Abstract
Background/Aims
Polyethylenimine-coated polyacrylonitrile (AN69ST) membrane is expected to improve the outcomes of critically ill patients treated by continuous renal replacement therapy (CRRT).
Methods
Using a Japanese health insurance claim database, we identified adult patients receiving CRRT in intensive care units (ICUs) from April 2014 to October 2015. We used a multivariable logistic regression model to assess in-hospital mortality and Fine and Gray's proportional subhazards model to assess the ICU length of stay (ICU-LOS) accounting for the competing risks.
Results
Of 2,469 ICU patients, 156 were treated by AN69ST membrane. Crude in-hospital mortality was 50.0% in the AN69ST group and 54.0% in the non-AN69ST group. Adjusted odds ratio (OR) of AN69ST membrane use for in-hospital mortality was 0.65 (95% CI 0.45–0.93). The use of AN69ST membrane was also independently associated with shorter ICU-LOS.
Conclusion
This retrospective observational study suggested that CRRT with AN69ST membrane might be associated with better in-hospital outcomes.
Keywords: Continuous renal replacement therapy, Acute kidney injury, Sepsis, Claims database, Hemofilter
Background
Cytokine removal using the extracorporeal blood purification technique has been expected to improve the outcomes of critically ill patients, especially in those with sepsis. Elevation in cytokine levels is observed in severe conditions, and significantly associated with poor outcomes [1]. The effect of cytokine removal by blood purification has been investigated using the interventions of extremely high volume hemofiltration (HVHF) for enhanced convective clearance, and high cut-off (HCO) membranes for enhanced diffusive clearance. Randomized controlled trials of HVHF show decreases in cytokine levels but no mortality benefit [2, 3]. There is preliminary experience with HCO membranes, with reported improvements of hemodynamic parameters and oxygenation [4].
Adsorption is another blood-purification strategy to remove middle-range molecules, including cytokines. Polymethylmethacrylate (PMMA) and polyacrylonitrile (PAN) membranes are known to have high adsorption capability [5]. Nakada et al. [6] reported that continuous renal replacement therapy (CRRT) with PMMA membrane showed a rapid decline of blood interleukin (IL)-6 levels in 43 septic shock patients together with improvement of hemodynamics and hyperlactemia. PAN membrane such as AN69 can also remove cytokines, mainly by the mechanism of adsorption [7]. The membrane is negatively charged from methallylsulfonate, adsorbing cytokines via ionic bonding between sulfonate groups and amino groups on cytokines. Newly developed polyethylenimine-coated AN69ST has a hydrogel structure, which enables adsorption not only on the membrane surface but also within the bulk layer, thereby exhibiting an increased capacity for cytokine removal. However, like HVHF and HCO membranes, there is only limited evidence that this intervention improves outcomes in critically ill patients.
The aim of this study is to investigate whether AN69ST-CRRT is associated with lower hospital mortality and intensive care unit (ICU) length of stay (ICU-LOS) in critically ill patients. Since 2014, the use of AN69ST membrane has been approved by the National Health Insurance System in Japan for severe sepsis in addition to acute kidney injury (AKI) or end-stage renal disease. We analyzed claims of critically ill patients who were treated by CRRT admitted from April 2014 to October 2015, and evaluated the impact of AN69ST-CRRT on outcomes with adjustment for potential confounders.
Materials and Methods
Study Design
We performed an observational retrospective cohort study comparing AN69ST-CRRT with standard-CRRT, using an “as-treated” framework (“did exposure that the patient actually receives affect outcomes?”) [8]. The data were sourced from Medical Data Vision Co., Ltd (https://www.mdv.co.jp), and contains Japanese diagnosis-procedure combination (DPC) codes and health insurance claims data (irrespective of insurer). Data are extracted directly from electronic information systems of hospital participating in the data-sharing networks (177 hospitals, consisting of 21 hospitals with <200 beds; 110 hospitals with 200–499 beds; and 46 hospitals with ≥500 beds). Medical Data Vision Co., Ltd is a well-established data source for epidemiological and pharmacoeconomic studies from that country [9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21]. Medical Data Vision Co., Ltd collects data only from consenting hospitals, and these data are de-identified at source with guaranteed anonymity.
Patient Selection and Data Source
We used a hospital-based composite database purchased by Baxter Japan, Ltd. (Tokyo, Japan). The source data contained information from patient admissions from April 1, 2014 to October 30, 2015. From this database, we identified patients treated with CRRT defined by the existence of either a CRRT hemofilter claim or a CRRT technical fee claim record. We extracted information on identified patients about their age, gender, diagnosis, DPC codes, International Classification of Diseases 10th version (ICD10) codes, surgical procedure, outpatient/inpatient status, drug claim record, ICU management fee, and renal replacement therapy history.
We analyzed clinical data from all patients satisfying the following inclusion criteria: (i) receiving CRRT; (ii) age at baseline ≥18 years. Exclusion criteria were (i) on maintenance dialysis as defined by pre-admission claims or chronic kidney disease at admission by ICD10 code; (ii) ICU-LOS greater than 60 days consistent with other studies [22, 23]. Patients were followed until they were discharged from the hospital.
