Abstract
Background
Poststroke fatigue (PSF) is a form of pathological fatigue that can develop after stroke and has a negative impact on functional outcome. PSF is associated with poststroke depression (PSD), which in turn shows similarities with poststroke apathy (PSA). This study aimed at disentangling the temporal associations between PSF and PSD and between PSF and PSA.
Methods
A total of 250 stroke patients were included, of which 243 completed the Fatigue Severity Scale, Montgomery-Åsberg Depression Rating Scale, and Apathy Evaluation Scale at 3 months poststroke, with follow-up measurements at 6 and 12 months after initial testing. Linear mixed models and linear regressions were performed to evaluate the temporal associations between PSF and PSD, and between PSF and PSA.
Results
PSF was present in 119 patients (49%), of whom 62 patients also had PSD (26%), and 21 patients (9%) also had PSA. At baseline, PSF patients showed higher depression levels, which remained stable at follow-up. PSD patients had higher fatigue levels compared with no-PSD patients at baseline, which remained stable at follow-up. No association between apathy and fatigue was found at baseline and no interaction with time was found. Change in fatigue from baseline to 12-month follow-up was associated with change in depression and with change in apathy.
Conclusions
Bidirectional associations were found between PSF and PSD. In treatment and rehabilitation programs, early focus on the presence of PSD and PSF is important, since these conditions tend to persist. As there are currently more treatment options for PSD, attention for PSD is important and might also have a beneficial effect on PSF.
Keywords: Stroke, Fatigue, Poststroke depression, Apathy
Introduction
Poststroke fatigue (PSF) can be defined as a form of pathological fatigue, characterized by a continuous feeling of weariness [1], independent of physical exertion [2], and can develop early after stroke or in the chronic stroke phase. It is a frequent complaint after stroke, with prevalence estimates between 35 and 92% [1]. PSF has a negative impact on daily functioning and rehabilitation [3, 4], resulting in higher dependency and poor quality of life [5]. PSF has been associated with both biological and psychological factors [6], and is therefore hypothesized to be of multifactorial origin [7]. Lack of acknowledgment of fatigue by others, including health professionals, due to its “invisible nature,” contributes to the difficulty to cope with PSF, and can cause irritability and mood changes [8].
A meta-analysis of 19 cross-sectional studies showed that PSF is associated with poststroke depression (PSD) [9]. Longitudinal studies found evidence that early PSD was associated with long-term PSF [10, 11], and long-term PSD was also associated with long-term PSF [7]. Early depressive symptoms correlated positively with both early fatigue and long-term fatigue, but no long-term depression data were available [12]. So, less is known about the effect of early PSF on long-term PSD, leaving the question whether a direct relationship between PSF and PSD exists, and if so, whether it is uni- or bidirectional [13]. In addition, there is large overlap in the symptoms of apathy and depression after stroke [14], and both often co-occur [15, 16, 17], suggesting they are closely related. Apathy can be defined as a condition of lack of interest and motivation, with diminished participation in activities [18]. Hence, poststroke apathy (PSA) might also be related to PSF. Therefore, the main aim of the present study is to disentangle the temporal relationships between PSF and PSD and between PSF and PSA.
Methods
Study Population
Participants of the present study took part in the Cognition and Affect after Stroke, a Prospective Evaluation of Risks study, a prospective clinical cohort study, which aims to examine predictors of poststroke cognitive impairment, depression, and apathy, and its design has been described in detail elsewhere [19]. Patients were approached to participate between June 2013 and November 2015; all patients were admitted to the Stroke Unit of Maastricht University Medical Center (MUMC+), or Zuyderland Hospital (The Netherlands). The study protocol was approved by the Medical Ethics Committee of MUMC+.
Patients with nonfatal first-ever ischemic stroke were included. Exclusion criteria were hemorrhage (n = 50), pre-stroke dementia, preexisting cognitive impairment, or mental retardation (n = 86), previous stroke (n = 54), age <40 years (n = 17), neurological or psychiatric diseases other than depression that are known to affect cognition (n = 75), insufficient knowledge of the Dutch language (n = 18), too severe aphasia to understand the study procedure (n = 35), Informant Questionnaire on Cognitive Decline in the Elderly score [20] ≥3.60 (n = 3), Mini-Mental State Examination Score [21] <15 (n = 1), no written informed consent (n = 1), blindness (n = 2), and post-surgery stroke/post-anoxic encephalopathy (n = 2). After 1 year, initial criteria were adapted to increase response rate by additionally including patients with hemorrhage (n = 15) and patients with a history of stroke ≥3 years, but without residual symptoms (n = 13). Patients with a history of stroke of <3 years or with residual symptoms from previous stroke were excluded (n = 68). Finally, 250 eligible patients gave their written informed consent and were enrolled in the present study (online suppl. Fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000481577).
