Abstract
Background
Androgenetic alopecia is a condition with a high prevalence worldwide and affects both males and females. Currently, only 2 approved treatments exist: finasteride (males only) and minoxidil 2 or 5% solution (males and females).
Methods
We conducted a randomized, open-label, multicenter noninferiority study to determine whether a caffeine-based 0.2% topical liquid would be no less effective than minoxidil 5% solution in males (n = 210) with androgenetic alopecia. The primary end point was the percentage change in the proportion of anagen hairs from baseline to 6 months using a frontal and occipital trichogram.
Results
At 6 months, the group of the 5% minoxidil solution showed a mean improvement in anagen ratio of the trichogram of 11.68%, and the group of the 0.2% caffeine solution had an anagen improvement of 10.59%. The difference of mean values between both groups was 1.09%. The statistical analysis was performed and reported in accordance with the CONSORT Guidelines 2010 for reporting of noninferiority and equivalence randomized trials.
Conclusion
A caffeine-based topical liquid should be considered as not inferior to minoxidil 5% solution in men with androgenetic alopecia.
Keywords: Androgenetic alopecia, Caffeine-based topical liquid, Anagen hairs, Frontal trichogram, Occipital trichogram
Introduction
Androgenetic alopecia (AGA) is the most common hair loss disorder worldwide, affecting up to 80% of Caucasian men aged older than 70 years. Although the prevalence of AGA is high in elderly subjects, the first signs often develop during puberty. Hair loss can have substantial impacts on quality of life, including negative effects on self-esteem and perceived physical attractiveness, and may even lead to depression. Despite these negative effects, many patients do not pursue preventative therapy for hair loss, and many of those that try therapies that promise hair regrowth are dissatisfied when they first go to see a specialist. Limited efficacy, poor tolerance, and lack of information on treatment duration or adverse events (AEs) may result in reduced compliance to a long-term therapeutic regimen [1].
The hair follicle has 3 main lifecycle stages. Anagen is the active growth phase of hair follicles in which the root of the hair is dividing rapidly and adding to the length of the hair shaft. Scalp hair normally stays in this active phase of growth for 2–6 years [2]. Following the anagen phase, the hair shaft enters into the catagen phase, and hair follicles undergo a highly controlled process of involution that largely reflects a burst of programmed cell death of the majority of follicular keratinocytes. The third and last stage is the telogen phase, during which the hair shaft matures into a club hair and is then shed from the follicle, usually during washing or combing.
Hair loss and unwanted hair growth both reflect aberrations in hair follicle cycling, and consequently, in principle, they may be reversed. AGA is caused by the progressive shortening of successive anagen phases as well as the gradual miniaturization of genetically predisposed follicles in the presence of androgens; in addition, large, pigmented hairs (terminal hairs) are replaced by thin lightly pigmented hair (vellus hair). Due to the presence of these cycling hair follicles, AGA is considered to be potentially reversible. However, simply removing androgens does not often result in the conversion of miniaturized follicles to terminal follicles. Therefore treatments utilizing other lines of approach are needed [3].
Currently, there are few approved treatments on the market for AGA. The only FDA-approved treatments for AGA are finasteride, taken orally (males only), and topical minoxidil 2 or 5% solution (females and males) [4, 5, 6, 7].
Minoxidil (Rogaine; H + H Pharmaceuticals) is an androgen-independent medication that counteracts AGA by causing premature termination of the telogen phase and likely prolongs the anagen phase [8]. There appear to be differences in efficacy between the 2 and 5% concentrations. In a study of 393 men with AGA, a 5% minoxidil solution demonstrated significantly greater efficacy, compared with the 2% minoxidil solution [8].
