Abstract
Pulse oximetry screening is safe, noninvasive, easy to perform and proven to enhance detection of critical congenital heart disease in newborns. However, this test has yet to be adopted as routine practice in Canada. The present practice point highlights essential details and recommendations for screening, which research has shown to be highly specific, with low false-positive rates. Optimal screening for critical congenital heart disease should include prenatal ultrasound, physical examination and pulse oximetry screening. Screening should be performed between 24 hours and 36 hours postbirth, using the infant’s right hand and either foot to minimize false-positive results. Newborns with abnormal results should undergo a thorough evaluation by the most responsible health care provider. When a cardiac diagnosis cannot be excluded, referral to a paediatric cardiologist for consultation and echocardiogram is advised.
Keywords: CCHD, NICU, POS
Pulse oximetry screening (POS) in newborns has been shown to enhance the detection of critical congenital heart disease (CCHD) (1–4). While many programs around the world have recommended and adopted screening, it is not yet standard practice in Canada. This practice point presents highlights and recommendations from a recently published, Canadian Paediatric Society-endorsed position statement from the Canadian Cardiovascular Society and Canadian Pediatric Cardiology Association (5). Throughout this practice point, the term ‘newborn’ includes both term and late preterm infants (born between 34 0/7 weeks and 36 6/7 weeks gestational age) being cared for in locations outside the neonatal intensive care unit (NICU).
WHAT IS CRITICAL CONGENITAL HEART DISEASE?
Congenital heart disease (CHD) is the most common congenital malformation, with a prevalence of 12/1000 live births in Canada. Approximately one-quarter of these newborns have CCHD, defined as more severe and often duct-dependant lesions that require intervention early in life for optimal outcome (3,6,7) (Box 1).
WHY DO WE NEED TO DO MORE?
Early diagnosis remains crucial for CCHD because delay increases morbidity, mortality and disability (7). One US study estimates that 30% of CCHDs are diagnosed more than 3 days after birth, while a study from northern England reports 25% of CCHDs are diagnosed following discharge from hospital (8,9). In a study from Sweden, deaths from unrecognized CCHD occurred at a rate of 4.6/100,000 live births (10). A one-time survey from the Canadian Paediatric Surveillance Program (CPSP) showed that 36% of responders had been involved in a late-presenting CCHD case, of which 52% of the responders recalled the case requiring resuscitation (11).
Current screening with prenatal ultrasound is limited by low sensitivity. In Alberta, from 2007 to 2010, only 50% of newborns with CHD requiring surgery before 1 year of age were diagnosed prenatally. This detection rate was similar to rates found in large studies in the USA and UK. Detection rates are improving overall but are influenced by regional expertise and other mediating factors (9,12,13).
Box 1. Examples of lesions detectable using pulse oximetry screening
Most consistently cyanotic
Hypoplastic left heart syndrome
Pulmonary atresia with intact ventricular septum
Total anomalous pulmonary venous return
Tetralogy of Fallot
Transposition of the great arteries
Tricuspid atresia
Truncus arteriosus
May be cyanotic
Coarctation of the aorta
Double outlet right ventricle
Ebstein’s anomaly
Interrupted aortic arch
Defects with single ventricle physiology
Adapted from ref. (3).
Physical examination findings may be limited by lack of examiner expertise or confidence, and some types of CCHD may not present with clinical features—such as a murmur, cyanosis, tachypnea or laboured breathing—before discharge (3). A study from Norway showed that hospitals without POS were only able to detect 77% of CCHDs by clinical features before discharge (1).
HOW CAN POS HELP?
POS can identify otherwise clinically undetectable degrees of cyanosis and should be used adjunctly with prenatal ultrasound and newborn physical examination to reduce the diagnostic gaps in detecting CCHDs (14). In one large, multicentre Swedish study, hospitals reported a seven times higher positive predictive value with POS compared with physical examination (20.69% versus 3.06%) and a much higher likelihood ratio for detecting CCHD (344.8 versus 32.4) (10). Sensitivities of 82% to 92% have been reported by adding POS to prenatal ultrasound and newborn physical examination (10,15).
Applying results from one study (15) that indicated a prenatal detection rate of 50% of infants with CCHDs, POS could potentially detect an additional 35/100,000 newborns with these heart anomalies. In Canada, where 388,729 births were reported in 2014, applying an incidence for CCHD of 3/1000 births and a prenatal detection rate of 50%, 583 cases of CCHD await detection after birth. The implementation of POS could detect an additional 136 cases of CCHD per year before the appearance of symptoms. The CPSP survey found that 83% of the general paediatrician responders were aware of POS but only 26% were screening (11).
HOW DOES POS MEASURE UP AS A SCREENING TEST?
