Abstract
Background
Bacillus cereus (B. cereus) rarely causes lower respiratory tract infections, although most reported cases of B. cereus pneumonia are fatal despite intensive antibiotic therapy. We present a case of B. cereus pneumonia in an immunocompetent patient.
Case presentation
An 81-year-old woman was transferred from a district general hospital to our hospital for treatment of congestive heart failure. The patient presented with a nonproductive cough, dyspnea, edema in both lower extremities, orthopnea, fever, and occult blood in the stool. A chest radiograph indicated bilateral pleural effusion and pulmonary congestion. After diuretic therapy and chest drainage, bilateral pleural effusion and pulmonary congestion improved. On day 2, she experienced severe respiratory distress. B. cereus was isolated from two blood sample cultures. On day 4, her condition had progressed to severe respiratory distress (PaO2/FiO2 ratio = 108). A chest radiograph and computed tomography indicated extensive bilateral infiltrates. She was transferred to the intensive care unit and was intubated. B. cereus was also isolated from five blood sample cultures at that time. After isolating B. cereus, we switched antibiotics to a combination of imipenem and levofloxacin, which were effective. She had no history of immunodeficiency, surgery, ill close contacts, risk factors for HIV or tuberculosis, recent central venous catheter insertion, or anthrax vaccination. She improved and was discharged from the intensive care unit after several days.
Conclusion
This is a rare case of B. cereus pneumonia in an immunocompetent patient, who subsequently recovered. Bacillus should be considered as a potential pathogen when immunocompetent patients develop severe pneumonia.
Keywords: Bacillus cereus, Pneumonia, Immunocompetent patient
Background
Bacillus cereus (B. cereus) is a Gram-positive, aerobic to facultative, spore-forming rod that is widely distributed in the environment [1]. B cereus is occasionally associated with food-borne illness, its presence in cultures is often considered a contaminant, and it is typically not further characterized beyond a descriptive identification and may not be reported at all [2]. However, severe hematogenous infections caused by B. cereus have been reported, especially in drug addicts, premature neonates, and patients with severe underlying diseases or compromised immunity [3]. B. cereus rarely causes lower respiratory tract infections, although most reported cases of B. cereus pneumonia are fatal despite intensive antibiotic therapy [3]. Here we present a case of B. cereus pneumonia in an immunocompetent patient.
Case presentation
An 81-year-old woman (160 cm, 60 kg) was transferred from a district general hospital to our hospital for treatment of congestive heart failure. At admission, the patient presented with a nonproductive cough, dyspnea, edema in both lower extremities, orthopnea, fever, and occult blood in the stool, all of which began to about 10 days prior to admission. Past medical history included atrial fibrillation (AF). She had a no drinking and smoking history, and activities of daily living were in the