Abstract
Purpose
COPD is a leading cause of morbidity and mortality. However, few studies have used spirometry to investigate its incidence, especially in Asia. In the present study, we analyzed the incidence and risk factors of COPD using a community cohort database in Korea.
Patients and methods
The study included 6,517 subjects aged 40–69 years from the Ansung–Ansan cohort database I–III (2001–2006). We calculated the crude incidence rate and the standardized incidence rate corrected for the Korean general population and the world population with COPD. We also determined the relative risks (RRs) for incident COPD and the attributable risks.
Results
In total, 329 new COPD cases were diagnosed during follow-up. The overall crude incidence rate per 100,000 person-years was 1,447. The standardized incidence rate corrected for the Korean general population was 1,550; this value was higher in men and increased with increasing age. Risk factors for incident COPD were age ≥60 years (adjusted RR [aRR] =2.52 vs age <60 years), male sex (aRR =2.02 vs female), heavy smoking (≥20 pack-years; aRR =2.54 vs never smoker), and lowest income group (first quartile; aRR =2.03 vs fourth quartile). The adjusted attributable risk was highest for education level of high school or lower (44.9%), followed by smoking history (25.8%), income (22.9%), and sex (12.0%).
Conclusion
In Korea, 15.5/1,000 people are diagnosed with COPD annually. The incidence rate increases with increasing age, heavier smoking, and decreasing income, with a higher rate in men than in women.
Keywords: chronic obstructive pulmonary disease, incidence rate, relative risk, attributable risk
Introduction
Chronic obstructive pulmonary disease is a common, preventable, and treatable airflow limitation disease that is usually progressive and associated with enhanced inflammation in the airways and lungs.1 COPD is a leading cause of chronic morbidity, mortality, and increased healthcare costs worldwide.2,3 It is currently the fourth leading cause of death globally, and the World Health Organization predicts that it will become the third leading cause by 2030.4
As COPD constitutes a major public health problem, precise and informative epidemiologic data about COPD are needed. However, reported prevalence estimates vary widely because of different definitions and methodologies.5 Furthermore, in contrast with many reports on the prevalence of COPD, there are fewer community-based studies on the incidence of COPD.6–10 A wide range of incidence rates can be observed among the studies, depending on the COPD definition used and the population studied.6–10 Despite the high burden of COPD in the Asia-Pacific region, there are few reports on the incidence rate of COPD in Asian countries.11
Although cigarette smoking is the most common risk factor for COPD, it is not the only one, and there is consistent evidence from epidemiologic studies that nonsmokers may also develop chronic airflow limitation.12–15 Moreover, most of the evidence concerning risk factors for COPD comes from cross-sectional epidemiologic studies that identify associations rather than cause-and-effect relationships. Thus, the current understanding of risk factors for COPD is still incomplete in many respects.
In this study, we investigated the incidence rate of COPD and risk factors for incident COPD, including age, sex, smoking status, and income, in a Korean patient population from the Ansung–Ansan cohort database.
Methods
Study populations
The Ansung–Ansan cohort study is an ongoing prospective study that was started in 2001 with support from the National Genome Research Institute (Korea Centers for Disease Control and Prevention, Cheongju, Korea). The study is a part of the Korean Genome and Epidemiology Study, a large community-based epidemiologic survey conducted to investigate chronic disease in Koreans. Detailed information on the study design and procedures has been published previously.16 Each study comprised a population-based sample of male and female Koreans aged 40–69 years and from the same ethnic background, but cohort members were enrolled at the following two different sites: Ansan, which is an urban community with a population of 555,000, and Ansung, which is a rural community including 133,000 residents, on the basis of the 2000 census. To enroll members for each cohort, the most efficient method was used on the basis of knowledge about characteristics of each community. For enrollment at the Ansan site, 10,957 eligible subjects were identified by telephone contact on the basis of a two-stage cluster-sampling method with the information of a governing district called Dong and demographic characteristics. Similarly, Ansung members were recruited from 5 of 11 governing districts called Myon by using a cluster-sampling method, and as a result, 7,192 eligible subjects were identified by mail or telephone contact and a door-to-door visit. For the baseline health examination from 18 June 2001 to 29 January 2003, 5,020 participants (2,523 men and 2,497 women) from Ansan and 5,018 (2,239 men and 2,779 women) from Ansung visited the Korea University Ansan Hospital and the Ajou University Medical Center, respectively. In these subjects, the results of pulmonary function test were available in 8,613 subjects.
Initial data were obtained from the 8,613 subjects aged 40–69 years who participated in Ansung–Ansan cohort I (2001–2002) and have valid lung function measurements. Follow-up examinations were conducted biennially. Data from a baseline survey and two subsequent biennial surveys (I–III: 2001–2006) were analyzed in our study. Of the 8,613 subjects in the study, 6,517 who underwent pulmonary function testing two or more times during I–III were included in this study (Figure 1).