POS is safe, noninvasive, easy to perform and widely available. In one systematic review of 229,421 neonates, POS was shown to have a high specificity (at 99.9%) and a moderately high sensitivity (at 76.5%) (4). Studies from the USA and UK also report that POS is cost-neutral to cost-effective for detecting CCHD (10,16–18).
Abnormal, particularly false-positive (FP) POS results can help with detection of other causes of hypoxemia, including important infections and respiratory disorders requiring intervention (19).
The potential reach of POS is comparable to more established newborn screening practices. CCHDs are as common in newborns as cystic fibrosis (0.5/1000 births), hearing loss (1 to 3/1000) and hypothyroidism (1/4000), and FP rates are similar or better for POS (at 0.05% to 0.5%) compared with universal newborn hearing screening (0.5% to 4%) and newborn thyroid screening (2%) (20).
WHO SHOULD BE SCREENED?
All term and late preterm infants should be routinely screened. POS has not been adequately studied in preterm newborns or in the NICU setting relative to cut-off values for normal and abnormal. While pulse oximetry is an important monitoring tool for newborns with signs of CHD, such as organic murmurs or other cardiac findings, the POS protocol described here is intended for use in asymptomatic newborns in nonacute care settings.
WHEN SHOULD NEWBORNS BE SCREENED?
POS can be performed at any time after birth but is recommended for infants 24 hours to 36 hours of age. One meta-analysis showed FP rates of 0.05% screening after 24 hours (versus 0.50% before 24 hours) without significant impact on sensitivity (4). This 10-fold increase in FP rate could significantly impact resource utilization, especially when transportation is required to access cardiology services.
Screening between 24 hours and 36 hours allows for flexibility, such that testing becomes part of the daily schedule but does not need to happen in the early morning hours, when a positive result could impact workload and resources unnecessarily. Some centres administer POS at the time of the newborn hearing assessment, the first bath, or alongside other routinely scheduled evaluations. Screening for infants discharged before 24 hours from hospitals or free-standing birthing centres, or for home births, would require special arrangements. Despite the risk for higher FP rates, screening before 24 hours is preferable to not screening at all. Centres having a care system sufficiently robust to ensure that early discharge newborns either return or are assessed by public health nurses at 24 hours to 36 hours postbirth could be an effective alternative. Similarly, midwives could screen during the routine home visit, at around 24 hours postbirth. Whatever the local practice may be, consistency and effective communication are critical, as well as having a tracking system in place so that newborns are not lost to screening.
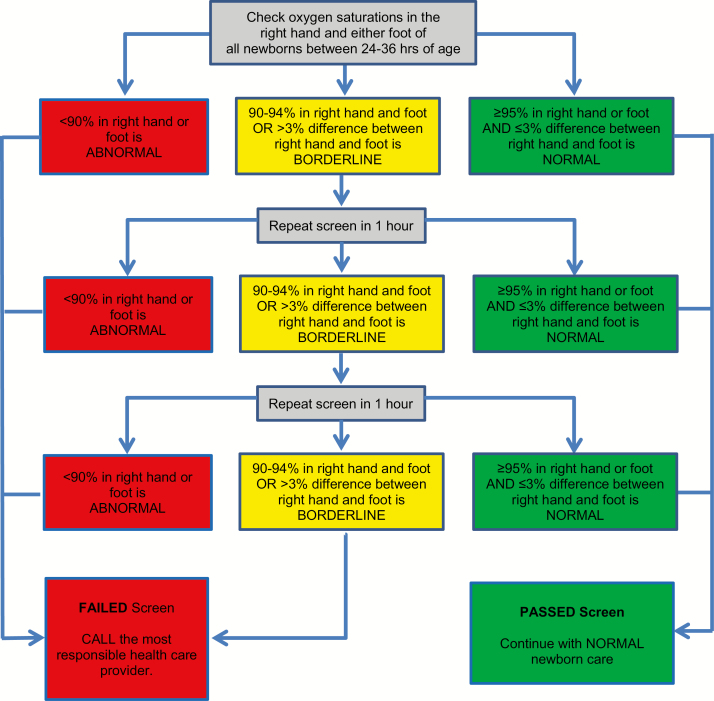
Figure 1 shows the protocol followed in the Canadian Cardiovascular Society/Canadian Pediatric Cardiology Association position statement (5). Screening can be completed in about 5 minutes (17).
Figure 1.
What screening protocol should be used? Adapted from ref. (5), with permission.
While protocols testing only one foot detect most CCHDs, additional sensitivity is gained by testing the right hand and one foot. Using the left hand is not recommended because of proximity to the ductus arteriosus. Local expertise should guide the use of pulse oximetry equipment and practices.