normal range. Her temperature was 37.3 °C, pulse rate was 133 beats per min with AF, respiratory rate was 24 breaths per min, and blood pressure was 115/58 mmHg. Laboratory findings were as follows: WBC count, 11,040 cells/mm3 without a left shift; hematocrit, 34.7%; platelets, 145,000/mm3; creatinine, 0.8 mg/dL; alanine aminotransferase, 69 U/mL; aspartate aminotransferase, 56 IU/mL; total bilirubin, 1.4 mg/dL; and respiratory alkalosis. A chest radiograph indicated bilateral pleural effusion and pulmonary congestion (Fig. 1). We performed transthoracic echocardiography (TTE). TTE showed an ejection fraction of 50%; verrucas were not detected. Intravenous ampicillin/sulbactam (6 g/day) therapy was initiated to treat a potential bacterial infection (Fig. 2). After diuretic therapy and chest drainage, bilateral pleural effusion and pulmonary congestion improved. On day 2, she experienced severe respiratory distress, with diminished breath sounds, bilateral pulmonary crackles, and an oxygen saturation of 98% while receiving 100% oxygen through a nonrebreather 4-L mask. B. cereus was isolated from two blood sample cultures (arterial blood and venous blood) collected at admission (Fig. 3), and intravenous levofloxacin (250–500 mg/day) therapy was initiated instead of ampicillin/sulbactam therapy (Fig. 2). No pathogenic organisms were identified by sputum and pleural effusion of chest drain cultures. On day 3, clindamycin (1200 mg/day) was combined with levofloxacin (Fig. 2). On day 4, her condition progressed to severe respiratory distress (PaO2/FiO2 ratio = 108). A chest radiograph (Fig. 4) and computed tomography (CT) (Fig. 5) indicated extensive bilateral infiltrates. Her temperature was 37.5 °C, pulse rate was 139 beats per min with AF, blood pressure was 78/47 mmHg, and oxygen saturation was 93% while receiving 100% oxygen by a rebreather 15-L mask. She was transferred to the intensive care unit (ICU) and intubated. She had pulmonary coarse crackles, and moderate amounts of frothy pale pink respiratory secretions were collected from the endotracheal tube. Dopamine, dobutamine, and noradrenaline were administered for hypotension. Laboratory findings were as follows: WBC count, 9860 cells/mm3 without a left shift; hematocrit, 37.0%; platelets, 147,000/mm3; creatinine, 1.47 mg/dL; alanine aminotransferase, 98 IU/mL; aspartate aminotransferase, 54 IU/mL; total bilirubin, 1.5 mg/dL; and respiratory alkalosis. The patient was unable to mount an adequate leukocyte count in light of her clinical condition. B. cereus was isolated from five blood sample cultures (four arterial blood cultures and one venous blood culture), but stool cultures were negative at that time.
Fig. 1.
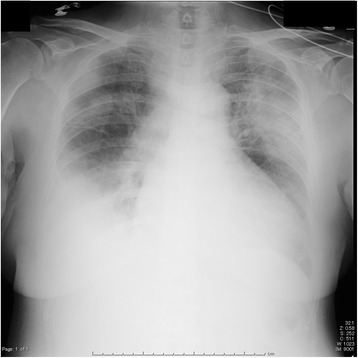
Chest radiograph indicated bilateral pleural effusion and pulmonary congestion
Fig. 2.
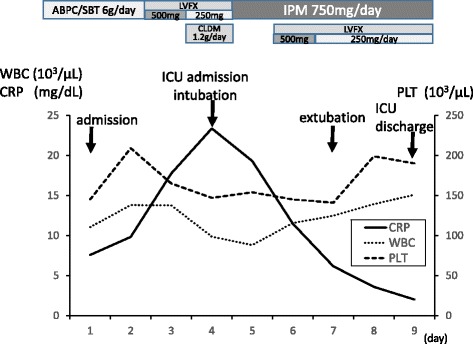
Time course of laboratory data and antibiotic treatment
Fig. 3.
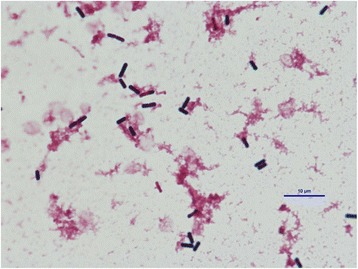
Bacillus cereus was isolated from two blood sample cultures collected at admission
Fig. 4.
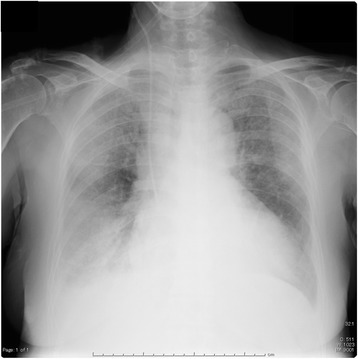
Chest radiograph indicated extensive bilateral infiltrates
Fig. 5.
