Abstract
Background
Regional cerebral oxygen saturation (rSO2) monitoring by near-infrared spectroscopy provides valuable information regarding cerebral oxygen delivery, and it has been increasingly used in cardiovascular surgery. Although it has been shown that dialysis-dependent patients [hemodialysis (HD) patients] suffer from low cerebral perfusion, limited information is available on cerebral tissue oxygenation levels in HD patients.
Findings
In this retrospective study, the preoperative rSO2 values in 9 HD patients undergoing coronary artery bypass graft surgery were compared with those in 40 non-HD patients. HD patients had lower preoperative rSO2 values than non-HD patients (median: 46 vs. 68%, respectively, P < 0.001). Despite adjusting for age, hemoglobin concentration, and left ventricular ejection fraction using multivariable linear regression, HD showed a strong association with low rSO2 (estimated coefficient: −20.4, P < 0.001).
Conclusions
HD showed a strong association with low preoperative rSO2 values in patients undergoing coronary artery bypass graft surgery, even after adjusting for known factors that affect rSO2 values, including age, hemoglobin concentration, and cardiac systolic function. Further research is required to elucidate the mechanisms decreasing rSO2 values in HD patients.
Keywords: Hemodialysis, Regional cerebral oxygen saturation, Cardiac surgery
Findings
Introduction
Regional cerebral oxygen saturation (rSO2) monitoring by near-infrared spectroscopy has been increasingly used to assess the adequacy of cerebral oxygen delivery in patients undergoing cardiovascular surgery [1, 2]. Previous studies have shown that rSO2, measured by near-infrared spectroscopy, is affected by several factors, including age, hemoglobin concentration, and cardiac function [3–7].
It has been shown that end-stage renal disease patients on hemodialysis (HD) suffer from low cerebral perfusion, which may contribute to cognitive deficits and high stroke prevalence observed in dialysis-dependent patients (HD patients) [8]. However, limited information is currently available on cerebral tissue oxygenation levels in HD patients.
Here, we hypothesized that the preoperative rSO2 values in HD patients undergoing cardiac surgery is lower than those in non-HD patients, reflecting low cerebral blood flow. The current study was designed to compare the preoperative rSO2 values in HD patients and non-HD patients undergoing coronary artery bypass grafting (CABG).
Methods
Our study was approved by the ethics committee of Kyoto University (approval number: R0719), and the requirement for written informed consent was waived. We included adult (≥20 years of age) patients who underwent isolated CABG at Kyoto University Hospital between January 1, 2012 and December 31, 2015. Patients who concurrently underwent cardiac valvular or aortic surgery were excluded. Patients without available rSO2 values taken before administration of oxygen or anesthetic drugs were also excluded.
Specific data from the electronic medical records of participants, including age, gender, body mass index, comorbidities, and preoperative examination findings, were retrospectively collected. Left ventricular ejection fraction (LVEF) was derived from preoperative transthoracic echocardiography records. For hemoglobin concentration values, the most recent value measured before the surgery was used.
Patients received no premedication. Upon entering the operating room, preoperative rSO2 and arterial oxygen saturation by pulse oximetry (SpO2) were obtained before administration of oxygen or anesthetic drugs. rSO2 values were detected using the INVOS 5100 system (Somanetics, Troy, MI) with probes containing light sources, each providing two continuous wavelengths of near-infrared light (730 and 810 nm) that reach a brain area corresponding to the junction between the anterior and middle cerebral artery vascularization territory. Two probes were attached to the right and the left sides of the forehead; the preoperative rSO2 values were defined as the mean of rSO2 values obtained from these two probes.
Data were analyzed using the statistical program R (http://cran.r-project.org). Continuous data are presented as median (interquartile range), and categorical variables are expressed as a number (percentage). Differences between groups were compared using the Mann–Whitney U test for continuous variables. For categorical variables, Pearson’s chi-square or Fisher’s exact tests were used as appropriate. Because it has been shown that rSO2 is affected by age, hemoglobin concentration, and cardiac function [3–7], we used multivariable linear regression analysis to assess the independent impact of HD on rSO2 values after adjustment for these factors. We constructed a linear regression model with rSO2 as a dependent variable and HD status, age, hemoglobin concentration, and LVEF as independent variables, and beta coefficients were calculated for all independent variables. All statistical tests were two-tailed, and the statistical significance was set at a P value of <0.05.