The 90% oxygen saturation (SaO2) threshold for failed screens is supported by data from one study where the CCHD group showed a median postductal SaO2 of 90% (10). A borderline screening result, with an SaO2 reading in any limb of 90% to 94% or >3% difference between limbs, has greater potential to be an FP than an SaO2 reading <90%. Studies usually repeated testing after 1 hour to allow transitional circulation to adapt and decrease the risk for an FP result. A third borderline result is considered a ‘fail’ because continuing to retest prolongs the screening process unnecessarily and clinical decompensation may occur.
WHAT TO DO WITH A FAILED POS SCREEN?
Newborns with a failed screen require thorough assessment by the most responsible health care provider, who may be a midwife, nurse, nurse practitioner or physician. An assessment that includes four limb blood pressures, an electrocardiogram and a chest x-ray may be helpful. If not already initiated, consult with a paediatrician, and when the most likely cause for a failed screening result appears to have a cardiac origin or remains unclear, a consult with paediatric cardiology followed by an echocardiogram is required to rule out CCHD. For many centres in Canada, the need for ground or air transport to access cardiac services, including an echocardiogram, underlines the importance of minimizing FP results.
WHAT ARE THE LIMITATIONS OF POS SCREENING?
While POS can help detect the cardiac anomalies listed in Text Box (1), it cannot identify all patients with CHD. The frequency of false negatives is low, reported in only 33/229,421 (0.014%) neonates screened in one meta-analysis (4). Because coarctation of the aorta is a challenge to diagnose by any detection method, a confident assessment of femoral pulses in the newborn period can be critical.
It is difficult to generalize the cost benefit of POS across all regions of Canada because prenatal ultrasound detection rates and access to echocardiography vary widely, but regions with high prenatal detection rates would probably benefit less from POS.
SUMMARY OF RECOMMENDATIONS FOR PRACTICE
Pulse oximetry screening improves detection rates for critical congenital heart disease and is recommended for all newborns in Canada, especially when used in conjunction with prenatal ultrasound and physical examination.
Recognizing that delivery and time of discharge practices vary across Canada, the timing of testing should be individualized for each centre and (ideally) occur after 24 hours postbirth to lower FP results. And because the intent is to screen newborns before they develop symptoms, the goal should be to perform screening before they reach 36 hours of age.
Testing using the right hand and one foot minimizes false-negative rates.
A rigorous care system should ensure that all newborns are screened and tracked for follow-up, as needed.
Newborns who FAIL screening should undergo a complete clinical evaluation by the most responsible health care provider, which could include consultation with a paediatrician if the initial assessment did not involve one. If a cardiac diagnosis cannot be excluded, referral to a paediatric cardiologist for consultation and echocardiogram is advised.
Acknowledgements
This practice point was reviewed by the Community Paediatrics Committee of the Canadian Paediatric Society. It was also reviewed by representatives from the College of Family Physicians of Canada and the Society for Obstetricians and Gynaecologists of Canada.
Footnotes
CPS FETUS AND NEWBORN COMMITTEE
Members: Mireille Guillot MD (Resident member), Leonora Hendson MD, Ann Jefferies MD (past Chair), Thierry Lacaze-Masmonteil MD (Chair), Brigitte Lemyre MD, Michael Narvey MD, Leigh Anne Newhook MD (Board Representative), Vibhuti Shah MD
Liaisons: Linda Boisvert RN, Canadian Association of Neonatal Nurses; Radha Chari MD, The Society of Obstetricians and Gynaecologists of Canada; James Cummings MD, Committee on Fetus and Newborn, American Academy of Pediatrics; Doris Sawatzky-Dickson, RN MN IBCLC, Canadian Association of Neonatal Nurses; William Ehman MD, College of Family Physicians of Canada; Roxanne Laforge RN, Canadian Perinatal Programs Coalition; Chantal Nelson PhD, Public Health Agency of Canada; Eugene H Ng MD, CPS Neonatal-Perinatal Medicine Section; Kristi Watterberg MD, Committee on Fetus and Newborn, American Academy of Pediatrics
Principal authors: Michael Narvey MD, Kenny K Wong MD, Anne Fournier MD
References
- 1. Meberg A, Andreassen A, Brunvand L et al. Pulse oximetry screening as a complementary strategy to detect critical congenital heart defects. Acta Paediatr 2009;98(4):682–6. [DOI] [PubMed] [Google Scholar]