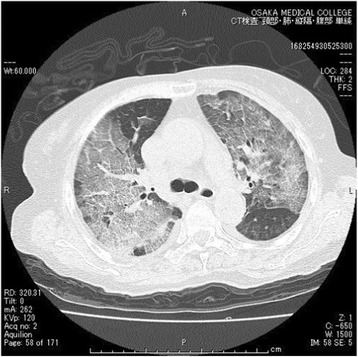
Computed tomography indicated extensive bilateral infiltrates
On day 4, intravenous imipenem (750 mg/day) therapy was initiated instead of levofloxacin and clindamycin therapy (Fig. 2). On day 5, levofloxacin (250–500 mg) was combined with imipenem (Fig. 2). She had no history of immunodeficiency, surgery, ill close contacts, risk factors for HIV or tuberculosis, recent central venous catheter insertion, or anthrax vaccination. On day 7, a chest radiograph and CT indicated a reduction in the size of bilateral infiltrates, suggesting that respiratory therapy and antibiotic therapy were effective. The patient was extubated and, on day 9, discharged from the ICU. No further remarkable changes were noted, and she was discharged from the hospital.
Discussion
The present case was unusual, given that the patient was not immunocompromised but succumbed to lung infiltrates associated with B. cereus. B. cereus is a well-known pathogen in food poisoning. It causes toxin-mediated, self-limited illness characterized by emetic or diarrheal syndromes [4, 5]. It is also known to cause bacteremia [6], endocarditis [7], meningitis [8], and pneumonia [2, 3, 9–20].
Previously reported cases of B. cereus pneumonia in adults are rare and usually associated with significant risk factors which are summarized in Table 1. Immunocompromised patients with B. cereus pneumonia frequently have septicemia and fatal illness. Most previously reported cases of infection occurred in patients with hematological disorders or alcohol abuse. However, four lethal cases of B. cereus pneumonia in immunocompetent welders and metalworkers have been reported [2, 10]. Our patient had no history of immunodeficiency, surgery, ill close contacts, risk factors for HIV or tuberculosis, recent central venous catheter insertion, or anthrax vaccination. It is interesting that our patient, who had none of the known risk factors for severe respiratory illness, survived the episode. However, the patient’s age (81 years) may have been a contributing factor. As age advances, the immune system undergoes profound remodeling and decline. This immune senescence predisposes older adults to a higher risk of acute viral and bacterial infections [21]. Compared to previously reported episodes of B. cereus pneumonia in adults (Table 1), our patient was much older and thus may have been more susceptible to B. cereus infection.
Table 1.
Reported episodes of B. cereus pneumonia in adults
| Patient | Age | Risk factor | Outcome | Reference |
|---|---|---|---|---|
| 1 | Not available | None | Died | 20 |
| 2 | 52 | Leukemia | Died | 19 |
| 3 | 63 | Leukemia | Died | 18 |
| 4 | 29 | Leukemia | Recovered | 17 |
| 5 | 60 | Alcohol abuse | Recovered | 16 |
| 6 | 18 | Alcohol abuse | Recovered | 15 |
| 7 | 54 | Leukemia | Recovered | 14 |
| 8 | 21 | Bronchiectasis | Recovered | 13 |
| 9 | 46 | None (welder) | Died | 2 |
| 10 | 41 | None (welder) | Died | 2 |
| 11 | 52 | Aplastic anemia | Died | 12 |
| 12 | 37 | Leukemia | Died | 11 |
| 13 | 39 | None (metal worker) | Died | 10 |
| 14 | 56 | None (metal worker) | Died | 10 |
| 15 | 60 | Leukemia | Died | 9 |
| 16 | 43 | Nephrotic syndrome | Recovered | 3 |
Non-anthracis Bacillus species might be dismissed as contaminants when isolated from clinical samples. Therefore, detection of the microorganism in multiple samples is usually necessary to make a definitive diagnosis of B. cereus pneumonia [3].