Figure 1.
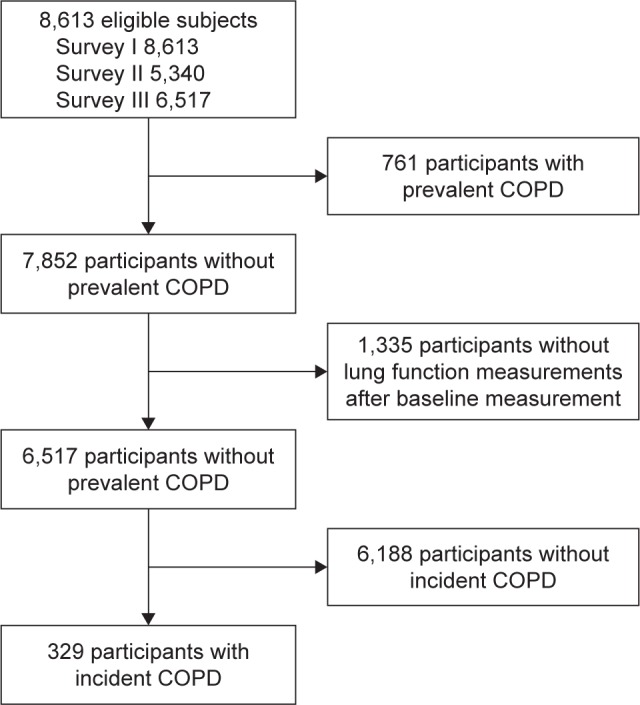
Flowchart of patient selection for the study.
The Korea Centers for Disease Control and Prevention obtained written informed consent from all participants, and the Institutional Review Board of Severance Hospital approved this study protocol (4-2016-0458).
Spirometry
Lung function was measured by spirometry (VMAX2130, SensorMedics Corporation, Yorba, CA, USA) at every visit (at baseline and at the first and second follow-up visits). COPD was defined as a pre-bronchodilator FEV1/FVC ratio <0.7.
Statistical analysis
COPD is a progressive disease, and we assumed that subjects were affected if they were diagnosed with COPD at least once during follow-up. Lung function was measured in all subjects up to three times, and if subjects who had been healthy at the previous visit became affected at the next visit, they were assumed to be affected at the mid time point between these two visits. On the basis of these definitions, we calculated crude, age-specific, and sex-specific incidence rates per 100,000 person-years (PY); CIs of incidence rates were obtained under the assumption that the number of events follows the Poisson distribution. Age-specific and sex-specific incidence rates were summed to obtain the standardized Korean incidence rates by weighting them with their proportions in the Korean general population.
Furthermore, we calculated relative risks (RRs) by using log-binomial regression.17 Response variables were assumed to follow a binomial distribution, and a logarithm was used as a link function. We included age, sex, smoking history, and income as covariates, and our generalized linear model for subject i was
The estimated exp(β) indicates the RRs when the corresponding covariate increases by 1 unit and the other covariates are the same.
Finally, we estimated attributable risks (ARs) with RRs. We assumed that there are k different groups and that the RR in each group j is . If we denote the proportion of cases in group j compared with all cases by ρj, the adjusted ARs can be estimated by .18
Results
Baseline characteristics
Of the 8,613 subjects, 761 (8.8%) had prevalent COPD at baseline, and 329 of the 6,517 at-risk subjects developed COPD during the follow-up period. The median follow-up duration was 4 years (interquartile range 3.0–4.0 years). Table 1 shows the baseline characteristics of the study population. Half of the subjects were aged 40–49 years, and 46.0% were men.
Table 1.
