Abstract
Background
In 2010, we published an often-cited case report describing smoking cessation and substantial weight loss after deep brain stimulation (DBS) for obsessive-compulsive disorder (OCD) in an obese patient. To test whether this single observation was also observed in the treated population at large, the weight changes of a larger cohort of patients who underwent DBS for OCD or major depressive disorder (MDD) were studied.
Results
Data were available for 46 patients (30 OCD and 16 MDD patients; mean age 46.2 years, SD 10.9) with an average baseline body mass index (BMI) of 28.0 (SD 7.3), 26 of whom (57%) were overweight (n = 11), obese (n = 12), or morbidly obese (n = 3). Mean follow-up was 3.8 years (range 10 months to 8.7 years, SD 2.3), after which the average BMI was 28.1 (SD 7.0), not significantly different from baseline. The average BMI of the 15 patients with (morbid) obesity at baseline decreased from 36.8 to 34.6 (ns), while the average BMI of the 31 normal or “only” overweight patients at baseline increased from 23.8 to 25.0 (ns).
Conclusion
There was no significant change in body weight on group level after DBS for either OCD or MDD.
Keywords: Body mass index, Deep brain stimulation, Depression, Obesity, Obsessive-compulsive disorder, Weight
Introduction
In 2010, we published a case report on substantial weight loss in an obese patient with intractable obsessive-compulsive disorder (OCD) treated by deep brain stimulation (DBS) [1]. This patient was amongst the first that we treated with DBS for OCD, and had a very good response reflected by an improvement of >90% on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS). Interestingly, after the OCD symptoms had disappeared, she quit smoking effortlessly, and, with the help of a dietician, started a program to lose weight. Her body mass index (BMI) went rapidly from 37 to 25, starting 10 months after surgery, with a further reduction to 21 which was retained during 7 years of follow-up.
Case reports can spark new ideas or shed light on a particular aspect of a disorder or treatment. Conversely, selective publication of single effects can lead to a distortion of the available evidence for treatment application or lead to duplication of research efforts [2]. Therefore, after our initial observation, we analyzed the changes in body weight over time in our entire sample of patients treated with DBS in the ventral part of the anterior limb of the internal capsule (vALIC) [3] for either OCD or major depressive disorder (MDD).
Methods
The records of all patients undergoing DBS surgery between April 2005 and June 2014 for OCD and MDD were evaluated. For baseline weight and BMI data, we used values measured at the department of anesthesiology before the initial electrode implantation. For follow-up data, we used the most recently available value for the BMI measured by anesthesiology before subsequent operations for stimulator replacements, or measured at the department of psychiatry during follow-up visits.
In order to study the relation between possible changes in BMI and the effect of therapy, patients were categorized as responders or nonresponders at the time of this follow-up for BMI. A responder was defined as having an improvement of >35% on the Y-BOCS for OCD, or >50% on the Hamilton Depression Rating Scale (HDRS) for MDD, in comparison to the preoperative baseline score. IBM SPSS Statistics for Windows, v20 was used for statistical analysis and the paired Student t test was carried out to assess the average change in BMI over time. ANOVA was performed for the comparison of BMI changes for different body weight groups (morbidly obese, obese, and nonobese) and the treatment response.
Results
In the stipulated time period, 68 patients underwent DBS for either OCD (n = 43) or MDD (n = 25). Sufficient details for this analysis were available for 46 patients (30 OCD and 16 MDD patients; 29 females and 17 males, mean age 46.2 years, SD 10.9). All patients received DBS treatment with active electrode contacts in the vALIC and there were no patients with active contacts in the nucleus accumbens (NAc). In 22 patients, there was no reliable recording of a postoperative measurement of body weight.
The average baseline BMI in our sample of 46 patients was 28.0 (SD 7.3), with 26 (57%) being overweight (BMI 25–30, n = 11), obese (BMI 30–40, n = 12), or morbidly obese (BMI ≥40, n = 3). There were no significant differences in baseline BMI between males and females or between the diagnostic groups.