In the present case, the pathogenic role of B. cereus in pneumonia was confirmed by seven different specimens of blood that were sampled aseptically. However, we did not perform a bronchoscopic assessment of lung infiltrates, and B. cereus was not confirmed from bronchial lavage fluid and transbronchial lung biopsy specimens. There were no episodes of infectious disease other than severe pneumonia during hospitalization. Respiratory symptoms such as cough and dyspnea, and gastrointestinal symptoms (occult blood in the stool) were present. Therefore, in the present case, B. cereus pulmonary infection may have resulted from transient bacteremia from a gastrointestinal infection. Indeed, we had suspected as such at a relatively early stage (on day 2). B. cereus produces β-lactamase and is therefore resistant to penicillin and cephalosporins [1]. B. cereus is usually susceptible to clindamycin, vancomycin, fluoroquinolones, carbapenems, and aminoglycosides. After isolating B. cereus, we switched antibiotics to a combination of imipenem and levofloxacin, which were effective.
Conclusions
In summary, we report a rare case of B. cereus pneumonia in an immunocompetent patient, who subsequently recovered. Healthcare providers should consider Bacillus species as a potential pathogen when immunocompetent patients develop severe pneumonia.
Acknowledgements
None
Funding
None
Availability of data and materials
Not applicable
Authors’ contributions
YS collected data and drafted the manuscript; OU, OY, TA, NK, and TM revised the manuscript. All authors read and approved the final manuscript for submission.
Authors’ information
None
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Ethics approval and consent to participate
Not applicable
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AF
Atrial fibrillation
- Bacillus cereus
B. cereus
- CT
Computed tomography
- ICU
Intensive care unit
- TTE
Transthoracic echocardiography
Contributor Information
Yuichiro Shimoyama, Phone: +81-72-683-1221, Email: shimocchiliebesfreud512@yahoo.co.jp.
Osamu Umegaki, Email: oumegaki@osaka-med.ac.jp.
Yukimasa Ooi, Email: y-ooi@osaka-med.ac.jp.
Tomoyuki Agui, Email: sur039@osaka-med.ac.jp.
Noriko Kadono, Email: ane069@osaka-med.ac.jp.
Toshiaki Minami, Email: ane022@osaka-med.ac.jp.
References
- 1.Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol. 2010;23:382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller JM, Hair JG, Hebert M, Hebert L, Roberts FJ, Jr, Weyant RS. Fulminating bacteremia and pneumonia due to Bacillus cereus. J Clin Microbiol. 1997;35:504–7. doi: 10.1128/jcm.35.2.504-507.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miyata J, Tasaka S, Miyazaki M, Yoshida S, Naoki K, Sayama KA, et al. Bacillus cereus necrotizing pneumonia in a patient with nephrotic syndrome. Intern Med. 2013;52:101–4. doi: 10.2169/internalmedicine.52.7282. [DOI] [PubMed] [Google Scholar]
- 4.Thompson NE, Ketterhagen MJ, Bergdoll MS, Schantz EJ. Isolation and some properties of an enterotoxin produced by Bacillus cereus. Infect Immun. 1984;43:887–94. doi: 10.1128/iai.43.3.887-894.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbull PC, Kramer JM, Jørgensen K, Gilbert RJ, Melling J. Properties and production characteristics of vomiting, diarrheal, and necrotizing toxins of Bacillus cereus. Am J Clin Nutr. 1979;32:219–28. doi: 10.1093/ajcn/32.1.219. [DOI] [PubMed] [Google Scholar]
