Abstract
Background
Patients with a recent ischemic stroke have a higher risk of recurrent stroke compared to (ocular) transient ischemic attack (TIA) patients. Plaque microvasculature is considered as a feature of plaque vulnerability and can be quantified with carotid dynamic contrast-enhanced MRI (DCE-MRI). The purpose of this cross-sectional study was to explore the association between plaque microvasculature and the type of recent cerebrovascular events in symptomatic patients with mild-to-moderate carotid stenosis.
Methods
A total of 87 symptomatic patients with a recent stroke (n = 35) or (ocular) TIA (n = 52) underwent carotid DCE-MRI examination. Plaque microvasculature was studied in the vessel wall and adventitia using DCE-MRI and the pharmacokinetic modeling parameter Ktrans. Statistical analysis was performed with logistic regression, correcting for associated clinical risk factors.
Results
The 75th percentile adventitial (OR 1.97, 95% CI 1.18–3.29) Ktrans was significantly associated with a recent ischemic stroke compared to (ocular) TIA in multivariate analysis, while clinical risk factors were not significantly associated with the type of event.
Conclusions
This study indicates a positive association of leaky plaque microvasculature with a recent ischemic stroke compared to (ocular) TIA. Prospective longitudinal studies are needed to investigate whether Ktrans or other plaque characteristics may serve as an imaging marker for predicting (the type of) future cerebrovascular events.
Keywords: Atherosclerosis, Dynamic contrast-enhanced MRI, Microvasculature, Magnetic resonance imaging, Ischemic stroke, Transient ischemic attack
Introduction
Annually 15 million people suffer from a stroke worldwide, from which 5 million people die and 5 million people are left permanently disabled [1]. Ischemic strokes account for approximately 80% of all strokes. The rupture of a vulnerable atherosclerotic plaque of the carotid artery is an important underlying cause for ischemic stroke. Epidemiological studies have identified various clinical risk factors associated with an increased risk of ischemic stroke [2, 3, 4, 5, 6, 7]. Previous studies have also shown a higher risk for recurrent stroke in patients with a ischemic stroke compared to patients with a transient ischemic attack (TIA). The risk for a recurrent stroke within 7 days was 5.2% after a TIA versus 11.5% after a stroke [8]. Additional research showed that patients with a minor stroke have an increased risk for a recurrent stroke compared to patients with a recent TIA or ocular TIA (also known as amaurosis fugax) [9]. Data from the European Carotid Surgery Trial also showed that the hazard ratio for a 5-year risk of ipsilateral ischemic stroke depends on the type of the presenting cerebrovascular event [10]. The underlying mechanisms responsible for the differences in risk for a recurrent event between the stroke subtypes are unclear.
Increased microvasculature within the atherosclerotic plaque has been proposed as a marker of plaque vulnerability [11, 12]. These microvessels grow from the adventitia into the plaque and generally have an impaired endothelial integrity, potentially providing an entry point for inflammatory cells and erythrocytes to the atherosclerotic lesion. It is believed that the growth of these microvessels into the plaque region is triggered by an increased activity of inflammatory cells, and subsequent hypoxia, within the plaque tissue [13].
Dynamic contrast-enhanced (DCE-)MRI has emerged as a non-invasive imaging technique to assess the plaque microvasculature [14, 15, 16]. With DCE-MRI, signal enhancement time curve after contrast medium (CM) injection is analyzed to quantify plaque microvasculature using pharmacokinetic modeling. We and others have shown that the volume transfer constant Ktrans (a reflection of the microvascular density, flow, and permeability) correlates with the amount of microvessels within the plaque determined from histology [14, 15, 16, 17] as a reference standard. We have also demonstrated a good interscan reproducibility for Ktrans[16].
It is not clear whether Ktrans is associated with the stroke subtype. Given the increased risk for recurrent stroke in stroke patients compared to (ocular) TIA patients, we hypothesized that plaque characteristics might differ between these patient groups.