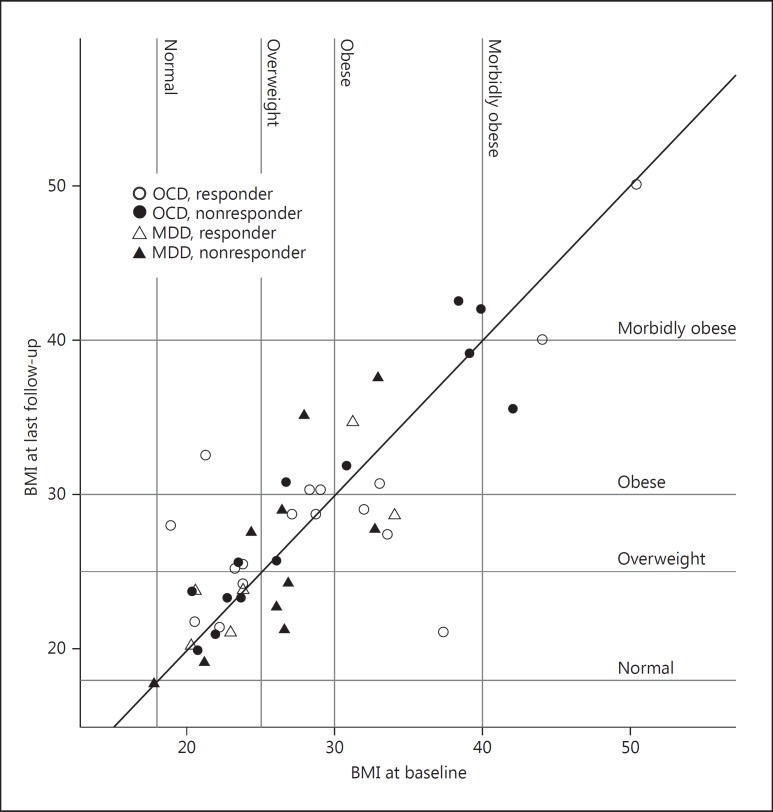
Follow-up time ranged from 10 months to 8.7 years (mean 3.8 years, SD 2.3). In the OCD group, 17 were classified as responders and 13 as nonresponders, using the criterium of >35% improvement on the Y-BOCS. In the MDD group, 6 were responders and 10 were nonresponders, using the criterium of >50% improvement on the HDRS. Figure 1 shows the individual BMI values at baseline and at the last follow-up (the patient from our earlier published case report is identifiable as the outlier at the bottom right on the graph).
Fig. 1.
Scatterplot of BMI at baseline (x axis) and at last follow-up (y axis) for all patients. The vertical distance to the diagonal represents the change in BMI. Dashed lines indicate the various BMI categories, so that a change in category after surgery can be seen for each subject. Patients below the diagonal had a decrease in BMI and those above the diagonal had an increase in BMI. In both OCD and MDD, there were no significant differences in the changes in BMI between the diagnostic groups, or between responders and nonresponders to DBS.
There were considerable changes in BMI in one-fifth of the sample; it increased by >5.0 in 3 patients and decreased by >5.0 in 6 patients. The average BMI at the last follow-up was 28.1 (SD 7.0), which was not significantly different from baseline (paired t test, ns). However, a subanalysis showed a two-way interaction for obesity as a small-effect modifier on BMI over time. The average BMI of the 15 patients with (morbid) obesity at baseline decreased slightly from 36.8 to 34.6 (ns), while the average BMI of the 31 normal or “only” overweight patients at baseline increased slightly from 23.8 to 25.0 (ns).
There was no significant difference in changes in BMI between the responders and nonresponders to DBS for either OCD or MDD in the open-label evaluations of symptoms. There was also no correlation between changes in body weight and the absolute values of Y-BOCS or HDRS score at baseline or score changes at the time of follow-up.
Discussion
There was no significant change in body weight on the group-level after vALIC DBS for either OCD or MDD. The potential of this therapy to change reward-related behavior did not result in significant weight loss in these patients, who were overweight on average at baseline. We did find a trend towards a decline in BMI in the subgroup of patients with (morbid) obesity, but we did not see a replication of the substantial weight loss previously described [1]. The vALIC is currently being explored as a potential target for DBS in obesity for its assumed role in reward-related behavior [4, 5, 6]. Evidence for the involvement of the NAc/vALIC in compulsive eating and obesity is limited to preclinical studies that show low D2-binding in the striatum in obese individuals after food-related sensory stimuli, and in animal studies that show reduced caloric intake and weight loss associated with an upregulation of the D2 receptor. There were increased DA levels in diet-induced obese rats treated with NAc shell DBS [7], whilst mice treated with NAc shell DBS were found to have a decrease in binge-eating and an increase in immediate D2 gene expression in the NAc shell. In diet-induced obese mice, chronic NAc shell DBS reduced caloric intake and led to weight loss [8].
In this study, weight loss or normalization of body weight was not a primary treatment target and the patients did not receive any motivational therapy targeted at losing weight. It is possible that if they had been coached specifically to lose weight in addition to combating the primary symptoms of OCD or MDD, this could have been effective with the reward system being under the influence of DBS. It is thus possible that we underestimated the possible effect of DBS on changes in BMI in morbidly obese patients. The patient in our previous study focused on losing weight out of intrinsic motivation without external prompting [1].
A severe limitation of this report is the amount of missing data on BMI during the follow-up of all patients treated with DBS, due to the fact that body weight was not routinely measured at fixed postoperative intervals. We cannot show that body weight did not fluctuate over time, with an initial significant weight loss followed by secondary weight gain, which is common with all weight loss methods available. However, from our frequent contact with these patients, we could ascertain that there were no such cases of strong fluctuations in BMI in our sample.
Further research is necessary to provide more insight into the possible effects of the modulation of the brain reward circuitry on food intake, energy balance, and body weight, and the possibility of a selective normalizing effect of vALIC DBS on body weight in eating disorders. Our findings may be of relevance for research groups working on DBS in eating disorders.
Disclosure Statement
P.R.S. acts as independent consultant on educational matters for Medtronic. All other authors report no competing interests. This research received no specific grant from any funding agency in the public, commercial, or non-profit sectors.
Author Contributions
R.S.N.L. and M.S.O. collected specific data used for this analysis. D.D. and P.R.S. designed the original trials in which the patients described here were treated. P.v.d.M. and P.R.S. performed the DBS implantation procedures. M.M. and D.D. performed the DBS settings and clinical follow-up of the patients. R.S.N.L., M.S.O., and P.R.S. analyzed the data and drafted the manuscript. All authors were involved in the final preparation and approval of the manuscript.
References
- 1.Mantione M, van de Brink W, Schuurman PR, et al. Smoking cessation and weight loss after chronic deep brain stimulation of the nucleus accumbens. Neurosurgery. 2010;66:E218. doi: 10.1227/01.NEU.0000360570.40339.64. [DOI] [PubMed] [Google Scholar]
- 2.Schlaepfer TE, Fins JJ. Deep brain stimulation and the neuroethics of responsible publishing: when one is not enough. JAMA. 2010;303:775–776. doi: 10.1001/jama.2010.140. [DOI] [PubMed] [Google Scholar]
- 3.van den Munckhof P, Bosch DA, Mantione MHM, et al. Active stimulation site of nucleus accumbens deep brain stimulation in obsessive-compulsive disorder is localized in the ventral internal capsule. Acta Neurochir Suppl. 2013;117:53–59. doi: 10.1007/978-3-7091-1482-7_9. [DOI] [PubMed] [Google Scholar]
- 4.Franco R, Fonoff ET, Alvarenga PL, et al. DBS for obesity. Brain Sci. 2016 doi: 10.3390/brainsci6030021. DOI: 10.3390/brainsci6030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nangunoori RK, Tomycz ND, Oh MY, Whiting DM. Deep brain stimulation for obesity: from a theoretical framework to practical application. Neural Plast. 2016;2016:7971460. doi: 10.1155/2016/7971460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho AL, Sussman ES, Pendharkar AV, et al. Deep brain stimulation for obesity: rationale and approach to trial design. Neurosurg Focus. 2015;38:E8. doi: 10.3171/2015.3.FOCUS1538. [DOI] [PubMed] [Google Scholar]
- 7.Zhang C, Wei N, Wanga Y, et al. Deep brain stimulation of the nucleus accumbens shell induces anti-obesity effects in obese rats with alteration of dopamine neurotransmission. Neurosci Lett. 2015;589:1–6. doi: 10.1016/j.neulet.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 8.Halpern CH, Tekriwal A, Santollo J, et al. Amelioration of binge eating by nucleus accumbens shell deep brain stimulation in mice involves D2 receptor modulation. J Neurosci. 2013;33:7122–7129. doi: 10.1523/JNEUROSCI.3237-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]