- 6.Cotton DJ, Gill VJ, Marshall DJ, Gress J, Thaler M, Pizzo PA. Clinical features and therapeutic interventions in 17 cases of Bacillus bacteremia in an immunosuppressed patient population. J Clin Microbiol. 1987;25:672–4. doi: 10.1128/jcm.25.4.672-674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steen MK, Bruno-Murtha LA, Chaux G, Lazar H, Bernard S, Sulis C. Bacillus cereus endocarditis: report of a case and review. Clin Infect Dis. 1992;14:945–6. doi: 10.1093/clinids/14.4.945. [DOI] [PubMed] [Google Scholar]
- 8.Barrie D, Wilson JA, Hoffman PN, Kramer JM. Bacillus cereus meningitis in two neurosurgical patients: an investigation into the source of the organism. J Infect. 1992;25:291–7. doi: 10.1016/0163-4453(92)91579-Z. [DOI] [PubMed] [Google Scholar]
- 9.Katsuya H, Takata T, Ishikawa T, Sasaki H, Ishitsuka K, Takamatsu Y, et al. A patient with acute myeloid leukemia who developed fatal pneumonia caused by carbapenem-resistant Bacillus cereus. J Infect Chemother. 2009;15:39–41. doi: 10.1007/s10156-008-0654-8. [DOI] [PubMed] [Google Scholar]
- 10.Avashia SB, Riggins WS, Lindley C, Hoffmaster A, Drumgoole R, Nekomoto T, et al. Fatal pneumonia among metalworkers due to inhalation exposure to Bacillus cereus containing Bacillus anthracis toxin genes. Clin Infect Dis. 2007;44:414–6. doi: 10.1086/510429. [DOI] [PubMed] [Google Scholar]
- 11.Frankard J, Li R, Taccone F, Struelens MJ, Jacobs F, Kentos A. Bacillus cereus pneumonia in a patient with acute lymphoblastic leukemia. Eur J Clin Microbiol Infect Dis. 2004;23:725–8. doi: 10.1007/s10096-004-1180-y. [DOI] [PubMed] [Google Scholar]
- 12.Strauss R, Mueller A, Wehler M, Neureiter D, Fischer E, Gramatzki M, et al. Pseudomembranous tracheobronchitis due to Bacillus cereus. Clin Infect Dis. 2001;33:E39–41. doi: 10.1086/322674. [DOI] [PubMed] [Google Scholar]
- 13.Gascoigne AD, Richards J, Gould K, Gibson GJ. Successful treatment of Bacillus cereus infection with ciprofloxacin. Thorax. 1991;46:220–1. doi: 10.1136/thx.46.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sliman R, Rehm S, Shlaes DM. Serious infections caused by Bacillus species. Medicine (Baltimore) 1987;66:218–23. doi: 10.1097/00005792-198705000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Bekemeyer WB, Zimmerman GA. Life-threatening complications associated with Bacillus cereus pneumonia. Am Rev Respir Dis. 1985;131:466–9. doi: 10.1164/arrd.1985.131.3.466. [DOI] [PubMed] [Google Scholar]
- 16.Panwalker AP, Trager GM. Necrotizing pneumonia and empyema caused by Bacillus cereus and Clostridium bifermentans. Am Rev Respir Dis. 1983;128:333–4. doi: 10.1164/arrd.1983.128.2.333b. [DOI] [PubMed] [Google Scholar]
- 17.Leff A, Jacobs R, Gooding V, Hauch J, Conte J, Stulbarg M. Bacillus cereus pneumonia. Survival in a patient with cavitary disease treated with gentamicin. Am Rev Respir Dis. 1977;115:151–4. doi: 10.1164/arrd.1977.115.1.151. [DOI] [PubMed] [Google Scholar]
- 18.Ihde DC, Armstrong D. Clinical spectrum of infection due to Bacillus species. Am J Med. 1973;55:839–45. doi: 10.1016/0002-9343(73)90266-0. [DOI] [PubMed] [Google Scholar]
- 19.Coonrod JD, Leadley PJ, Eickhoff TC. Bacillus cereus pneumonia and bacteremia. A case report. Am Rev Respir Dis. 1971;103:711–4. doi: 10.1164/arrd.1971.103.5.711. [DOI] [PubMed] [Google Scholar]
- 20.Stopler T, Camuescu V, Voiculescu M. Bronchopneumonia with lethal evolution determined by a microorganism of the genus bacillus (B. cereus) Rum Med Rev. 1965;19:7–9. [PubMed] [Google Scholar]
- 21.Simon AK, Hollander GA, McMichael A. Evolution of the immune system in humans from infancy to old age. Proc Biol Sci. 2015;282:20143085. doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable


