Abstract
Introduction
Owing to the shared embryonic origin, defects in development of optic nerves are often seen in conjunction with defects affecting the surrounding brain and pituitary gland. Optic nerve hypoplasia (ONH) and septo-optic dysplasia (SOD) represent a clinical spectrum associated with visual, pituitary and severe central nervous system structural abnormalities (SODplus). Based on changing clinical patterns, our primary objective was to examine trends in annual incidence of ONH/SOD and geographical clustering in Manitoba.
Methods
This was a retrospective 1996 to 2015 chart review with extraction of anthropometric measures, radiologic findings, parental characteristics, endocrinopathies and neurologic symptoms from all involved in care. Postal codes were used to assign map co-ordinates and identify relevant census-based deprivation indices.
Results
Ninety-three children were identified in our catchment area; Poisson regression confirmed a striking 1.11-fold annual increase (95% confidence interval 1.07 to 1.16) or ~800% over two decades. The annual incidence (averaged 2010 to 2014 chart data) reached 53.3 per 100,000, affecting 1 in 1875 live births. Most (~55%) had SODplus. Common presenting features were hypoglycemia, nystagmus, seizures and developmental delay; 40% had hormone deficiencies; 80% had reduced visual acuity, typically bilateral. Many were premature with young, primiparous mothers. Unhealthy maternal lifestyles and severe material deprivation were noted. There was disproportionate clustering in individuals from Northern Manitoba at three times the average provincial rate.
Conclusion
We noted a dramatic rise in the annual incidence of ONH/SOD, which was strongly associated with poverty and northern communities. The pattern was consistent with environmental or nutritional etiologies. Many children were severely affected with increased morbidity and health care burdens.
Keywords: Blindness, Hypopituitarism, Incidence, Poverty, Septo-optic dysplasia
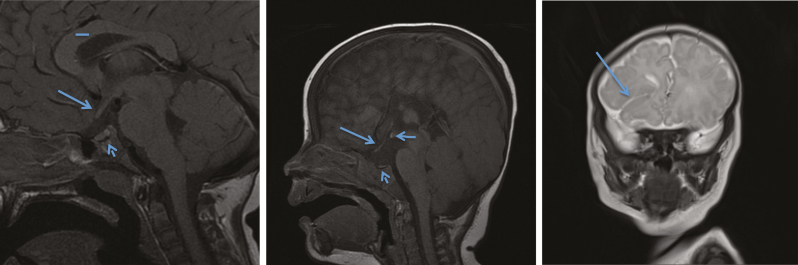
Optic nerves and the pituitary gland develop together embryologically. Optic nerve hypoplasia (ONH) is a leading cause of blindness in North American children (1). ONH is part of the spectrum of septo-optic dysplasia (SOD), which differs from isolated ONH with the addition of structural pituitary abnormalities (hypoplasia and/or ectopic posterior pituitary) and/or absence of the septum pellucidum (Figure 1b: Normal 1a). SODplus is further associated with cortical dysplasia or other structural brain malformations (Figure 1c) (2). Children within this spectrum often present in infancy with pituitary hormone deficiencies (3); however, later onset endocrinopathies are common. Additionally, many have mild to severe visual disturbances (legal blindness), developmental delay or seizures attributed to central nervous system (CNS) malformations.
Figure 1.
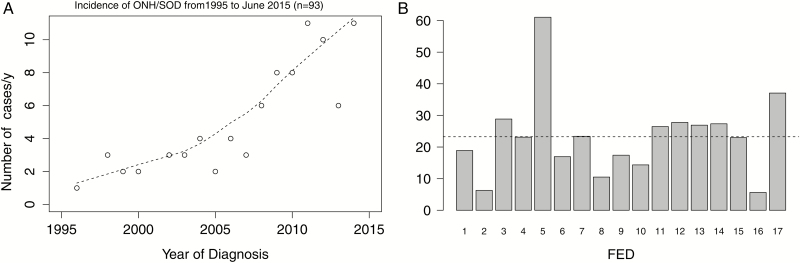
(a) Normal optic nerve (arrow) and pituitary (short arrow), normal corpus callosum (line). (b) Thin optic nerve (arrow) and very small anterior pituitary (short arrow), ectopic posterior pituitary (horizontal arrow) and thin corpus callosum. (c) Closed lip schizencephaly (arrow) often found in SODplus. (d) Incidence of ONH/SOD from 1995 to June 2015 (n=93).
Although the exact cause of ONH/SOD is unknown, both genetic and environmental factors play a role. Viral infections, medications and vascular disruption have been suggested as possible environmental contributors (4). It has also been associated with primipara, young maternal age, preterm delivery and prenatal exposure to alcohol and smoking (5,6). Rarely ONH/SOD is caused by mutations in HESX1, OTX2 and SOX2 (5,6).
Clinically, we observed an increase in the number of cases with SOD, particularly in families from remote communities. Several studies have also reported a rise in ONH/SOD in other jurisdictions (e.g., Sweden) (7,8). The primary objective of this study was to examine temporal trends in the annual incidence of ONH/SOD and identify any geographical clustering of cases or putative etiological factors.
