Abstract
Background/Aims
Childhood stunting is a prevalent problem in low- and middle-income countries and is associated with long-term adverse neurodevelopment and health outcomes. In this review, we define indicators of growth, discuss key challenges in their analysis and application, and offer suggestions for indicator selection in clinical research contexts.
Methods
Critical review of the literature.
Results
Linear growth is commonly expressed as length-for-age or height-for-age z-score (HAZ) in comparison to normative growth standards. Conditional HAZ corrects for regression to the mean where growth changes relate to previous status. In longitudinal studies, growth can be expressed as ΔHAZ at 2 time points. Multilevel modeling is preferable when more measurements per individual child are available over time. Height velocity z-score reference standards are available for children under the age of 2 years. Adjusting for covariates or confounders (e.g., birth weight, gestational age, sex, parental height, maternal education, socioeconomic status) is recommended in growth analyses.
Conclusion
The most suitable indicator(s) for linear growth can be selected based on the number of available measurements per child and the child's age. By following a step-by-step algorithm, growth analyses can be precisely and accurately performed to allow for improved comparability within and between studies.
Keywords: Stunting, Anthropometry, Environmental enteric dysfunction, Biomarker, Growth
Introduc.tion
Many children in low- and middle-income countries (LMICs) live in conditions characterized by poverty, in utero insults, inadequate dietary intake, high infectious disease burden, and contaminated environments. These factors can result in poor weight gain and/or a deceleration of linear growth (growth faltering), particularly in the first 2 years of life. A z-score of supine length (in the first 2 years of life) or standing height (≥2 years of age) below −2 is defined as stunting. Stunting is an important risk factor for poor development, reduced educational attainment and adult economic productivity, and noncommunicable illnesses (e.g., diabetes) in adulthood [1, 2]. Despite global reductions in stunting [3], stunting prevalence among children under 5 years of age remains high and in 2011 was 26%, corresponding to 165 million children [4].
While adequate nutrition is an important prerequisite to prevent stunting, nutritional approaches, by themselves, are insufficient. Furthermore, dietary nutrient provision only partially resolves growth failure in resource-poor settings [5]. Interventions beyond traditional dietary approaches, such as those targeting environmental factors, may be critical, for example the availability of basic sanitation and safe water, control of infections, and maternal education and employment [6, 7, 8].
Environmental enteric dysfunction (EED) is thought to be an important underlying cause of stunting in LMICs [9]. It is a condition characterized by small bowel villous blunting and crypt hyperplasia and a T-cell-mediated inflammatory response, likely caused by recurrent or repeated enteric infections or exposure to environments heavily contaminated with fecal organisms. Resultant malabsorption, intestinal permeability leading to bacterial translocation and subsequent local and systemic inflammation can each or in concert result in linear growth faltering with or without effects on ponderal growth. The EED Biomarker Initiative (EEDBI) is a Consortium of 8 projects with cohorts in South America, sub-Saharan Africa, and South Asia and additional laboratory capacity in North America and Europe. The goal of the EEDBI Consortium is to discover and validate biomarkers for EED, especially EED that attenuates linear growth. As such, the work of the Consortium includes identification of associations between candidate biomarkers and linear growth, both within (as part of discovery) and across (as part of validation) individual projects/cohorts.
Many indicators and analytic options are available to use when assessing growth, and in fact many different indicators of growth have been used in research and clinical practice. Based on the context of the EEDBI Consortium, we aimed at reviewing optimal linear growth indicators that can be used in analysis across various populations and research settings. While this paper may have relevance for patient care, its focus is for researchers assessing child growth. We focused on linear growth (rather than on weight) for 3 reasons: (1) stunting is a more tenacious and intractable problem; (2) while processes and etiologies that contribute to weight shortfalls can overlap with those that contribute to linear growth shortfall (e.g., dietary insufficiency), others uniquely and relevantly contribute to linear faltering (e.g., via systemic inflammatory effects on bone growth); and (3) stunting is associated with important long-term adverse neurodevelopment and health outcomes (e.g., chronic noncommunicable disesases).
In the first part of this review, we define indicators of growth and discuss key challenges in the analysis of these measures in children in LMICs. We then describe the various linear growth indicators that can be used in analyses of biomarkers, offer suggestions for indicator selection in various analysis contexts, and discuss their advantages and disadvantages. The considerations in this guidance are applicable to other researchers studying stunting, whether in relation to risk factors, interventions, or biomarkers.
Challenges in the Ascertainment and Analysis of Anthropometric Measures in LMICs
Calculation of Height-for-Age z-Score
A variety of anthropometric indicators are used in clinical, population, and research settings (Table 1). Linear growth in the first 2 years of life is measured as recumbent length and thereafter as standing height, and is usually expressed as a z-score (standard deviation units; also referred to as standard deviation scores [SDS] in clincial studies) for age in comparison with appropriate sex-specific population reference curves. For simplicity, in this document the term “height” is used to refer to both length and height, and z-scores of both are referred to as height-for-age z-score (HAZ).
Table 1.
Commonly used anthropometric indicators
| Anthropometric indicator | Common expression | Use |
|---|---|---|
| Weight-for-age | WAZ <−2 = underweight | Weight is strongly related to height as well as to nutrient intake and health status; thus, low WAZ is a composite measure of stunting and thinness |
| Weight-for-height | WHZ <−2 = wasted | Measure of acute malnutrition, usually as a consequence of food insecurity or severe disease |
| Length-for-age | LAZ <−2 = stunted | Measure of linear growth in 0- to 1.99-year-olds |
| Height-for-age | HAZ <−2 = stunteda | Measure of linear growth in ≥2-year-olds |
| Body mass index | BMIZ | Measure of the degree of leanness or overweight |
| Mid-upper arm circumference | MUAC <115 mm = severe acute malnutrition | Measure of acute malnutrition, especially in emergency settings |
| Head circumference | HCZ | A measure of brain growth; deceleration of which is spared except in instances of severe malnutrition or cerebral injury or disease |
BMIZ, body mass index z-score; HAZ, height-for-age z-score; HCZ, head circumference z-score; LAZ, length-for-age z-score; MUAC, mid-upper arm circumference; WAZ, weight-for-age z-score; WHZ, weight-for-height z-score. a In this paper, as in many others, for convenience we refer to LAZ and HAZ as HAZ.
Though outside of the scope of this article, we wish to emphasize the critical importance of accurate anthropometeric measurements by trained and supervised personnel using standardized methods and equipment [10, 11]. In addition, data quality checks are essential prior to interpreting growth data.
The World Health Organization (WHO) developed global normative growth charts (standards) in 2006 [12, 13]. These charts are based on longitudinal growth patterns of children aged 0–24 months and cross-sectional data from 18- to 71-month-old children from Brazil, Ghana, India, Norway, Oman and the United States, living in favorable environmental conditions, exclusively or predominantly breastfed for at least 4 months, in whom complementary foods were introduced by 6 months of age, and who continued breastfeeding to at least 12 months of age. While there is some disagreement regarding the use of one global standard which does not take into account genetic influences of growth, the WHO growth standards have been commonly adopted for routine use in LMICs as well as in some high-income countries (e.g., the US). Software programs to compute z-scores have been published by the WHO, including macros for several statistical packages (http://www.who.int/childgrowth/software/en). The US Centers for Disease Control and Prevention's (CDC) open access EpiInfo Program allows for z-score calculation using either the WHO or CDC growth standards (www.cdc.gov/epiinfo).