In recent years, caffeine has demonstrated potential as a treatment for AGA [9]. Due to being a phosphodiesterase inhibitor, caffeine increases cyclic adenosine monophosphate levels in cells and consequently promotes cell proliferation through stimulating cell metabolism - a mechanism that may counteract testosterone/dihydrotestosterone-induced miniaturization of the hair follicle [10]. In a male skin organ culture model, caffeine reversed the inhibiting effect of testosterone on keratinocyte proliferation [11]. In an in vitro study, testosterone-induced hair follicle growth suppression was reversed with addition of caffeine at concentrations of 0.001 and 0.005%; moreover, caffeine alone led to significant stimulation of hair follicle growth [10]. In another in vitro study of male and female hair follicles, caffeine was found to enhance hair shaft elongation, prolong anagen duration and stimulate hair matrix keratinocyte proliferation. In addition, hair follicles from females appeared to be more sensitive to caffeine than hair follicles from males, and caffeine counteracted testosterone-induced transforming growth factor-β2 expression, a major antagonistic hair growth regulatory factor, in male hair follicles. Caffeine also resulted in increased expression of insulin-like growth factor-1, a promoter of hair growth, in both male and female hair follicles [12]. Notwithstanding that these findings are results of in vitro experiments, the topical treatment of caffeine in vivo is also promising because of its good absorption and follicular penetration [13].
Caffeine has also been shown to penetrate the hair follicle even when applied as a shampoo formulation. In a study of 6 male volunteers, a caffeine-based shampoo (Alpecin; Dr. Kurt Wolff GmbH) resulted in penetration of caffeine into both the stratum corneum and hair follicles after 2-min application, with the highest values being found 2 h after application [14].
The potential efficacy of a caffeine-based topical formulation has been previously demonstrated in a prospective study of 40 men with AGA. In this study, daily use of the caffeine-based lotion resulted in an 8.14% reduction in hairs extracted at 2 months and a 15.33% reduction in hairs extracted at 4 months. In addition, at 2 and 4 months, 75 and 83% of participants were considered to have had a positive response (decrease in number of pull test hairs) [15]. However, this study only had one arm and, consequently, was noncomparative, and therefore one cannot draw too many conclusions from the results.
Here, results are reported from a noninferiority study that aimed to determine whether a caffeine-based topical 0.2% liquid is no less effective than minoxidil 5% solution in males with AGA.
The design of a noninferiority study, to demonstrate the efficacy of the caffeine solution, seemed more appropriate than a placebo-controlled study design, because the reference product is the FDA-approved ingredient minoxidil. Hence the effect of the test product can be directly compared with an approved reference.
Methods
Study Design
This was a registered, prospective, open-label, randomized, active-controlled study conducted at 5 centers in India (CTRI/ 2014/07/004768, registered on July 25, 2014): Lokmanya Tilak Municipal General Hospital, Mumbai; St. John Medical College and Hospital, Bengaluru; Sri Ramachandra Medical College and Hospital, Chennai; Jehangir Clinical Development Center, Pune; Chennai Meenakshi Multi Specialty Hospital, Chennai. Ethical votes have been approved for all study sites. The study period included 4 visits to the study center: visit 1 (screening, conducted 3 days prior to baseline visit), visit 2 (baseline, day 1), visit 3 (treatment visit; day 90/month 3), and visit 4 (end-of-study visit; day 180/month 6). There was a 3-day hair wash quarantine before visits 3 and 4.
Subjects were randomized in a 1:1 ratio to either a caffeine-based topical liquid (treatment group A; test product) or minoxidil 5% solution (treatment group B; reference product).
Subjects
This study included males aged between 18 and 55 years with AGA. Additional inclusion criteria were: balding stage of III-V on the Hamilton-Norwood scale [16] and use of global photography to confirm the Hamilton-Norwood stage of balding, at least 20% telogen ratio in the trichogram, and a willingness to participate and provide signed informed consent. Exclusion criteria were: diagnosis of pathological forms of alopecia (such as alopecia areata, trichotillomania, scarring alopecia, and alopecia due to medication), diagnosis of dermatological conditions (such as eczema, fungal scalp conditions, seborrheic dermatitis, recurrent herpes, pityriasis versicolor, psoriasis, pigmentary disorders, chronic lupus erythematosus), history of chemotherapy, and known hypersensitivity to minoxidil 5% solution or Alpecin that could interfere directly/indirectly with the study.