METHODS
This was a retrospective chart review of patients with ONH/SOD in the databases of any of the following subspecialty clinics: medical genetics, endocrinology, neurology, ophthalmology or radiology at the Health Sciences Center (HSC), a tertiary care hospital in Winnipeg, between April 1996 and June 2015. All subjects were confirmed to have small optic nerves identified by magnetic resonance imaging (MRI; i.e., less than 2.57 mm to 2.81 mm) (9) and by fundoscopic examination. Subjects with isolated ONH were categorized as ONH, SOD or SODplus.
A total of 93 patients with ONH/SOD from our longstanding catchment areas (Manitoba, North West Ontario and West Nunavut) were identified (‘chart review’ dataset) during the study period. All 93 were included when examining the clinical phenotype. Only patients residing within Manitoba at the time of diagnosis were eligible for the calculation of incidence. Data extracted included sex, anthropometric measurements at presentation and last clinical encounter, maternal exposures, pregnancy complications, parental age, self-declared ancestry and postal code. Pertinent radiological findings included the size of the optic nerves, unilateral or bilateral involvement and other CNS abnormalities. Head computerized tomography (CT) scan was the only radiologic examination performed in three cases. Forty-seven MRIs reporting ONH/SOD were selected at random and reviewed by a single radiologist (M.B.), who was blind to the past imaging reports; his readings were 100% concordant.
Ophthalmologic findings included presence of nystagmus, decreased visual acuity (<20 of 50 but >20 of 200) and legal blindness (≤20 of 200). Neurologic findings included seizures, developmental delay or autism. Endocrinopathies were identified through positive laboratory evaluation or treatment with replacement hormone therapy and included deficiencies of growth hormone (GH), thyroid-stimulating hormone (TSH), adrenocorticotropic hormone (ACTH), luteinizing hormone, follicle-stimulating hormone or antidiuretic hormone. From 2011 onward, microarrays were considered standard of care.
A Quality Assurance (QA) sub-study looked at all paediatric radiology reports from 2010 to 2014 for the words ‘optic nerve’ and identified 567 individual reports; 43 of whom had ONH and 2 had SOD not found by chart review. None had apparent endocrinopathies; most had unilateral ONH.
The frequency of diagnosis (‘chart review’ only, n = 93) is plotted by year; a non-parametric LOESS smooth was used to fit the curve. A Poisson regression model was applied to assess the impact of time on the annual incidence in Manitoba. Incidences were calculated based on the number of Manitoba children <19 years (n=313,507 based on 2011 census) or per 100,000 live births (~15,000/year in Manitoba) (10).
Clinical, biochemical and radiographic features were tabulated (‘chart data’); medians with range or percentage and 95% confidence intervals (CIs) were calculated. These were compared using non-parametric testing (Fisher’s exact or Kruskal–Wallis) given their non-normal distribution or small cell size. Data were analyzed using STATA 12 (College Station, Texas, USA).
Individual six-digit postal codes were mapped to longitude, latitude, census dissemination area (DA) and federal electoral district (FED) using the PCCF+ (postal code conversion file, 2015) provided by Statistics Canada through the Data Liberation Initiative. FEDs have roughly the same population (80,000 to 100,000, except for Nunavut at 31,000). Census DAs are an administrative unit from Statistics Canada, each containing 400 to 700 individuals. The Raymond–Pampalon deprivation indices were assigned for each DA based on 2006 census data (11). Within each DA, the material deprivation quintile is a composite score based on household income, unemployment and high school completion rates. Similarly, social deprivation quintiles are based on the proportion of single parent families; adults who are separated, divorced or widowed; and adults living alone (11). Only 85 postal codes were matched for deprivation indices, while 90 were available for geomapping; we did not delete children from analyses whose residences could not be geomapped. Maps were created using the QGIS GeoInformation System version 2.8. Chi-squared and Fisher exact tests were used to test the null hypothesis of homogeneity in the distribution of incidence across FEDs or deprivation quintiles.
Ethics
Ethical approval was obtained from the Research Ethics Board at the University of Manitoba.
RESULTS
We noted a striking increase in annual incidence of ONH/SOD over the past decade (Figure 1d, ‘chart review’ only). By Poisson regression, a significant increase of 1.11-fold per year (95% CI 1.07 to 1.16) was observed representing an 800% increase over 20 years. The annual incidence in Manitoba averaged over the past 5 years is 53.3 per 100,000 live births (n=40, ‘chart data’). Including additional cases from the QA project (60 per 100,000 live births [n=45]), this totalled 113.3 per 100,000. This translated to 1 in 1875 live births (chart data) or 1 in 892 live births (chart and QA data) in Manitoba with ONH/SOD.
