. The Sertoli cell (SC) barrier (also known as the blood–testis barrier) creates a unique microenvironment required for the completion of spermatogenesis and sequesters the majority of the advanced germ cells from the immune system. Given that an intact SC barrier is necessary for spermatogenesis and that disruption of the SC barrier results in loss of advanced germ cells independent of an immune response, this dual role of the SC barrier makes it difficult to directly test the importance of the SC barrier in immune privilege. The ability of SCs to survive and protect co-grafted cells when transplanted ectopically (outside the testis) across immunological barriers is well-documented. Here, we will discuss the use of a SC transplantation model to investigate the role of SC and the SC barrier in immune privilege. Additionally, the formation of cord/tubule like structures in this model, containing both SCs and myoid cells, further extends its application to study testis morphogenesis. We will also discuss the potential use of this model to study the effects of drugs/environmental toxins on testis morphogenesis, tight junction formation and SC–myoid cell interactions.
Keywords: Sertoli cell, blood–testis barrier, Sertoli cell barrier, immune privilege, testis, Sertoli cell transplantation, testis morphogenesis model
Introduction
Sir Peter Medawar first coined the term “immune privilege” in the 1940s when he observed that skin allografts from rabbits transplanted into the brain or anterior chamber of the eye did not elicit an immune response. He believed that these immune privileged sites were “immunologically ignorant” as tissue transplanted to these sites was isolated behind blood tissue barriers and lacked lymphatics, and thereby failed to evoke an immune response [1]. Later in the 1970s, seminal findings by J. Wayne Streilein and others demonstrated that the immune system is not ignorant to the antigens place in privileged sites [1–3]. Instead, immune privilege results from education of the immune system through active regulatory processes that results in tolerance to the transplanted antigens. The first evidence that the testis is an immune privileged site was provided over two centuries ago. However, it was not until the 1970s, when a series of studies describing the survival and functionality of various tissues transplanted into the testis, that strong support for the immune privileged status of the testis was provided (extensively reviewed in [4, 5]). Here, we will describe results from several studies using a Sertoli cell (SC) transplantation model that support the concept that the immune privilege environment in the testis is more than just the blood–testis barrier (BTB). In addition, we will discuss the potential use of this model to study SC immune regulation, testis morphogenesis and the effect of external factors such as environmental toxins on these processes.
Why Testis Immune Privilege Is Critical?
The testis, central to the function of the male reproductive system, consists of seminiferous tubules surrounded by the interstitium. Spermatogenesis takes place inside the seminiferous tubules, which are comprised of germ cells and SCs surrounded by one (rodents) or multiple (large animals) layers of contractile peritubular myoid cells [6, 7]. The interstitium, site of androgen production, consists of Leydig cells, leukocytes, blood vessels, lymphatic vessels and fibroblasts. At the onset of puberty, advanced germ cells expressing novel cell surface and intracellular proteins [8, 9] first differentiate from spermatogonia within the testis. Since these cells first appear after the establishment of central tolerance they are in danger of eliciting an immune response and yet these auto-antigenic germ cells fail to evoke an immune response due to the immune privileged environment within the testis.
The Blood–Testis Barrier and Its Role in Germ Cell Development and Testis Immune Privilege
Similar to other organs, testis immune privilege was first attributed to the unique physical or anatomical components of the testis such as impaired lymphatic drainage, the BTB and the lower temperature or higher Zinc concentration of the testis (reviewed in [4]). In an attempt to identify the mechanism(s) responsible for the unique immune environment of the testis, the contribution of these components was explored. Examination of the testicular lymphatic drainage demonstrated that it was fully functional [10, 11]. Likewise, testes placed into the abdominal cavity, at the normal body temperature, maintained the ability to protect transplanted cells [12, 13]. Furthermore, parathyroid allografts transplanted into the prostate, which contains the highest Zinc concentration of any organ, were rejected suggesting that these properties of the testis do not contribute to testis immune privilege [14]. Additionally, the cellular components of the testis (somatic and germ cells) were examined. The survival of allografts in the testis depleted of Leydig cells [15, 16] and germ cells [12, 13, 17] demonstrated that these cells are not required for testis immune privilege.
Initially it was assumed that, similar to the blood–brain barrier, the BTB exists at the vascular endothelium and thus restricts the exit of molecules from the blood flow into the testis. However, while dyes and radiolabeled substances are completely excluded from the seminiferous tubules, they freely entered the interstitial space suggesting that the BTB is present in or around the seminiferous tubules [18–20]. Later, Fawcett et al., demonstrated that in rodent testis peritubular myoid cells act as a semipermeable barrier by excluding the entry of large molecules (e.g. carbon particles) and limiting the access of small molecules (e.g. lanthum) in the majority of the tubules [7, 21]. At the same time, tight junctions formed between adjacent SCs completely prevented the entrance of molecules (e.g. colloidal thorium, ferritin, horse-radish peroxidase and lanthum) from entering the lumen of the seminiferous tubules. Thus, in testis tight junctions formed between adjacent SCs along with the SC body constitute an effective and impermeable BTB. These tight junctions between SCs, which are located in the basal third of the seminiferous epithelium, divide the seminiferous epithelium into basal (containing spermatogonia and preleptotene spermatocytes) and adluminal (containing advanced germ cells) compartments. Russell and Peterson, used the term the “Sertoli cell barrier” to more adequately define the barrier location at the seminiferous epithelium instead of at the blood vessels [22]. So, from this point onwards we will refer to the BTB as the SC barrier.
The SC barrier should not be perceived as an impenetrable wall as the presence of specific transporters located along the basolateral membrane of the SCs allow the passage of selective molecules while restricting the entry of others. For instance, physiological studies performed on the rete testis fluid demonstrated that the concentration of certain amino acids and ions was significantly different between the rete testis fluid and testicular lymph [23]. This physiological barrier is vital for the development and maturation of advanced germ cells. The role of SCs in germ cell survival, maintenance of spermatogenesis and the spermatogonial stem cell niche, as well as survival of adult Leydig cells is highlighted by SC ablation studies [24–26]. Depletion of SCs at post-natal day 50, after a single injection of diphtheria toxin in mice expressing the diphtheria toxin receptor only in SCs, resulted in extensive loss of germ cells as by 30 days post-injection the lumen of most tubules was acellular. Leydig cell number was significantly reduced compared to controls (37 and 25% of control by 30 and 90 days post-SC ablation, respectively) [25]. One year after diphtheria toxin injection, the tubules had shrunk and contained calcium deposits [25] indicating loss of spermatogenesis and the spermatogonial stem cell niche.