Interventions
The test product, 0.2% caffeine solution, is marketed as Alpecin Liquid by Dr. Kurt Wolff and distributed by the study sponsor Fullife Healthcare Pvt Ltd. The control product is a 5% minoxidil solution, marketed as Mx-5 by H + H Pharmaceuticals PvT Ltd. Subjects in the 0.2% caffeine solution arm were instructed to apply 2 mL of the test product to the scalp twice a day (once in the morning and once in the evening), and subjects in the minoxidil 5% solution arm were instructed to apply 1 mL of the reference product to the scalp twice a day (once in the morning and once in the evening). The active treatment period duration was 6 months.
Primary Outcome
The primary end point was the percentage change in proportion of anagen hairs, or the anagen rate (AR), from baseline to 6 months using frontal and occipital trichograms in the per-protocol (PP) population.
In this study, hair loss was assessed with trichograms in the frontal and occipital areas of the scalp. A trichogram involves the microscopic examination of hairs plucked from the scalp and provides information about both the hair root and the hair tip. A set number of hairs are removed and examined per trichogram. The hairs are arranged side by side on a glass slide and taped, and are then examined under a microscope. Examination of the proximal end of the hair shaft (the hair root) can help determine whether the hair is in anagen, telogen, or catagen phase and whether it is normal or dystrophic [17].
Secondary Outcomes
Secondary end points were the separate percentage changes in AR from baseline to 3 and 6 months using frontal and occipital trichograms, changes in number of subjects with an increase in anagen hair (and decrease in telogen hair) using a frontal trichogram from baseline to 3 and 6 months, changes in number of subjects with increase in anagen hair (and decrease in telogen hair) using an occipital trichogram from baseline to 3 and 6 months, change in subject's perception of effect of the caffeine-based topical 0.2% liquid or minoxidil 5% solution on hair loss management, assessed with a subject questionnaire from baseline to 3 and 6 months, and change in clinical effect as assessed by a dermatologist questionnaire from baseline to 3 and 6 months.
Safety was also assessed: incidence of AEs during the study period and changes in vital signs from baseline to 6 months.
Sample Size Calculation
The calculation of the sample size was based on the noninferiority margin for the difference in ARs of 5% using a 2-sided t test (assuming a common standard deviation of AR by 10%). A sample size of 176 subjects was calculated to be sufficient to reject the hypothesis of inferiority with a 90% power and an alpha error level of 0.05%. In total, 210 subjects were required, assuming a dropout rate of approximately 15%.
Randomization and Blinding
The randomization list (balanced randomization with a block size of 6) was generated by Dr. Kurt Wolff GmbH & Co. KG using a validated computer program (RANCODE, IDV Gauting) and sent to Fullife Healthcare Pvt Ltd., who then provided envelopes to the study sites containing the subject randomization details.
A blinding of probands and examiners was not intended, because the dosing in both arms was different. In the control arm of 5% minoxidil the regular dose was 1 mL twice per day, in the 0.2% caffeine solution arm the dosing was 2 mL twice per day.
Statistical Analysis
The statistical analysis was performed and reported in accordance with the CONSORT Guidelines 2010 for reporting of noninferiority and equivalence randomized trials [18]. The primary aim of this study was to demonstrate noninferiority of a caffeine-based topical 0.2% liquid to minoxidil 5% solution in subjects with AGA. The primary outcome measure was analyzed primarily for the PP population and repeated for sensitivity reasons for the intention-to-treat (ITT) population. The 2-sided 95% confidence interval approach was used to test noninferiority [18, FDA Guidelines 2016]. In addition, analyses of covariance (ANCOVAs) were performed to evaluate the difference between treatment arms in ARs after 6 months (using AR at baseline as a covariate).
Analyses of secondary efficacy end points were based on nonparametric tests (2-sided Mann-Whitney test and the Friedman test), due to the nonnormal distribution of the data, as well as the Fisher exact text (for categorical data) at the 5% level of significance (p < 0.05).
All randomized probands were included in these analyses, and missing values were not replaced. Statistical tests were performed as 2-sided tests with an alpha error of 0.05. However, statistical testing of all secondary end points was explorative and not confirmatory.