Of note, few children (12 of 93) had isolated ONH while the majority had SODplus (52 of 93). Roughly half of all children were born prematurely (<37 weeks); most were diagnosed in infancy, especially for SOD or SODplus (P=0.005). Infants commonly presented with hypoglycemia, often with underlying hormone deficiencies, developmental delay or ocular findings. Only about 20% of children had normal bilateral visual acuity; 33% of all children were legally blind bilaterally (Tables 1 and 2). Besides age, there was no apparent difference in presenting features across the three categories. Approximately 60% of the children lacked a septum pellucidum, frequently detected by cranial ultrasounds.
Table 1.
Patient characteristics at diagnosis
| All (93) | ONH (12) | SOD (29) | SODplus (52) | P value | |
|---|---|---|---|---|---|
| Median age (month) | 10 (0–202) | 33 (1–119) | 7.5 (0–202) | 8.5 (0–168) | 0.005 |
| Sex |
1.0 |
||||
| % female | 44.1 (41/93) [33.8–54.8] | 41.7 (5/12) [15.1–72.3] | 48.5 (14/29) [29.4–67.5] | 42.3 (22/52) [28.7–56.8] | |
| Hypoglycemia, % | 29.4 (25/85) [20.0–40.2] | 20.0 (2/10) [2.5–55.6] | 33.3 (9/27) [16.5- 53.9] | 29.2 (14/48) [17.0–44.1] | 0.7 |
| Prematurity, % | 53.6 (30/56) [40.0–67.0] | 66.7 (4/6) [22.3–95.6] | 58.8 (10/17) [32.9–81.6] | 48.5 (16/33) [30.8–66.5] | 1.0 |
| Seizures, % | 20.0 (17/87) [12.1–30.1] | 0 (0/12) | 7.1 (2/28) [0.9–23.5] | 31.9 (15/47) [9.1–47.1] | 0.2 |
| Developmental delay, % | 32.9 (28/85) [23.1–43.9] | 20.0 (2/10) [2.5–55.6] | 25.0 (7/28) [10.7–44.9] | 40.4 (19/47) [26.4–55.7] | 0.5 |
| Bilateral reduced vision, % | 58.1 (50/86) [47.0–68.7] | 58.3 (7/12) [27.7–84.5] | 56.0 (14/25) [34.9–75.6] | 61.2 (30/49) [46.2–74.8] | 0.1 |
| Bilateral legal blindness, %* | 33.7 (29/86) [23.9–44.7] | 33.3 (4/12) [9.9–65.1] | 28.0 (7/25) [12.1–49.4] | 36.7 (18/49) [23.4–51.7] | 0.8 |
| Bilateral normal vision, % | 17.4 (15/86) [10.1–27.1] | 0 (0/12) | 20.0 (5/25) [6.8–40.7] | 20.4 (10/49) [10.2–34.3] | 0.5 |
| Nystagmus, % | 36.5 (31/85) [26.3–47.6] | 45.5 (5/11) [16.8–76.6] | 35.7 (10/28) [18.6–55.9] | 34.7 (16/46) [21.4–50.2] | 0.2 |
| Hormone deficiencies | |||||
| GH, % | 9.3 (8/86) [4.1–17.5] | 20.0 (2/10) [2.5–55.6] | 10.7, (3/28) [2.2–28.2] | 6.3 (3/48) [1.3–17.2] | 0.2 |
| Cortisol, % | 25.6 (22/86) [16.8–36.1] | 10.0 (1/10) [0.0–44.5] | 32.1 (9/28) [15.8–52.4] | 25.0 (12/48) [13.6–39.6] | 0.4 |
| TSH, % | 20.9 (18/86) [12.9–31.0] | 0 (0/12) | 35.1 (10/28) [18.6–55.9] | 16.7 (8/48) [7.5–30.2] | 0.1 |
| LH/FSH or micropenis, % | 5.4 (5/93) [1.8–12.1] | 8.3 (1/12) [0.2–38.5] | 3.5 (1/29) [0.0–17.8] | 5.7(3/52) [1.2–15.9] | 0.5 |
| ADH, % | 2.4 (2/85) [0.2–8.2] | 0 (0/12) | 0 (0/28) | 4.3 (2/47) [0.5–14.5] | 0.8 |
Values are median (range), % (n/total) [95% CI]. ADH Antidiuretic hormone; FSH Follicle-stimulating hormone; GH Growth hormone; LH Luteinizing hormone; ONH Optic nerve hypoplasia; SOD Septo-optic dysplasia; SODplus SOD associated with visual, pituitary and severe central nervous system structural abnormalities; TSH Thyroid-stimulating hormone. *Subgroup of bilateral reduced vision
Table 2.