Study Aim
The aim of the present cross-sectional observational study was to explore the association between leaky plaque microvasculature and the subtype of recent cerebrovascular symptoms (ischemic stroke versus [ocular] TIA) in patients with a symptomatic mild-to-moderate carotid artery stenosis.
Material and Methods
Study Population
The present study was performed as a substudy of the baseline data of the Plaque At RISK (PARISK) study [18] (http://ClinicalTrials.gov unique identifier NCT01208025). The overall hypothesis of the PARISK study is that the assessment of imaging markers of plaque vulnerability improves the identification of a subgroup of patients in the <70% carotid artery stenosis group with an increased risk of recurrent ischemic stroke. Consecutive symptomatic patients with a recent (<3 months) minor ischemic stroke, or (ocular) TIA with a mild-to-moderate ipsilateral stenosis of the carotid artery were eligible for inclusion. The clinical symptoms were diagnosed by a neurologist. The definition of ischemic stroke was the clinical evidence of the sudden onset of a new neurological deficit, persisting for more than 24 h, with no other cause and without evidence of an intracerebral hemorrhage on index imaging. No specific imaging marker (e.g., diffusion restriction on MRI) was required to confirm an ischemic stroke. TIA and ocular TIA were defined as the sudden onset of a new neurological deficit or monocular loss of vision, respectively, with no other cause and lasting less than 24 h. A mild-to-moderate stenosis was defined as a plaque of at least 2 mm to carotid stenosis <70% (based on NASCET criteria [19]).
For the present study, patients from a single center (Maastricht University Medical Center) underwent additional DCE-MRI and were included for analysis. Exclusion criteria were standard contraindications for MRI, such as ferromagnetic or other electronic implants. Patients with severe renal disease (creatinine clearance <30 mL/min) were not eligible for contrast-enhanced MRI and were therefore excluded from the current analysis. Clinical history and medication use were ascertained at the time of subject enrolment. Approval of the local Institutional Ethical Review Board was obtained, and all patients provided written informed consent before study inclusion.
MR Imaging
MR imaging was performed on a 3T whole body MRI system (Achieva, Philips Healthcare, Best, The Netherlands) using a dedicated 8-channel carotid RF coil (Shanghai Chenguan Medical Technologies Co., Shanghai, China). A previously described multi-contrast MR protocol [18] was used, which included the following sequences: 3D time-of-flight, 2D T1weighted (T1w) inversion recovery turbo field echo (TFE), T2 weighted turbo spin echo (TSE), and pre- and post-contrast T1w double/quadruple inversion recovery (QIR) TSE. Fifteen adjoining transversal slices with 2 mm slice thickness and an acquired and reconstructed resolution of 0.62 × 0.62–0.67 and 0.30 × 0.24–0.30, respectively, were acquired. For DCE-MRI, an end-diastolic ECG-gated 3D T1-TFE MRI pulse sequence, centered at the position of highest plaque burden was acquired with the following parameters: repetition/echo time 11.6/5.7 ms, flip angle 35°, Field of view 130 × 130 mm, acquisition and reconstruction matrix 208 × 206 and 512 × 512, respectively, 5 consecutive transversal slices, slice thickness 2 mm [20]. The temporal resolution was approximately 20 s per time frame (dependent on heart rate). At the beginning of the third time frame, 0.1 mmol/kg of Gadobutrol (Gadovist, Bayer HealthCare, Berlin, Germany) was injected as CM with a power injector (Spectris Solaris, Medrad, Warrendale, PA, USA) at 0.5 mL/s followed by a 20 mL saline chaser at the same rate.