Procedure
Baseline measurements were scheduled approximately 3 months poststroke to avoid confounding effects caused by the acute stroke state [22]. At baseline (T0), sociodemographic information was recorded and all patients underwent a neuropsychiatric interview in order to evaluate the presence and severity of fatigue, depression, and apathy, which was repeated at 6-month (T1) and 12-month (T2) follow-up.
Measurements
The Fatigue Severity Scale (FSS), a 9-item self-report questionnaire, was used to assess the presence and severity of PSF [23]. The FSS has been validated in stroke patients [24], and based on previous studies, a cut-off score ≥4 was applied to indicate PSF [5, 25].
The Apathy Evaluation Scale (AES) was used to assess the presence and severity of PSA [26, 27], which consists of 18 items measured on a 4-point scale, with a higher total score representing a higher level of apathy. The clinician-rated AES (AES-C) version was used as outcome measure of apathy, and patients with a score ≥37 on either the AES-C or informant-rated AES were classified as having clinically relevant apathy [28].
The Mini International Neuropsychiatric Interview, a semi-structured interview based on DSM-IV criteria, was used to diagnose major depressive disorder (MDD) and minor depression (MIND) [29]. The “Montgomery-Åsberg Depression Rating Scale (MADRS), a 10-item clinician-rated questionnaire, was used as outcome measure of depression [30]. The MADRS was evaluated as a valid instrument for PSD, as it focuses less on somatic symptoms and is sensitive to change [31]. Patients having MDD or MIND according to the Mini International Neuropsychiatric Interview, or having a MADRS score ≥7 were classified as having clinically relevant depression.
The Barthel Index was used to measure impairment in activities of daily living [32], and the NEO Five Factor Inventory was used to assess the personality trait neuroticism, which was included as potential covariate in the analyses [33].
Statistical Analyses
All statistical analyses were performed with Stata version 13.1 for Mac OS X (StataCorp, USA). An alpha level of 0.05 (2-sided) was used for all analyses. Baseline differences between PSF groups were tested using χ2 tests for qualitative variables and t-tests for quantitative variables. Pearson, point-biserial, phi, and Spearman rho correlations were used to assess correlations between variables. Variables that correlated significantly with both fatigue and depression, or fatigue and apathy were analyzed in a linear regression model. Variables that were significantly associated with both fatigue and depression, or fatigue and apathy in the linear regression models were included as potential confounders in the analyses.
Linear mixed models were performed to measure the effect of baseline PSF on depression and apathy scores at baseline, and to test whether PSF predicts change in depression and apathy scores over time from baseline to 12-month follow-up. A group (2-levels: no-PSF, PSF) by time (3-levels: T0, T1, T2) interaction was included to study differences in the course of depression between the groups. An χ2 test test of homogeneity with 2 degrees of freedom (d.f.) tested the null hypothesis of no difference in rate of change over time between the groups by examining whether there is a significant group-by-time interaction. The models included a random intercept and random slope with an unstructured correlation matrix, as this resulted in the best fit and the analyses were corrected for relevant confounders.
Furthermore, we tested whether change in FSS from baseline to T1 or T2 was associated with change in PSD or PSA during the same period. For this, a change score of the FSS was calculated by regressing FSS scores at follow-up on baseline FSS scores, thereby accounting for different FSS baseline levels (autoregression). The FSS change score was then used as the independent variable in a linear regression in which MADRS or AES-C scores at follow-up were regressed on baseline MADRS or AES-C scores.
Next, the same analytic approach was adopted with FSS as outcome to measure the effect of baseline PSD or PSA on fatigue scores at baseline, and to test whether PSD or PSA predicted change in fatigue scores over time from baseline to 12-month follow-up. We additionally adjusted for the PSA-by-time or PSD-by-time interaction to study the independence of these effects. The linear mixed models only included a random intercept, as this resulted in the best fit.