Results
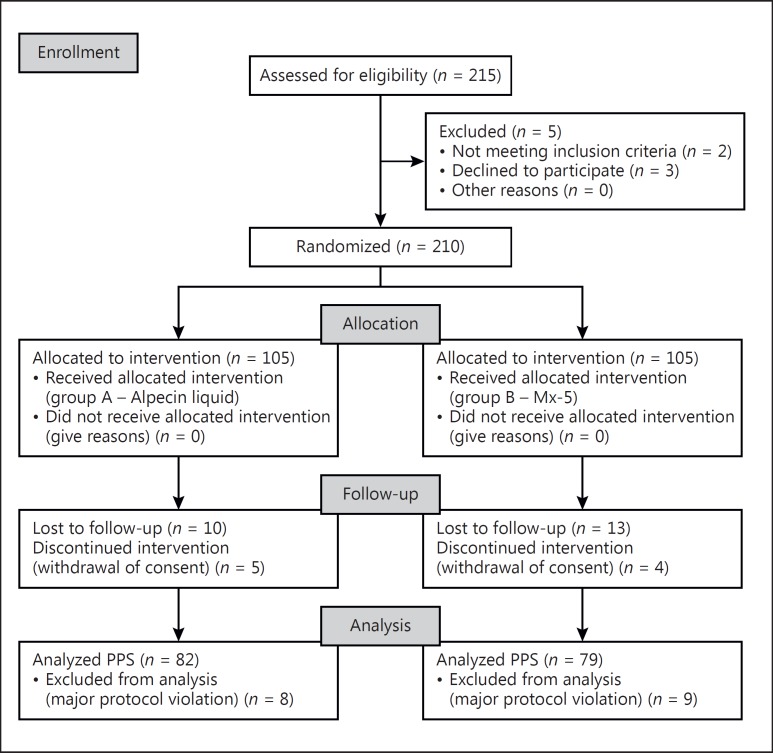
Overall, 210 subjects were enrolled and randomized to treatment arms; the PP population comprised 161 subjects. For a CONSORT flow diagram, see Figure 1.
Fig. 1.
CONSORT flow diagram. PPS, per protocol set.
Patient Characteristics
The median age of the subjects was 31.0 (range 19–50) and 29.0 (range 21–51) years in the caffeine-based topical 0.2% liquid and minoxidil 5% solution arms, respectively; the mean age was 32.2 (standard deviation 7.3) and 31.1 (7.6) years, respectively. There were no significant differences in baseline characteristics between treatment arms (Table 1); however, the number of anagen, telogen, and dystrophic hairs extracted from subjects at baseline significantly differed (p < 0.01) in 1 study center from the other 4 centers. Reasons for that could be differences in the study population. In 1 center the percentage of young and working people was higher. These people could have experienced psychological stress or nutritional deficiencies in addition to their predisposition of AGA which could explain the higher percentage of telogen hairs. The higher telogen rate in the differing center did not change the outcome of the noninferiority between both study arms.
Table 1.