Image Review
MR Image Review
Plaque contours were drawn by experienced observers, as described previously, using dedicated vessel wall analysis software (VesselMASS, Leiden, The Netherlands) [21]. In case of doubt, a second highly experienced observer was consulted. There was a minimum period of 2 months between patient inclusion and MR contour drawing to assure that reviewers were blinded for clinical characteristics and other test results. Per slice, luminal contours were drawn on the 3D time-of-flight MR images. Outer vessel wall contours were drawn using the pre-contrast T1w QIR TSE, post-contrast T1w QIR TSE, T1w TFE, or T2 weighted TSE MR images, in subsequent order. Luminal and outer vessel wall contours were transferred to the DCE-MRI images and, if necessary, contours were manually adjusted. The images acquired at each individual time frame were inspected and these images were shifted to correct for small patient displacements during the dynamic acquisition, if necessary. Outer plaque contours were corrected to include the adventitial vasa vasorum, which shows hyperenhancement after contrast material administration, while inner vessel wall contours were adjusted to avoid luminal partial volume effects. The adventitial region of the entire vessel wall region was automatically selected according to previously introduced criteria [22], that is, all pixels within 0.625 mm of the outer wall contour in a region of the vessel wall with plaque (defined as having a wall thickness in excess of 1.5 mm). Contour drawing and adjustment was performed blinded for clinical characteristics.
Data Analysis
DCE-MRI Pharmacokinetic Modeling
Tissue CM concentrations were determined from the signal intensity time course, as previously described [16]. In brief, the CM concentrations were calculated using the Ernst equation, literature values for the longitudinal and transversal relaxation times for tissue [23] and the r1 and r2 relaxivity rates of the CM [24]. Subsequently, Ktrans was determined for each vessel wall voxel separately with the Patlak model [25]. Fitting was performed using an adapted population averaged arterial input function [26] that was previously determined in the carotid artery from dynamic phase MR images [20]. The Patlak model consists of 2 compartments, that is, the plasma compartment and the extracellular extravascular space. In this model, it is assumed that only leakage from the plasma compartment to the extracellular extravascular space occurs in the first few minutes after CM injection. The resulting Ktrans distribution in the vessel wall (i.e., region-of-interest between the inner and outer vessel walls) and adventitial region was analyzed by calculating the 75th percentile of the vessel wall and adventitial Ktrans distribution for the entire plaque region, respectively.
Statistical Analysis
Univariate binary logistic regression was performed to select clinical risk factors with a relevant association (OR ≥1.2) with the clinically diagnosed stroke subtype.
Multivariable logistic regression analysis was performed to study the independent association of plaque microvasculature characteristic with the clinically diagnosed type of event when adjusted for the selected clinical risk factors.
The dependent variable in these models was the clinically diagnosed type of event ([ocular] TIA versus stroke), coded as 0 ([ocular] TIA) or 1 (stroke), respectively. All dichotomous risk factors were entered with the presence or absence of the parameter coded as 1 and 0, respectively. For logistic regression, the 95% CI was determined. An OR ≥1 indicates a positive association of the clinical risk factors/plaque microvasculature with a recent ischemic stroke compared to a recent (ocular) TIA.
Statistical significance was set at p ≤0.05. Since it was an explorative study, all statistical tests were 2-tailed. Data are presented as mean ± SD or percentage, as appropriate.
Results
Patient Inclusion
A total of 104 symptomatic patients with a recent (ocular) TIA or minor stroke were included. In 13 patients, DCE-MRI was aborted due to ECG abnormalities. Four patients were excluded from final analysis due to low image quality, leaving 87 patients (70.1 ± 8.4 year, 56 males) for final analysis. Patient characteristics are shown in Table 1. No significant difference in clinical risk factors between patients with (ocular) TIA or minor stroke was found.
Table 1.
Patient characteristics
| (Ocular) TIA | Stroke | t test, p value | |
|---|---|---|---|
| Number of patients, n (%) | 52 (60)b | 35 (40) | |
| Age, years | 70.0±9.1 | 70.1±7.3 | 0.97 |
| Gender, male, n (%) | 33 (55) | 23 (66) | 0.83 |
| Body mass index, kg/m2 | 27.0±4.3 | 26.4±3.6 | 0.42 |
| Current smoking status, n (%) | 10 (17) | 6 (17) | 0.85 |
| Diabetes mellitus, n (%) | 7 (12) | 7 (20) | 0.42 |
| Hypertension, n (%) | 27 (45) | 23 (66) | 0.20 |
| Hypercholesterolemiaa, n (%) | 23 (38) | 15 (43) | 0.99 |
| Prior statin usea, n (%) | 24 (40) | 17 (49) | 0.89 |
| Prior platelet usea, n (%) | 18 (35) | 14 (40) | 0.66 |
| Time between event and MRI, days | 41±19 | 42±20 | 0.86 |
Clinical characteristics for all patients and (ocular) TIA versus stroke patients.