Results
A total of 80 patients underwent isolated CABG during the study period. Of these, 31 patients were excluded because of the lack of rSO2 measurements before administration of oxygen or anesthetic drugs; the remaining 49 patients were included in the analysis. Among these 49 patients, 9 patients (18.4%) received HD preoperatively. Duration of HD in HD patients ranged from 2 to 14 years; 4 patients (44.4%) underwent HD for ≥10 years. Patient characteristics stratified by HD status are presented in Table 1. HD patients had significantly higher prevalence of diabetes mellitus and arteriosclerosis obliterans and significantly lower hemoglobin concentrations than non-HD patients. Although it did not reach statistical significance, LVEF in HD patients tended to be lower than that in non-HD patients.
Table 1.
Patient characteristics stratified by HD
| Non-HD patients (n = 40) |
HD patients (n = 9) |
P value | |
|---|---|---|---|
| Age (years) | 73 (64–78) | 73 (69–77) | 0.846 |
| Female gender | 7 (17.5%) | 2 (22.2%) | 0.741 |
| Body mass index (kg/m2) | 23.4 (21.7–25.3) | 22.2 (19.4–24.2) | 0.245 |
| Hypertension | 30 (75.0%) | 7 (77.8%) | 0.861 |
| Diabetes mellitus | 13 (32.5%) | 9 (100.0%) | <0.001 |
| ASO | 1 (2.5%) | 4 (44.4%) | <0.001 |
| Hemoglobin concentration (g/dL) | 12.7 (11.9–14.1) | 10.1 (9.7–11.8) | 0.001 |
| LVEF (%)a | 66.0 (55.3–72.0) | 50.0 (29.9–73.9) | 0.398 |
HD hemodialysis, ASO arteriosclerosis obliterans, LVEF left ventricular ejection fraction, rSO 2 regional cerebral oxygen saturation
aLVEF was missing in one non-HD patient
Distributions of preoperative rSO2 and SpO2 values stratified by HD status are shown in Fig. 1. HD patients had significantly lower rSO2 values than non-HD patients [46% (43–50%) vs. 68% (64–73%), P < 0.001; Fig. 1a]. No patients had an rSO2 value of ≤50% in the non-HD group, whereas 7 out of 9 HD patients (77.8%) had an rSO2 value of ≤50%. SpO2 values of study participants ranged from 94–100% and were comparable between groups (P = 0.625; Fig. 1b).
Fig. 1.
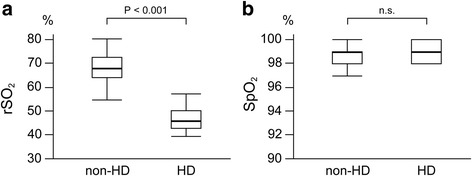
Box plots of preoperative rSO2 (a) and SpO2 (b) values stratified by HD. Horizontal bold lines represent median values. rSO 2 regional cerebral oxygen saturation, SpO 2 arterial oxygen saturation of pulse oximetry, HD hemodialysis, n.s. not significant
Multivariable linear regression analysis was performed to assess the impact of HD on preoperative rSO2 values after adjustment for age, hemoglobin concentration, and LVEF, which have been shown to affect rSO2 in previous studies [3–7]. HD maintained a strong association with low rSO2, even after adjusting for age, hemoglobin concentration, and LVEF. The estimated coefficient for HD in the multivariable model was −20.4 (95% confidence interval: −26.3 to −14.4) (Table 2).
Table 2.