Results
Of the 250 participants, 243 completed the FSS, MADRS, and AES-C at T0 (97.2%), of which 210 patients (86.4%) completed T1 and 215 patients (88.5%) completed T2 (online suppl. Fig. 1). Patients lost to follow-up at one or 2 time measurements (n = 27) did not differ from patients who completed all measurements (n = 216; online suppl. Table 1).
Table 1 shows the baseline characteristics of the total sample (n = 243), and also separately for the PSF groups at baseline. PSF was present in 119 patients at T0 (49.0%). Patients with PSF were significantly younger compared to the no-PSF group, more often reported sleep apnea, and showed higher levels of neuroticism, depression, and apathy. PSD was present in 94 patients at T0 (38.7%), of which 13 (5.3%) fulfilled criteria for MDD, 15 (6.2%) for MIND, and 66 (27.2%) had a MADRS score ≥7. A significantly higher frequency of PSD and MDD was found in the PSF group in comparison with the no-PSF group, and 62 (25.5%) of the patients experienced both PSD and PSF. PSA was present in 40 patients at T0 (16.5%). The presence of both PSA and PSD at baseline was seen in 26 patients (10.7%), whereas the presence of both PSA and PSF at baseline was seen in 21 patients (8.6%).
Table 1.
Baseline characteristics
| Variable | Overall | No PSF | PSF |
|---|---|---|---|
| n (%) | 243 (100.0) | 124 (51.0) | 119 (49.0) |
| Age, years | 67.41 (11.76) | 69.44 (11.33) | 65.30 (11.87)b |
| Gender, male, n (%) | 157 (64.6) | 83 (66.9) | 74 (62.2) |
| Low education, n (%) | 100 (41.3) | 50 (40.3) | 50 (42.4) |
| Middle education, n (%) | 85 (35.1) | 38 (30.7) | 47 (39.8) |
| High education, n (%) | 57 (23.6) | 36 (29.0) | 21 (17.8) |
| Time since stroke, months | 2.93 (0.43) | 2.90 (0.41) | 2.96 (0.46) |
| First-ever stroke, n (%) | 227 (93.4) | 119 (96.0) | 108 (90.8) |
| Barthel index | 19.44 (1.47) | 19.40 (1.74) | 19.48 (1.14) |
| Sleep apnea, n (%) | 18 (7.4) | 2 (1.6) | 16 (13.5)b |
| High neuroticism, n (%) | 115 (48.7) | 69 (59.0) | 46 (38.7)b |
| Pre-stroke depression, n (%) | 55 (22.6) | 23 (18.6) | 32 (26.9) |
| Family history of depression, n (%) | 34 (14.1) | 15 (12.2) | 19 (16.0) |
| MADRS | 6.07 (5.84) | 4.24 (4.43) | 7.96 (6.49)b |
| AES-C | 26.36 (7.87) | 25.26 (6.88) | 27.50 (8.66)a |
| FSS | 3.78 (1.59) | 2.47 (0.92) | 5.14 (0.79)b |
| MINI minor or major depression, n (%) | 28 (11.5) | 10 (8.1) | 18 (15.1) |
| MDD | 13 (5.4) | 2 (1.6) | 11 (9.2)b |
| MIND | 15 (6.2) | 8 (6.5) | 7 (5.9) |
| PSA, n (%) | 40 (16.5) | 19 (15.3) | 21 (17.7) |
| PSD, n (%) | 94 (38.7) | 32 (25.8) | 62 (52.1)b |
p < 0.05
p < 0.01.
AES-C, Apathy Evaluation Scale Clinician-rated; FSS, Fatigue Severity Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; MDD, major depressive disorder; MIND, minor depression; MINI, Mini International Neuropsychiatric Interview; PSA, poststroke apathy; PSD, poststroke depression; PSF, poststroke fatigue; data are mean (SD), unless otherwise specified.
Based on correlation analyses (online suppl. Table 2) and linear regression models, only sleep apnea was associated with all outcomes, whereas neuroticism and history of depression were significantly associated with fatigue and depression scores. As sleep apnea has a direct influence on fatigue level, it was included in the primary analyses, together with baseline age, gender, and highest level of education. In the secondary analyses, also neuroticism and depression history were included to see how they influence the relationship between depression and fatigue, and apathy and fatigue.