Data known for 86 out of 87 patients.
Seven patients experienced an ocular TIA.
Association between Event Type versus Clinical Risk Factors and Plaque Characteristics
Fifty-two patients (60%) were diagnosed with an ocular TIA (7 patients) or TIA (45 patients), while 35 (40%) were diagnosed with a stroke.
An example of DCE-MR images is shown in Figure 1. Univariate analysis (Table 2) showed a non-significant positive association of diabetes mellitus, hypertension, and prior antiplatelet use with OR ≥1.2.
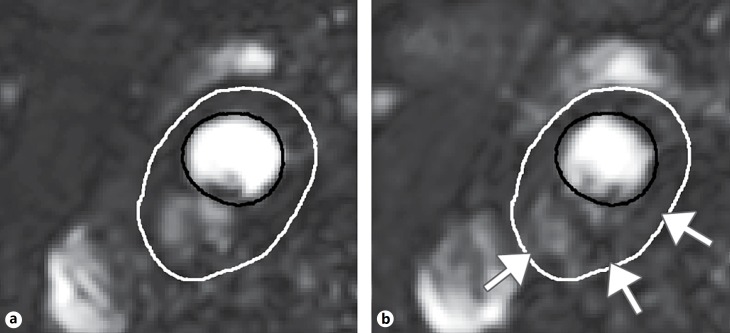
Fig. 1.
Example DCE-MR images of the carotid artery before (a) and after (b) injection of the contrast medium. The lumen contour is depicted by a solid black line, while the outer vessel wall is depicted by the white solid line. After injection of the contrast medium, subtle enhancement of the MR signal is seen in the vessel wall with a stronger enhancement in the adventitial region of the vessel wall (indicated by white arrows in b).
Table 2.
Univariate analysis
| Clinical risk factor/plaque characteristic | OR (95% CI; p value) |
|---|---|
| Age, per 5 years | 0.99 (0.76–1.29; 0.92) |
| Gender, male | 1.10 (0.45–2.71; 0.83) |
| Body mass index, per 1 SD | 0.86 (0.56–1.33; 0.50) |
| Current smoking status | 0.87 (0.29–2.66; 0.81) |
| Diabetes mellitus | 1.61 (0.51–5.07; 0.42) |
| Hypertension | 1.78 (0.73–4.3; 0.20) |
| Hypercholesterolemia | 1.00 (0.42–2.38; 0.99) |
| Prior statin use | 1.06 (0.45–2.51; 0.89) |
| Prior antiplatelet use | 1.22 (0.50–2.97; 0.66) |
OR (95% CI) and p value of univariate analysis of clinical risk factors and plaque characteristics on MRI with the type of recent clinically diagnosed event ([ocular] TIA versus stroke).
NWI, normalized wall index; IPH, intraplaque hemorrhage; LRNC, lipid-rich necrotic core; FC, fibrous cap.
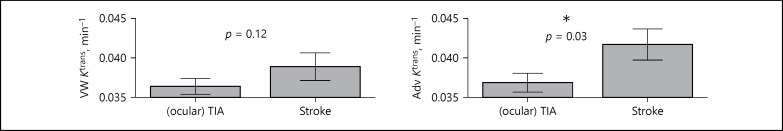
Multivariate analysis of the association between Ktrans and the type of clinically diagnosed event adjusted for associated clinical risk factors showed a significant association of adventitial Ktrans (OR 1.68, 95% CI 1.06–2.68; p = 0.03) with the clinically diagnosed type of event (Table 3; Fig. 2). No significant association was found between stroke subtype and vessel wall Ktrans (Table 3).