MRI findings of patients
| All (93) | ONH (12) | SOD (29) | SODplus (52) | P value | |
|---|---|---|---|---|---|
| Small optic nerves | U: 14.0 (13/93) [7.6–22.7] | U: 16.7 (2/12) [2.0–48.4] | U: 17.2 (5/29) [5.8–35.8] | U: 11.5 (6/52) [4.4–23.4] | 0.6 |
| B: 86.0% (80/93) [77.2–92.3] | B: 83.3 (10/12) [51.6–97.9] | B: 82.8 (24/ 29) [64.2–94.2] | B: 88.5 (46/52) [76.6–95.6] |
Values are median (min to max), % (n/total) [95% CI]. B Bilateral; MRI Magnetic resonance imaging; U Unilateral
No family history was apparent for ONH/SOD; consanguinity was noted in four families. Twenty-one children had microarray analyses; all were non-informative. Included in this were 14 (11 SODplus; 3 SOD) with other congenital abnormalities; these were cleft palate, cardiac defects (e.g., ventricular septal defect (VSD), atrial septal defect (ASD)) and dysmorphic facial features (e.g., Hallermann Streiff syndrome).
Table 3 summarizes characteristics at last encounter. The median age at last visit was older in patients with isolated ONH (146 months). Roughly 10% of children had bilateral normal vision and ~1/4 had bilateral legal blindness. Developmental delay was found in 49.5% of all patients and 55.8% of the SODplus patients (P=0.027). ACTH and TSH deficiencies were most common (34.4%), followed by deficiencies of GH (26.9 %) and antidiuretic hormone (4.3%). Of the isolated ONH group, five patients had evidence of GH deficiency, four had TSH deficiency and two had deficiency in ACTH. Overall, ~ 40% of all children had endocrine deficiencies at some point. There were three deceased patients; all had SODplus.
Table 3.
Patient characteristics—age at last visit plus features that developed at any time during follow-up
| Category | All (93) | Isolated ONH only (12) | SOD only (29) | SODplus only (52) | P value |
|---|---|---|---|---|---|
| Median age at last visit (month) | 57 (0.23–238) | 146 (84–238) | 33.5 (5–210) | 51 (0.23–220) | <0.005 |
| Seizures, % | 24.7 (23/93) [16.4–34.8] | 0 (0/12) | 10.3 (3/29) [2.1–27.4] | 38.5 (20/52) [25.3–53.0] | 0.03 |
| Autism, % | 3.2 (3/93) [0.7–9.14] | 8.3 (1/12) [0.2–38.5] | 0 (0/29) | 3.9 (2/52) [0.5–13.2] | 0.3 |
| Developmental delay, % | 49.5 (46/93) [38.9–60.0] | 16.7 (2/12) [2.1–48.4] | 51.7 (15/29) [32.5–70.6] | 55.8 (29/52) [41.3–69.5] | 0.03 |
| Deceased, % | 3.4 (3/88) [0.8–10.6] | 0 (0/12) | 0 (0/29) | 6.4 (3/47) [1.3–17.5] | 0.7 |
| Bilateral reduced vision, % | 57.5 (42/73) [45.4–69.0] | 45.5 (5/11) [16.7–76.6] | 59.1 (13/22) [36.4–79.3] | 60.0 (24/40) [43.4–75.1] | 0.6 |
| Bilateral legal blindness, %* | 27.4 (20/73) [17.6–39.1] | 27.2 (3/11) [6.0–61.0] | 18.2 (4/22) [5.2–40.3] | 32.5 (13/40) [18.6–49.1] | 0.5 |
| Bilateral normal vision, % | 12.3 (9/73) [5.8–22.1] | 0 (0/12) | 9.1 (2/22) [1.1–29.2] | 17.5 (7/40) [7.3–32.8] | 0.3 |
| Nystagmus, % | 38.7 (36/93) [28.8–49.4] | 50.0 (6/12) [21.1–78.9] | 37.9 (11/29) [20.7–57.7] | 36.5 (19/52) [23.6–51.0] | 0.5 |
| GH deficiency, % | 26.9 (25/93) [18.2–37.1] | 41.7 (5/12) [15.2–72.3] | 27.6 (8/29) [12.7–47.2] | 23.1 (12/52) [12.5–36.8] | 0.3 |
| ACTH deficiency, % | 34.4 (32/93) [24.9–45.0] | 16.7 (2/12) [2.1–48.4] | 44.8 (13/29) [26.4–64.3] | 32.7 (17/52) [20.3–47.1] | 0.2 |
| TSH deficiency, % | 34.4 (32/93) [24.9–45.0] | 33.3 (4/12) [9.9–65.1] | 44.8 (13/29) [26.4–64.3] | 28.9 (15/52) [17.1–43.1] | 1.0 |
| ADH deficiency, % | 4.3 (4/93) [1.2–10.6] | 0 (0/12) | 6.9 (2/29) [0.9–22.8] | 3.9 (2/52) [0.50–13.2] | 1.0 |
| One or more hormone deficiencies, % | 41.4 (36/87) [30.2–52.4] | 60.0 (6/10) [26.2–87.8] | 37.9 (11/29) [20.7–57.7] | 39.6 (19/48) [25.2–53.8] | 0.6 |
Values are median (range), % (n/total) [95% CI]. ACTH Adrenocorticotropic hormone; ADH Antidiuretic hormone; GH Growth hormone; ONH Optic nerve hypoplasia; SOD Septo-optic dysplasia; SODplus SOD associated with visual, pituitary and severe central nervous system structural abnormalities; TSH Thyroid-stimulating hormone. *Subgroup of bilateral reduced vision
Maternal characteristics are shown in Table 4. Their median age was 21 years (interquartile range 19 to 26 years); about 50% were primiparous. About 1/3 of the pregnancies had in utero exposure to smoking, alcohol or illicit drugs (37%). Gestational diabetes and hypertension were only noted in pregnancies of those with SOD/SODplus. There was a significantly disproportionate number of SOD cases born to mothers whose self-declared ethnicity was First Nations (~60%, n=45).