Characteristics of study subjects at baseline
| Caffeine-based topical 0.2% liquid (n = 105) | Minoxidil 5% solution (n = 105) | ||
|---|---|---|---|
| Hamilton-Norwood stage, % subjects | |||
| Grade III | 28.6 | 28.6 | |
| Grade III vertex | 17.1 | 22.9 | |
| Grade IIIA | 7.6 | 15.2 | |
| Grade IV | 28.6 | 20.0 | |
| Grade IVA | 3.8 | 2.9 | |
| Grade V | 14.3 | 10.5 | |
| Telogen hairs, mean % | 36.5 | 36.2 | |
| Frontal trichogram | |||
| Anagen hairs | 36.4 (13.7)/39 (0–60) | 35.3 (15.7)/41 (0–62) | |
| Telogen hairs | 29.2 (9.5)/28 (13–69) | 28.7 (9.6)/27 (10–75) | |
| Dystrophic hairs | 8.2 (7.6)/6 (0–29) | 9.6 (9.6)/7 (0–42) | |
| Total hair count | 73.8 (8.2)/73 (60–100) | 73.6 (8.0)/73 (60–97) | |
| Occipital trichogram | |||
| Anagen hairs | 40.7 (16.4)/46 (1–74) | 39.4 (18.3)/44 (0–74) | |
| Telogen hairs | 25.0 (10.5)/23 (8–76) | 24.8 (10.3)/24 (6–65) | |
| Dystrophic hairs | 9.6 (10.1)/7 (0–44) | 10.7 (11.4)/8 (0–50) | |
| Total hair count | 75.4 (8.0)/74 (61–95) | 74.9 (8.0)/74 (61–95) | |
| Frontal + occipital trichogram | |||
| Anagen hairs | 77.2 (28.8)/85 (1–134) | 74.7 (33.3)/87 (1–135) | |
| Telogen hairs | 54.2 (17.3)/51 (26–145) | 53.5 (18.0)/51 (27–140) | |
| Dystrophic hairs | 17.8 (16.4)/16 (0–64) | 20.4 (20.3)/16 (0–80) | |
| Total hair count | 149.1 (13.4)/147 (123–180) | 148.6 (13.1)/148 (123–177) | |
For trichogram data, mean numbers with standard deviations in parentheses are given, followed by medians with ranges in parentheses.
Efficacy Outcomes
Primary Efficacy Analysis
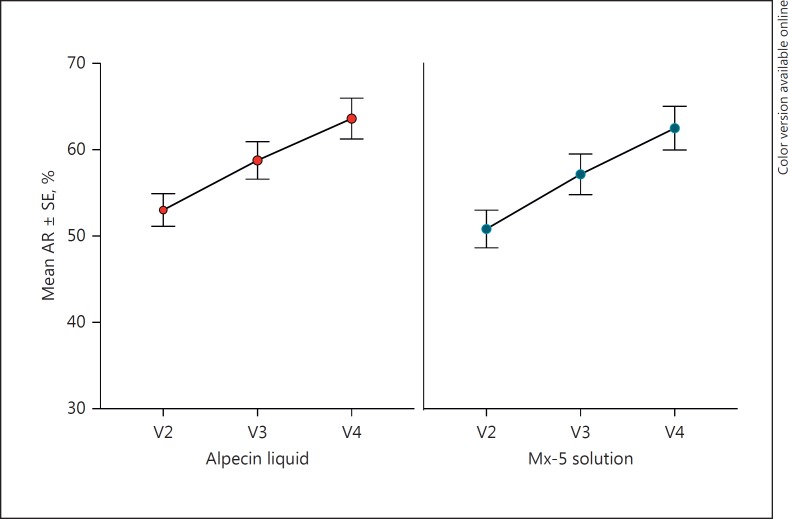
At 6 months, minoxidil 5% solution was associated with a nonsignificantly higher increase from baseline in AR, compared with the caffeine-based topical 0.2% liquid (11.68 ± 12.44 vs. 10.59 ± 12.02% in the PP population; p = 0.574), using frontal and occipital trichograms (Table 2). The 2-sided 95% CI for the difference in the mean increase in AR between treatment arms (1.09%) ranged from −2.72 to 4.89%. The upper limit of the 95% CI below the predetermined margin for noninferiority of 5% was not exceeded, and consequently the caffeine-based topical 0.2% liquid was determined to be noninferior to the minoxidil 5% solution (Fig. 2).
Table 2.
Change in percentage of anagen hairs from baseline to 3 and 6 months in the per-protocol population using frontal and occipital trichograms
| Caffeine-based topical 0.2% liquid (n = 82) | Minoxidil 5% solution (n = 79) | |
|---|---|---|
| Baseline | 53.01 (17.13)/59.37 (0.7–76.6) | 50.81 (19.33)/56.86 (0.8–77.3) |
| 3 months | 58.76 (19.75)/67.42 (3.8–83.1) | 57.15 (20.94)/65.45 (2.4–80.0) |
| 6 months | 63.60 (21.43)/74.75 (5.7–82.1) | 62.49 (22.47)/72.86 (4.8–83.2) |
Results are expressed as means with standard deviations in parentheses, followed by medians with ranges in parentheses.