Under optimal conditions, the growth curve demonstrates greatest height velocity in utero (50 cm in 9 months), rapidly declines in the first 2 postnatal years (from 25 to 10.5 cm/year) as well as through childhood to puberty (8.5–5 cm/year), and is followed by a final and relatively short pubertal growth spurt. While average height velocity for age is commonly portrayed as a smooth continuous line, individual child growth is actually a saltatory process which can be depicted if very precise measurements are obtained over short intervals [14].
Pattern of Linear Growth in LMICs
A picture of contemporary growth patterns of 1- to 59-month-olds in LMICs can be gleaned from a review of cross-sectional data from 54 countries, clustered into 5 geographic regions [15]. Average birth weight and length z-scores varied between −0.7 and −1 and growth faltering occurred in the first 24 months of life, followed by stabilization, with considerable HAZ differences between regions (ranging from −0.7 in Europe and Central Asia to −2.2 in South Asia). Data from The Gambia and 5 LMICs within the COHORTS study demonstrated significant recovery (∼0.75 HAZ) between 2 and 5 years of life [16, 17], but this pattern is not universal [15]. Two longitudinal studies showed that after the first or second year of age HAZ recovery was limited, in the order of 0.2 SD [18, 19]. This suggests that the window of opportunity for preventing linear growth faltering usually ends at about 2 years of age.
Age-related growth trajectories imply that the interpretation of growth depends on the age of the child being assessed. For example, in an LMIC setting, a child with a stable length z-score in the first 2 years of life would be considerably better than average, while after 2 years of age a stable height z-score is the expected pattern.
Indicators of Growth
We believe that there is no “gold standard” measure of growth; however, based on study aims and given the availability of measurements, the time intervals between the measurements, and the age at which these are collected, the most suitable indicator(s) should be selected. Growth indicators in infancy and childhood can be studied for different purposes. First, population-based anthropometric surveys can be used to describe national and subnational nutritional status. Second, growth can serve as an independent variable in assessments such as those that have demonstrated growth during the first 2 years of life as a predictor of cognitive and motor development [20, 21], schooling trajectories [22] or various adult consequences [23, 24]. Third, growth indicators can be used as a dependent/outcome variable, for example as an outcome parameter of a nutritional intervention.
A fourth purpose of growth measures is as indicators of clinical disorders, for example of EED in LMICs. Growth indicators can therefore be used as correlates of potential biomarkers of EED (or other conditions causing growth-related outcomes). We recognize the limitations of cross-sectional associations of a single-point biomarker with any parameter of growth, which is of course a longitudinal and multifactorial phenomenon. A single HAZ measured at the same time as a candidate biomarker reflects growth from birth to that time point, but does not provide information about the growth trajectory preceding it over a shorter time. Ideally, a biomarker would be used to predict a child at risk for linear growth shortfalls due to EED or to predict EED recovery in the context of intervention response. However, cautious interpretation is needed, as the duration of the interval to accurately assess changes in linear growth may vary based on etiology and confounding factors.
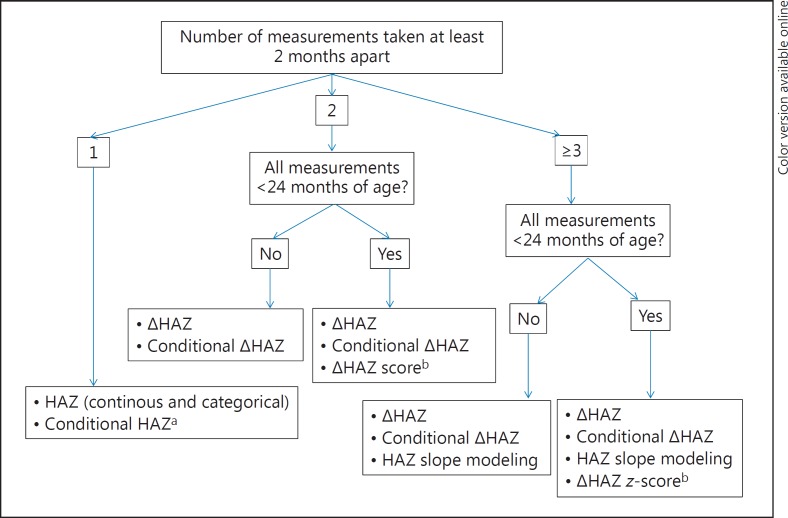
We present an overview of various indicators that can be used in studies of growth in LMICs and suggest the indicator(s) that may customarily provide highest order value. A summary of what we believe are best practices for growth indicator selection is offered in Table 2. Ability to utilize higher-order indicators relies on 2 simple specifications. The first is the number of available observations: 1 height measurement; 2 measurements at least 2 months apart; or more than 2 observations (again, at least 2 months apart). The second specification is whether all anthropometric measurements were carried out before 24 months of age. Our suggested strategy for selecting growth indicators to use in analyses is shown in Figure 1. We advocate a step-by-step approach, from relatively simple to more complicated analyses, depending on available data, age of participants and study objectives. We recommend starting with the basic analyses, i.e., we do not advise skipping forward to the more advanced analyses as the initial, more basic steps are informative and can allow comparison to data published in the literature. For more details about statistical analytical strategies in human growth research, the interested reader is referred to a recent review by Johnson [25].
Table 2.
Summary of best practices for growth indicator selection in biomarker association studies
| Studies | Recommended best practices1 |
|---|---|
| Descriptive analysis | |
| All | a. Perform descriptive analysis of HAZ as mean ± SD and median (interquartile range) using WHO child growth standards. b. Express proportion stunted (i.e., HAZ <−2); consider analysis using more categories if sample size and HAZ spread allows. |
| Analyses to look for biomarker association with growth2 | |
| All | a. Linear regression with HAZ: i. A continuous approach can be used for initial analysis. ii. Categorical approaches are advised for more definitive analysis preferably with more than 2 categories, if sample size and HAZ spread allows. iii. More advanced techniques for analyzing nonlinear relations (e.g., quantile regression, regression trees or generalized additive models) are preferred. b. If birth length (or weight) is known, conditional length can be used as an indicator of postnatal growth. |
| Additionally consider when 2 height measurements at least 2 months apart are available3 | a. Change in z-scores of attained height (ΔHAZ), over a time interval of at least 2 months, adjusted for HAZ at either time 1 (forward analysis) or time 2 (backward analysis). b. Conditional height velocity. |
| Additionally consider in studies with >2 height measurements at least 2 months apart3 | a. Change in z-scores of attained height, over a time interval of 3–6 months, adjusted for HAZ at one of the time points. b. Conditional height velocity. c. Random-effects regression models modeling ΔHAZ. |
HAZ, height-for-age z-score; SD, standard deviation.
Unadjusted and adjusted models should be run.
Principles in this table also apply to weight measurements, although shorter intervals of at least 1-month duration are acceptable when more than one measure is available.
If all measurements are among children <24 months of age, in theory the z-score of the increment in length could be a valuable indicator. However, given the lack of availability of validated statistical tools to calculate conditional velocity z-score (adjusted for baseline status), this approach should be considered experimental at this time.
Fig. 1.
Suggested strategy for linear growth indicator selection for use in analyses according to the number of measurements and maximum age, in ascending order of potential utility (and complexity). Similar strategies are applicable for weight indicators. HAZ, height-for-age z-score. a If birth length or birth weight are known, it is preferable to use these conditional indicators as they adjust for previous growth, the biggest determinant of current growth. Birth length is preferred to birth weight. Early infancy length (or weight) can be used as proxies if birth measures are not available. b While z-score of length velocity may be a theoretically preferred indicator, the current lack of appropriate statistical tools for straightforward calculation of such z-scores prohibit its widespread use.