Table 4.
Maternal characteristics during pregnancy
| Category | All | ONH | SOD | SODplus | P value |
|---|---|---|---|---|---|
| Smoking, % | 35.2 (19/53) [23.1–50.2] | – | 37.5 (6/16) [15.2–64.6] | 41.9 (13/31) [24.5–60.9] | 0.08 |
| Alcohol use, % | 21.4 (12/56) [11.6–34.4] | 16.7 (1/6) [0.4–64.1] | 16.7 (3/18) [3.6–41.4] | 25.0 (8/32) [11.5–43.4] | 1.0 |
| Illicit drug use, % | 20.0 (11/55) [10.4–33.0] | 16.7 (1/6) [0.4–64.1] | 5.6 (1/18) [0.1–27.3] | 28.1 (9/31) [14.2–48.0] | 1.0 |
| Any maternal exposures, % | 36.9 (24/65) [25.3–49.8] | 14.2 (1/7) [0.4–57.9] | 45.5 (10/22) [24.9–67.8] | 36.1 (13/36) [20.8–53.8] | 0.2 |
| Hypertension, % | 11.3 (7/62) [5.7–23.9] | – | 14.3 (3/21)[3.0–36.3] | 11.4 (4/35) [3.2–26.7] | 1.0 |
| Diabetes, % | 12.3 (8/65) [5.5–22.8] | – | 17.4 (4/23) [5.0–38.8] | 11.1 (4/36) [3.1–26.1] | 1.0 |
| Percentage of primigravida, % | 53.6 (30/56) [39.7–67.0] | 66.7 (4/6) [22.2–95.7] | 58.8 (10/17) [32.9–81.6] | 48.5 (16/33) [30.8–66.5] | 1.0 |
| Median maternal age (years) | 21 (14–45) | 22.5 (15–45) | 21.5 (16–33) | 21 (14–38) | 0.3 |
| Consanguinity, % | 8.9 (4/45) [2.5–21.2] | – | 7.1 (1/14) [0.18–33.9] | 12.0 (3/25) [2.5–31.2] | 1.0 |
| Ethnicity (n) | (45) | (6) | (14) | (25) | |
| White, % | 22.2 (10) [11.2–37.1] | 66.7 (4) [22.3–95.7] | 14.3 (2) [1.8–42.8] | 16.0 (4) [4.5–36.1] | <0.001 |
| First Nations, % | 60.0 (28) [46.5–76.2] | – | 71.4 (10) [41.9–91.2] | 68.0 (17) [46.5–85.1] | |
| Inuit, % | 11.1 (5) [3.7–24.1] | – | 7.1 (1) [0.2–33.9] | 16.0 (4) [4.5–36.1] | |
| Asian, % | 6.7 (3) [1.4–18.3] | 33.3 (2) [4.3–77.7] | 7.1 (1) [0.2–33.9] | – |
Values are median (range), % (n) and [95% CI]. ONH Optic nerve hypoplasia; SOD Septo-optic dysplasia; SODplus SOD associated with visual, pituitary and severe central nervous system structural abnormalities
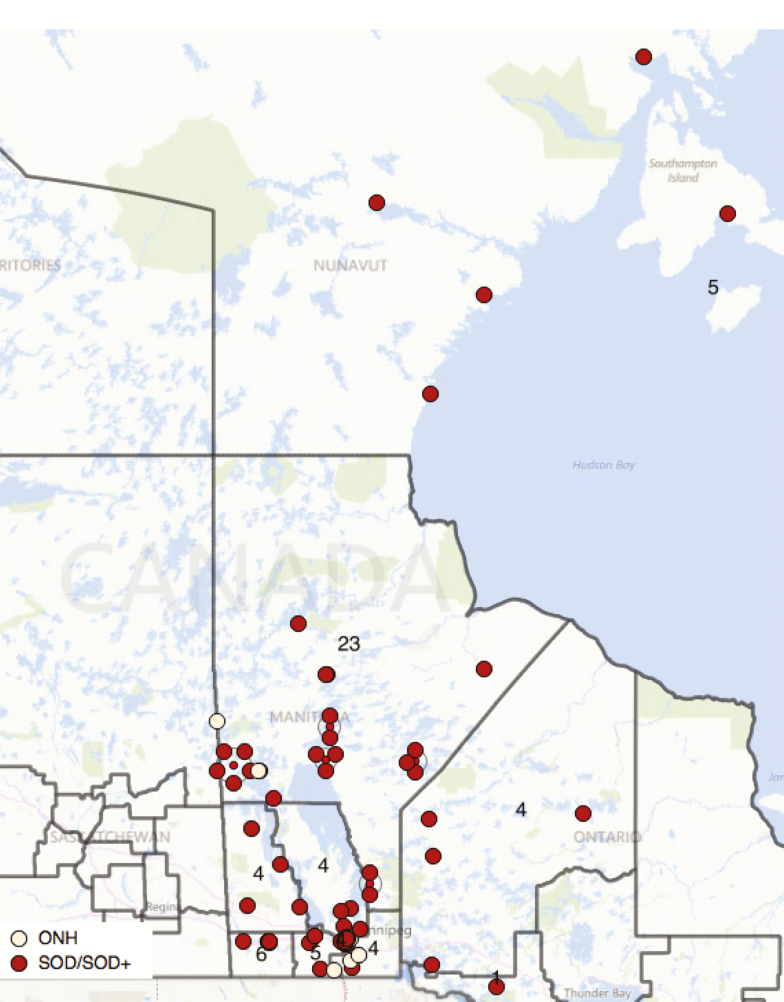
There was clear geographical clustering in the North: Churchill (with 66.9 cases per 100,000 youths <19 years) and Nunavut (37.1 per 100,000) over the 20-year period, compared with 26.8 for Manitoba as whole (Figure 2a and 2b). Between 2010 and 2014, the mean annual incidence is 2.6 per 100,000 youths <19 years. Over half of the infants lived in neighbourhoods in quintiles 4 or 5 for material deprivation (54 of 85, P<0.001). Similar inhomogeneity was seen within urban Winnipeg (19 of 33, P=0.003). Across Manitoba, the distribution of material deprivation quintiles between patients and neighbourhoods (DAs) differed significantly (P=0.01). A similar disparity was seen if northern Churchill and Nunavut were examined separately (P=0.04). There was no statistically significant association with social deprivation index (P=0.4).
Figure 2.