Baseline characteristics of the study population stratified according to age, sex, smoking status, and income
| Characteristics | Total
|
Male
|
Female
|
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Age (n=6,517) | |||
| 40–49 | 3,334 (51.2) | 1,686 (56.2) | 1,648 (46.8) |
| 50–59 | 1,733 (26.6) | 775 (25.9) | 958 (27.2) |
| 60–69 | 1,450 (22.2) | 536 (17.9) | 914 (26.0) |
| Sex (n=6,517) | |||
| Male | 2,997 (46.0) | – | – |
| Female | 3,520 (54.0) | – | – |
| Smoking history (n=6,517) | |||
| Never smokers | 4,018 (61.7) | 643 (21.5) | 3,375 (95.9) |
| Former smokers | 1,005 (15.4) | 965 (32.2) | 40 (1.1) |
| Current smokers | 1,494 (22.9) | 1,389 (46.3) | 105 (3.0) |
| Income (n=6,514) | |||
| First quartile | 2,064 (31.6) | 657 (21.9) | 1,409 (40.0) |
| Second quartile | 1,941 (29.8) | 928 (31.0) | 1,013 (28.8) |
| Third quartile | 1,972 (30.2) | 1,087 (36.3) | 886 (25.2) |
| Fourth quartile | 537 (8.2) | 325 (10.8) | 212 (6.0) |
| Education (n=6,485) | |||
| Elementary school or lower | 1,963 (30.3) | 464 (15.5) | 1,499 (42.9) |
| Middle school | 1,440 (22.2) | 611 (20.5) | 829 (23.7) |
| High school | 2,118 (32.7) | 1,168 (39.1) | 950 (27.1) |
| College/university or higher | 964 (14.9) | 744 (24.9) | 220 (6.3) |
Incidence rate of COPD
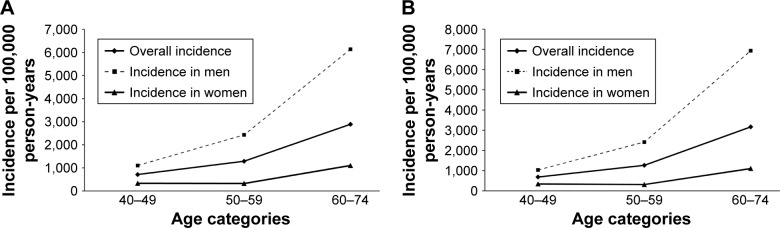
During follow-up, 329 new cases of COPD were diagnosed. The overall crude incidence of COPD in persons aged ≥40 years was 1,447/100,000 PY (Table 2). The incidence of COPD was higher in men than in women (2,541/100,000 PY vs 564/100,000 PY, respectively). The incidence rate increased almost fourfold from 713/100,000 PY at the age of 40–49 years to 2,888/100,000 PY at the age of 60–69 years (Table 2, Figure 2A). The overall standardized incidence rate corrected for the Korean general population was 1,550/100,000 PY. The standardized incidence rate for COPD was higher in men than in women and increased with increasing age (Table 3; Figure 2B).
Table 2.
Overall, age-specific, and sex-specific crude incidence rates of COPD per 100,000 person-years
| Age groups, years | Overall, COPD IR |
Men, COPD IR |
Women, COPD IR |
|---|---|---|---|
| 40–49 | 713.9 | 1,104.5 | 332.9 |
| 50–59 | 1,288.4 | 2,430.3 | 327.3 |
| 60–69 | 2,888.5 | 6,137.6 | 1,105.3 |
| All age categories | 1,447.8 | 2,541.6 | 564.7 |
Abbreviation: IR, incidence rate.
Figure 2.
Age-specific and sex-specific (A) incidence rates of COPD per 100,000 person-years, and (B) standardized incidence rates of COPD per 100,000 person-years (standardized for the Korean general population).
Table 3.
Overall, age-specific, and sex-specific standardized incidence rates of COPD per 100,000 person-years (standardized for the Korean general population)
| Age groups, years | Overall
|
Men
|
Women
|
|||
|---|---|---|---|---|---|---|
| COPD IR | 95% CI | COPD IR | 95% CI | COPD IR | 95% CI | |
| 40–49 | 684.8 | 523.8–845.9 | 1,033.6 | 755.5–1,311.7 | 341.6 | 176.4–506.8 |
| 50–59 | 1,263.0 | 999.0–1,527.0 | 2,412.0 | 1,873.4–2,950.7 | 311.2 | 134.9–487.5 |
| 60–69 | 3,165.4 | 2,628.5–3,702.3 | 6,933.1 | 5,624.5–8,241.7 | 1,104.1 | 724.0–1,484.3 |
| All age categories | 1,550.0 | 1,368.2–1,731.8 | 3,007.8 | 2,609.7–3,405.9 | 551.6 | 412.4–690.8 |
Abbreviation: IR, incidence rate.
Risk factors for incident COPD
Table 4 shows the risk factors for incident COPD. After adjusting for other variables, the risk factors for incident COPD were age ≥60 years (adjusted RR 2.52; 95% CI 2.23–2.85), male sex (adjusted RR 2.02; 95% CI 1.64–2.48), heavy smoking (≥20 pack-years; adjusted RR 2.54; 95% CI 2.09–3.08), and lowest income group (first quartile; adjusted RR 2.03; 95% CI 1.64–2.50). Table S1 demonstrates the risk factors for incident COPD including smoking history of never, former, and current smokers. The adjusted RRs were 1.90 (95% CI 1.55–2.33) for the former smokers and 2.48 (95% CI 2.05–2.99) for the current smokers. Table 5 shows the adjusted AR for incident COPD. When adjusted for the risk factors, the AR for education level of high school or lower predominated (adjusted AR 44.9%), followed by smoking history (adjusted AR 25.8%), income in the first to third quartiles (adjusted AR 22.9%), and male sex (adjusted AR 12.0%).
Table 4.