In addition to creating an environment adequate for germ cell development, the SC barrier prevents the passage of antibodies into the adluminal compartment of the seminiferous epithelium [27, 28]. For instance, the immunoglobulin concentration in the rete testis fluid was 0.2% the concentration in serum [27] and immunoglobulins were not detected in the tubule fluid indicating a lower level of immunoglobulins in the seminiferous tubules than in the rete testis fluid [29]. Interestingly, despite the presence of immune cells in the testis interstitium [30], lymphocytes are not detected beyond the myoid cell layer suggesting that the semipermeable barrier created by peritubular myoid cells is sufficient to inhibit the entry of immune cells into the seminiferous epithelium [31]. SC ablation studies further support the role of peritubular myoid cells in restricting the entry of immune cells into the seminiferous tubules. For example, after depletion of SCs at puberty or in adult mice, peritubular myoid cells were still present and maintained the basement membrane [25]. After loss of SCs an immune response was generated in the testis of these mice, as evident by an increase in interstitial macrophages and pro-inflammatory cytokine TNF-α by 10 and 30 days post-injection, respectively [25]. Macrophage infiltration was not detected in the seminiferous tubules with an intact myoid layer suggesting that the myoid cells and underlying extracellular matrix is sufficient to exclude immune cells from the tubules. Collectively, lack of antibodies and immune cells in the seminiferous tubules demonstrates that the SC barrier plays a role in testis immune privilege. However, given that an intact SC barrier is necessary to maintain the unique environment necessary for germ cell development and that disruption of the SC barrier results in loss of advanced germ cells independent of an immune response, this dual role of the SC barrier makes it difficult to directly test the importance of the SC barrier in testis immune privilege. Therefore, a model that could separate these functions is needed.
Understanding the Immunological Role of SCs and the SC Barrier through SC Transplantation Studies
The initial studies on testis immune privilege pointed to a role for SCs. Therefore, Selawry et al. [32], co-grafted allogeneic pancreatic islets with syngeneic or allogeneic SC-enriched fractions underneath the kidney capsule of diabetic rats. In this study, 65% of the co-grafted animals remained normoglycemic for over 100 days, while none of the animals receiving islets alone became normoglycemic. However, a short course of immune suppression (cyclosporine for 3 days) was required for the SCs to prolong survival of allogeneic islets. Korbutt et al. [33], extended these results by modifying the SC isolation method and adding a recovery period by culturing the cells in vitro as aggregates for 48 h. Electron microscopy revealed that tight junctions were formed between adjacent SCs during this recovery period. Co-transplantation of allogeneic islets with the aggregated SCs resulted in 100% islet graft survival (based on normoglycemia) for at least 100 days without the requirement of immune suppression. Double immunostaining the grafts for insulin (islet cell marker) and vimentin (SC marker) demonstrated that the islets were present in close proximity to SCs. Korbutt et al. [33], concluded that “The aggregated state of SCs, which allows the formation of intercellular tight junctions, promotes intercellular cooperation and creates a more functional effector unit, more closely resembling the organization of SCs within the seminiferous tubules”. Subsequent studies demonstrated that Sertoli cellular aggregates can protect co-grafted islets from an autoimmune response [34, 35] and xenogeneic rejection [36–38] (also reviewed in [39]). These studies primarily focused on investigating the importance of immunoregualtory factors expressed by SCs in protecting the islets while the role of the SC barrier in this protection was largely overlooked.
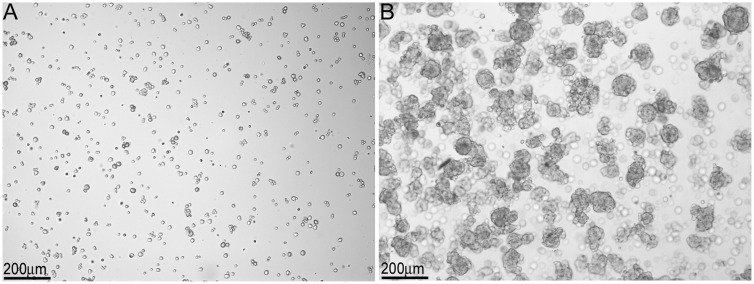
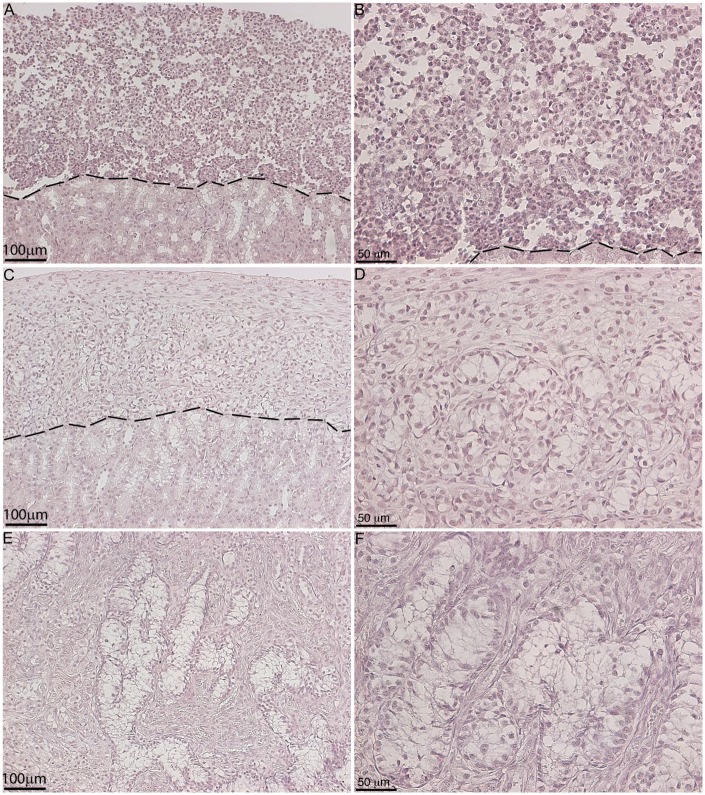
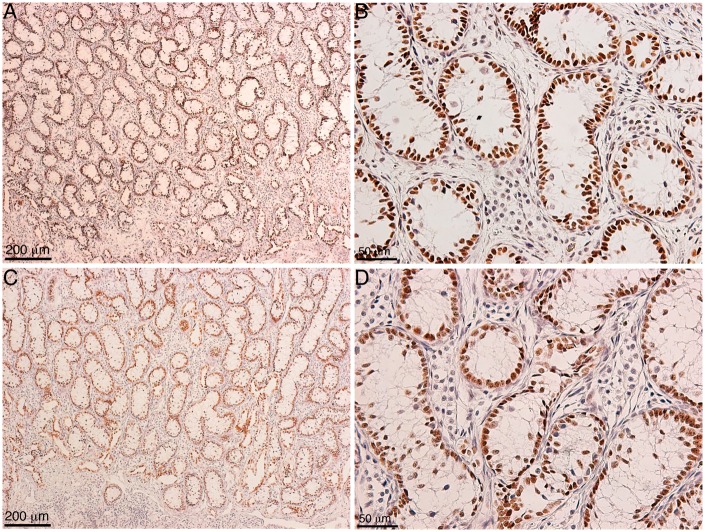
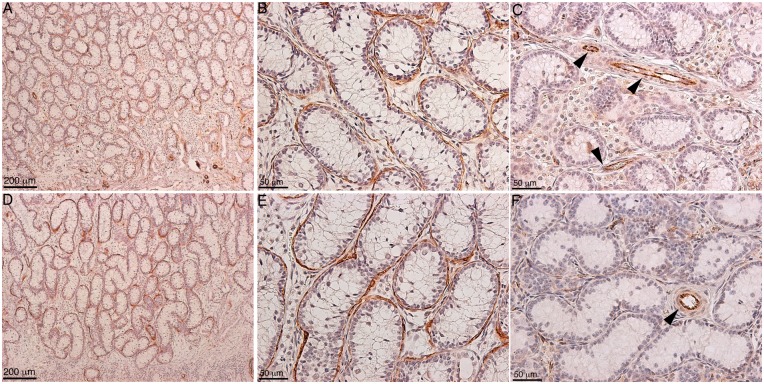