The Course of Depression by PSF Group
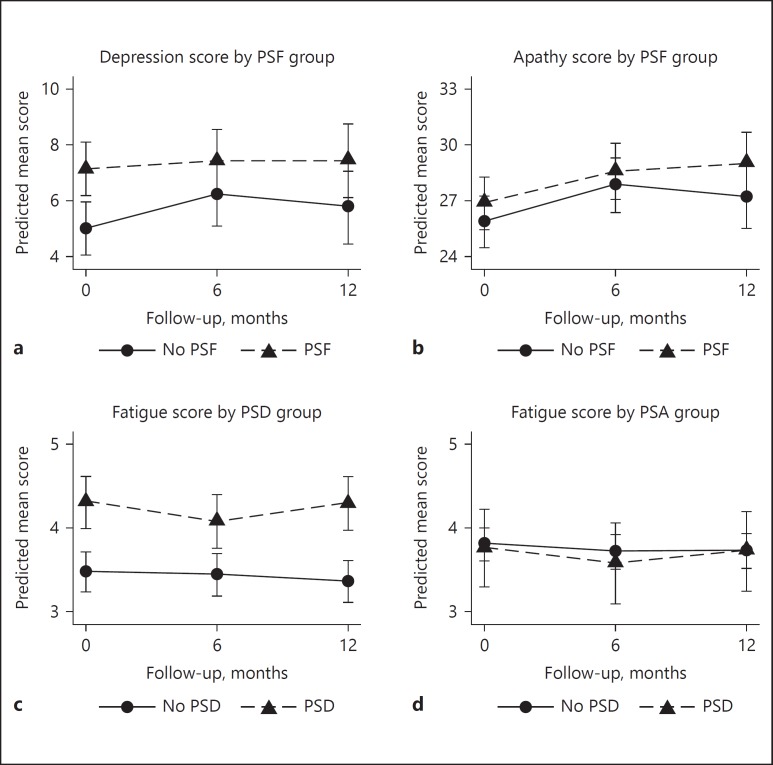
In the PSF group, a significantly higher MADRS score was found at T0 (χ2 = 10.68; d.f. = 1; p = 0.001), which remained stable at T1 (p = 0.044) and T2 (p = 0.008). There was no significant interaction between PSF and MADRS score over time (χ2 = 1.37; d.f. = 2; p = 0.504; Fig. 1a). Adding neuroticism and history of depression to the model did not change the results (Table 2).
Fig. 1.
Course of apathy and depression by poststroke fatigue (PSF) group and course of fatigue by poststroke depression (PSD) and poststroke apathy (PSA) group. a Course of depression scores by PSF group. b Course of apathy scores by PSF group. c Course of fatigue scores by PSD group. d Course of fatigue scores by PSA group. Based on random-effects analyses adjusted for age at baseline, gender, highest level of education, sleep apnea, history of depression, and high neuroticism. Predicted mean scores are estimated marginal means of time by group, with all covariates fixed at their means. Higher mean scores indicate a higher level of depression, apathy, or fatigue respectively. PSA, poststroke apathy; PSD, poststroke depression; PSF, poststroke fatigue.
Table 2.
Mean differences (and 95% CIs) in depression, apathy, and fatigue scores and in the rate of change (slopes) from baseline to follow-up
| Time |
||||||
|---|---|---|---|---|---|---|
| baseline |
change T0–T1 |
change T0–T2 |
||||
| Parameter | difference | 95% CI | change | 95% CI | change | 95% CI |
| MADRS by PSF | ||||||
| Model 1c | 2.75b | 1.10 to 4.40 | −0.99 | −2.65 to 0.67 | −0.44 | −2.09 to 1.21 |
| Model 2d | 2.14b | 0.76 to 3.52 | −0.95 | −2.59 to 0.69 | −0.46 | −2.21 to 1.29 |
| AES-C by PSF | ||||||
| Model 1c | 1.57 | −0.42 to 3.56 | −0.22 | −2.07 to 1.63 | 0.91 | −1.28 to 3.10 |
| Model 2d | 0.99 | −1.04 to 3.03 | −0.26 | −2.12 to 1.59 | 0.90 | −1.25 to 3.05 |
| FSS by PSD | ||||||
| Model 1c | 1.08b | 0.67 to 1.49 | −0.17 | −0.57 to 0.23 | 0.13 | −0.27 to 0.53 |
| Model 2d | 0.83b | 0.42 to 1.24 | −0.19 | −0.60 to 0.22 | 0.10 | −0.30 to 0.51 |
| FSS by PSA | ||||||
| Model 1c | −0.12 | −0.64 to 0.41 | −0.04 | −0.57 to 0.49 | 0.07 | −0.45 to 0.59 |
| Model 2d | −0.05 | −0.56 to 0.46 | −0.09 | −0.62 to 0.45 | 0.04 | −0.48 to 0.57 |
a p < 0.05.
p < 0.01.