Incidence per 100,000 youth <19y by Federal Electoral District (FED). P < 0.001 by Fiser Exact test (dotted line = average for all Manitoba districts). 1 Kenora; 2 Thunder Bay & Rainy River, ON; 3 Brandon-Souris; 4 Charleswood; 5 Churchill; 6 Dauphin; 7 Elmwood; 8 Kildonan; 9 Portage; 10 Provencher; 11 Saint Boniface; 12 Selkirk; 13 Winnipeg Centre; 14 Winnipeg North; 15 Winnipeg South; 16 Winnipeg South Centre; 17 Nunavut.
DISCUSSION
Main findings
This study confirms our clinical suspicion that the incidence of ONH/SOD has been increasing dramatically; the Poisson regression confirmed a 1.11-fold annual increase since 1996. The averaged Manitoba annual incidence (2010 to 2014) of ONH/SOD (chart review and QA) confirms high values of 113.3 per 100,000 live births compared with other reports (7,8). This implies that 1 in 892 live births in Manitoba are affected with ONH/SOD (chart review and QA), whereas 1 in 1875 live births present early with features of ONH/SOD (chart data). Half have the severe SODplus phenotype (chart data). Children with SOD or SODplus presented at a younger age than those with ONH. Seizures, developmental delay and bilateral visual impairment were common and consistent findings over time; given the symptomatic phenotype, we do not believe that missed cases early on explain the changing incidence. Mothers were young, 60% self-identified as Aboriginal or Inuit and lived in remote areas; 1/3 consumed alcohol, cigarettes or street drugs during the pregnancy. The families were impoverished in terms of neighbourhood material deprivation.
Few studies have evaluated contemporary temporal evolution; Blohmé et al. reported a quadrupling of incidence of ONH to 7 per 100,000 live births in Sweden from 1980 to 1999 (7). Recent reports from Olmsted County place their annual incidence at 2.4 per 100,000 youths <19 years with a doubling from 1988 to 2013. This meant that ONH/SOD was diagnosed 1 in 2200 live births (8). Likewise in the UK, the incidence was 10.9 per 100,000 youths <15 years in the Manchester region (12). All of these are lower than our current report with the exception of Olmsted County. Olmsted County did not have as dramatic a rise in incidence, perhaps due to their smaller population.
Reported incidences are recognized as underestimates due to undiagnosed cases of mild ONH. We have attempted to address this through our QA sub-study to identify all radiologic occurrences of ONH. However, even if we were to exclude the children with ONH/SOD detected by the QA sub-study, our annual incidence would still be higher than that previously reported at 53 per 100,000 live births. Similar to our findings, most children have bilateral ONH and significant visual problems (8), although many manuscripts identified cases via registries for children with visual impairment (13,14).