Within our SC–islet co-grafts [40], we observed that the SCs were arranged in tubule-like structures similar to those in the testis. This suggested us that transplanted SCs could be used to study testis function. Therefore, in 2002 we developed a model to study testicular morphogenesis. [41]. In this model, SCs were isolated from neonatal pig testes. The isolation method resulted in dissociated SCs (Fig. 1A), which were then cultured in vitro for 48 h on non-tissue culture treated petri dishes in Ham’s F10 media with supplements and 10% heat-inactivated neonatal pig serum [41]. These culture conditions resulted in reaggregation of the dissociated SCs (Fig. 1B). These Sertoli cellular aggregates, containing 92.5 ± 3.5% SCs and 2.2 ± 0.7% myoid cells, were transplanted underneath the kidney capsule of naïve severe combined immunodeficient (SCID) mice. Morphological and histological analysis of graft bearing kidneys, collected between 0 and 150 days post-transplantation, was performed to analyze the progressive development of structures resembling testicular cords. Immediately after transplantation, Sertoli cellular aggregates were randomly arranged and by day 3 post-transplantation the SCs and myoid cells had begun to organize into clusters forming precursors to cords (Fig. 2A–D). With progression of time, cord/tubule like structures similar to those found in germ cell depleted (SC only) seminiferous tubules were detected (Fig. 2E and F). Analysis of grafts, collected at days 90 and 150 post-transplantation, for Wilms’ Tumor 1 (WT1; SC marker) and smooth muscle alpha actin (myoid cell marker) revealed that the SCs were arranged with their nuclei along the basal edge adjacent to the myoid cells that were surrounding the tubules (Figs 3 and 4). Additionally, numerous blood vessels were also detected within the grafts (Fig. 4C and F). The vessels were located outside of the tubule-like structures, consistent with a recent study describing the potential role of pertubular myoid cells in inhibiting vascular growth resulting in the avascularity of the seminiferous tubules within the testis [42].
Figure 1:
Isolation and in vitro culture of neonatal pig SCs. Male neonatal pigs (1–3 days old) were used as testis donors. Testes were surgically removed and placed in cold Hanks balanced salt solution (HBSS) supplemented with 0.25% (w/v) fraction V bovine serum albumin (Sigma Chemical Company, St Louis, MO). The testes were cut into small fragments, digested for 10 min at 37 °C with collagenase type V (2.5 mg/ml; Sigma), and then washed with cold HBSS. The tissue was further suspended in calcium-free medium supplemented with 1mM ethyleneglycoltetraacetic acid (EGTA) and digested with trypsin (25 μg/ml; Boehringer Mannheim, Laval, Canada) and DNase (4 μg/ml, Boehringer) for 10 min at 37 °C. The tissue was then passed through a nylon mesh, washed with HBSS, and cultured in nontreated petri dishes in Ham’s F10 media with supplements (10 mmol/L D-glucose, 2mmol/L L-glutamine, 50 μmol/L isobutylmethylxanthine, 0.5% bovine serum albumin, 10 mmol/L nicotinamide, 100U/ml penicillin and 100 μg/ml streptomycin) and 10% heat-inactivated neonatal pig serum. (A) Immediately after isolation, dissociated NPSC were detected in the media and (B) after 48 h of culture the formation of Sertoli cellular aggregates was observed.
Figure 2:
Cord-like structures and tubule formation by transplanted SCs. Eleven million neonatal pig Sertoli cellular aggregates, containing myoid cells, were transplanted underneath the kidney capsule of male SCID mice (6–8 weeks old; Taconic Farms). Graft bearing kidneys were collected at 0 (A, B), 3 (C, D), and 21 (E, F) days post-transplantation, immersed in Z-fix and embedded in paraffin. The tissue sections were stained with hematoxylin and eosin. B, D and F represents higher magnification images of A, C and E, respectively.
Figure 3:
Immunolocalization of transplanted SCs in the cord/tubule like structures. Eleven million neonatal pig Sertoli cellular aggregates, containing myoid cells, were transplanted underneath the kidney capsule of male SCID mice (6-8 weeks old; Taconic Farms). Grafts were collected at 90 (A, B) and 150 (C, D) days post-transplantation. Tissue sections were immunostained with WT1 (SC marker, brown color, A-D; 1:10 dilution; Dako/Agilent Technologies, Santa Clara, CA) antibody. The sections were counterstained with hematoxylin to detect cell nuclei (blue color, A–D). B and D are higher magnification images of A and C, respectively.
Figure 4:
Immunolocalization of myoid cells in the cord/tubule like structures. Eleven million neonatal pig Sertoli cellular aggregates, containing myoid cells, were transplanted underneath the kidney capsule of male SCID mice (6–8 weeks old; Taconic Farms). Grafts were collected at 90 (A–C) and 150 (D–F) days post-transplantation. Tissue sections were immunostained with smooth muscle alpha-actin (myoid cell marker, brown color, A, B, D and E; 1:50 dilution; Dako/Agilent Technologies) or CD31/PECAM1 (endothelial cell marker, brown color, C and F; 1:10 dilution; Abcam, Cambridge, MA) antibodies. The sections were counterstained with hematoxylin to detect cell nuclei (blue color, A–F). B and E are higher magnification images of A and D, respectively. The brown peritubular myoid cells can be seen surrounding the tubule structures (A, B, D and E). Arrowheads in C and F represent endothelial cells lining the blood vessels.