Risk factors and adjusted relative risk of incident COPDa
| Risk factors | Total
|
Male
|
Female
|
|||
|---|---|---|---|---|---|---|
| Adjusted relative riska | 95% CI | Adjusted relative riska | 95% CI | Adjusted relative riska | 95% CI | |
| Age | ||||||
| <60 years | Reference | Reference | Reference | |||
| ≥60 years | 2.52 | 2.23–2.85 | 2.60 | 2.26–2.98 | 2.18 | 1.68–2.84 |
| Sex | ||||||
| Female | Reference | – | – | – | – | |
| Male | 2.02 | 1.64–2.48 | – | – | – | – |
| Smoking history | ||||||
| Never smoker | Reference | Reference | Reference | |||
| 1–19 pack-years | 1.78 | 1.44–2.20 | 1.58 | 1.25–1.99 | 2.66 | 1.77–3.99 |
| ≥20 pack-years | 2.54 | 2.09–3.08 | 2.30 | 1.88–2.81 | 3.94 | 2.12–7.31 |
| Income | ||||||
| Fourth quartile | Reference | Reference | Reference | |||
| Third quartile | 1.29 | 1.01–1.65 | 1.14 | 0.87–1.49 | 2.48 | 1.27–4.86 |
| Second quartile | 1.56 | 1.26–1.93 | 1.48 | 1.18–1.86 | 2.18 | 1.15–4.15 |
| First quartile | 2.03 | 1.64–2.50 | 1.89 | 1.51–2.37 | 2.99 | 1.61–5.57 |
Note:
Adjusted for other factors (age, sex, smoking history, and income).
Table 5.
Adjusted attributable risk of incident COPD
| Risk factors | Total
|
Male
|
Female
|
|---|---|---|---|
| Adjusted attributable riska | Adjusted attributable riskb | Adjusted attributable riskb | |
| Sex, male | 0.120 | – | – |
| Ever-smokers | 0.258 | 0.244 | 0.365 |
| Income of first to third quartiles | 0.229 | 0.208 | 0.323 |
| Education of high school or lower | 0.449 | 0.458 | 0.393 |
Notes:
Adjusted for other factors (sex, smoking status, income, and education level).
Adjusted for other factors (smoking status, income, and education level).
Discussion
In our study, the overall crude incidence rate of COPD was 1,447/100,000 PY and the standardized incidence rate corrected for the Korean general population was 1,550/100,000 PY. The incidence increased with older age, heavier smoking, lower income, and male sex. After adjustment for the other three variables, the AR for smoking history was the highest, followed by low income, older age, and male sex.
Previous epidemiologic studies of COPD were mainly focused on the prevalence of COPD because they often applied a cross-sectional spirometry-based approach to obtain the true prevalence of COPD. In the present study, the prevalence of COPD at baseline was found to be 8.8%, which is lower than that reported previously for Korea.19 The subjects in the present study were aged 40–69 years, whereas the previous study included subjects aged ≥45 years, with 20% of the subjects aged >65 years.
Several studies have reported the incidence of COPD in various populations and countries using different diagnostic methods, including spirometry and healthcare databases (Table 6).7–9,11,20–27 In a Swedish report based on the Obstructive Lung Disease in Northern Sweden cohort, Lindberg et al estimated the incidence rate of COPD at 13.1/1,000 PY according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) spirometric criteria (FEV1/FVC <0.7).7 In that study, a random sample of 963 subjects aged 46–77 years were invited for a structured interview and spirometry.7 The findings of Lindberg et al are similar to ours, which may be because of the similar age categories and spirometry-based criteria for COPD used in the two studies. In a study by de Marco et al the incidence rate of COPD was found to be 2.8/1,000 PY in an international cohort of 5,002 subjects aged 20–44 years.20 The lower incidence rate in their study when compared with that in ours may be explained by the younger age of their study population. In a study performed by van Durme et al in the Netherlands, the incidence of COPD was evaluated in a large prospective population-based cohort of 7,983 subjects aged ≥55 years.8 Their study was part of the Rotterdam Study, an ongoing population-based cohort study that is assessing the occurrence of and risk factors for chronic diseases in the elderly. COPD was diagnosed using an algorithm based on validation of hospital discharge letters, files from the general practitioner, and spirometry reports. Only 44.5% (3,550/7,983) of participants underwent spirometry. In the absence of spirometry, the investigators reviewed all medical information for subjects who had used respiratory medication for at least 6 months and all hospital discharge letters or mortality reports with a coded diagnosis of COPD. The overall incidence rate was 9.2/1,000 PY, which is much lower than that in our study, although their study was conducted in an elderly population. Detection of COPD on the basis of medical records and healthcare utilization may have resulted in an underestimation of COPD incidence. Respiratory symptoms are often absent in patients with early COPD, and not all patients with respiratory symptoms seek medical services because of low public awareness of COPD.28 Therefore, the incidence of physician-diagnosed (coded diagnosis) COPD can be lower than the incidence of COPD defined by spirometry.8,29
Table 6.