Table 31.
Multivariate analysis correcting for associated clinical risk factors
| Plaque characteristic | OR (95% CI; p value) |
|---|---|
| Vessel wall Ktrans, per 1 SD | 1.45 (0.91–2.29; 0.12) |
| Diabetes mellitus | 1.52 (0.46–5.04; 0.49) |
| Hypertension | 1.74 (0.69–4.42; 0.24) |
| Prior antiplatelet use | 0.82 (0.48–1.41; 0.48) |
| Adventitial Ktrans, per 1 SD | 1.68 (1.06–2.68; 0.03)* |
| Diabetes mellitus | 1.78 (0.53–6.01; 0.35) |
| Hypertension | 1.65 (0.64–4.23; 0.30) |
| Prior antiplatelet use | 0.82 (0.47–1.44; 0.50) |
p < 0.05.
OR (95% CI) and corresponding p values from multivariate analysis of adventitial and vessel wall Ktrans including associated clinical risk factors (OR ≥1.2; shown in italics) with clinically diagnosed type of event ([ocular] TIA versus stroke, coded as 0 and 1, respectively).
Fig. 2.
Distribution of vessel wall (VW) and adventitial (Adv) Ktrans based on clinically diagnosed event (amaurosis fugax/TIA versus stroke). * p < 0.05.
Discussion
In the present observational study in symptomatic patients with carotid plaque, a positive association of higher adventitial Ktrans was found in patients who recently experienced a minor ischemic stroke compared to patients who were diagnosed with a recent TIA or ocular TIA independent of associated clinical risk factors. Previous studies have shown that stroke patients are at increased risk for a recurrent stroke compared to patients with an (ocular) TIA [8, 9, 10]. The results from the present study suggest that differences between adventitial microvasculature of symptomatic patients with (ocular) TIA and minor stroke might exist between these 2 groups. Stroke patients demonstrated increased leaky plaque microvasculature compared to (ocular) TIA patients.
Although numerous studies demonstrated that symptomatic lesions differ from asymptomatic lesions [27, 28, 29, 30], there are only a few studies that investigated differences between plaques from TIA and stroke patients. From a large histological study, it is known that carotid plaques show a tendency for plaque inflammation and overall instability to persist with time after a TIA, while the inflammation decreases with time after a stroke. In this study, it was also shown that plaques of stroke patients that are removed ≤60 days after the last ischemic cerebrovascular events were more unstable and inflammatory than those of TIA patients. These findings are in line with the present study, since an increased activity of macrophages may lead to hypoxia, which in turn triggers intraplaque angiogenesis [31]. This may explain the difference in leaky adventitial microvasculature between stroke and (ocular) TIA patients. Another large study on carotid endarterectomy specimens demonstrated a higher prevalence of plaques with acute thrombosis in stroke than in TIA patients [32]. Together these results suggest a difference in the underlying pathology of both patient groups, potentially leading to different or smaller emboli for TIA patients compared to stroke patients [33]. Alternatively, the stroke itself may lead to secondary plaque inflammation. In contrast to plaque inflammation, acute thrombosis, and adventitial microvasculature, other features of plaques, that is, plaque stress [34], plaque morphology (determined by echolucency), and plaque surface (smooth, irregular, or ulcerated) were reported to be similar in stroke and TIA patients [35].
The cross-sectional design of our study does not allow studying causality. Whether there is a causal relationship between differences in plaque type and the type of cerebrovascular event and whether Ktrans can be used as an additional imaging marker to identify patients at risk needs to be studied in prospective serial studies. A recent DCE-MRI study [27] showed that patients with cerebrovascular events had an increased adventitial Ktrans compared to asymptomatic patients.