Multivariable linear regression analysis for the association of HD and rSO2, adjusting for age, hemoglobin concentration, and LVEF
| Beta (95% confidence interval) | P value | |
|---|---|---|
| Intercept | 60.9 (39.0 to 82.8) | |
| HD | −20.4 (−26.3 to −14.4) | <0.001 |
| age | −0.03 (−0.22 to 0.16) | 0.753 |
| Hemoglobin concentration | 0.38 (−0.78 to 1.55) | 0.510 |
| LVEFa | 0.08 (−0.05 to 0.20) | 0.236 |
HD hemodialysis, rSO 2 regional cerebral oxygen saturation, LVEF left ventricular ejection fraction
aOne non-HD patient with missing LVEF data was excluded from this analysis
Discussion
This study demonstrated that HD patients had significantly lower preoperative rSO2 values, even after adjusting for known factors that affect rSO2, including age, hemoglobin concentration, and LVEF.
Several reports have suggested that patients undergoing HD have low rSO2 values, reflecting low cerebral perfusion [9, 10]; however, they did not assess cardiac function, which is known to affect rSO2 values [7]. In our study, we were able to compare rSO2 values in HD and non-HD patients after adjusting for LVEF because most patients underwent preoperative transthoracic echocardiography. Although HD patients had significantly lower hemoglobin concentrations than non-HD patients, and LVEF in HD patients tended to be lower, the estimated coefficient for HD in the multivariable linear regression analysis, adjusting for age, hemoglobin concentration, and LVEF, was −20.4. This means that HD patients had 20.4% lower rSO2 values than non-HD patients after adjusting for these factors, indicating that low rSO2 values in HD patients are, at least in part, because of factors other than age, anemia, or cardiac systolic function. Although it is not possible to determine the specific cause of low rSO2 values in HD patients in our study, possible explanations are as follows: (1) metabolic acidosis frequently seen in HD patients decreases affinity between hemoglobin and oxygen [11] and decreases microcirculatory oxygen saturation. (2) The acute intravascular volume loss and fluid shifts that occur during dialysis induce cerebral edema and decrease intracerebral blood pressure, blood velocity, and cerebral perfusion [12]. (3) Cerebral atrophy seen in HD patients [13] might increase the thickness of the cerebrospinal fluid layer, which decreases the intensity of near-infrared light that the detector can receive, thereby, decreasing rSO2 values [5]. Further research is required to elucidate the mechanisms by which the rSO2 values are decreased in HD patients.
It is important to determine adequate target values of rSO2 to guide intraoperative management in HD patients. rSO2 monitoring may be used to guide hemodynamics and CPB management during cardiac surgery by adjusting therapy based on relative changes from the preoperative baseline (i.e., to maintain relative rSO2 > 80% of baseline) [14]. However, this concept might allow too low rSO2 values and be harmful for majority of HD patients, who frequently have abnormally low baseline rSO2 values. Future research should be conducted to establish the appropriate target value of rSO2 in HD patients.
This study had certain limitations, primarily based on its retrospective design. We used only preoperative LVEF to evaluate cardiac function because data on diastolic function or cardiac output were not available in most patients. In addition, we could not adjust for the influence of some factors, which are suggested to be related to rSO2, including partial pressure of carbon dioxide in arterial blood, central venous pressure, skull thickness, and area of cerebrospinal fluid layer [4, 5]. The number of patients was small; however, it was still sufficient to support our hypothesis.
Conclusions
In conclusion, this study demonstrated that HD showed strong associations with low preoperative rSO2 values in patients undergoing CABG, even after adjusting for known factors that affect rSO2 values, including age, hemoglobin concentration, and cardiac systolic function.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for the English language review.
Funding
This work was in part supported by JSPS KAKENHI (grant number 16K20092; TM, principle investigator).