Fig. 2.
Mean (±standard error, SE) of the rate of anagen hair (AR; %) by treatment and visit (baseline, after 3 months, and after 6 months).
A similar outcome was observed in the ITT population. At 6 months, increases in AR were not significantly different between the caffeine-based topical 0.2% liquid and minoxidil 5% solution arms (10.18 ± 11.83 vs. 11.34 ± 12.03%; p = 0.504). The 2-sided 95% CI for the difference in mean increase in AR (1.16%) ranged from −2.25 to 4.56%. The upper limit of the 95% CI was lower than the prespecified noninferiority margin of 5%.
ANCOVAs of the AR (in percent) after 6 months (using baseline values as covariate) confirmed that there was no significant treatment effect (difference between groups) in both population sets (PP and ITT).
In addition, ANCOVAs were performed including “center” as factor. In both population sets (PP and ITT), the effect of “center” was highly significant. However, neither in the PP nor in the ITT population was the margin of noninferiority violated.
Secondary Efficacy Analysis
Using only the frontal trichogram, there was no significant difference in the increase from baseline in AR at 6 months between the caffeine-based topical 0.2% liquid and minoxidil 5% solution arms (11.27 ± 13.02 vs. 11.89 ± 11.78%; p = 0.740). The corresponding 2-sided 95% CI for the difference in mean increases in AR (0.62%) ranged from −3.06 to 4.30%.
Using only the occipital trichogram, there was no significant difference in the increase from baseline in AR at 6 months between the caffeine-based topical 0.2% liquid and minoxidil 5% solution arms (9.15 ± 13.14 vs. 11.08 ± 14.33%; p = 0.349). The corresponding 2-sided 95% CI for the difference in mean increases in AR (1.94%) ranged from −2.13 to 6.00%.
In addition, there were no significant differences at 6 months in the number of subjects for whom anagen hair increased and telogen hair decreased from baseline, using both the frontal trichogram (p = 1.000) and the occipital trichogram (p = 0.310) assessments.
With respect to subject assessments, subjects in both treatment arms considered the intensity of hair loss, the number of hairs falling out while combing, and hair thickness to be significantly improved from baseline at 6 months (all p < 0.01), with no significant differences between treatment arms at 6 months. At 3 months, subjects using minoxidil 5% solution reported significantly higher treatment satisfaction, compared with subjects using the caffeine-based topical 0.2% liquid (p = 0.020); however, by 6 months this difference was no longer significant (p = 0.090). Subjects using the caffeine-based topical 0.2% liquid reported significant improvement from baseline in scalp itchiness at 6 months (p = 0.003) whereas subjects using minoxidil 5% solution reported no such improvement (p = 0.211). However, the difference between arms was not significant at 6 months. Subjects in both groups reported a significant improvement in scalp tension/dryness (p < 0.05), with no significant difference between groups at 6 months.
With respect to investigator assessments, hair strength, balding progression, and extent of hair loss were considered to be significantly improved from baseline in both treatment arms at 6 months (all p < 0.01), with no significant differences between treatment arms. At 6 months, investigators recommended the study product in 97.8% of subjects using the caffeine-based topical 0.2% liquid and in 100% of subjects using minoxidil 5% solution. Investigators considered scalp redness and scaling/dandruff to be significantly improved from baseline in both treatment arms at 6 months (all p < 0.05), with no significant differences between treatment arms.
Safety
One patient (1%) in the minoxidil 5% solution arm reported an AE (mild headache). This AE was not considered to be related to treatment.
Discussion
This study provides the first comparative in vivo proof of the noninferiority of caffeine against 5% minoxidil solution for AGA in males. In this study, a caffeine-based topical 0.2% liquid was found to be no less effective than minoxidil 5% solution regarding the percentage change from baseline in AR at 6 months in both the PP population (primary end point) and ITT population, using frontal and occipital trichograms. In addition, similar outcomes between treatment arms were observed regarding the increase from baseline in AR at 6 months using either the frontal trichogram or the occipital trichogram alone and the change from baseline in the number of subjects with an increase in anagen hair (and decrease in telogen hair) using a frontal or occipital trichogram at 3 and 6 months.