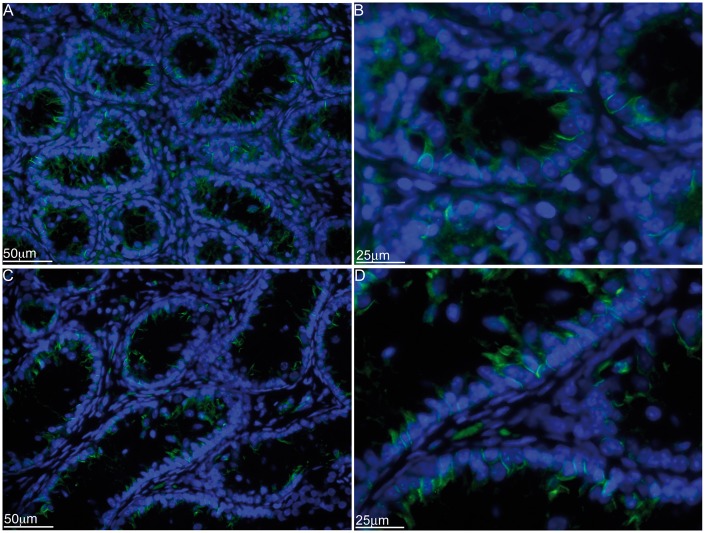
Given the similar arrangement to testicular seminiferous tubules, we further analyzed the grafts for the tight junction protein, claudin-11, which was detected between the adjacent SCs arranged in tubule-like structures (Fig. 5). Collectively, this demonstrated that the transplanted SCs and myoid cells can orient themselves into testis like tubules ectopically and thus this in vivo transplantation model can be used to study testis development/morphogenesis such as seminiferous cord formation and Sertoli–myoid cell interactions. Moreover, detection of claudin-11 between SCs suggests the presence of tight junctions between transplanted SCs, further validating that the transplantation model can be used to study the immunological role of the SC barrier in SC immune privilege.
Figure 5:
Immunolocalization of claudin-11 in the cord/tubule like structures. Eleven million neonatal pig Sertoli cellular aggregates, containing myoid cells, were transplanted underneath the kidney capsule of male SCID mice (6–8 weeks old; Taconic Farms). Graft bearing kidneys were collected at 90 (A and B) and 150 (C and D) days post-transplantation and immunostained for claudin-11 (tight junction protein, green color, A–D; 1:100 dilution; Santa Cruz Biotechnology, Inc., Dallas, TX). The sections were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; ThermoFisher Scientific, Waltham, MA) to detect cell nuclei (blue color, A-D). B and D are higher magnification images of A and C, respectively.
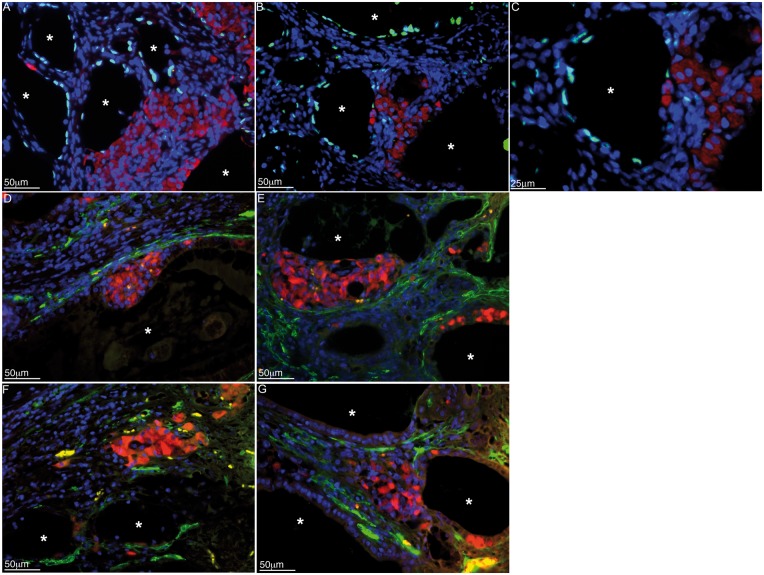
Thus, we analyzed SC/islet co-grafts for cellular localization and ectopic tubule formation. Allogeneic BALB/c SCs and islets were co-transplanted underneath the kidney capsule of diabetic C3H mice without any immunosuppression [43, 44]. The survival time of co-transplanted islets was significantly prolonged compared to controls (containing islets only), with 61.5% of grafts surviving beyond 60 days. Double immunostaining the successful grafts, collected at over 100 days post-transplantation, for WT1 and insulin revealed that the majority of the transplanted islets were located outside the tubules (Fig. 6A–C) indicating immune protection of islets within the grafts is not dependent on sequestering the islets behind the SC barrier. However, since myoid cells alone are sufficient to restrict the entry of immune cells into the seminiferous tubules in the testis, it is plausible that co-transplanted islets are protected behind the myoid cell layer surrounding the tubules. Double immunostaining of the grafts for insulin and smooth muscle alpha actin demonstrated that the myoid cell barrier is also not critical for islet cell survival as islets were present both inside (Fig. 6D and E) and outside (Fig. 6F and G) the myoid cell layer. Collectively, these data suggest that rather than sequestering the transplanted islets within the SC barrier, the barrier plays an indirect role in protecting the islets. For instance, primary SCs co-cultured with peritubular myoid cells stimulated clusterin (an immunoregulatory factor) secretion by SCs (2–3-fold) compared to controls (SCs cultured alone) [45]. Peritubular myoid cells, cultured alone, did not produce considerable amounts of clusterin [45]. Thus, SC interactions with other SCs or myoid cells, achieved through tubule like arrangement, could be important for production of immunoregualtory factors and protection of co-grafted cells. Overall, the transplanted cells create the appropriate immunoregulatory environment necessary for protection of co-grafted cells.
Figure 6:
Immunolocalization of islets, SCs and myoid cells in the grafts. Primary mouse SCs were isolated from BALB/c mice (8–10 days old; Charles Rivers Laboratories; Wilmigton, MA) and cultured in vitro as described in Fig. 1. Islets were isolated from male BALB/c mice (6–8 weeks old; Charles Rivers Laboratories) by collagenase digestion of the pancreas and Ficoll density gradient purification. The isolated islets were cultured in nontreated petri dishes in Ham’s F10 media with supplements at 37 °C. Three million BALB/c SCs were co-transplanted with 500 BALB/c islets underneath the kidney capsule of diabetic C3H mice as allografts. The graft bearing kidneys were collected from normoglycemic mice at day 102 post-transplantation. The tissue sections were double immunostained for WT1 (SC marker, green, A–C) or smooth muscle alpha-actin (myoid cell marker, green, D–G) and insulin (islet cell marker, red, A–G; 1:1000 dilution; Dako/Agilent Technologies). Sections were counterstained with DAPI to detect cell nuclei (blue color, A–G; ThermoFisher Scientific). Asterisks represent SC tubules.