Authors’ contributions
SM conceptualized and designed the study, collected the data, performed the statistical analysis, and drafted the first version of the manuscript. MH helped draft the manuscript. TM helped with the statistical analysis and critically revised the manuscript. All authors read and approved the manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- CABG
Coronary artery bypass grafting
- HD
Hemodialysis
- LVEF
Left ventricular ejection fraction
- rSO2
Regional cerebral oxygen saturation
- SpO2
Arterial oxygen saturation by pulse oximetry
References
- 1.Fischer GW. Recent advances in application of cerebral oximetry in adult cardiovascular surgery. Semin Cardiothorac Vasc Anesth. 2008;12:60–9. doi: 10.1177/1089253208316443. [DOI] [PubMed] [Google Scholar]
- 2.Murkin JM, Arango M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br J Anaesth. 2009;103:i3–13. doi: 10.1093/bja/aep299. [DOI] [PubMed] [Google Scholar]
- 3.Kishi K, Kawaguchi M, Yoshitani K, Nagahata T, Furuya H. Influence of patient variables and sensor location on regional cerebral oxygen saturation measured by INVOS 4100 near-infrared spectrophotometers. J Neurosurg Anesthesiol. 2003;15:302–6. doi: 10.1097/00008506-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Moritz S, Rochon J, Völkel S, Hilker M, Hobbhahn J, Graf BM, Arlt M. Determinants of cerebral oximetry in patients undergoing off-pump coronary artery bypass grafting: an observational study. Eur J Anaesthesiol. 2010;27:542–9. doi: 10.1097/00003643-201006121-00393. [DOI] [PubMed] [Google Scholar]
- 5.Yoshitani K, Kawaguchi M, Miura N, Okuno T, Kanoda T, Ohnishi Y, Kuro M. Effects of hemoglobin concentration, skull thickness, and the area of the cerebrospinal fluid layer on near-infrared spectroscopy measurements. Anesthesiology. 2007;106:458–62. doi: 10.1097/00000542-200703000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Papadopoulos G, Karanikolas M, Liarmakopoulou A, Berris A. Baseline cerebral oximetry values in elderly patients with hip fractures: a prospective observational study. Injury. 2011;42:1328–32. doi: 10.1016/j.injury.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Paquet C, Deschamps A, Denault AY, Couture P, Carrier M, Babin D, Levesque S, Piquette D, Lambert J, Tardif JC. Baseline regional cerebral oxygen saturation correlates with left ventricular systolic and diastolic function. J Cardiothorac Vasc Anesth. 2008;22:840–6. doi: 10.1053/j.jvca.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Prohovnik I, Post J, Uribarri J, Lee H, Sandu O, Langhoff E. Cerebrovascular effects of hemodialysis in chronic kidney disease. J Cereb Blood Flow Metab. 2007;27:1861–9. doi: 10.1038/sj.jcbfm.9600478. [DOI] [PubMed] [Google Scholar]
- 9.Hoshino T, Ookawara S, Goto S, Miyazawa H, Ito K, Ueda Y, Kaku Y, Hirai K, Nabata A, Mori H, Yoshida I, Tabei K. Evaluation of cerebral oxygenation in patients undergoing long-term hemodialysis. Nephron Clin Pract. 2014;126:57–61. doi: 10.1159/000358432. [DOI] [PubMed] [Google Scholar]
- 10.Papadopoulos G, Dounousi E, Papathanasiou A, Papathanakos G, Tzimas P. Cerebral oximetry values in dialyzed surgical patients: a comparison between hemodialysis and peritoneal dialysis. Ren Fail. 2013;35:855–9. doi: 10.3109/0886022X.2013.794675. [DOI] [PubMed] [Google Scholar]
- 11.Opdahl H, Strømme TA, Jørgensen L, Bajelan L, Heier HE. The acidosis-induced right shift of the HbO2 dissociation curve is maintained during erythrocyte storage. Scand J Clin Lab Invest. 2011;71:314–21. doi: 10.3109/00365513.2011.565366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murray AM, Tupper DE, Knopman DS, Gilbertson DT, Pederson SL, Li S, Smith GE, Hochhalter AK, Collins AJ, Kane RL. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67:216–23. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 13.Drew DA, Bhadelia R, Tighiouart H, Novak V, Scott TM, Lou KV, Shaffi K, Weiner DE, Sarnak MJ. Anatomic brain disease in hemodialysis patients: a cross-sectional study. Am J Kidney Dis. 2013;61:271–8. doi: 10.1053/j.ajkd.2012.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denault A, Deschamps A, Murkin JM. A proposed algorithm for the intraoperative use of cerebral near-infrared spectroscopy. Semin Cardiothorac Vasc Anesth. 2007;11:274–81. doi: 10.1177/1089253207311685. [DOI] [PubMed] [Google Scholar]