- 2. Zhao QM, Ma XJ, Ge XL et al. ; Neonatal Congenital Heart Disease screening group Pulse oximetry with clinical assessment to screen for congenital heart disease in neonates in China: A prospective study. Lancet 2014;384(9945):747–54. [DOI] [PubMed] [Google Scholar]
- 3. Mahle WT, Newburger JW, Matherne GP et al. ; American Heart Association Congenital Heart Defects Committee of the Council on Cardiovascular Disease in the Young, Council on Cardiovascular Nursing, and Interdisciplinary Council on Quality of Care and Outcomes Research; American Academy of Pediatrics Section on Cardiology and Cardiac Surgery; Committee On Fetus and Newborn Role of pulse oximetry in examining newborns for congenital heart disease: A scientific statement from the AHA and AAP. Pediatrics 2009;124(2):823–36. [DOI] [PubMed] [Google Scholar]
- 4. Thangaratinam S, Brown K, Zamora J, Khan KS, Ewer AK. Pulse oximetry screening for critical congenital heart defects in asymptomatic newborn babies: A systematic review and meta-analysis. Lancet 2012;379(9835):2459–64. [DOI] [PubMed] [Google Scholar]
- 5. Wong KK, Fournier A, Fruitman DS et al. Canadian Cardiovascular Society/Canadian Pediatric Cardiology Association position statement on pulse oximetry screening in newborns to enhance detection of critical congenital heart disease. Can J Cardiol 2017;33(2):199–208. [DOI] [PubMed] [Google Scholar]
- 6. Marelli AJ, Mackie AS, Ionescu-Ittu R, Rahme E, Pilote L. Congenital heart disease in the general population: Changing prevalence and age distribution. Circulation 2007;115(2):163–72. [DOI] [PubMed] [Google Scholar]
- 7. Brown KL, Ridout DA, Hoskote A, Verhulst L, Ricci M, Bull C. Delayed diagnosis of congenital heart disease worsens preoperative condition and outcome of surgery in neonates. Heart 2006;92(9):1298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peterson C, Ailes E, Riehle-Colarusso T et al. Late detection of critical congenital heart disease among US infants: Estimation of the potential impact of proposed universal screening using pulse oximetry. JAMA Pediatr 2014;168(4):361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wren C, Reinhardt Z, Khawaja K. Twenty-year trends in diagnosis of life-threatening neonatal cardiovascular malformations. Arch Dis Child Fetal Neonatal Ed 2008;93(1):F33–5. [DOI] [PubMed] [Google Scholar]
- 10. de-Wahl Granelli A, Wennergren M, Sandberg K et al. Impact of pulse oximetry screening on the detection of duct dependent congenital heart disease: A Swedish prospective screening study in 39,821 newborns. BMJ 2009;338:a3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dow KE, Wong KK. Neonatal Pulse Oximetry Screening: 2016 Results of a Canadian Paediatric Surveillance Program Survey. 2016. CPSP Annual Results (in press). <www.cpsp.cps.ca/uploads/publications/CPSP-2016-Results.pdf> (Accessed February 2016). [Google Scholar]
- 12. Trines J, Fruitman D, Zuo KJ, Smallhorn JF, Hornberger LK, Mackie AS. Effectiveness of prenatal screening for congenital heart disease: Assessment in a jurisdiction with universal access to health care. Can J Cardiol 2013;29(7):879–85. [DOI] [PubMed] [Google Scholar]
- 13. Quartermain MD, Pasquali SK, Hill KD et al. Variation in prenatal diagnosis of congenital heart disease in infants. Pediatrics 2015;136(2):e378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine—results from a prospective multicenter study. Eur J Pediatr 2010;169(8):975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ewer AK, Middleton LJ, Furmston AT et al. ; PulseOx Study Group Pulse oximetry screening for congenital heart defects in newborn infants (pulseox): A test accuracy study. Lancet 2011;378(9793):785–94. [DOI] [PubMed] [Google Scholar]
- 16. Kochilas LK, Lohr JL, Bruhn E et al. Implementation of critical congenital heart disease screening in Minnesota. Pediatrics 2013;132(3):e587–94. [DOI] [PubMed] [Google Scholar]
- 17. Roberts TE, Barton PM, Auguste PE, Middleton LJ, Furmston AT, Ewer AK. Pulse oximetry as a screening test for congenital heart defects in newborn infants: A cost-effectiveness analysis. Arch Dis Child 2012;97(3):221–6. [DOI] [PubMed] [Google Scholar]
- 18. Peterson C, Grosse SD, Glidewell J et al. A public health economic assessment of hospitals’ cost to screen newborns for critical congenital heart disease. Public Health Rep 2014;129(1):86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meberg A, Brügmann-Pieper S, Due R Jr et al. First day of life pulse oximetry screening to detect congenital heart defects. J Pediatr 2008;152(6):761–5. [DOI] [PubMed] [Google Scholar]
- 20. Nelson HD, Bougatsos C, Nygren P; 2001 US Preventive Services Task Force Universal newborn hearing screening: Systematic review to update the 2001 US preventive services task force recommendation. Pediatrics 2008;122(1):e266–76. [DOI] [PubMed] [Google Scholar]