Model 1: corrected for age, gender, highest level of education, and sleep apnea.
Model 2: corrected for age, gender, highest level of education, sleep apnea, high neuroticism, and history of depression.
AES-C, Apathy Evaluation Scale Clinician-rated; FSS, Fatigue Severity Scale; MADRS, Montgomery-Asberg Depression Rating Scale; PSA, poststroke apathy; PSD, poststroke depression; PSF, poststroke fatigue; T0, 3 months poststroke; T1, 6-month follow-up; T2, 12-month follow-up.
The Course of Apathy by the PSF Group
No significant group effect at T0 (p = 0.121; Table 2) or interaction by time effect (χ2 = 1.37; d.f. = 2; p = 0.505) was found between PSF and AEC-C score (Fig. 1b). While groups scored similar at T0 or T1 (p = 0.218), a significant difference in apathy score between the PSF and no-PSF group was found at T2 (p = 0.046). By the addition of neuroticism and history of depression to the model, the group difference in AES-C score at T2 was not significant anymore (p = 0.119).
The Course of Fatigue by PSD Group
At T0, a significantly higher FSS score was found in the PSD group (χ2 = 27.25; d.f. = 1; p < 0.001; Table 2), which remained stable at T1 (p < 0.001) and T2 (p < 0.001). The overall interaction between PSD and FSS scores over time was not significant (χ2 = 2.02; d.f. = 2; p = 0.365; Fig. 1c).
The Course of Fatigue by the PSA Group
No significant group difference in FSS score between the PSA and no-PSA group was found at T0 (χ2 = 0.20; d.f. = 1; p = 0.659; Table 2), T1 (p = 0.571) or T2 (p = 0.865; Fig. 1d), and the overall interaction between PSA and FSS scores over time was not significant either (χ2 = 0.17; d.f. = 2; p = 0.921).
Association between Change of Fatigue and Change of Depression
Linear regression analyses showed that change in FSS score from T0 to T1 was not significantly associated with change in MADRS score from T0 to T1 (B = 0.07, p = 0.820). However, from T0 to T2, change in FSS score was significantly associated with change in MADRS score (B = 1.47, p < 0.001). When neuroticism and history of depression were added to this model, change in FSS score remained significant (B = 1.35, p < 0.001).
Association between Change of Fatigue and Change of Apathy
There was no significant association between change in FSS score and change in AES-C score from T0 to T1 (B = 0.62, p = 0.086). However, from T0 to T2, change in FSS score was significantly associated with change in AES-C score (B = 1.21, p = 0.004). When neuroticism and history of depression were added to this model, change in FSS score remained significant (B = 0.91, p = 0.027).
Discussion
This study aimed to disentangle the temporal relationship between PSF and PSD, and between PSF and PSA. Patients with PSF at baseline had higher depression scores that remained stable throughout time, and patients with PSD at baseline had higher fatigue scores that remained stable throughout time as well. No association between PSF and the baseline level or course of apathy symptoms was found, and neither in the reverse direction, as no association between PSA and the baseline level or course of fatigue symptoms was found. Additionally, change in fatigue score from baseline to 12-month follow-up was significantly associated with change in depression and change in apathy from baseline to 12-month follow-up. No associations were found when looking at change from baseline to 6-month follow-up.
Half of the stroke patients experienced PSF at baseline, which is in agreement with a previous meta-analysis that showed a range between 35 and 92% [1]. PSD at baseline was present in almost 40%, which is somewhat higher than the estimated prevalence of 31% reported in an earlier meta-analysis [34], and baseline PSA was present in less than 20%, which is lower than the reported estimated prevalence of 35% in the meta-analysis by van Dalen et al. [15]. However, this can be explained partially by the variability in the time range between the measurements and the stroke date, the cut-off scores that were applied, and the use of different questionnaires.