HAZ as a Continuous Variable
Attained HAZ is a frequently used indicator in cross-sectional and longitudinal studies of growth [18, 26, 27, 28] and in serial cross-sectional assessments of its association with various biomarkers [29, 30, 31].
In such analyses, HAZ cannot be considered as a predictor of EED, but rather as an associated factor present for an unknown duration, possibly including the prenatal phase. It is highly recommended to adjust for independent covariates of growth, of which birth size is probably the most important. This is true for all indicators discussed in this paper, but it is particularly relevant for HAZ, since it does not take into account growth trajectory over time. Based on HAZ and birth weight SDS, the residual postnatal increase in height z-score can be calculated (see conditional height below).
Advantages of HAZ include its ease of calculation, and its extensive use in the literature, allowing for comparison to other studies that have used this approach. It is also easy to use in linear regression analyses. Disadvantages of a single HAZ include that it is a static, cross-sectional measure that does not capture longitudinal growth. HAZ expressed as a continuous variable is particularly useful in initial exploratory analyses, so that each cohort interrogation should ideally start with calculations of HAZ, using WHO standards (and computerized tools, e.g., http://www.who.int/childgrowth/software/en/). z-scores provide a common reference against which cohort-specific growth can be assessed. Reporting of summary statistics should be conveyed as means and standard deviations (SD) or medians and interquartile ranges of HAZ. The analysis can be stratified (e.g., by age and sex), if appropriate.
While HAZ expression as a continuous variable is a good starting point, it should be noted that it confers as much emphasis to values across the “normal” range (e.g., HAZ greater than −1 or 0) as it does to values at the lower extremes of clinical concern (e.g., HAZ lower than −2 or −3). That is to say, the dynamic range of growth related to pathology might actually not be linear. This means that there is often a range of values across which there is little or no variation in the occurrence of the adverse health outcome of interest. Drawing an example from another field, antihypertensives might have only an infinitesimal effect on reducing stroke risk in individuals with systolic blood pressures in the 100–130 mm Hg range, but have a profoundly greater effect among those blood pressures from 140 to 180 mm Hg. Similarly, intervening to prevent serious illness or death is unlikely to be very effective among children with HAZ scores of 0 or −1. Still, even in the range that is classified as “normal”, there could be negative health consequences, if the whole population distribution is shifted [32].
HAZ as a Categorical Variable
For each child, at any point in time, length cannot only be expressed as a continous variable HAZ, but this HAZ can also be categorized, in the sense that categories are defined based on values of HAZ at a predefined threshold.
Proportions of children with stunting (HAZ lower than −2), as well as underweight (weight-for-age z-score [WAZ] lower than −2) and wasting (weight-for-height z-score lower than −2) (Table 1) are commonly used to describe the nutritional status of a population, for example from nutritional surveys. The main advantage of these indicators is that they have been widely used, so that results can be easily compared to prior studies (if the same reference/standard is used). Often, this categorical representation is reported in combination with mean HAZ.
However, dichotomizing populations into stunted versus nonstunted ignores important variations in HAZ. The main disadvantages of categorical representations of HAZ are that they decrease potential inferential power because of the inability to capture total HAZ variation, and they use an arbitrary (even if conventional) cutoff point to define groups (i.e., the expected severity of stunting does not meaningfully differ between 2 infants with length z-scores of −1.95 and −2.05, but they would be assigned to 2 different categories). Further, dichotomous expression would not differentiate the biologic magnitude of very severe stunting (e.g., HAZ lower than −4) compared to moderate stunting (e.g., HAZ ≥3 and lower than −2).
Multilevel categorization can mitigate this issue. For example, some studies have defined 3 classes: greater than or equal to −2 (“normal”), −2.01 to −3.0 (moderate stunting), and lower than −3 (severe stunting) [33, 34, 35, 36]. Other studies have distinguished an additional category (very severe stunting defined as HAZ lower than −4) [37]. While dichotomous assessment is a usual first step in analysis (e.g., comparing stunted to nonstunted), analysis of more than 2 HAZ categories is preferred if sample size and HAZ spread permit, because it enables a more nuanced assessment of the effect of different grades of stunting severity.
While adjacent growth categories are typically contrasted (e.g., stunted versus nonstunted), comparisons of noncontiguous growth categories can generate better separation between diseased and healthy children. For example, a recent study from Zimbabwe compared insulin-like growth factor I and inflammatory markers between stunted (HAZ lower than −2.0) infants to nonstunted controls defined as HAZ greater than −0.5 [38].
While we acknowledge that categorization is still commonly used, and may be appropriate for public health surveillance, we believe that from a purely methodological point of view categorization of continuous data, in particular dichotomization, is unnecessary if specific statistical methods, such as quantile regression, semiparametric regression, regression trees and general additive smoothing models, are utilized as alternatives for assessing nonlinear relations with continuous HAZ [39]. However, discretization into categories (e.g., 3) could still be effective in aiding the communication of such regression results [40, 41].
Categories of stunting or continuous HAZ can be compared with a categorical or continuous distribution of a biomarker or risk factor, which might be most meaningful at extremes of abnormality, as it can be used to focus on children within ranges that are most likely to be related to a consequential host process or disease.
Conditional HAZ
Height (and weight) at any age are associated with birth length and weight, as well as with prior measurements at younger ages. To analyze the effect of postnatal pathologic processes such as EED on growth, conditional height can be used, which is defined as current height accounting for previous height(s). In such analyses, standardized residuals are estimated by regressing current HAZ on all previous measures to produce conditional measures, using the pertinent study sample for calculating HAZ, in lieu of national reference charts or international standards (e.g., WHO charts).
Thus, conditional height represents children's deviation from the expected size based on their own previous measures and the growth of other children in the pertinent cohort, and thus represents a child's deviation from his or her expected size given the pattern of growth in that population. For example, a child with a positive value for mid-childhood conditional height is taller than expected in view of previous size and thus had a faster rate of linear growth than would be expected. This approach has been used in a number of studies [42, 43], and a similar approach was used in a multicountry study examining factors that influence growth [1, 17, 23, 44]. This approach is not only suitable for studies of the association of early growth on adult consequences, but also for separating prenatal from postnatal growth in a certain age interval, or for interpreting longitudinal growth in a time interval preceding or following biomarker measurement, as illustrated in an analysis of 5 birth cohort studies [1].
The advantages of conditional HAZ include that it corrects for regression to the mean where changes in growth may be appreciably related to previous status. It requires at least 2 measurements at least 2 months apart, but longer intervals and additional measurements are desirable, in view of saltatory growth patterns, which can introduce ascertainment artifacts.
Change in z-Scores of Attained Height
Growth of a group of children can be expressed as change in height in centimeters, but this is only useful if the age interval and age at start are similar among all subjects. In general, it is better to first calculate HAZ and then calculate the difference between HAZ at 2 time points. This has been used particularly in intervention studies, for example in trials examining the impact of education, zinc [45] or complementary feeding [46, 47] on growth. In a study on the possible predictive value for motor development [48], HAZ at 6 months minus length at birth was used as a marker of postnatal stunting. The general pattern of longitudinal growth in LMICs (growth faltering in the first 2 years, followed by stabilization) implies that the change in HAZ is primarily useful in this age range, where interventions to mitigate growth faltering are particularly important [30]. The main advantages of this method is that it reflects a dynamic assessment of growth and can be used over the whole growth trajectory, although the same difference in z-score may not necessarily have the same effect or meaning at different ages.