Of additional relevance to protection of germ cells from an autoimmune attack, SCs also provide immune protection to islets in a non-obese diabetic (NOD) mouse model of autoimmune type 1 diabetes. In these mice, syngeneic islets are destroyed by an autoimmune mechanism. Using this model, SCs co-transplanted with pancreatic islets were capable of prolonging syngeneic islet graft survival regardless of whether the islets were co-grafted with the SCs within the same kidney or transplanted to separate kidneys, demonstrating systemic tolerance independent of the SC barrier. Overall islet graft survival was significantly prolonged to over 60 days in 40–64% of the recipients, while all recipients that received islets only (no SCs) rejected the islet grafts within 14 days [34, 35]. Moreover, transplantation of encapsulated neonatal porcine SCs into the intraperitoneal cavity of diabetic or prediabetic NOD mice resulted in the return to normoglycemia or prevention of diabetes in 81 or 88% of the mice, respectively. Further analysis indicated that the SCs reestablished systemic immune tolerance within the mice, thus, preventing the autoimmune attack on the islets, while also promoting beta cell regeneration [46, 47]. These studies support the ability of SCs to modulate the whole immune environment rather than just protecting cells sequestered behind the SC barrier.
Additional support that testis immune privilege is more than just the SC barrier comes from several studies. For instance, foreign tissue engrafted into the testis interstitium outside the SC barrier enjoyed extended survival (reviewed in [4, 48]). Preleptotene spermatocytes, located in the basal compartment outside the SC barrier, are immunogeneic and yet are not attacked by the immune system [49]. Routine fine needle biopsies, causing local injury to the seminiferous tubules, fail to elicit an immune response against auto-immunogeneic germ cells [50]. Also, in seasonal breeders, the SC barrier is cyclically disrupted during the non-breeding period and yet the development of meiotic spermatocytes is possible in the absence of this barrier [51]. Collectively, these studies suggest that the whole testis, rather than the adluminal compartment, is immune privileged.
In the testis, SCs create a unique microenvironment for germ cell development by sequestering the advanced germ cells behind the SC barrier and by expressing immunoregualtory molecules that protect these auto-antigeneic germ cells from immune attack (reviewed in [4, 48]). Interestingly, SCs are capable of creating a comparable immune modulatory environment that provides immune protection to co-transplanted cells when they are transplanted ectopically. With this model, our lab has demonstrated that SCs survive allo- and xeno-transplantation by inducing regulatory T cells and regulatory macrophages (M2 macrophages). Similar modifications to immune cells by SCs is likely how they protect germ cells within the testis. In an attempt to identify factors important for SC immune regulation, we performed microarray analysis on the enriched primary SC preparations that have been shown to survive as allografts after transplantation in mice [52]. The gene expression profile was compared between these SCs and MSC-1 cells, a mouse Sertoli cell line that is rejected when allotransplanted. Two thousand three hundred and sixty nine genes were differentially expressed by at least 4-fold, including 340 immune related genes. For instance, thrombospondin 1 and 2, which convert latent transforming growth factor (TGF)β into active TGFβ, are upregulated 4.3 and 11.2-fold, respectively, in enriched primary SCs compared to MSC-1 cells. Active TGF-β is important for inducing regulatory immune cells such as regulatory T cells and regulatory macrophages [53, 54]. Additionally, several potential immune regulatory pathways were identified including inhibition of complement activation and membrane associated cell lysis, suppression of inflammation by specific cytokines and prostanoid molecules, and slowing of leukocyte migration by controlled cell junctions and actin polymerization.
Differential expression of these genes could be due to epigenetic differences between the primary SCs and MSC-1 cells as it was shown that the lack of follicle stimulating hormone receptor (FSHr) expression in MSC-1 cells is due to an epigenetic change when compared with primary SCs. Comparison of the DNA methylation pattern in the FSHr promoter in MSC-1 cells and SCs identified four methylated CpG dinucleotides in the MSC-1 cells. This methylation is thought to explain the SC specific expression of FSHr in the male. Consistently the FSHr promoter was also found to be methylated in the promoter region in other tissues that do not express FSHr [55]. Further comparison of the specific epigenetic modifications regulating the immune regulatory pathways in SCs and MSC-1 cells would be interesting but requires further study.
Other Potential Uses of This SC Transplantation Model
Testicular tissue grafting, implanting pieces of testicular tissue into rodents, is currently utilized to study regulation of spermatogenesis, effect of drugs on testicular function and pathophysiology of testicular tissue [56–58]. However, the effects of drugs/environmental toxins on testis morphogenesis, testicular cell interactions as they come together to form cords and tight junction formation are difficult to investigate using the testis tissue grafting technique as the seminiferous tubules are already established. Our SC transplantation model utilizes SCs and myoid cells that are dissociated during the isolation procedure. Furthermore, we have demonstrated that dissociated SCs and myoid cells can reaggregate in vitro, and reform cords/tubules in vivo suggesting this model could be useful to answer these questions.
During fetal testis development SCs aggregate with primordial germ cells, begin to differentiate and become polarized, and peritubular myoid cells surround the aggregating SCs and primordial germ cells [59]. Thus, SC–peritubular myoid cell interactions promote seminiferous cord formation during early testis development. At the onset of puberty these cords then give rise to seminiferous tubules [59]. Utilizing the SC transplantation model, containing myoid cells, the effects of environmental toxins on SC specific gene expression/epigenetics, SC–peritubular myoid cell interactions and cord/tubule formation could be analyzed ectopically. A system to analyze these effects independent of the other testicular cells would help identify their individual contributions. Additionally, the isolation protocol could be modified to include other testicular cells such as Leydig cells and spermatogonia in the isolated testicular cellular aggregates. Thereby, interactions among other testicular cells and spermatogenesis can also be studied.
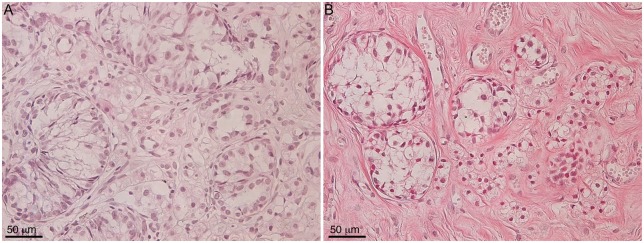
Historically, the kidney capsule is used as a transplantation site to study cell survival. However, it cannot be used to study spermatogenesis due to its high temperature (37 °C versus 32 °C required for spermatogenesis). In a preliminary attempt to study whether isolated Sertoli cellular aggregates can orient themselves into testis like tubules at lower temperature, Sertoli cellular aggregates (isolated from neonatal pigs) were transplanted subcutaneously under the skin. The transplanted cells were collected at days 34 and 61 post-transplantation and analyzed for tubule formation (Fig. 7). Similar to the kidney transplantation site, the SCs transplanted subcutaneously were arranged in tubule like structures suggesting that transplantation of Sertoli cellular aggregates, containing spermatogonia, can be used to study spermatogenesis ectopically. In 2006, a three-dimensional culture system containing dissociated testicular cells plated on extracellular matrix gel to study testicular development was also utilized [60–62]. Here, testicular cells isolated from postnatal rats were plated on extracellular matrix gel and after 3 days spherical cell aggregates were observed in vitro [62]. Xenografting of extracellular matrix gel-enclosed spherical testicular cell aggregates was required for further progression of the morphogenetic cascade [62]. Although, this model offers advantages over testis tissue grafting, the use of matrigel in three-dimensional culture system has some pitfalls. For instance, matrigel contains murine laminin 1, collagen IV, heparin sulfate proteoglycans and TGF-β, which can interfere with experimental analysis when testing specific factors [63]. Nonetheless, together these studies suggest that Sertoli cellular aggregates can be utilized to study testis morphogenesis after transplantation.