The present findings suggest that both PSF and PSD are frequent early after stroke and remain relatively stable throughout time. Half of the patients with PSF at baseline also had PSD, suggesting a strong bidirectional association between the 2 symptoms. These results are in agreement with the study of Lerdal et al. [35] who also found an association between early fatigue and early depressive symptoms. In addition, baseline depression scores were associated with high fatigue scores at follow-up. This is in line with a previous longitudinal study that suggested that baseline depression was associated with long-term fatigue [10].
With respect to the temporal relationship between PSD and PSF, the results of this study indicate that fatigue and depression show a similar course over time and co-occur in a quarter of the stroke patients 3 months after stroke. Patients with PSD had high FSS scores, which remained stable over time, whereas patients with PSF had high MADRS scores that also remained stable over time. This suggests a bidirectional relationship, as the results also suggest that change in depression over time was associated with change in fatigue over time.
Yet, some patients experience PSF in the absence of PSD, and vice versa, suggesting that PSD and PSF should be considered distinct consequences of stroke [36]. Early PSD, together with other factors, contributes to early PSF and vice versa. According to the PSF model of Wu et al. [6], potential factors that might be involved in this relationship include lesion location, inflammatory responses, personality traits, coping styles, and sleep apnea. These factors differ among individuals, which explains the inter-patient variability in the development of fatigue [1]. Patient-tailored treatment is therefore important, as individual differences have to be taken into account when targeting PSD or PSF. With respect to treatment and rehabilitation programs, this suggests that an early focus on both PSD and PSF is important, as these conditions tend to persist. Since there are more treatment options available for depression, early screening for depression might have a positive effect on clinical outcome [37], and might also affect fatigue symptoms. Future longitudinal studies are needed to give more insight into the complex multifactorial origin of PSF. This requires large cohort studies taking into account the various factors that influence the complex relationship between PSD and PSF.
An important strength of the present study is the longitudinal design, which made it possible to study temporal associations. This is the first study that examined associations between fatigue and depression in both directions, and that also included the influence of apathy on this relationship. Well-validated instruments were used to measure fatigue, depression, and apathy, and adequate statistical models for repeated measures were used to account for missing data. In addition, sleep apnea, neuroticism, and history of depression were included as they might influence the relationships between depression, apathy, and fatigue. Particularly, the role of sleep apnea is important, as it has been shown to be a risk factor for incident stroke [38], and is associated with worse cognitive and functional status [39]. Also in multiple sclerosis [40, 41], associations between sleep apnea and fatigue have been found. Treatment of sleep apnea may have a beneficial effect on PSF, though future studies are needed to examine this. However, some limitations of the study should be noted. The exclusion of patients with severe aphasia and other neurological or psychiatric conditions made the study sample less representative, and it is reasonable to assume that stroke patients with a worse clinical outcome, who were unable to participate, have an even higher degree of PSF, PSD, or PSA, resulting in an underestimation of the presence of these conditions.
Conclusions
PSD and PSF were strongly associated throughout time, and the association seemed to be bidirectional. Although apathy scores were correlated with the level of fatigue throughout time, this appeared largely to be due to comorbid depression. The present results suggest that immediate screening for both PSD and PSF after stroke is important, as it might help to prevent the development of these symptoms at an early or later stage. This will result in better participation in rehabilitation programs, improving clinical outcome after stroke.
Disclosure Statement
The authors declare that there are no conflicts of interest to disclose.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
Acknowledgments
The authors thank the participants of the Cognition and Affect after Stroke, a Prospective Evaluation of Risks study and their proxies for their important contributions and Mr. Nico Rozendaal, for expert design and management of the electronic database. This project was funded by Maastricht University, Health Foundation Limburg, and the Adriana van Rinsum-Ponsen Stichting.