The primary disadvantage of this method (if the correlation between 2 consecutive measurements is not considered) is that it does not provide information about the normality of an observed change. For example, ΔHAZ = 0 can be appropriate if HAZ is normal or high for genetic target or inappropriate if HAZ is below target. In a relatively long infant (e.g., HAZ >2), HAZ may slightly decrease with time because of regression to the mean and relatively short parents, while a decrease of identical magnitude may be quite deleterious for the already stunted infant. For example, the biological meaning of ΔHAZ = 0 over the preceding 6 months for a 1.9-year-old child with a present HAZ of −2.5 might be different than for a child with a HAZ = 0. A further disadvantage is that this marker has an “asymmetric” range: positive changes in HAZ have biologic limitations and mostly occur if the child is in a situation where catch-up growth is possible, while negative changes occur within a greater range.
When this growth indicator over a time period from T1 to T2 is used in biomarker studies, it is useful to adjust for HAZ at either T1 or T2. In a forward analysis, aimed at investigating if the biomarker at T1 is predictive for growth in the subsequent time interval, the change in HAZ is adjusted for HAZ at T1. This was, for example, performed in a recent study, showing that a fecal biomarker of environmental enteropathy (myeloperoxidase) was predictive for subsequent 3-month growth [49]. In a backward analysis, the change in HAZ is adjusted for HAZ at T2.
The proper interpretation of ΔHAZ requires a further mathematical step as described by Cole [50]. Cole, who used the term standard deviation score (SDS) instead of z-score, first explained that “in the reference population the mean of ΔSDS = 0 and the SD of ΔSDS = √2(1 – r), where r is the correlation between SDS1 and SDS2. This allows ΔSDS to be expressed as an SD score for SD change: SD score for ΔSDS: ΔSDS/√2(1 – r).” The value of r varies with the measurement interval. At short intervals, for example from 5 to 6 years of age in a French longitudinal study, the r was 0.981. In a later paper, Cole [51] elaborated more on this method and introduced the concept of “thrive lines.” This method was also used to compare weight gain in sudden infant death syndrome versus controls [52]. This method presents the important conceptual issue of the impact of correlated measurements in evaluating growth increments. A disadvantage is that the additional manipulations of data require fairly sophisticated statistical techniques.
The z-Score of the Change in Height
An expression of height velocity (if corrected for age and sex) represents an attractive measure of growth in longitudinal studies. For example, urinary lactulose: mannitol and fecal neopterin were correlated with growth rate, expressed as centimeters/month, and corrected for age-related changes [53, 54]. Similarly, height velocity (in cm/year) and HAZ were used as outcome parameters in a study on the impact of micronutrient fortification of yogurt [55], and in a recent Indian study, growth velocity (in cm/month) was associated with maternal height and exclusive breastfeeding [56].
However, given the impact of age and time intervals on height velocity, growth velocity as a z-score would theoretically better portray a more symmetrical parameter of growth velocity than crude differences in HAZ, because height velocity can be positive or negative with equivalent likelihoods. In fact, the WHO has published tables allowing comparison of length (cm) and weight increments (kg) at certain age intervals with WHO reference data. These values are provided for intervals of 2, 3, 4, and 6 months (note that 1-month interval values are also provided for the z-score of weight velocity). This approach was used to show that child mortality is predicted by nutritional status (BMI-for-age) and recent weight velocity in children under 2 in rural Africa [36]. Weight increment values between the first and second contact were linearly extrapolated to the exact target interval of 3 months. The authors developed an SPSS syntax macro for calculating z-scores for weight velocity. In developing that macro, they followed the z-score calculation algorithm suggested by the WHO. In a study using the same study sample, Schwinger et al. [57] showed that despite their higher variability weight and length velocity z-scores were better to predict death within the next 3 months than length-for-age z-score or WAZ, even without taking nutritional status at the beginning of the assessment period into account. The predictive value improved after conditioning on WAZ or length-for-age z-score at T1. Another recent study showed that successive 1-month weight increments in infancy can be used to screen for faltering linear growth [58].
Despite the theoretical advantages of this method, there are important disadvantages. It is complicated to calculate the z-score if the age is between an integer number of months (e.g., 2.4 months) or if the measurement interval is not one used in the WHO reference set (e.g., 2.5 or 5 months). In such cases, the z-score must be interpolated using starting age and time interval, which is only feasible with a suitable computer algorithm. Currently, the statistical tools to calculate z-scores of growth velocity (e.g., SPSS macros) are still under development. Furthermore, the calculation of height velocity z-score is only currently possible for anthropometric measures obtained before age 24 months because WHO reference standard values for older children are not available.
As with other indicators, this growth velocity measure is influenced by baseline HAZ, genetic factors and regression to the mean. For example, for a boy to maintain a HAZ of −2 from 12 to 24 months of age, a length velocity of 10.7 cm/year is required, while 13.4 cm/year is need ed to maintain a HAZ of +2 (http://www.who.int/childgrowth/standards/LFA_boys_0_2_zscores.pdf?ua=1). A potential improvement considering this approach could be achieved by using regression models to calculate conditional velocity z-scores [51]. However, because the longitudinal correlation structure of the WHO standards is unpublished, such conditional velocity z-scores are not an option at this time. We conclude that at the present time, this approach is not sufficiently mature to be used widely.
HAZ Slope Modeling
When multiple measurements are available (at least 3, but preferably 4 or more [59]) during a time period of interest, multilevel modeling of longitudinal growth data provides an opportunity to take a more detailed account of the growth trajectories and smooth out irregularities. Essentially, ΔHAZ is then estimated by fitting a line through the points, and using the slope for the individual as the measure of change. The change in HAZ is usually nonlinear, particularly if there is a large discrepancy between birth size and genetic target. For example, in fully breastfed babies with low birth weights and tall parents, ΔHAZ can increase in the first 6 months and then decrease because of poor nutritional intake, disease, and/or environmental factors. A linear regression through these measurements would result in a slope of 0, which would not reflect the actual clinical course. Fitting of curvilinear patterns to growth can capture nonlinear aspects, but requires biostatistical sophistication.
Many different modeling approaches have been described including linear spline, complex spline functions, and fractional polynomial linear regression models. For example, in a recent study, 6 models were fitted to growth data from children in an urban African setting aged 0–10 years using this technique, showing that the Berkey-Reed model fitted well beyond infancy into childhood [60], and the technique of fitting mean growth curves using fractional polynomial linear regression models was described in a Vietnamese study [61]. In a study aimed at finding critical periods for the development of later overweight, the “broken stick model” was proposed [62]. In an analysis of growth and disease surveillance data from 7 cohort studies, an association between age-specific diarrhea burden and modeled growth velocity in length and weight was measured [63]. Diarrhea was cross-sectionally associated with slower linear and ponderal growth, and this was followed by a period of catch-up growth. For this purpose, growth was modeled as a piecewise linear function of age with knots at 3, 6, 12, and 18 months. After adding various covariates, diarrhea burden was added to the model as an interaction term with age for each of the time periods. The advantages and disadvantages of the linear spline approach compared with other growth modeling methods such as fractional polynomials, more complex spline functions, and other nonlinear models have recently been discussed [64].