In this study, both the caffeine-based topical 0.2% liquid and minoxidil 5% solution appeared to be well tolerated. However, AEs associated with topical minoxidil at the lower FDA-approved concentration of 2% have been previously reported, including irritant and allergic contact dermatitis as well as allergic reactions to the nonactive ingredient propylene glycol - a compound found in some topical solutions, particularly if they are galenic in nature [19]. In our study, the 0.2% caffeine solution resulted in a significant improvement from baseline in scalp itchiness at 6 months whereas no such improvement was observed for minoxidil 5% solution.
The subject information of the control product among other AEs associated with topical solutions at both the 2 and 5% concentrations includes headache, feeling faint or dizzy, chest pains, rapid heartbeat, increased weight, and fluid retention [20]. However, these AEs were not reported by subjects in our study.
A limitation of our study included its open-label design, which may have resulted in unintentional bias on the part of both investigators and subjects. With respect to generalizability of the study findings, the relatively broad inclusion criteria suggest external validity in the general population of men with AGA.
In conclusion, the results of this study are promising because they show that an effective natural ingredient like caffeine can be considered as an effective alternative treatment to common drug therapies against hair loss. As well as the fact that genetically predisposed hair loss can hardly be classified as a hair disease, its treatment with drugs might be of concern when taking risk/benefit profiles into account. Such ethical considerations become even more important when the treatment is applied or administered daily for the rest of the subject's life and has to be done lifelong and not just only within a shorter therapy period. Well-tolerated ingredients are prerequisites for safe treatment against hereditary alopecia, and the caffeine solution assessed in this study seemed to meet this requirement.
Statement of Ethics
From all study participants, a signed informed consent was obtained.
Disclosure Statement
Adolf Klenk is an employee of the sponsor Dr. Kurt Wolff GmbH & Co. KG. Theodor May is a consultant of Dr. Kurt Wolff GmbH & Co KG.
Acknowledgments
The authors would like to thank the following team members for their contributions to the success of this trial: study management and monitoring was supported by JSS Medical Research India Pvt Ltd., Chennai, India; medical writing assistance was provided by Melody Watson, at co.medical, Berlin.
Study sites were: Lokmanya Tilak Municipal General Hospital, Mumbai; India St. Johns Medical College and Hospital, Bengaluru; Sri Ramachandra Medical College and Hospital, Chennai; Jehangir Clinical Development Center, Pune; Chennai Meenakshi Multi Specialty Hospital, Chennai, India.
We also thank Dr. Kurt Wolff GmbH & Co. KG for funding this study.
References
- 1.Blumeyer A, Tosti A, Messenger A, Reygagne P, Del Marmol V, Spuls PI, et al. Evidence-based (S3) guideline for the treatment of androgenetic alopecia in women and in men. J Dtsch Dermatol Ges. 2011;9((suppl 6)):S1–S57. doi: 10.1111/j.1610-0379.2011.07802.x. [DOI] [PubMed] [Google Scholar]
- 2.Stenn K, Parimoo S, Zheng Y, Barrows T, Boucher M, Washenik K. Bioengineering the hair follicle. Organogenesis. 2007;3:6–13. doi: 10.4161/org.3.1.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paus R, Cotsarelis G. The biology of hair follicles. N Engl J Med. 1999;341:491–497. doi: 10.1056/NEJM199908123410706. [DOI] [PubMed] [Google Scholar]
- 4.Torres F. Androgenetic, diffuse and senescent alopecia in men: practical evaluation and management. Curr Probl Dermatol. 2015;47:33–44. doi: 10.1159/000369403. [DOI] [PubMed] [Google Scholar]
- 5.US Food and Drug Administration Drug facts - minoxidil 2% solution. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078877.pdf (accessed March 1, 2017).