References
- 1.Duncan F, Wu S, Mead GE. Frequency and natural history of fatigue after stroke: a systematic review of longitudinal studies. J Psychosom Res. 2012;73:18–27. doi: 10.1016/j.jpsychores.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Visser-Keizer AC, Hogenkamp A, Westerhof-Evers HJ, Egberink IJ, Spikman JM. Dutch multifactor fatigue scale: a new scale to measure the different aspects of fatigue after acquired brain injury. Arch Phys Med Rehabil. 2015;96:1056–1063. doi: 10.1016/j.apmr.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Christensen D, Johnsen SP, Watt T, Harder I, Kirkevold M, Andersen G. Dimensions of post-stroke fatigue: a two-year follow-up study. Cerebrovasc Dis. 2008;26:134–141. doi: 10.1159/000139660. [DOI] [PubMed] [Google Scholar]
- 4.Wondergem R, Pisters MF, Wouters EJ, Olthof N, de Bie RA, Visser-Meily JM, et al. The course of activities in daily living: who is at risk for decline after first ever stroke? Cerebrovasc Dis. 2017;43:1–8. doi: 10.1159/000451034. [DOI] [PubMed] [Google Scholar]
- 5.van de Port IG, Kwakkel G, Schepers VP, Heinemans CT, Lindeman E. Is fatigue an independent factor associated with activities of daily living, instrumental activities of daily living and health-related quality of life in chronic stroke? Cerebrovasc Dis. 2006;23:40–45. doi: 10.1159/000095757. [DOI] [PubMed] [Google Scholar]
- 6.Wu S, Mead G, Macleod M, Chalder T. Model of understanding fatigue after stroke. Stroke. 2015;46:893–898. doi: 10.1161/STROKEAHA.114.006647. [DOI] [PubMed] [Google Scholar]
- 7.Naess H, Lunde L, Brogger J, Waje-Andreassen U. Fatigue among stroke patients on long-term follow-up. The Bergen Stroke Study. J Neurol Sci. 2012;312:138–141. doi: 10.1016/j.jns.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Ormstad H, Eilertsen G. A biopsychosocial model of fatigue and depression following stroke. Med Hypotheses. 2015;85:835–841. doi: 10.1016/j.mehy.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Wu S, Barugh A, Macleod M, Mead G. Psychological associations of poststroke fatigue a systematic review and meta-analysis. Stroke. 2014;45:1778–1783. doi: 10.1161/STROKEAHA.113.004584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Passier PE, Post MW, van Zandvoort MJ, Rinkel GJ, Lindeman E, Visser-Meily JM. Predicting fatigue 1 year after aneurysmal subarachnoid hemorrhage. J Neurol. 2011;258:1091–1097. doi: 10.1007/s00415-010-5891-y. [DOI] [PubMed] [Google Scholar]
- 11.Snaphaan L, Van Der Werf S, de Leeuw FE. Time course and risk factors of post-stroke fatigue: a prospective cohort study. Eur J Neurol. 2011;18:611–617. doi: 10.1111/j.1468-1331.2010.03217.x. [DOI] [PubMed] [Google Scholar]
- 12.Lerdal A, Gay CL. Fatigue in the acute phase after first stroke predicts poorer physical health 18 months later. Neurology. 2013;81:1581–1587. doi: 10.1212/WNL.0b013e3182a9f471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naess H, Lunde L, Brogger J. The triad of pain, fatigue and depression in ischemic stroke patients: the Bergen Stroke Study. Cerebrovasc Dis. 2012;33:461–465. doi: 10.1159/000336760. [DOI] [PubMed] [Google Scholar]
- 14.Withall A, Brodaty H, Altendorf A, Sachdev PS. A longitudinal study examining the independence of apathy and depression after stroke: the Sydney Stroke Study. Int Psychogeriatr. 2011;23:264–273. doi: 10.1017/S1041610209991116. [DOI] [PubMed] [Google Scholar]
- 15.van Dalen JW, van Charante EP, Nederkoorn PJ, van Gool WA, Richard E. Poststroke apathy. Stroke. 2013;44:851–860. doi: 10.1161/STROKEAHA.112.674614. [DOI] [PubMed] [Google Scholar]
- 16.Caeiro L, Ferro JM, Costa J. Apathy secondary to stroke: a systematic review and meta-analysis. Cerebrovasc Dis. 2013;35:23–39. doi: 10.1159/000346076. [DOI] [PubMed] [Google Scholar]
- 17.Hackett ML, Köhler S, T O’Brien JT, Mead GE. Neuropsychiatric outcomes of stroke. Lancet Neurol. 2014;13:525–534. doi: 10.1016/S1474-4422(14)70016-X. [DOI] [PubMed] [Google Scholar]
- 18.Marin RS. Differential diagnosis and classification of apathy. Am J Psychiatry. 1990;147:22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- 19.Douven E, Schievink SH, Verhey FR, van Oostenbrugge RJ, Aalten P, Staals J, et al. The cognition and affect after stroke – a prospective evaluation of risks (CASPER) study: rationale and design. BMC Neurol. 2016;16:1–11. doi: 10.1186/s12883-016-0588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jorm AF. A short form of the Informant questionnaire on cognitive decline in the elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145–153. doi: 10.1017/s003329170002691x. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Lees R, Stott DJ, Quinn TJ, Broomfield NM. Feasibility and diagnostic accuracy of early mood screening to diagnose persisting clinical depression/anxiety disorder after stroke. Cerebrovasc Dis. 2014;37:323–329. doi: 10.1159/000360755. [DOI] [PubMed] [Google Scholar]
- 23.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 24.Valko PO, Bassetti CL, Bloch KE, Held U, Baumann CR. Validation of the fatigue severity scale in a Swiss cohort. Sleep. 2008;31:1601–1607. doi: 10.1093/sleep/31.11.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi-Kwon S, Ko M, Jun SE, Kim J, Cho KH, Nah HW, et al. Post-stroke fatigue may be associated with the promoter region of a monoamine oxidase a gene polymorphism. Cerebrovasc Dis. 2016;43:54–58. doi: 10.1159/000450894. [DOI] [PubMed] [Google Scholar]
- 26.Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation Scale. Psychiatry Res. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- 27.Clarke DE, Ko JY, Kuhl EA, van Reekum R, Salvador R, Marin RS. Are the available apathy measures reliable and valid? A review of the psychometric evidence. J Psychosom Res. 2011;70:73–97. doi: 10.1016/j.jpsychores.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caeiro L, Ferro JM, Pinho e Melo T, Canhão P, Figueira ML. Post-stroke apathy: an exploratory longitudinal study. Cerebrovasc Dis. 2013;35:507–513. doi: 10.1159/000350202. [DOI] [PubMed] [Google Scholar]
- 29.Overbeek I, Schruers K, Griez E. Mini International Neuropsychiatric Interview: Nederlandse Versie 5.0.0. DSM-IV [Dutch version]. Maastricht: Universiteit Maastricht; 1999. [Google Scholar]
- 30.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 31.Kang HJ, Stewart R, Kim JM, Jang JE, Kim SY, Bae KY, et al. Comparative validity of depression assessment scales for screening poststroke depression. J Affect Disord. 2013;147:186–191. doi: 10.1016/j.jad.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 32.Mahoney FI, Barthel DW. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- 33.Costa P, McCrae R. NEO Five-Factor Inventory (NEO-FFI). Odessa: Psychological Assessment Resources; 1989. [Google Scholar]
- 34.Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9:1017–1025. doi: 10.1111/ijs.12357. [DOI] [PubMed] [Google Scholar]
- 35.Lerdal A, Bakken LN, Rasmussen EF, Beiermann C, Ryen S, Pynten S, et al. Physical impairment, depressive symptoms and pre-stroke fatigue are related to fatigue in the acute phase after stroke. Disabil Rehabil. 2011;33:334–342. doi: 10.3109/09638288.2010.490867. [DOI] [PubMed] [Google Scholar]
- 36.Schepers VP, Visser-Meily AM, Ketelaar M, Lindeman E. Poststroke fatigue: course and its relation to personal and stroke-related factors. Arch Phys Med Rehabil. 2006;87:184–188. doi: 10.1016/j.apmr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Towfighi A, Ovbiagele B, El Husseini N, Hackett ML, Jorge RE, Kissela BM, et al. Poststroke depression: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2017;48:30–43. doi: 10.1161/STR.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 38.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10:355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aaronson JA, van Bennekom CA, Hofman WF, van Bezeij T, van den Aardweg JG, Groet E, et al. Obstructive sleep apnea is related to impaired cognitive and functional status after stroke. Sleep. 2015;38:1431–1437. doi: 10.5665/sleep.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaminska M, Kimoff RJ, Benedetti A, Robinson A, Bar-Or A, Lapierre Y, et al. Obstructive sleep apnea is associated with fatigue in multiple sclerosis. Mult Scler. 2012;18:1159–1169. doi: 10.1177/1352458511432328. [DOI] [PubMed] [Google Scholar]
- 41.Braley TJ, Segal BM, Chervin RD. Obstructive sleep apnea and fatigue in patients with multiple sclerosis. J Clin Sleep Med. 2014;10:155–162. doi: 10.5664/jcsm.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data