A similar result could be accomplished by random-effects regression modeling, such as was done in a mixed model for repeated measurements in a study of REG1B as a predictor of childhood stunting in Bangladesh and Peru [65] and in a study on the association of fecal markers of intestinal inflammation and permeability on subsequent acquisition of linear growth deficits in infants [66]. In a recent paper, several methods for analyzing longitudinal growth to evaluate both short- and long-term associations between risk factors and growth trajectories over the first 2 years of life in the MAL-ED study were described [67]. We believe that random-effects regression modeling is most appropriate for studying the association between a biomarker and growth, although the disadvantage is that it needs great computational effort and due to higher complexity, results can be unstable if sample size is not sufficient. Additional adjustment for baseline HAZ, birth weight and maternal height might offer further improvements in predictive analyses.
Potential Covariates and Confounders
Given the many factors that influence growth and the relatively imprecise nature of its assessment, one would expect that the strength of association of any single growth parameter with a disease biomarker will likely be weak. Therefore, for most analyses of growth, whatever the indicator used, it is important to include potential determinants of growth that are not directly associated with the pathological condition of interest (e.g., EED). In other words, it is appropriate to adjust for covariates or confounders.
As discussed above, preceding growth status is an important, if not the most important, predictor of future growth. As such, birth length represents an important factor when assessing biomarker (or intervention or risk factor) association with linear growth outcomes. However, as this is usually not available in field conditions, birth weight can be used as a proxy. If birth weight is unavailable, WAZ (or even better, HAZ) in early infancy (e.g., before the age of 3 months) can be used as a proxy for birth length [37]. Gestational age is another useful parameter, so that preterm babies can be distinguished from babies born small for gestational age (SGA), defined as birth length and/or birth weight below the 3rd percentile. However, in LMICs, good estimates of gestational age are often difficult to obtain. In high-income countries, approximately 80% of SGA born babies show catch-up growth in the sense that their HAZ becomes greater than −2 [68], but their mean adult height is still an estimated 3.6–4.0 cm lower compared to those born with lengths appropiate for gestational age [69]. A low birth length for gestational age may have a stronger effect on adult height than a low birth weight [70].
Fetal growth restriction is common in LMICs; in a recent review, it was estimated that 27% of all neonates in LMICs were born SGA in 2010 [4]. Fetal growth restriction strongly contributes to stunting, underweight and wasting [71, 72, 73]. Relative to children born appropiate for gestational age and at term, the mean odds ratio for stunting associated with preterm and/or SGA birth was 1.9–4.5; similar magnitudes of risk were also observed for wasting and underweight. The population attributable risk related to overall SGA on outcomes of childhood stunting and wasting was 20 and 30%, respectively [72]. This implies that a child's height is influenced by prenatal as well as postnatal factors. Indeed, in a study in Guatemala, prenatal and postnatal growth were equally important determinants of height, weight and fat-free mass [42].
Another relevant determinant is parental height, which can be used as a proxy parameter of genetic factors influencing height. However, in LMICs, the contribution of nongenetic factors on adult height is probably larger than in other countries, and the treatment of parental height as a biological factor in studies of birth weight and childhood growth is complex [74]. The proportion of variability explained by mid-parental height in the WHO Multicentre Gowth Reference Study ranged from 11% in Ghana to 21% in India, higher than maternal or paternal height alone [75]. Prepregnancy maternal height is a composite indicator representing genetic and environmental effects on the growing period of childhood [4] and is a strong determinant of low birth weight, SGA, and child stunting [72, 76, 77, 78]. BMI and maternal weight gain during pregnancy are also associated with birth weight [72]. There may also be an intergenerational cycle of growth failure that links small maternal size to her mother's stature and birth weight, and her growth in childhood [79, 80, 81, 82].
Other important contributors to stunting may include low maternal education and socioeconomic status, poor breastfeeding and responsive feeding practices, and, of course, food insecurity. Age and sex are other critical factors. Even though age- and sex-specific HAZ standards are utilized, residual relationships often remain because the sample does not precisely comport with reference standards.
Factors contributing to stunting will vary between and within countries. Therefore, a standard set of covariates to be used across all settings may not be appropriate. We encourage adjustment and stratification analysis plans based on setting-specific factors, data availability, and study aims.
Conclusions
There are many challenges in the analysis of linear growth of children in LMICs. Difficulties include accurate measurement and recording of anthropometric data, careful assessment of data quality, the irregularity of linear growth, uncertainties about the causality and pathophysiology of stunting and their variation across settings, and multiple potential covariates and confounders. This has led to a lack of a standardized approach for growth analysis.
With regard to the interval over which growth is measured, ideally, balance should be sought between 2 objectives: the interval should be long enough to minimize the effect of measurement errors and short enough to both detect fast changing features of growth and reflect biomarker (or risk factor or intervention) effect. The choice of the interval is thus a trade-off between noise (measurement error) on the one hand and signal (ability to detect growth faltering) on the other [51]. Age is a critical consideration, because in infancy, length measurement inaccuracy is more pronounced than for older children, and length velocity is much greater. We speculate that for the age group older than 12 months, linear growth is best assessed over a time interval of at least 6 months to allow for detection of significantly measurable velocity. Before 12 months of age, we propose that 3- to 4-month intervals are best used, possibly augmented with 1 or 2 extra measurements in the first 3 months. We suggest that investigators model various intervals to find the most suitable time points for their respective studies and cohorts.
For studies aimed at associating growth with a biomarker, HAZ is a static measure resulting from both prenatal and postnatal growth. If birth length (or at least birth weight) is known, conditional length can be calculated, and used as a surrogate of postnatal growth. If more than one measure of an anthropometric parameter is available, an indicator of growth over a time interval can be calculated. For an analysis of the association between environmental factors (e.g., EED) and growth, modeling techniques are probably the best approach (Fig. 1).
Despite the challenges in assessment and interpretation of early child growth, several methods are available, and by following a step-by-step algorithm, as proposed in this paper, the precision, accuracy, and comparibility of growth assessment analyses can be improved.
Disclosure Statement
The authors have nothing to disclose.
Author Contributions
J.M.W., J.H.H., D.M.D., and P.S.S. were responsible for the study concept and design; J.M.W., D.M.D., and P.S.S. drafted the various versions of the manuscript, which was critically revised by all authors. All authors approved the submitted manuscript.
Acknowledgments
The work on this review was funded by the Bill & Melinda Gates Foundation. The findings and conclusions contained in this report are those of the authors and do not necessarily reflect positions of the Bill & Melinda Gates Foundation. We thank Drs. Elizabeth Lundeen, Phillip Tarr, Kelley Van Buskirk, and Catherine Schwinger for helpful comments.
References
- 1.Adair LS, Fall CH, Osmond C, Stein AD, Martorell R, Ramirez-Zea M, Sachdev HS, Dahly DL, Bas I, Norris SA, Micklesfield L, Hallal P, Victora CG. Associations of linear growth and relative weight gain during early life with adult health and human capital in countries of low and middle income: findings from five birth cohort studies. Lancet. 2013;382:525–534. doi: 10.1016/S0140-6736(13)60103-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perez-Escamilla R. Post-1000 days growth trajectories and child cognitive development in low- and middle-income countries. Am J Clin Nutr. 2013;98:1375–1376. doi: 10.3945/ajcn.113.074757. [DOI] [PubMed] [Google Scholar]
- 3.Stevens GA, Finucane MM, Paciorek CJ, Flaxman SR, White RA, Donner AJ, Ezzati M. Trends in mild, moderate, and severe stunting and underweight, and progress towards MDG 1 in 141 developing countries: a systematic analysis of population representative data. Lancet. 2012;380:824–834. doi: 10.1016/S0140-6736(12)60647-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, Uauy R. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 5.Dewey KG, Adu-Afarwuah S. Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Matern Child Nutr. 2008;4((suppl 1)):24–85. doi: 10.1111/j.1740-8709.2007.00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remans R, Pronyk PM, Fanzo JC, Chen J, Palm CA, Nemser B, Muniz M, Radunsky A, Abay AH, Coulibaly M, Mensah-Homiah J, Wagah M, An X, Mwaura C, Quintana E, Somers MA, Sanchez PA, Sachs SE, McArthur JW, Sachs JD, Millennium Villages Study Group Multisector intervention to accelerate reductions in child stunting: an observational study from 9 sub-Saharan African countries. Am J Clin Nutr. 2011;94:1632–1642. doi: 10.3945/ajcn.111.020099. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt CW. Beyond malnutrition: the role of sanitation in stunted growth. Environ Health Perspect. 2014;122:A298–A303. doi: 10.1289/ehp.122-A298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prendergast AJ, Humphrey JH. Stunting persists despite optimal feeding: are toilets part of the solution? Nestle Nutr Inst Workshop Ser. 2015;81:99–110. doi: 10.1159/000365807. [DOI] [PubMed] [Google Scholar]
- 9.Keusch GT, Rosenberg IH, Denno DM, Duggan C, Guerrant RL, Lavery JV, Tarr PI, Ward HD, Black RE, Nataro JP, Ryan ET, Bhutta ZA, Coovadia H, Lima A, Ramakrishna B, Zaidi AK, Burgess DC, Brewer T. Implications of acquired environmental enteric dysfunction for growth and stunting in infants and children living in low- and middle-income countries. Food Nutr Bull. 2013;34:357–364. doi: 10.1177/156482651303400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cogill B. Anthropometric Indicators Measurement Guide. Washington: Food and Nutrition Technical Assistance (FANTA) Project; 2003. [Google Scholar]
- 11.World Health Organization . Training Course on Child Growth Assessment. Geneva: World Health Organization; 2008. [Google Scholar]
- 12.WHO Multicentre Growth Reference Study Group WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- 13.de Onis M, Onyango A, Borghi E, Siyam A, Blossner M, Lutter C. Worldwide implementation of the WHO Child Growth Standards. Public Health Nutr. 2012;15:1603–1610. doi: 10.1017/S136898001200105X. [DOI] [PubMed] [Google Scholar]
- 14.Heinrichs C, Munson PJ, Counts DR, Cutler GB, Jr, Baron J. Patterns of human growth. Science. 1995;268:442–447. doi: 10.1126/science.7716552. [DOI] [PubMed] [Google Scholar]
- 15.Victora CG, de Onis M, Hallal PC, Blossner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–e80. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- 16.Prentice AM, Moore SE, Fulford AJ. Growth faltering in low-income countries. World Rev Nutr Diet. 2013;106:90–99. doi: 10.1159/000342563. [DOI] [PubMed] [Google Scholar]
- 17.Stein AD, Wang M, Martorell R, Norris SA, Adair LS, Bas I, Sachdev HS, Bhargava SK, Fall CH, Gigante DP, Victora CG. Growth patterns in early childhood and final attained stature: data from five birth cohorts from low- and middle-income countries. Am J Hum Biol. 2010;22:353–359. doi: 10.1002/ajhb.20998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundeen EA, Behrman JR, Crookston BT, Dearden KA, Engle P, Georgiadis A, Penny ME, Stein AD. Growth faltering and recovery in children aged 1–8 years in four low- and middle-income countries: young lives. Public Health Nutr. 2014;17:2131–2137. doi: 10.1017/S1368980013003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adair LS. Filipino children exhibit catch-up growth from age 2 to 12 years. J Nutr. 1999;129:1140–1148. doi: 10.1093/jn/129.6.1140. [DOI] [PubMed] [Google Scholar]
- 20.Siegel EH, Stoltzfus RJ, Kariger PK, Katz J, Khatry SK, LeClerq SC, Pollitt E, Tielsch JM. Growth indices, anemia, and diet independently predict motor milestone acquisition of infants in south central Nepal. J Nutr. 2005;135:2840–2844. doi: 10.1093/jn/135.12.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sudfeld CR, Charles MD, Danaei G, Fink G, Ezzati M, Andrews KG, Fawzi WW. Linear growth and child development in low- and middle-income countries: a meta-analysis. Pediatrics. 2015;135:e1266–e1275. doi: 10.1542/peds.2014-3111. [DOI] [PubMed] [Google Scholar]
- 22.Daniels MC, Adair LS. Growth in young Filipino children predicts schooling trajectories through high school. J Nutr. 2004;134:1439–1446. doi: 10.1093/jn/134.6.1439. [DOI] [PubMed] [Google Scholar]
- 23.Hoddinott J, Behrman JR, Maluccio JA, Melgar P, Quisumbing AR, Ramirez-Zea M, Stein AD, Yount KM, Martorell R. Adult consequences of growth failure in early childhood. Am J Clin Nutr. 2013;98:1170–1178. doi: 10.3945/ajcn.113.064584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu YK, Tilling K, Sterne JA, Gilthorpe MS. A critical evaluation of statistical approaches to examining the role of growth trajectories in the developmental origins of health and disease. Int J Epidemiol. 2013;42:1327–1339. doi: 10.1093/ije/dyt157. [DOI] [PubMed] [Google Scholar]
- 25.Johnson W. Analytical strategies in human growth research. Am J Hum Biol. 2015;27:69–83. doi: 10.1002/ajhb.22589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prentice AM, Ward KA, Goldberg GR, Jarjou LM, Moore SE, Fulford AJ, Prentice A. Critical windows for nutritional interventions against stunting. Am J Clin Nutr. 2013;97:911–918. doi: 10.3945/ajcn.112.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis. 2014;59((Suppl 4)):S193–S206. doi: 10.1093/cid/ciu653. [DOI] [PubMed] [Google Scholar]
- 28.Krishna A, Oh J, Lee JK, Lee HY, Perkins JM, Heo J, Ro YS, Subramanian SV. Short-term and long-term associations between household wealth and physical growth: a cross-comparative analysis of children from four low- and middle-income countries. Glob Health Action. 2015;8:26523. doi: 10.3402/gha.v8.26523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olusanya BO, Renner JK. Pattern and characteristics of growth faltering in early infancy in an urban sub-Saharan African setting. Pediatr Neonatol. 2013;54:119–127. doi: 10.1016/j.pedneo.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Lin A, Arnold BF, Afreen S, Goto R, Huda TM, Haque R, Raqib R, Unicomb L, Ahmed T, Colford JM, Jr, Luby SP. Household environmental conditions are associated with enteropathy and impaired growth in rural Bangladesh. Am J Trop Med Hyg. 2013;89:130–137. doi: 10.4269/ajtmh.12-0629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu JR, Sheng XY, Hu YQ, Yu XG, Westcott JE, Miller LV, Krebs NF, Hambidge KM. Fecal calprotectin levels are higher in rural than in urban Chinese infants and negatively associated with growth. BMC Pediatr. 2012;12:129. doi: 10.1186/1471-2431-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Briend A, Van den Broeck J, Fadnes LT. Target weight gain for moderately wasted children during supplementation interventions – a populations-based approach. Public Health Nutr. 2011;14:2117–2123. doi: 10.1017/S1368980011001777. [DOI] [PubMed] [Google Scholar]
- 33.Mendez MA, Adair LS. Severity and timing of stunting in the first two years of life affect performance on cognitive tests in late childhood. J Nutr. 1999;129:1555–1562. doi: 10.1093/jn/129.8.1555. [DOI] [PubMed] [Google Scholar]
- 34.Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, Ashorn P. Complementary feeding with fortified spread and incidence of severe stunting in 6- to 18-month-old rural Malawians. Arch Pediatr Adolesc Med. 2008;162:619–626. doi: 10.1001/archpedi.162.7.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, Ashorn P. Postintervention growth of Malawian children who received 12-mo dietary complementation with a lipid-based nutrient supplement or maize-soy flour. Am J Clin Nutr. 2009;89:382–390. doi: 10.3945/ajcn.2008.26483. [DOI] [PubMed] [Google Scholar]
- 36.O’Neill SM, Fitzgerald A, Briend A, Van den Broeck J. Child mortality as predicted by nutritional status and recent weight velocity in children under two in rural Africa. J Nutr. 2012;142:520–525. doi: 10.3945/jn.111.151878. [DOI] [PubMed] [Google Scholar]
- 37.Maleta K, Virtanen SM, Espo M, Kulmala T, Ashorn P. Childhood malnutrition and its predictors in rural Malawi. Paediatr Perinat Epidemiol. 2003;17:384–390. doi: 10.1046/j.1365-3016.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- 38.Prendergast AJ, Rukobo S, Chasekwa B, Mutasa K, Ntozini R, Mbuya MN, Jones A, Moulton LH, Stoltzfus RJ, Humphrey JH. Stunting is characterized by chronic inflammation in zimbabwean infants. PLoS One. 2014;9:e86928. doi: 10.1371/journal.pone.0086928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Royston P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 40.Rosyton P, Altman DG, Sauerbrei W. Dichotomizing continuous predictors in multiple regression: a bad idea. Stat Med. 2006;25:127–141. doi: 10.1002/sim.2331. [DOI] [PubMed] [Google Scholar]
- 41.Gelman A, Park DK. Splitting a predictor at the upper quarter or third and the lower quarter or third. Am Stat. 2008;62:1–8. [Google Scholar]
- 42.Li H, Stein AD, Barnhart HX, Ramakrishnan U, Martorell R. Associations between prenatal and postnatal growth and adult body size and composition. Am J Clin Nutr. 2003;77:1498–1505. doi: 10.1093/ajcn/77.6.1498. [DOI] [PubMed] [Google Scholar]
- 43.Keijzer-Veen MG, Euser AM, van Montfoort N, Dekker FW, Vandenbroucke JP, Van Houwelingen HC. A regression model with unexplained residuals was preferred in the analysis of the fetal origins of adult diseases hypothesis. J Clin Epidemiol. 2005;58:1320–1324. doi: 10.1016/j.jclinepi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Stein AD, Barros FC, Bhargava SK, Hao W, Horta BL, Lee N, Kuzawa CW, Martorell R, Ramji S, Stein A, Richter L. Birth status, child growth, and adult outcomes in low- and middle-income countries. J Pediatr. 2013;163:1740–1746. doi: 10.1016/j.jpeds.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2002;75:1062–1071. doi: 10.1093/ajcn/75.6.1062. [DOI] [PubMed] [Google Scholar]
- 46.Thakwalakwa CM, Ashorn P, Jawati M, Phuka JC, Cheung YB, Maleta KM. An effectiveness trial showed lipid-based nutrient supplementation but not corn-soya blend offered a modest benefit in weight gain among 6- to 18-month-old underweight children in rural Malawi. Public Health Nutr. 2012;15:1755–1762. doi: 10.1017/S1368980012003023. [DOI] [PubMed] [Google Scholar]
- 47.Lassi ZS, Das JK, Zahid G, Imdad A, Bhutta ZA. Impact of education and provision of complementary feeding on growth and morbidity in children less than 2 years of age in developing countries: a systematic review. BMC Public Health. 2013;13((suppl 3)):S13. doi: 10.1186/1471-2458-13-S3-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheung YB, Yip PS, Karlberg JP. Fetal growth, early postnatal growth and motor development in Pakistani infants. Int J Epidemiol. 2001;30:66–72. doi: 10.1093/ije/30.1.66. [DOI] [PubMed] [Google Scholar]
- 49.Arndt MB, Richardson BA, Ahmed T, Mahfuz M, Haque R, John-Stewart GC, Denno DM, Petri WA, Jr, Kosek M, Walson JL, in coordination with the MAL-ED Network Project Fecal markers of environmental enteropathy and subsequent growth in Bangladeshi children. Am J Trop Med Hyg. 2016;95:694–701. doi: 10.4269/ajtmh.16-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cole TJ. Growth charts for both cross-sectional and longitudinal data. Stat Med. 1994;13:2477–2492. doi: 10.1002/sim.4780132311. [DOI] [PubMed] [Google Scholar]
- 51.Cole TJ. Presenting information on growth distance and conditional velocity in one chart: practical issues of chart design. Stat Med. 1998;17:2697–2707. doi: 10.1002/(sici)1097-0258(19981215)17:23<2697::aid-sim36>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 52.Blair PS, Nadin P, Cole TJ, Fleming PJ, Smith IJ, Platt MW, Berry PJ, Golding J. Weight gain and sudden infant death syndrome: changes in weight z scores may identify infants at increased risk. Arch Dis Child. 2000;82:462–469. doi: 10.1136/adc.82.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet. 1991;338:907–910. doi: 10.1016/0140-6736(91)91772-m. [DOI] [PubMed] [Google Scholar]
- 54.Campbell DI, McPhail G, Lunn PG, Elia M, Jeffries DJ. Intestinal inflammation measured by fecal neopterin in Gambian children with enteropathy: association with growth failure, Giardia lamblia, and intestinal permeability. J Pediatr Gastroenterol Nutr. 2004;39:153–157. doi: 10.1097/00005176-200408000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Sazawal S, Habib A, Dhingra U, Dutta A, Dhingra P, Sarkar A, Deb S, Alam J, Husna A, Black RE. Impact of micronutrient fortification of yoghurt on micronutrient status markers and growth – a randomized double blind controlled trial among school children in Bangladesh. BMC Public Health. 2013;13:514. doi: 10.1186/1471-2458-13-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kattula D, Sarkar R, Sivarathinaswamy P, Velusamy V, Venugopal S, Naumova EN, Muliyil J, Ward H, Kang G. The first 1,000 days of life: prenatal and postnatal risk factors for morbidity and growth in a birth cohort in southern India. BMJ Open. 2014;4:e005404. doi: 10.1136/bmjopen-2014-005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwinger C, Fadnes LT, Van den Broeck J. Using growth velocity to predict child mortality. Am J Clin Nutr. 2016;103:801–807. doi: 10.3945/ajcn.115.118679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Onyango AW, Borghi E, de OM, Frongillo EA, Victora CG, Dewey KG, Lartey A, Bhandari N, Baerug A, Garza C. Successive 1-month weight increments in infancy can be used to screen for faltering linear growth. J Nutr. 2015;145:2725–2731. doi: 10.3945/jn.115.211896. [DOI] [PubMed] [Google Scholar]
- 59.Curran PJ, Obeidat K, Losardo D. Twelve frequently asked questions about growth curve modeling. J Cogn Dev. 2010;11:121–136. doi: 10.1080/15248371003699969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chirwa ED, Griffiths PL, Maleta K, Norris SA, Cameron N. Multi-level modelling of longitudinal child growth data from the Birth-to-Twenty Cohort: a comparison of growth models. Ann Hum Biol. 2014;41:166–177. doi: 10.3109/03014460.2013.839742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nguyen HT, Eriksson B, Petzold M, Bondjers G, Tran TK, Nguyen LT, Ascher H. Factors associated with physical growth of children during the first two years of life in rural and urban areas of Vietnam. BMC Pediatr. 2013;13:149. doi: 10.1186/1471-2431-13-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Kroon ML, Renders CM, Van Wouwe JP, van Buuren S, Hirasing RA. The Terneuzen Birth Cohort: BMI change between 2 and 6 years is most predictive of adult cardiometabolic risk. PLoS One. 2010;5:e13966. doi: 10.1371/journal.pone.0013966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Richard SA, Black RE, Gilman RH, Guerrant RL, Kang G, Lanata CF, Molbak K, Rasmussen ZA, Sack RB, Valentiner-Branth P, Checkley W. Catch-up growth occurs after diarrhea in early childhood. J Nutr. 2014;144:965–971. doi: 10.3945/jn.113.187161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Howe LD, Tilling K, Matijasevich A, Petherick ES, Santos AC, Fairley L, Wright J, Santos IS, Barros AJ, Martin RM, Kramer MS, Bogdanovich N, Matush L, Barros H, Lawlor DA. Linear spline multilevel models for summarising childhood growth trajectories: a guide to their application using examples from five birth cohorts. Stat Methods Med Res. 2016;25:1854–1874. doi: 10.1177/0962280213503925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peterson KM, Buss J, Easley R, Yang Z, Korpe PS, Niu F, Ma JZ, Olortegui MP, Haque R, Kosek MN, Petri WA., Jr REG1B as a predictor of childhood stunting in Bangladesh and Peru. Am J Clin Nutr. 2013;97:1129–1133. doi: 10.3945/ajcn.112.048306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kosek M, Haque R, Lima A, Babji S, Shrestha S, Qureshi S, Amidou S, Mduma E, Lee G, Yori PP, Guerrant RL, Bhutta Z, Mason C, Kang G, Kabir M, Amour C, Bessong P, Turab A, Seidman J, Olortegui MP, Quetz J, Lang D, Gratz J, Miller M, Gottlieb M. Fecal markers of intestinal inflammation and permeability associated with the subsequent acquisition of linear growth deficits in infants. Am J Trop Med Hyg. 2013;88:390–396. doi: 10.4269/ajtmh.2012.12-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richard SA, McCormick BJ, Miller MA, Caulfield LE, Checkley W. Modeling environmental influences on child growth in the MAL-ED cohort study: opportunities and challenges. Clin Infect Dis. 2014;59((suppl 4)):S255–S260. doi: 10.1093/cid/ciu436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hokken-Koelega ACS, de Ridder MAJ, Lemmen RJ, den Hartog H, de Muinck Keizer-Schrama SM, Drop SLS. Children born small for gestational age: do they catch up? Pediatr Res. 1995;38:267–271. doi: 10.1203/00006450-199508000-00022. [DOI] [PubMed] [Google Scholar]
- 69.Leger J, Limoni C, Collin D, Czernichow P. Prediction factors in the determination of final height in subjects born small for gestational age. Pediatr Res. 1998;43:808–812. doi: 10.1203/00006450-199806000-00015. [DOI] [PubMed] [Google Scholar]
- 70.Karlberg J, Albertsson-Wikland K. Growth in full-term small-for-gestational-age infants: from birth to final height. Pediatr Res. 1995;38:733–739. doi: 10.1203/00006450-199511000-00017. [DOI] [PubMed] [Google Scholar]
- 71.Schmidt MK, Muslimatun S, West CE, Schultink W, Gross R, Hautvast JG. Nutritional status and linear growth of Indonesian infants in West Java are determined more by prenatal environment than by postnatal factors. J Nutr. 2002;132:2202–2207. doi: 10.1093/jn/132.8.2202. [DOI] [PubMed] [Google Scholar]
- 72.Christian P, Lee SE, Donahue AM, Adair LS, Arifeen SE, Ashorn P, Barros FC, Fall CH, Fawzi WW, Hao W, Hu G, Humphrey JH, Huybregts L, Joglekar CV, Kariuki SK, Kolsteren P, Krishnaveni GV, Liu E, Martorell R, Osrin D, Persson LA, Ramakrishnan U, Richter L, Roberfroid D, Sania A, Ter Kuile FO, Tielsch J, Victora CG, Yajnik CS, Yan H, Zeng L, Black RE. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int J Epidemiol. 2013;42:1340–1355. doi: 10.1093/ije/dyt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mukhopadhyay K, Mahajan R, Louis D, Narang A. Longitudinal growth of very low birth weight neonates during first year of life and risk factors for malnutrition in a developing country. Acta Paediatr. 2013;102:278–281. doi: 10.1111/apa.12113. [DOI] [PubMed] [Google Scholar]
- 74.Spencer NJ, Logan S. The treatment of parental height as a biological factor in studies of birth weight and childhood growth. Arch Dis Child. 2002;87:184–187. doi: 10.1136/adc.87.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garza C, Borghi E, Onyango AW, de Onis M, WHO Multicentre Growth Reference Study Group Parental height and child growth from birth to 2 years in the WHO Multicentre Growth Reference Study. Matern Child Nutr. 2013;9((suppl 2)):58–68. doi: 10.1111/mcn.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kramer MS. The epidemiology of adverse pregnancy outcomes: an overview. J Nutr. 2003;133:1592S–1596S. doi: 10.1093/jn/133.5.1592S. [DOI] [PubMed] [Google Scholar]
- 77.Ozaltin E, Hill K, Subramanian SV. Association of maternal stature with offspring mortality, underweight, and stunting in low- to middle-income countries. JAMA. 2010;303:1507–1516. doi: 10.1001/jama.2010.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Christian P. Maternal height and risk of child mortality and undernutrition. JAMA. 2010;303:1539–1540. doi: 10.1001/jama.2010.469. [DOI] [PubMed] [Google Scholar]
- 79.Ramakrishnan U, Martorell R, Schroeder DG, Flores R. Role of intergenerational effects on linear growth. J Nutr. 1999;129:544S–549S. doi: 10.1093/jn/129.2.544S. [DOI] [PubMed] [Google Scholar]
- 80.Emanuel I, Kimpo C, Moceri V. The association of grandmaternal and maternal factors with maternal adult stature. Int J Epidemiol. 2004;33:1243–1248. doi: 10.1093/ije/dyh268. [DOI] [PubMed] [Google Scholar]
- 81.Martin RM, Smith GD, Frankel S, Gunnell D. Parents’ growth in childhood and the birth weight of their offspring. Epidemiology. 2004;15:308–3016. doi: 10.1097/01.ede.0000120042.16363.e3. [DOI] [PubMed] [Google Scholar]
- 82.Morton SM. Commentary: the child is the mother of the woman: intergenerational associations in maternal anthropometry. Int J Epidemiol. 2004;33:1249–1251. doi: 10.1093/ije/dyh355. [DOI] [PubMed] [Google Scholar]