- 6.US Food and Drug Administration Approval letter - minoxidil 5% solution 2001. http://www.accessdata.fda.gov/drugsatfda_docs/anda/2001/075598Orig1s000.pdf (accessed March 1, 2017).
- 7.Pierard GE, Pierard-Franchimont C, Marks R, Elsner P. EEMCO guidance for the assessment of hair shedding and alopecia. Skin Pharmacol Physiol. 2004;17:98–110. doi: 10.1159/000076020. [DOI] [PubMed] [Google Scholar]
- 8.Olsen EA, Dunlap FE, Funicella T, Koperski JA, Swinehart JM, Tschen EH, et al. A randomized clinical trial of 5% topical minoxidil versus 2% topical minoxidil and placebo in the treatment of androgenetic alopecia in men. J Am Acad Dermatol. 2002;47:377–385. doi: 10.1067/mjd.2002.124088. [DOI] [PubMed] [Google Scholar]
- 9.Herman A, Herman AP. Caffeine's mechanisms of action and its cosmetic use. Skin Pharmacol Physiol. 2013;26:8–14. doi: 10.1159/000343174. [DOI] [PubMed] [Google Scholar]
- 10.Fischer TW, Hipler UC, Elsner P. Effect of caffeine and testosterone on the proliferation of human hair follicles in vitro. Int J Dermatol. 2007;46:27–35. doi: 10.1111/j.1365-4632.2007.03119.x. [DOI] [PubMed] [Google Scholar]
- 11.Tsianakas A, Hüsing B, Moll I. An ex vivo model of male skin - caffeine counteracts testosterone effects. Arch Dermatol Res. 2005;296:450. [Google Scholar]
- 12.Fischer TW, Herczeg-Lisztes E, Funk W, Zillikens D, Biro T, Paus R. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor-beta2/insulin-like growth factor-1-mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br J Dermatol. 2014;171:1031–1043. doi: 10.1111/bjd.13114. [DOI] [PubMed] [Google Scholar]
- 13.Trauer S, Lademann J, Knorr F, Richter H, Liebsch M, Rozycki C, et al. Development of an in vitro modified skin absorption test for the investigation of the follicular penetration pathway of caffeine. Skin Pharmacol Physiol. 2010;23:320–327. doi: 10.1159/000313514. [DOI] [PubMed] [Google Scholar]
- 14.Otberg N, Teichmann A, Rasuljev U, Sinkgraven R, Sterry W, Lademann J. Follicular penetration of topically applied caffeine via a shampoo formulation. Skin Pharmacol Physiol. 2007;20:195–198. doi: 10.1159/000101389. [DOI] [PubMed] [Google Scholar]
- 15.Bussoletti C, Mastropietro F, Tolaini MV, Celleno L. Use of a cosmetic caffeine lotion in the treatment of male androgenetic alopecia. J Appl Cosmetol. 2011;29:167–180. [Google Scholar]
- 16.Norwood OT. Male pattern baldness: classification and incidence. South Med J. 1975;68:1359–1365. doi: 10.1097/00007611-197511000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Serrano-Falcon C, Fernandez-Pugnaire MA, Serrano-Ortega S. Hair and scalp evaluation: the trichogram. Actas Dermosifiliogr. 2013;104:867–876. doi: 10.1016/j.ad.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 18.Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG, CONSORT Group Reporting of noninferiority and equivalence randomized trials. Extension of the CONSORT 2010 statement. JAMA. 2012;308:2594–2604. doi: 10.1001/jama.2012.87802. [DOI] [PubMed] [Google Scholar]
- 19.Rossi A, Cantisani C, Melis L, Iorio A, Scali E, Calvieri S. Minoxidil use in dermatology, side effects and recent patents. Recent Pat Inflamm Allergy Drug Discov. 2012;6:130–136. doi: 10.2174/187221312800166859. [DOI] [PubMed] [Google Scholar]
- 20.DrugsUpdate: Minoxidil information from DrugsUpdate. DrugsUpdatecom. http://www.drugsupdate.com/generic/view/318/Minoxidil (accessed April 21, 2017).