The present study is an explorative study. Therefore, the results need to be confirmed by an independent study and also in patients with severe carotid artery stenosis. Further, we cannot exclude that the results are influenced by the difficulty of diagnosing a TIA. Agreement on diagnosis among physicians is considerably lower for (ocular) TIA compared to stroke [36, 37]. Since the mean interval between the clinical event and the MRI examination was 41 ± 19 (for [ocular] TIA patients) and 42 ± 20 (for stroke patients) days, brain diffusion-weighted imaging could not be used in our study to distinguish between transient and permanent ischemic events [38]. Therefore, the results of the current study may partly be explained by the heterogeneity among patients diagnosed with an (ocular) TIA.
Conclusions
The present observational study has shown a positive association of leaky adventitial microvasculature in patients with a recent ischemic stroke compared to (ocular) TIA. Results suggest that differences of atherosclerotic plaques of patients with different risk profiles may exist, though confirmation of these results in future prospective studies is needed for more definitive conclusions. Further longitudinal research in larger cohorts is needed to investigate whether DCE-MRI model parameters of the plaque microvasculature may serve as additional imaging markers to predict (the type of) future cerebrovascular events.
Acknowledgements
M.E.K. is supported by Aspasia Grant 015.008.047 from the Netherlands Organization for Scientific Research. This research was performed within the framework of CTMM, the Center for Translational Molecular Medicine (www.ctmm.nl), project PARISK (grant 01C-202), and supported by the Dutch Heart Foundation. J.E.W. and M.E.K. are supported by Stichting De Weijerhorst. The authors thank R.J. van der Geest (Department of Radiology, Leiden University Medical Center, Leiden, The Netherlands) for providing the VesselMASS analysis software package.
References
- 1.Mackay J, Mensah G. The Atlas of Heart Disease and Stroke. Global burden of Stroke. 2004 [Google Scholar]
- 2.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 3.Arboix A. Cardiovascular risk factors for acute stroke: Risk profiles in the different subtypes of ischemic stroke. World J Clin Cases. 2015;3:418–429. doi: 10.12998/wjcc.v3.i5.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacMahon S, Peto R, Cutler J, Collins R, Sorlie P, Neaton J, et al. Blood pressure, stroke, and coronary heart disease. Part 1, Prolonged differences in blood pressure: prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 5.Jarrett RJ. Epidemiology and public health aspects of non-insulin-dependent diabetes mellitus. Epidemiol Rev. 1989;11:151–171. doi: 10.1093/oxfordjournals.epirev.a036034. [DOI] [PubMed] [Google Scholar]
- 6.Higa M, Davanipour Z. Smoking and stroke. Neuroepidemiology. 1991;10:211–222. doi: 10.1159/000110272. [DOI] [PubMed] [Google Scholar]
- 7.Suk SH, Sacco RL, Boden-Albala B, Cheun JF, Pittman JG, Elkind MS, et al. Abdominal obesity and risk of ischemic stroke: the Northern Manhattan Stroke Study. Stroke. 2003;34:1586–1592. doi: 10.1161/01.STR.0000075294.98582.2F. [DOI] [PubMed] [Google Scholar]
- 8.Pendlebury ST, Rothwell PM. Risk of recurrent stroke, other vascular events and dementia after transient ischaemic attack and stroke. Cerebrovasc Dis. 2009;27((suppl 3)):1–11. doi: 10.1159/000209260. [DOI] [PubMed] [Google Scholar]