Given that most children in our population had SOD or SODplus, it is not surprising that many of them had significant neurological sequelae. This has also been described in other cohorts (Sweden, Olmsted County) where at least 50% of the children had developmental delay (8,13). Rates of endocrinopathy may vary with severity of the central lesions. Approximately 40% in our study had an endocrine deficiency diagnosed by their last visit. There are conflicting reports as to whether children with isolated ONH develop endocrine deficiencies (8,15). Small sample sizes and varying case definitions may account for variable incidences or phenotype including the apparently high number of children with ACTH/TSH deficiencies. Importantly, we found a significant proportion of our children with isolated ONH had associated endocrine disorders suggesting that even isolated ONH demands ongoing follow-up and screening (8,15).
The etiology of ONH/SOD is not yet clear; single gene mutations in HESX1 appear to be uncommon (16). Environmental, nutritional or lifestyle factors are potentially important. Others have reported that primiparous and/or young women, often of Indigenous descent, seem to be at highest risk (8,12,14). Poverty as determined by area-based measures has been strongly associated with ONH in Manchester, UK (12). The mothers of the children in our study were younger than the Canadian average (Statistics Canada – median age 29 years) and were more likely primiparous (53.6% versus 44.1 % for the national average in 2008, P<0.005) (17). Although we had chart data on ancestry for only about half of the mothers, there was still a disproportionate number of mothers of self-declared Indigenous ancestry. In 2011 Statistics Canada reported 16% of Manitobans were of First Nations descent; in our study, approximately 60% of children with identifiable ethnicity (50% of cases) were of First Nations descent (18). Interestingly, the rapid rise in frequency of ONH after about 2005 coincides with a parallel increase in the number of Northern Manitoba mothers who had inadequate prenatal care over the period 2001 to 2008 (19). In utero deficiencies, such as iron, folic acid or vitamin D or exposure to alcohol or smoking could be potential contributors. Unfortunately, Indigenous Canadian populations have higher rates of smoking and alcohol abuse compared with non-Indigenous (20,21). This may play a role in the higher rates; however, a case series cannot determine causal effects.
Strengths and limitations
This study has several strengths; we are the sole referral centre for Manitoba, West Nunavut and much of North West Ontario for paediatric genetics, ophthalmology, endocrinology and radiology. It is unlikely that significantly affected children would go undetected. Our comprehensive search through multiple databases likely ensured good case detection. A random sample of MRIs was re-read by a single radiologist in a blinded manner and he confirmed the diagnosis in all cases; we also included those without ONH/SOD. It is unlikely that the increase was due to better radiologic equipment, as MRI was the standard method of evaluation for most of the study period (2000 to 2015) (22). Because many of the children were symptomatic at diagnosis (hypoglycemia or seizures), it is unlikely that similar cases would have been missed in the early part of this study period. Moreover, by being able to search all radiographic reports, we were able to find a truer incidence of ONH/SOD (chart review and QA). The limitations include the potential underestimation despite these efforts; inflation of incidence because of relatively small numbers is possible. Additionally, data such as maternal characteristics were not always available in the medical charts. Ecological measures of socioeconomic status may not be as reliable as family-level indicators.
CONCLUSIONS
In conclusion, we demonstrate a striking increase in the incidence of ONH/SOD, which surpasses previous reports. Many children were severely affected (blindness, CNS abnormalities) and therefore carry high morbidity, which poses a significant burden of care on families and communities. It behooves clinicians to note early clinical changes (nystagmus, early hypoglycemia, absent septum pellucidum on head ultrasound) and consider this diagnosis. More detailed imaging (MRI) and referrals to paediatric centres are required for early detection of life-threatening endocrine deficiencies. Additionally, our findings suggest that SOD spectrum of disorders are rooted in poverty; the underlying etiology is likely nutritional or environmental.
Figure 3.
Distribution of ONH/SOD/SODplus in Manitoba, West Nunamvut or North West Ontario. Dark boundaries represent Federal Electoral Districts (FEDs) with comparable populations of 80-100k (except for Nunavut at 31k). Annotations represent the number of children with ONH/SOD in each. Individual points have been jittered to improve visibility, but each dot in the urban Winnipeg area represents multiple cases.
Conflict of Interest
None of the authors have any conflict of interest.
Acknowledgements
We wish to thank the Faculty of Medicine, University of Manitoba for providing funding allowing us to undertake this project.