Figure 7:
Immunolocalization of SCs in the cord/tubule like structures. Eleven million neonatal pig Sertoli cellular aggregates, containing myoid cells, were transplanted subcutaneously in male SCID mice (6–8 weeks old; Taconic Farms). Grafted cells were collected at 34 (A) and 61 (B) days post-transplantation, immersed in Z-fix and embedded in paraffin. The tissue sections were stained with hematoxylin and eosin.
Spermatogenesis requires a controlled hormonal environment and thus the testis is vulnerable to endocrine-disrupting chemicals (EDC) such as phthalates, pesticides, phytoestrogens, xenoestrogens and fungicides. SCs and myoid cells express androgen receptor thereby putting these cells at higher risk from EDC [64]. Dobrinski et al., evaluated the effects of phthalates on testis maturation and function by transplanting testis fragments from 6 months old rhesus macaques subcutaneously into SCID mice that were exposed to different doses of phthalates [65]. Utilizing this model, it was demonstrated that exposure to a high dose (500 mg/kg, used as a positive control) impaired tubule lumen formation, germ cell differentiation and reduced the number of spermatogonia, while a low dose of phthalates (10 mg/kg, physiological dose) did not affect testis maturation but reduced SC number [65]. Phthalate exposure is associated with increased inflammation, oxidative stress and macrophage activation [66–68]. Thus, a decreased observance of detrimental effects on testis morphology, utilizing the low dose of phthalates, could be attributed to the use of immune deficient mice in this study. The SC transplantation model using testicular cells transplanted into a syngeneic immune competent recipient (e.g. BALB/c testicular cells into a BALB/c mouse) could be used to determine the combined effects of phthalates and the immune response on testis cord formation, SC tight junctions, myoid cell barrier and SC–myoid cell interactions.
Heavy metals such as lead and cadmium chloride disrupt seminiferous tubules thereby adversely affecting spermatogenesis [69, 70]. These heavy metals affect SCs directly by breaking down SC tight junctions resulting in increased SC barrier permeability and damaging the SC mitochondria and endoplasmic reticulum resulting in apoptosis [69, 70]. For instance, primary SCs plated on reconstituted basement membrane were utilized to study the effects of cadmium on SC tight junctions in vitro [71] by measuring the tight junction assembly using transepithelial electrical resistance (TER). An increase in TER across the SC epithelia indicated the formation of inter-SC tight junctions. Exposure of these cells to cadmium chloride perturbed the inter-SC tight junction assembly in a dose dependent manner which was accompanied by a steady decline in occludin expression [71]. In addition to the in vitro cultured SC model, the SC transplantation model can further provide insight in determining the effects of heavy metals on SC–myoid cell interactions, SC tight junctions and permeability of the SC–myoid cell barrier.
Another advantage of this model is the ability to genetically manipulate or treat the isolated cells in vitro prior to transplantation. For example, we have engineered isolated mouse, rat, and pig SCs to express insulin as a potential treatment for type 1 diabetes [72–74]. Initially using an adenoviral vector containing furin-modified human proinsulin cDNA, we found that the engineered SCs were able to transiently decrease blood glucose levels after transplantation into diabetic SCID mice. More recently using a lentiviral vector, we have demonstrated the long-term stable expression of insulin by SCs after allotransplantation into BALB/c mice. Other investigators have genetically modified SCs to express chemokine receptor 7 (CCR7) to direct SCs transplanted into the abdominal cavity to the secondary lymphoid organs or inhibited the expression of indoleamine, 2, 3-dioxygenase (IDO) by SCs using siRNA to test the importance of IDO in SC protection from diabetes in NOD mice [46, 75]. In the later study, survival was prolonged to over 130 days in over 80% of mice that received SCs transduced with control siRNA, while all mice transplanted with SCs lacking IDO expression developed diabetes. These studies demonstrate the feasibility of engineering SCs in vitro prior to transplantation. This approach could be used to manipulate the cells to either express or inhibit other factors related to testis morphogenesis or immune regulation in order to investigate the individual role of these factors.
Collectively, the SC transplantation model provides an additional method to shed light on SC immune regulation and the role of the SC barrier in testis immune privilege. This model is advantageous in that it allows for the modification of the cells in vitro prior to transplantation as well as a method to separate the role of SCs and myoid cells from the other testicular cells such as Leydig cells or macrophages that may play a role in immune modulation. The formation of cord like structures and tubules in this model, which contains both SCs and myoid cells, further extends its application to study testis morphogenesis, tight junction formation and SC–myoid cell interactions. Furthermore, the effects of environmental toxins or other treatments on SC gene expression/epigenetics, immune privilege, testis morphogenesis, and the SC–myoid cell barrier could also be tested utilizing this model.
Funding
This work was supported in part by NIAID grant Al109398, and funding from The Jasper L. and Jack Denton Wilson Foundation and Ted Nash Long Life Foundation.
Conflict of interest statement. None declared.