- 9.Stromberg S, Nordanstig A, Bentzel T, Osterberg K, Bergstrom GM. Risk of early recurrent stroke in symptomatic carotid stenosis. Eur J Vasc Endovasc Surg. 2015;49:137–144. doi: 10.1016/j.ejvs.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Rothwell PM, Mehta Z, Howard SC, Gutnikov SA, Warlow CP. Treating individuals 3: from subgroups to individuals: general principles and the example of carotid endarterectomy. Lancet. 2005;365:256–265. doi: 10.1016/S0140-6736(05)17746-0. [DOI] [PubMed] [Google Scholar]
- 11.Fleiner M, Kummer M, Mirlacher M, Sauter G, Cathomas G, Krapf R, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation. 2004;110:2843–2850. doi: 10.1161/01.CIR.0000146787.16297.E8. [DOI] [PubMed] [Google Scholar]
- 12.Koole D, Heyligers J, Moll FL, Pasterkamp G. Intraplaque neovascularization and hemorrhage: markers for cardiovascular risk stratification and therapeutic monitoring. J Cardiovasc Med (Hagerstown) 2012;13:635–639. doi: 10.2459/JCM.0b013e3283590cd2. [DOI] [PubMed] [Google Scholar]
- 13.Sluimer JC, Daemen MJ. Novel concepts in atherogenesis: angiogenesis and hypoxia in atherosclerosis. J Pathol. 2009;218:7–29. doi: 10.1002/path.2518. [DOI] [PubMed] [Google Scholar]
- 14.Kerwin W, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation. 2003;107:851–856. doi: 10.1161/01.cir.0000048145.52309.31. [DOI] [PubMed] [Google Scholar]
- 15.Kerwin WS, O'Brien KD, Ferguson MS, Polissar N, Hatsukami TS, Yuan C. Inflammation in carotid atherosclerotic plaque: a dynamic contrast-enhanced MR imaging study. Radiology. 2006;241:459–468. doi: 10.1148/radiol.2412051336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaens ME, Backes WH, Rozel S, Lipperts M, Sanders SN, Jaspers K, et al. Dynamic contrast-enhanced MR imaging of carotid atherosclerotic plaque: model selection, reproducibility, and validation. Radiology. 2013;266:271–279. doi: 10.1148/radiol.12120499. [DOI] [PubMed] [Google Scholar]
- 17.Kerwin WS, Oikawa M, Yuan C, Jarvik GP, Hatsukami TS. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magn Reson Med. 2008;59:507–514. doi: 10.1002/mrm.21532. [DOI] [PubMed] [Google Scholar]
- 18.Truijman MT, Kooi ME, van Dijk AC, de Rotte AA, van der Kolk AG, Liem MI, et al. Plaque At RISK (PARISK): prospective multicenter study to improve diagnosis of high-risk carotid plaques. Int J Stroke. 2014;9:747–754. doi: 10.1111/ijs.12167. [DOI] [PubMed] [Google Scholar]
- 19.Clinical alert. benefit of carotid endarterectomy for patients with high-grade stenosis of the internal carotid artery. National Institute of Neurological Disorders and Stroke Stroke and Trauma Division. North American Symptomatic Carotid Endarterectomy Trial (NASCET) investigators. Stroke. 1991;22:816–817. doi: 10.1161/01.str.22.6.816. [DOI] [PubMed] [Google Scholar]
- 20.van Hoof RH, Hermeling E, Truijman MT, van Oostenbrugge RJ, Daemen JW, van der Geest RJ, et al. Phase-based vascular input function: Improved quantitative DCE-MRI of atherosclerotic plaques. Med Phys. 2015;42:4619. doi: 10.1118/1.4924949. [DOI] [PubMed] [Google Scholar]
- 21.Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051–2056. doi: 10.1161/hc4201.097839. [DOI] [PubMed] [Google Scholar]
- 22.Sun J, Song Y, Chen H, Kerwin WS, Hippe DS, Dong L, et al. Adventitial perfusion and intraplaque hemorrhage: a dynamic contrast-enhanced MRI study in the carotid artery. Stroke. 2013;44:1031–1036. doi: 10.1161/STROKEAHA.111.000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanisz GJ, Odrobina EE, Pun J, Escaravage M, Graham SJ, Bronskill MJ, et al. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magn Reson Med. 2005;54:507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- 24.Pintaske J, Martirosian P, Graf H, Erb G, Lodemann KP, Claussen CD, et al. Relaxivity of gadopentetate dimeglumine (magnevist), gadobutrol (gadovist), and gadobenate dimeglumine (multiHance) in human blood plasma at 0.2, 1.5, and 3 Tesla. Invest Radiol. 2006;41:213–221. doi: 10.1097/01.rli.0000197668.44926.f7. [DOI] [PubMed] [Google Scholar]