References
- 1. Steinkuller PG, Du L, Gilbert C, Foster A, Collins ML, Coats DK. Childhood blindness. J Aapos 1999;3(1):26–32. [DOI] [PubMed] [Google Scholar]
- 2. Miller SP, Shevell MI, Patenaude Y, Poulin C, O’Gorman AM. Septo-optic dysplasia plus: A spectrum of malformations of cortical development. Neurology 2000;54(8):1701–3. [DOI] [PubMed] [Google Scholar]
- 3. Oatman OJ, McClellan DR, Olson ML, Garcia-Filion P. Endocrine and pubertal disturbances in optic nerve hypoplasia, from infancy to adolescence. Int J Pediatr Endocrinol 2015;2015(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garvin J, Sampath V, Karody VR. Gastroschisis complicated by septo-optic dysplasia: Two distinct anomalies with a common origin. AJP Rep 2016;6(1):e15–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dattani MT, Martinez-Barbera JP, Thomas PQ et al. . Mutations in the homeobox gene HESX1/HESX1 associated with septo-optic dysplasia in human and mouse. Nat Genet 1998;19(2):125–33. [DOI] [PubMed] [Google Scholar]
- 6. McCabe MJ, Alatzoglou KS, Dattani MT. Septo-optic dysplasia and other midline defects: The role of transcription factors: HESX1 and beyond. Best Pract Res Clin Endocrinol Metab 2011;25(1):115–24. [DOI] [PubMed] [Google Scholar]
- 7. Blohmé J, Bengtsson-Stigmar E, Tornqvist K. Visually impaired Swedish children. Longitudinal comparisons 1980–1999. Acta Ophthalmol Scand 2000;78(4):416–20. [DOI] [PubMed] [Google Scholar]
- 8. Mohney BG, Young RC, Diehl N. Incidence and associated endocrine and neurologic abnormalities of optic nerve hypoplasia. JAMA Ophthalmol 2013;131(7):898–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoffmann EM, Zangwill LM, Crowston JG, Weinreb RN. Optic disk size and glaucoma. Surv Ophthalmol 2007;52(1):32–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Population Report Manitoba. Manitoba Health, Healthy Living & Seniors June 1, 2014. <www.gov.mb.ca/health/population/> (Accessed September 10, 2016).
- 11. Denis H, Pampalon R. Traumatismes et defavorisation au Quebec 2002. <www.inspq.qc.ca/pdf/publications/085_TraumatismeDefavorisation.pdf> (Accessed September 10, 2016).
- 12. Patel L, McNally RJ, Harrison E, Lloyd IC, Clayton PE. Geographical distribution of optic nerve hypoplasia and septo-optic dysplasia in Northwest England. J Pediatr 2006;148(1):85–8. [DOI] [PubMed] [Google Scholar]
- 13. Tornqvist K, Ericsson A, Källén B. Optic nerve hypoplasia: Risk factors and epidemiology. Acta Ophthalmol Scand 2002;80(3):300–4. [DOI] [PubMed] [Google Scholar]
- 14. Goh YW, Andrew D, McGhee C, Dai S. Clinical and demographic associations with optic nerve hypoplasia in New Zealand. Br J Ophthalmol 2014;98(10):1364–7. [DOI] [PubMed] [Google Scholar]
- 15. Birkebaek NH, Patel L, Wright NB et al. . Endocrine status in patients with optic nerve hypoplasia: Relationship to midline central nervous system abnormalities and appearance of the hypothalamic-pituitary axis on magnetic resonance imaging. J Clin Endocrinol Metab 2003;88(11):5281–6. [DOI] [PubMed] [Google Scholar]
- 16. McNay DE, Turton JP, Kelberman D et al. . HESX1 mutations are an uncommon cause of septooptic dysplasia and hypopituitarism. J Clin Endocrinol Metab 2007;92(2):691–7. [DOI] [PubMed] [Google Scholar]
- 17. Milan A. Fertility: Overview, 2008. Statistics Canada; <www.statcan.gc.ca/pub/91-209-x/2011001/article/11513-eng.htm> (Accessed September 11, 2016). [Google Scholar]
- 18. Statistics Canada. Number and Distribution of the Population Reporting an Aboriginal Identity and Percentage of Aboriginal People in the Population, Canada, Provinces and Territories, 2011. <www12.statcan.gc.ca/nhs-enm/2011/as-sa/99-011-x/2011001/tbl/tbl02-eng.cfm>. (Accessed November 20, 2016). [Google Scholar]
- 19. Heaman M, Kingston D, Helewa M et al. . Perinatal Services and Outcomes in Manitoba. 2012. Manitoba Centre for Health Policy 1-413. <http://mchp-appserv.cpe.umanitoba.ca/reference/perinatal_report_WEB.pdf> (Accessed September 11, 2016). [Google Scholar]
- 20. Muckle G, Laflamme D, Gagnon J, Boucher O, Jacobson JL, Jacobson SW. Alcohol, smoking, and drug use among Inuit women of childbearing age during pregnancy and the risk to children. Alcohol Clin Exp Res 2011;35(6):1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Statistics Canada. Select Health Indicators of First Nations People Living Off Reserve, Metis and Inuit. <www.statcan.gc.ca/pub/82-624-x/2013001/article/11763-eng.html> (Accessed November 21, 2016). [Google Scholar]
- 22. Brodsky MC, Glasier CM, Pollock SC, Angtuago EJ. Optic nerve hypoplasia. Identification by magnetic resonance imaging. Arch Ophthalmol 1990;108(11):1562–7. [DOI] [PubMed] [Google Scholar]