Overview of studies that investigated the incidence of COPD
| Author | Source population | Country | COPD definitiona | Year of study | Cohort size | Follow-up time (years) | Number of incident COPD patients (%) | Age range in years | IR COPD (per 1,000 PY) | Men (per 1,000 PY) | Women (per 1,000 PY) | Smoking prevalence, males (% of adults)b
|
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2000 | 2012 | ||||||||||||
| Huhti et al21 | Non-selected population | Finland | Spirometry | 1961–1971 | 1,476 | 10 | 1,163 (78.7) | 40–64 | 2.0 and 10.0 for smokers | – | – | 34 | 25 |
| Krzyzanowski et al23 | Longitudinal data, random sample | Poland | Spirometry | 1968–1981 | 4,612 | 13 | 112 (2.4) | 19–70 | 5.0 | – | – | 47 | 35 |
| Vestbo and Lange25 | Population based | Denmark | Spirometry | 1976–1978 1981–1983 1992–1994 | 14,223 | 9 | – | ≥20 | 19 (5 years) and 9 (15 years) | – | – | 42 | 21 |
| Johannessen et al22 | Participants selected via postal questionnaire (1985), a questionnaire (1987–1988), and spirometry (1996–1997) | Norway | Spirometry | 1985, 1987–1988, 1996–1997 | 908 | 12 | 40 (4.4) | 18–74 | 7 | – | – | 43 | 26 |
| Terzikhan et al24 | Population based; embedded within the Rotterdam study | the Netherlands | Spirometry | RSI: 1989–1992 RSII: 2000–2003 RSIII: 2006–2009 | 14,619 | 10.7 | 1,304 (8.9) | ≥45 | 8.9 | 13.3 | 6.1 | 37 | 28 |
| van Durme et al8 | Population based | the Netherlands | Spirometry/discharge letters | 1990–2004 | 7,983 | 11 | 648 (8.1) | ≥55 | 9.2 | 14.4 | 6.2 | 37 | 28 |
| de Marco et al20 | ECRHS on random sample of young adults. Participants were invited by questionnaire. Of the responders, a random sample of 20% were invited for spirometry | Belgium, Denmark, Estonia, France, Germany, Iceland, Italy, the Netherlands, Norway, Spain, Sweden, Switzerland, UK | Spirometry | ECRHSI: 1991–1993 ECRHSII: 1999–2002 | 5,002 | 8.9 | 123 (2.4) | 20–44 | 2.8 | 3.2 | 2.4 | – | – |
| Lindberg et al7 | Participants selected via postal questionnaire and a random sample was invited to a structured interview and spirometry in 1996 and 2003 | Sweden | Spirometry | Participated in 1996 and 2003 | 963 | 7 | 45 (≥GOLD II) (4.6) 91 (≥GOLD I) (9.4) | 46–77 | 6.7 GOLD II 13.5 GOLD I and more | – | – | 33 | 22 |
| Kojima et al11 | Participants subjected to health checkups including spirometry | Japan | Spirometry | 1997–2005 | 17,106 | 8 | 466 (2.7) | 25–74 | 8.1 | 3.1 | 51 | 36 | |
| Garcia Rodriguez et al26 | Population based | UK | Based on COPD-specific OXMIS and Read Codesc | 1996 | 808,513 | – | 1,927 (0.2) | 40–89 | 2.6 | – | – | 31 | 22 |
| Gershon et al27 | Population based | Canada | ICD codes | 1996–2007 | 5–6.4 million | 12 | 61,998–55,903 | ≥35 | 1996: 11.8 2007: 8.5 | 1996: 13.9 2007: 9.4 | 1996: 10.4 2007: 7.8 | 29 | 20 |
| Afonso et al9 | Population based | the Netherlands | Spirometry/medical records | 2000–2007 | 7,308 | 7 | 1,713 (23.4) | ≥40 | 2.92 | 3.54 | 2.34 | 37 | 28 |
| Leem et al (current study) | Population based | Korea | Spirometry | 2001–2006 | 8,613 | 4 | 329 (3.8) | 40–69 | 15.50 | 30.08 | 5.52 | 62 | 52 |
Notes:
BTS criteria: FEV1/FVC ratio <0.70 and FEV1 <80% predicted; GOLD criteria: FEV1/FVC ratio <0.70.
Data from World Health Organization, Global Health Observatory Data Repository.
READ codes, current UK coding system for diseases.
Abbreviations: BTS, British Thoracic Society; ECRHS, European Community Respiratory Health Survey; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICD, International Classification of Diseases; OXMIS, Oxford Medical Information System; PY, person-years; RS, Rotterdam Study.
In our study, we used spirometry as a screening tool for the detection of COPD in all subjects. Therefore, we could detect asymptomatic patients with COPD, resulting in a higher but more accurate incidence rate than that in other studies. In a study by Kojima et al in Japan, the incidence rates per 1,000 PY were 8.1 in men and 3.1 in women.11 In this study, participants were aged 25–74 years at the time of health check-ups, including spirometry, at a single regional medical center. Differences in the study populations may be the reason why the incidence rates in Korea and Japan are different, even though the follow-up duration was similar. Moreover, high rate of smoking, especially in male Korean adults (62% in 2000 and 52% in 2012, decreasing down to 43.1% in 2014), is attributable to the relatively high incidence rate of COPD in Korea compared with that in most European countries.30,31
In the present study, we confirmed known risk factors for COPD, including smoking history, male sex, and increasing age. Cigarette smoking is a widely proven risk factor for COPD. The proportion of smokers in COPD patients is two-thirds, while age, sex, exposure to particles including organic and inorganic dusts, socioeconomic status, and bronchial hyperreactivity have been regarded as possible risk factors for COPD.1 However, limited longitudinal data are available on other risk factors and RRs associated with COPD development.6–9,25 In the Swedish study by Lindberg et al, incident COPD according to the GOLD criteria (FEV1/FVC <0.7) was significantly associated with increasing age and the smoking status of current smokers, but not with sex in a general population sample.7 In the study by van Durme et al, male sex and smoking status of current smokers were related to occurrence of COPD.8 In a study by Afonso et al in the Netherlands, male sex, increasing age, and smoking history were strong risk factors for COPD, and these associations remained significant after adjustment for other variables.9 In our study, increasing age, heavier smoking, lower income, and male sex were risk factors for incident COPD. This is the first study to identify income as a risk factor for incident COPD. Furthermore, no previous reports exist on the AR of each risk factor for incident COPD. In our study, the adjusted AR was highest for low education level of high school or lower, followed by smoking history, low income, and male sex. Low income included the first to third quartiles, suggesting that subjects with an intermediate income are also at high risk of COPD.
The strengths of this study are its prospective population-based design, large cohort size, and the use of spirometric results for all cohort participants. However, the study also has several limitations. First, the follow-up period was relatively short. Because half of the subjects were aged 40–49 years, their age was <55 years even after a median follow-up duration of 4 years. However, the Ansung–Ansan cohort study is ongoing, and we expect to be able to analyze the data further with longer follow-up. Second, we used pre-bronchodilator spirometric values instead of post-bronchodilator values to define COPD. However, previous study reported that the assessment of prognostic risk factors for COPD was not influenced by the type of lung function assessment (before or after bronchodilation), even if the prevalence of COPD defined without bronchodilation may be overestimated.32 Third, information on mortality of subjects was not available. Further study is warranted to investigate mortality rates and mortality-related factors in a longitudinal cohort of Koreans with COPD. Lastly, the data on smoking cessation have not been confirmed objectively using biological method.
In conclusion, in the Korean general population, around 15.5/1,000 individuals are newly diagnosed with COPD every year. The incidence rate increases with increasing age, increasing amount of smoking, and decreasing income and is higher in men than in women.
Supplementary material
Table S1.
Risk factors and adjusted relative risk of incident COPDa
| Risk factors | Total
|
Male
|
Female
|
|||
|---|---|---|---|---|---|---|
| Adjusted relative riska | 95% CI | Adjusted relative riska | 95% CI | Adjusted relative riska | 95% CI | |
| Age | ||||||
| <60 years | Reference | Reference | Reference | Reference | Reference | Reference |
| ≥60 years | 2.66 | 2.35–3.01 | 2.78 | 2.42–3.2 | 2.21 | 1.69–2.89 |
| Sex | ||||||
| Female | Reference | Reference | – | – | – | – |
| Male | 2.15 | 1.76–2.63 | – | – | – | – |
| Smoking history | ||||||
| Never smokers | Reference | Reference | Reference | Reference | Reference | Reference |
| Former smokers | 1.90 | 1.55–2.33 | 1.79 | 1.44–2.23 | 1.02 | 0.34–3.01 |
| Current smokers | 2.48 | 2.05–2.99 | 2.28 | 1.86–2.80 | 4.04 | 2.84–5.75 |
| Income | ||||||
| Fourth quartile | Reference | Reference | Reference | Reference | Reference | Reference |
| Third quartile | 1.27 | 0.99–1.62 | 1.14 | 0.87–1.49 | 2.29 | 1.16–4.53 |
| Second quartile | 1.56 | 1.26–1.93 | 1.48 | 1.18–1.86 | 2.19 | 1.15–4.15 |
| First quartile | 2.03 | 1.64–2.5 | 1.91 | 1.52–2.39 | 2.90 | 1.56–5.42 |
Note:
Adjusted for other factors (age, sex, smoking history, and income).
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1B03933125). This research was also supported by a fund (code: 2014ER560600) by Research of Korea Centers for Disease Control and Prevention and the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2014S1A2A2028559). Ah Young Leem and Boram Park contributed equally to this work as first authors. Sungho Won and Ji Ye Jung contributed equally to this work as corresponding authors.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting, and critically revising the paper and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Global strategy for the diagnosis m, and prevention of chronic obstructive pulmonary disease Global Initiative for Chronic Obstructive Lung Disease website. [Accessed November 10, 2016]. Available from: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosismanagement.html.
- 2.Mannino DM, Higuchi K, Yu TC, et al. Economic burden of COPD in the presence of comorbidities. Chest. 2015;148(1):138–150. doi: 10.1378/chest.14-2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mannino DM, Buist AS. Global burden of COPD: risk factors, prevalence, and future trends. Lancet. 2007;370(9589):765–773. doi: 10.1016/S0140-6736(07)61380-4. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization . Chronic obstructive pulmonary disease (COPD) Geneva, Switzerland: WHO; [Accessed November 10, 2016]. p. 2015. Available from: http://www.who.int/respiratory/copd/en/ [Google Scholar]
- 5.Halbert RJ, Natoli JL, Gano A, Badamgarav E, Buist AS, Mannino DM. Global burden of COPD: systematic review and meta-analysis. Eur Respir J. 2006;28(3):523–532. doi: 10.1183/09031936.06.00124605. [DOI] [PubMed] [Google Scholar]
- 6.Lindberg A, Jonsson AC, Ronmark E, Lundgren R, Larsson LG, Lundback B. Ten-year cumulative incidence of COPD and risk factors for incident disease in a symptomatic cohort. Chest. 2005;127(5):1544–1552. doi: 10.1378/chest.127.5.1544. [DOI] [PubMed] [Google Scholar]
- 7.Lindberg A, Eriksson B, Larsson LG, Ronmark E, Sandstrom T, Lundback B. Seven-year cumulative incidence of COPD in an age-stratified general population sample. Chest. 2006;129(4):879–885. doi: 10.1378/chest.129.4.879. [DOI] [PubMed] [Google Scholar]
- 8.van Durme YMTA, Verhamme KMC, Stijnen T, et al. Prevalence, incidence, and lifetime risk for the development of COPD in the elderly: the Rotterdam study. Chest. 2009;135(2):368–377. doi: 10.1378/chest.08-0684. [DOI] [PubMed] [Google Scholar]
- 9.Afonso AS, Verhamme KM, Sturkenboom MC, Brusselle GG. COPD in the general population: prevalence, incidence and survival. Respir Med. 2011;105(12):1872–1884. doi: 10.1016/j.rmed.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 10.de Marco R, Accordini S, Marcon A, et al. European Community Respiratory Health Survey (ECRHS) Risk factors for chronic obstructive pulmonary disease in a European cohort of young adults. Am J Respir Crit Care Med. 2011;183(7):891–897. doi: 10.1164/rccm.201007-1125OC. [DOI] [PubMed] [Google Scholar]
- 11.Kojima S, Sakakibara H, Motani S, et al. Incidence of chronic obstructive pulmonary disease, and the relationship between age and smoking in a Japanese population. J Epidemiol. 2007;17(2):54–60. doi: 10.2188/jea.17.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behrendt CE. Mild and moderate-to-severe COPD in nonsmokers: distinct demographic profiles. Chest. 2005;128(3):1239–1244. doi: 10.1378/chest.128.3.1239. [DOI] [PubMed] [Google Scholar]
- 13.Celli BR, Halbert RJ, Nordyke RJ, Schau B. Airway obstruction in never smokers: results from the Third National Health and Nutrition Examination Survey. Am J Med. 2005;118(12):1364–1372. doi: 10.1016/j.amjmed.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 14.Eisner MD, Anthonisen N, Coultas D, et al. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182(5):693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 15.Lamprecht B, McBurnie MA, Vollmer WM, et al. BOLD Collaborative Research Group COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;;139(4):752–763. doi: 10.1378/chest.10-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baik I, Cho NH, Kim SH, Han BG, Shin C. Genome-wide association studies identify genetic loci related to alcohol consumption in Korean men. Am J Clin Nutr. 2011;93(4):809–816. doi: 10.3945/ajcn.110.001776. [DOI] [PubMed] [Google Scholar]
- 17.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 18.Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol. 1985;122(5):904–914. doi: 10.1093/oxfordjournals.aje.a114174. [DOI] [PubMed] [Google Scholar]
- 19.Kim DS, Kim YS, Jung KS, et al. Korean Academy of Tuberculosis and Respiratory Diseases Prevalence of chronic obstructive pulmonary disease in Korea: a population-based spirometry survey. Am J Respir Crit Care Med. 2005;172(7):842–847. doi: 10.1164/rccm.200502-259OC. [DOI] [PubMed] [Google Scholar]
- 20.de Marco R, Accordini S, Cerveri I, et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med. 2007;175(1):32–39. doi: 10.1164/rccm.200603-381OC. [DOI] [PubMed] [Google Scholar]
- 21.Huhti E, Ikkala J, Hakulinen T.Chronic respiratory disease, smoking and prognosis for life. An epidemiological studyScand J Respir Dis 1977583170–180. [PubMed] [Google Scholar]
- 22.Johannessen A, Omenaas E, Bakke P, Gulsvik A. Incidence of GOLD-defined chronic obstructive pulmonary disease in a general adult population. Int J Tuberc Lung Dis. 2005;9(8):926–932. [PubMed] [Google Scholar]
- 23.Krzyzanowski M, Jedrychowski W, Wysocki M. Factors associated with the change in ventilatory function and the development of chronic obstructive pulmonary disease in a 13-year follow-up of the Cracow Study. Risk of chronic obstructive pulmonary disease. Am Rev Respir Dis. 1986;134(5):1011–1019. doi: 10.1164/arrd.1986.134.5.1011. [DOI] [PubMed] [Google Scholar]
- 24.Terzikhan N, Verhamme KM, Hofman A, Stricker BH, Brusselle GG, Lahousse L. Prevalence and incidence of COPD in smokers and nonsmokers: the Rotterdam Study. Eur J Epidemiol. 2016;31(8):785–792. doi: 10.1007/s10654-016-0132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vestbo J, Lange P. Can GOLD Stage 0 provide information of prognostic value in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2002;166(3):329–332. doi: 10.1164/rccm.2112048. [DOI] [PubMed] [Google Scholar]
- 26.Garcia Rodriguez LA, Wallander MA, Tolosa LB, Johansson S. Chronic obstructive pulmonary disease in UK primary care: incidence and risk factors. COPD. 2009;6(5):369–379. doi: 10.1080/15412550903156325. [DOI] [PubMed] [Google Scholar]
- 27.Gershon AS, Wang C, Wilton AS, Raut R, To T. Trends in chronic obstructive pulmonary disease prevalence, incidence, and mortality in Ontario, Canada, 1996 to 2007: a population-based study. Arch Intern Med. 2010;170(6):560–565. doi: 10.1001/archinternmed.2010.17. [DOI] [PubMed] [Google Scholar]
- 28.Seo JY, Hwang YI, Mun SY, et al. Awareness of COPD in a high risk Korean population. Yonsei Med J. 2015;56(2):362–367. doi: 10.3349/ymj.2015.56.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frank TL, Hazell ML, Linehan MF, Frank PI. The diagnostic accuracies of chronic obstructive pulmonary disease (COPD) in general practice: the results of the MAGIC (Manchester Airways Group Identifying COPD) study. Prim Care Respir J. 2006;15(5):286–293. doi: 10.1016/j.pcrj.2006.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization Global Health Observatory Data Repository. [Accessed November 10, 2016]. Available from: http://data.worldbank.org/indicator/SH.PRV.SMOK.MA?end=2012&start=2000&view=chart.
- 31.Korean National Health and Nutrition Examination Survey, Smoking Prevalence. [Accessed November 10, 2016]. Available from: http://knhanes.cdc.go.kr/knhanes/index.do.
- 32.Johannessen A, Omenaas ER, Bakke PS, Gulsvik A. Implications of reversibility testing on prevalence and risk factors for chronic obstructive pulmonary disease: a community study. Thorax. 2005;60(10):842–847. doi: 10.1136/thx.2005.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Risk factors and adjusted relative risk of incident COPDa
| Risk factors | Total
|
Male
|
Female
|
|||
|---|---|---|---|---|---|---|
| Adjusted relative riska | 95% CI | Adjusted relative riska | 95% CI | Adjusted relative riska | 95% CI | |
| Age | ||||||
| <60 years | Reference | Reference | Reference | Reference | Reference | Reference |
| ≥60 years | 2.66 | 2.35–3.01 | 2.78 | 2.42–3.2 | 2.21 | 1.69–2.89 |
| Sex | ||||||
| Female | Reference | Reference | – | – | – | – |
| Male | 2.15 | 1.76–2.63 | – | – | – | – |
| Smoking history | ||||||
| Never smokers | Reference | Reference | Reference | Reference | Reference | Reference |
| Former smokers | 1.90 | 1.55–2.33 | 1.79 | 1.44–2.23 | 1.02 | 0.34–3.01 |
| Current smokers | 2.48 | 2.05–2.99 | 2.28 | 1.86–2.80 | 4.04 | 2.84–5.75 |
| Income | ||||||
| Fourth quartile | Reference | Reference | Reference | Reference | Reference | Reference |
| Third quartile | 1.27 | 0.99–1.62 | 1.14 | 0.87–1.49 | 2.29 | 1.16–4.53 |
| Second quartile | 1.56 | 1.26–1.93 | 1.48 | 1.18–1.86 | 2.19 | 1.15–4.15 |
| First quartile | 2.03 | 1.64–2.5 | 1.91 | 1.52–2.39 | 2.90 | 1.56–5.42 |
Note:
Adjusted for other factors (age, sex, smoking history, and income).