- 25.Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 26.Parker GJ, Roberts C, Macdonald A, Buonaccorsi GA, Cheung S, Buckley DL, et al. Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magn Reson Med. 2006;56:993–1000. doi: 10.1002/mrm.21066. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Chen H, Sun J, Hippe DS, Zhang H, Yu S, et al. Dynamic contrast-enhanced MR imaging of carotid vasa vasorum in relation to coronary and cerebrovascular events. Atherosclerosis. 2017;263:420–426. doi: 10.1016/j.atherosclerosis.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Lindsay AC, Biasiolli L, Lee JM, Kylintireas I, MacIntosh BJ, Watt H, et al. Plaque features associated with increased cerebral infarction after minor stroke and TIA: a prospective, case-control, 3-T carotid artery MR imaging study. JACC Cardiovasc Imaging. 2012;5:388–396. doi: 10.1016/j.jcmg.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Cappendijk VC, Kessels AG, Heeneman S, Cleutjens KB, Schurink GW, Welten RJ, et al. Comparison of lipid-rich necrotic core size in symptomatic and asymptomatic carotid atherosclerotic plaque: initial results. J Magn Reson Imaging. 2008;27:1356–1361. doi: 10.1002/jmri.21359. [DOI] [PubMed] [Google Scholar]
- 30.Saam T, Cai J, Ma L, Cai YQ, Ferguson MS, Polissar NL, et al. Comparison of symptomatic and asymptomatic atherosclerotic carotid plaque features with in vivo MR imaging. Radiology. 2006;240:464–472. doi: 10.1148/radiol.2402050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sluimer JC, Gasc JM, van Wanroij JL, Kisters N, Groeneweg M, Sollewijn Gelpke MD, et al. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol. 2008;51:1258–1265. doi: 10.1016/j.jacc.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 32.Mauriello A, Sangiorgi GM, Virmani R, Trimarchi S, Holmes DR, Jr, Kolodgie FD, et al. A pathobiologic link between risk factors profile and morphological markers of carotid instability. Atherosclerosis. 2010;208:572–580. doi: 10.1016/j.atherosclerosis.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 33.Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: the Oxford plaque study. Circulation. 2006;113:2320–2328. doi: 10.1161/CIRCULATIONAHA.105.589044. [DOI] [PubMed] [Google Scholar]
- 34.Sadat U, Teng Z, Young VE, Graves MJ, Gaunt ME, Gillard JH. High-resolution magnetic resonance imaging-based biomechanical stress analysis of carotid atheroma: a comparison of single transient ischaemic attack, recurrent transient ischaemic attacks, non-disabling stroke and asymptomatic patient groups. Eur J Vasc Endovasc Surg. 2011;41:83–90. doi: 10.1016/j.ejvs.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Golledge J, Cuming R, Ellis M, Davies AH, Greenhalgh RM. Carotid plaque characteristics and presenting symptom. Br J Surg. 1997;84:1697–1701. [PubMed] [Google Scholar]
- 36.Castle J, Mlynash M, Lee K, Caulfield AF, Wolford C, Kemp S, et al. Agreement regarding diagnosis of transient ischemic attack fairly low among stroke-trained neurologists. Stroke. 2010;41:1367–1370. doi: 10.1161/STROKEAHA.109.577650. [DOI] [PubMed] [Google Scholar]
- 37.Leppala JM, Virtamo J, Heinonen OP. Validation of stroke diagnosis in the National Hospital Discharge Register and the Register of Causes of Death in Finland. Eur J Epidemiol. 1999;15:155–160. doi: 10.1023/a:1007504310431. [DOI] [PubMed] [Google Scholar]
- 38.van Rooij FG, Vermeer SE, Goraj BM, Koudstaal PJ, Richard E, de Leeuw FE, et al. Diffusion-weighted imaging in transient neurological attacks. Ann Neurol. 2015;78:1005–1010. doi: 10.1002/ana.24539. [DOI] [PubMed] [Google Scholar]