References
- 1. Streilein JW. Unraveling immune privilege. Science 1995;270:1158–9. [DOI] [PubMed] [Google Scholar]
- 2. Kaplan HJ, Streilein JW.. Immune response to immunization via the anterior chamber of the eye. I. F. lymphocyte-induced immune deviation. J Immunol 1977;118:809–14. [PubMed] [Google Scholar]
- 3. Streilein JW, Wegmann Tg.. Immunologic privilege in the eye and the fetus. Immunol Today 1987;8:362–6. [DOI] [PubMed] [Google Scholar]
- 4. Kaur G, Mital P, Dufour JM.. Testisimmune privilege - assumptions versus facts. Anim Reprod 2013;10:3–15. [PMC free article] [PubMed] [Google Scholar]
- 5. Setchell Bp. The testis and tissue transplantation: historical aspects. J Reprod Immunol 1990;18:1–8. [DOI] [PubMed] [Google Scholar]
- 6. Dym M. The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood-testis barrier. Anat Rec 1973;175:639–56. [DOI] [PubMed] [Google Scholar]
- 7. Dym M, Fawcett DW.. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod 1970;3:308–26. [DOI] [PubMed] [Google Scholar]
- 8. O'Rand MG, Romrell LJ.. Appearance of cell surface auto- and isoantigens during spermatogenesis in the rabbit. Dev Biol 1977;55:347–58. [DOI] [PubMed] [Google Scholar]
- 9. Tung PS, Fritz Ib.. Specific surface antigens on rat pachytene spermatocytes and successive classes of germinal cells. Dev Biol 1978;64:297–315. [DOI] [PubMed] [Google Scholar]
- 10. Fawcett DW, Neaves WB, Flores MN.. Comparative observations on intertubular lymphatics and the organization of the interstitial tissue of the mammalian testis. Biol Reprod 1973;9:500–32. [DOI] [PubMed] [Google Scholar]
- 11. Head JR, Neaves WB, Billingham RE.. Reconsideration of the lymphatic drainage of the rat testis. Transplantation 1983;35:91–5. [DOI] [PubMed] [Google Scholar]
- 12. Head JR, Billingham RE.. Immune privilege in the testis. II. Evaluation of potential local factors. Transplantation 1985;40:269–75. [DOI] [PubMed] [Google Scholar]
- 13. Selawry HP, Whittington K.. Extended allograft survival of islets grafted into intra-abdominally placed testis. Diabetes 1984;33:405–6. [DOI] [PubMed] [Google Scholar]
- 14. Whitmore WF, Gittes RF.. Intratesticular grafts: the testis as an exceptional immunologically privileged site. Trans Am Assoc Genitourin Surg 1978;70:76–80. [PubMed] [Google Scholar]
- 15. Cameron DF, et al. Successful islet/abdominal testis transplantation does not require Leydig cells. Transplantation 1990;50:649–53. [DOI] [PubMed] [Google Scholar]
- 16. Selawry HP, Whittington KB.. Prolonged intratesticular islet allograft survival is not dependent on local steroidogenesis. Horm Metab Res 1988;20:562–5. [DOI] [PubMed] [Google Scholar]
- 17. Whitmore Wf 3rd, Karsh L, Gittes RF.. The role of germinal epithelium and spermatogenesis in the privileged survival of intratesticular grafts. J Urol 1985;134:782–6. [DOI] [PubMed] [Google Scholar]
- 18. Kormano M. Dye permeability and alkaline phosphatase activity of testicular capillaries in the postnatal rat. Histochemie 1967;9:327–38. [DOI] [PubMed] [Google Scholar]
- 19. Kormano M. Penetration of intravenous trypan blue into the rat testis and epididymis. Acta Histochem 1968;30:133–6. [PubMed] [Google Scholar]
- 20. Setchell BP. The blood-testicular fluid barrier in sheep. J Physiol (Lond) 1967;189:63P–5P. [PubMed] [Google Scholar]
- 21. Fawcett DW, Leak LV, Heidger PM.. Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil 1970;10:105–22. [PubMed] [Google Scholar]
- 22. Russell LD, Peterson RN.. Sertoli cell junctions: morphological and functional correlates. Int Rev Cytol 1985;94:177–211. [DOI] [PubMed] [Google Scholar]
- 23. Setchell BP, Voglmayr JK, Waites GM.. A blood-testis barrier restricting passage from blood into rete testis fluid but not into lymph. J Physiol 1969;200:73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith LB, O'Shaughnessy PJ, Rebourcet D.. Cell-specific ablation in the testis: what have we learned?. Andrology 2015;3:1035–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rebourcet D, et al. Sertoli cells maintain Leydig cell number and peritubular myoid cell activity in the adult mouse testis. PLoS One 2014;9:e105687.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shinomura M, et al. A novel Amh-Treck transgenic mouse line allows toxin-dependent loss of supporting cells in gonads. Reproduction 2014;148:H1–9. [DOI] [PubMed] [Google Scholar]
- 27. Johnson MH, Setchell BP.. Protein and immunoglobulin content of rete testis fluid of rams. J Reprod Fertil 1968;17:403–6. [DOI] [PubMed] [Google Scholar]
- 28. Johnson MH. The distribution of immunoglobulin and spermatozoal autoantigen in the genital tract of the male guinea pig: its relationship to autoallergic orchitis. Fertil Steril 1972;23:383–92. [DOI] [PubMed] [Google Scholar]
- 29. Koskimies Ai, Kormano M, Lahti A.. A difference in the immunoglobulin content of seminiferous tubule fluid and rete testis fluid of the rat. J Reprod Fertil 1971;27:463–5. [DOI] [PubMed] [Google Scholar]
- 30. Hedger MP, Hales DB.. Immunophysiology of the male reproductive tract In: Neill JD. (ed.), Knobil and Neill’s Physiology of Reproduction. Academic Press, 2006, 1195–286. [Google Scholar]
- 31. Dym M, Romrell LJ.. Intraepithelial lymphocytes in the male reproductive tract of rats and rhesus monkeys. J Reprod Fertil 1975;42:1–7. [DOI] [PubMed] [Google Scholar]
- 32. Selawry HP, Cameron DF.. Sertoli cell-enriched fractions in successful islet cell transplantation. Cell Transplant 1993;2:123–9. [DOI] [PubMed] [Google Scholar]
- 33. Korbutt GS, Elliott JF, Rajotte RV.. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes 1997;46:317–22. [DOI] [PubMed] [Google Scholar]
- 34. Korbutt GS, et al. Testicular Sertoli cells exert both protective and destructive effects on syngeneic islet grafts in non-obese diabetic mice. Diabetologia 2000;43:474–80. [DOI] [PubMed] [Google Scholar]
- 35. Suarez-Pinzon W, et al. Testicular sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta1-dependent mechanism. Diabetes 2000;49:1810–8. [DOI] [PubMed] [Google Scholar]
- 36. Dufour JM, et al. Immunoprotection of rat islet xenografts by cotransplantation with sertoli cells and a single injection of antilymphocyte serum. Transplantation 2003;75:1594–6. [DOI] [PubMed] [Google Scholar]
- 37. Luca G, et al. Improved function of rat islets upon co-microencapsulation with Sertoli's cells in alginate/poly-L-ornithine. AAPS PharmSciTech 2001;2:E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang H, Wright J. R. Jr., Co-encapsulation of Sertoli enriched testicular cell fractions further prolongs fish-to-mouse islet xenograft survival. Transplantation 1999;67:815–20. [DOI] [PubMed] [Google Scholar]
- 39. Mital P, Kaur G, Dufour JM.. Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction 2010;139:495–504. [DOI] [PubMed] [Google Scholar]
- 40. Kin T, et al. Development of an immunoprivileged site to prolong islet allograft survival. Cell Transplant 2002;11:547–52. [PubMed] [Google Scholar]
- 41. Dufour JM, Rajotte RV, Korbutt GS.. Development of an in vivo model to study testicular morphogenesis. J Androl 2002;23: 635–44. [PubMed] [Google Scholar]
- 42. Windschuttl S, et al. Human testicular peritubular cells secrete pigment epithelium-derived factor (PEDF), which may be responsible for the avascularity of the seminiferous tubules. Sci Rep 2015;5:12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dufour JM, et al. Sertoli cell line lacks the immunoprotective properties associated with primary Sertoli cells. Cell Transplant 2008;17:525–34. [DOI] [PubMed] [Google Scholar]
- 44. Dufour JM, et al. Comparison of successful and unsuccessful islet/Sertoli cell cotransplant grafts in streptozotocin-induced diabetic mice. Cell Transplant 2008;16:1029–38. [PubMed] [Google Scholar]
- 45. Zwain IH, et al. Regulation of Sertoli cell alpha 2-macroglobulin and clusterin (SGP-2) secretion by peritubular myoid cells. Biol Reprod 1993;48:180–7. [DOI] [PubMed] [Google Scholar]
- 46. Fallarino F, et al. Therapy of experimental type 1 diabetes by isolated Sertoli cell xenografts alone. J Exp Med 2009;206:2511–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Luca G, et al. Xenograft of microencapsulated sertoli cells reverses T1DM in NOD mice by inducing neogenesis of beta-cells. Transplantation 2010;90:1352–7. [DOI] [PubMed] [Google Scholar]
- 48. Kaur G, Thompson LA, Dufour JM.. Sertoli cells–immunological sentinels of spermatogenesis. Semin Cell Dev Biol 2014;30:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yule TD, et al. Autoantigenic germ cells exist outside the blood testis barrier. J Immunol 1988;141:1161–7. [PubMed] [Google Scholar]
- 50. Mallidis C, Baker HW.. Fine needle tissue aspiration biopsy of the testis. Fertil. Steril 1994;61:367–75. [PubMed] [Google Scholar]
- 51. Pelletier RM. Cyclic formation and decay of the blood-testis barrier in the mink (Mustela vison), a seasonal breeder. Am J Anat 1986;175:91–117. [DOI] [PubMed] [Google Scholar]
- 52. Doyle TJ, et al. Immunoprotective properties of primary Sertoli cells in mice: potential functional pathways that confer immune privilege. Biol Reprod 2012;86:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cao Q, et al. IL-10/TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol 2010;21:933–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Worthington JJ, et al. Regulation of TGFbeta in the immune system: an emerging role for integrins and dendritic cells. Immunobiology 2012;217:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Griswold Md, Kim Js.. Site-specific methylation of the promoter alters deoxyribonucleic acid-protein interactions and prevents follicle-stimulating hormone receptor gene transcription. Biol Reprod 2001;64:602–10. [DOI] [PubMed] [Google Scholar]
- 56. Arregui L, Dobrinski I.. Xenografting of testicular tissue pieces: 12 years of an in vivo spermatogenesis system. Reproduction 2014;148:R71–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Honaramooz A, et al. Sperm from neonatal mammalian testes grafted in mice. Nature 2002;418:778–81. [DOI] [PubMed] [Google Scholar]
- 58. Johnson L., et al. Heterotopic transplantation as a model to study the regulation of spermatogenesis; some histomorphological considerations about sperm decline in man. Contracept Fertil Sex, 1997;25:549–55. [PubMed] [Google Scholar]
- 59. Skinner MK, Anway MD.. Seminiferous cord formation and germ-cell programming: epigenetic transgenerational actions of endocrine disruptors. Ann N Y Acad Sci 2005;1061:18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dores C, Dobrinski I.. De novo morphogenesis of testis tissue: an improved bioassay to investigate the role of VEGF165 during testis formation. Reproduction 2014;148:109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gassei K, Ehmcke J, Schlatt S.. Initiation of testicular tubulogenesis is controlled by neurotrophic tyrosine receptor kinases in a three-dimensional Sertoli cell aggregation assay. Reproduction 2008;136:459–69. [DOI] [PubMed] [Google Scholar]
- 62. Gassei K, Schlatt S, Ehmcke J.. De novo morphogenesis of seminiferous tubules from dissociated immature rat testicular cells in xenografts. J Androl 2006;27:611–8. [DOI] [PubMed] [Google Scholar]
- 63. Benelli R. et al. Models of inflammatory processes in cancer In: Stevenson CS, Marshall LA, Morgan DW (ed.), In Vivo Models of Inflammation. Die Deutsche Bibliothek, 2006, 83–102. [Google Scholar]
- 64. Wang RS, et al. Androgen receptor roles in spermatogenesis and fertility: lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev 2009;30:119–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rodriguez-Sosa JR, et al. Phthalate esters affect maturation and function of primate testis tissue ectopically grafted in mice. Mol Cell Endocrinol 2014;398:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Campioli E, Martinez-Arguelles DB, Papadopoulos V.. In utero exposure to the endocrine disruptor di-(2-ethylhexyl) phthalate promotes local adipose and systemic inflammation in adult male offspring. Nutr Diab 2014;4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ferguson KK, et al. Urinary phthalate metabolite associations with biomarkers of inflammation and oxidative stress across pregnancy in Puerto Rico. Environ Sci Technol 2014;48:7018–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Murphy CJ, Stermer AR, Richburg JH.. Age, species-dependent infiltration of macrophages into the testis of rats and mice exposed to mono-(2-Ethylhexyl) phthalate (MEHP). Biol Reprod 2014;91:18.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Marettova E, Maretta M, Legath J.. Toxic effects of cadmium on testis of birds and mammals: a review. Anim Reprod Sci 2015;155:1–10. [DOI] [PubMed] [Google Scholar]
- 70. Siu ER, et al. Cadmium-induced testicular injury. Toxicol Appl Pharmacol 2009;238:240–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chung NP, Cheng CY.. Is cadmium chloride-induced inter-sertoli tight junction permeability barrier disruption a suitable in vitro model to study the events of junction disassembly during spermatogenesis in the rat testis?. Endocrinology 2001;142:1878–88. [DOI] [PubMed] [Google Scholar]
- 72. Halley K, et al. Delivery of a therapeutic protein by immune-privileged Sertoli cells. Cell Transplant 2010;19:1645–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kaur G, et al. Sustained expression of insulin by a genetically engineered sertoli cell line after allotransplantation in diabetic BALB/c mice. Biol Reprod 2014;90:109.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mital P, et al. Nondividing, postpubertal rat sertoli cells resumed proliferation after transplantation. Biol Reprod 2014;90:13.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lim HG, et al. Cell-mediated immunomodulation of chemokine receptor 7-expressing porcine sertoli cells in murine heterotopic heart transplantation. J Heart Lung Transplant 2009;28:72–8. [DOI] [PubMed] [Google Scholar]