Variables
The primary endpoint was in-hospital mortality. The secondary outcome was the length of ICU stay (ICU-LOS). In case of multiple ICU admissions (different admissions defined by a gap in ICU technical fee records of 3 or more days), only the first admission was modeled and the rest were excluded.
The primary exposure was the type of CRRT, dichotomized to AN69ST-CRRT at any time during the ICU admission versus standard-only-CRRT. The patients in the standard-only-CRRT group were treated with the hemofilter membranes of polysulfone, cellulose triacetate, and PMMA but not with AN69ST. In case of multiple continuous sessions of CRRT (consecutive sessions defined by a gap in CRRT technical fee or CRRT hemofilter claim records of 3 or more days), only the first session was taken and the rest were excluded.
We included the following covariates in models: patient age at admission; gender; recorded diagnoses of sepsis, disseminated intravascular coagulation (DIC), and AKI; modified Charlson Comorbidity Index (CCI) calculated from pre- or at-admission ICD10 codes [24, 25, 26]; presence and type of surgery (open abdominal surgery, open cardiac surgery, non-invasive abdominal surgery); emergency versus elective surgery; emergency versus elective hospital admission; number of vasopressors at first hemofilter use; mechanical ventilation at first hemofilter use; administration of blood products between hospital and first hemofilter use; administration of albumin between ICU admission and first hemofilter use; hospital size; hospital DPC classification (DPC I, university hospital; DPC II, tertiary hospital; DPC III, other acute care hospital); and year of admission. The specific definitions of these covariates and the details of their data mapping and abstraction from source datasets are provided in online supplementary Appendix 1 (see www. karger.com/doi/10.1159/000476052).
Statistical Analysis
We constructed 3 multivariable time-varying exposure-outcome models to estimate the effect of the type of CRRT membranes on the outcomes. In all the multivariable models, we initially included all covariates in the models, which we then removed in a backward stepwise fashion beginning with the covariate with the highest p value from 2-tailed Wald tests of the individual coefficients. We based the final confounder selection upon both biological plausibility and contribution to the comprehensibility of the model, and also the significance of the covariate within the model as assessed by the 2-tailed partial likelihood ratio test p value at a level of 0.2 when jointly adjusted for other covariates [27].
For the primary outcome of in-hospital mortality, we used logistic regression. For the secondary outcome of ICU-LOS, we used 2 approaches. First, we used generalized linear modeling assuming a gamma distribution of ICU-LOS utilizing a log link, adjusting for ICU mortality as a measure of illness severity [28, 29, 30]. Because ICU death is a competing risk to ICU discharge, we also used competing risks regression based on the Fine and Gray's proportional subhazards model, modeling ICU-LOS accounting for the competing outcome of ICU death [31]. Subhazard ratios (SHRs) calculated using this approach can be interpreted as actual rather than actuarial probabilities: time to “ICU discharge without death” accounting for the risk of “death at ICU discharge” [32].
We used 2-way interaction terms in all the main-effects models to test effect modification by sepsis and AKI. We chose these interactions as being clinically plausible, on the basis of both published literature as well as cumulative clinical experience [33, 34]. Interactions were assessed using the 2-tailed Wald test p values as a guide to selecting interaction terms for testing, with significance within the model using the likelihood ratio test (p value <0.05). Where necessary, we made comparisons between groups using the Fisher's exact test and Mann-Whitney U (or Kruskal-Wallis) tests as appropriate.
The claim dataset does not include a number of factors that are therefore not included in this study (e.g., physiological measures such as blood pressure, process measures such as antibiotic levels etc.), which might confound the relationship between hemofilter and outcomes. We therefore performed sensitivity analyses to estimate the extent to which bias due to unmeasured, hence uncontrolled, confounding may have over- or underestimated the true effect [35, 36, 37, 38]. We varied the prevalence of the unmeasured confounder among patients on standard-only-CRRT (reference group) and those treated with AN69ST-CRRT (comparator group), as well as the strength of the association between the unmeasured confounder and mortality risk reflecting various plausible scenarios.
Analyses were performed using Stata Intercooled MP/14.1 (StataCorp, www.stata.com).
Results
Participants
We identified a cohort of 2,469 adult patients with sufficient data for modeling, with 19,494 patient-ICU-days. Due to the de-identified nature of the data, we were not able to ascertain how many hospitals (out of the 177 participating hospitals in the Medical Data Vision's database) contributed information about patients to this dataset.
Descriptive Data
The mean (SD) age of all patients was 70.4 (14.2) years, 1,643 (66.3%) of whom were males. The mean (SD) CCI was 2.3 (2.1). Associated sepsis diagnosis was recorded in 1,196 (48.3%) patients, DIC in 1,166 (47.1%) and AKI in 1,935 (78.1%). Of the cohort, 156 (6.3%) received AN69ST-CRRT and 2,322 (93.7%) standard-only-CRRT. Consistent with the results of other investigators, the patients in our cohort had a high probability of ICU death (659 patients, 26.6%) and hospital death (1,331 patients, 53.7%). The median (interquartile range) ICU-LOS was 7 (3–14) days.
Table 1 shows the characteristics of the study cohort based on the type of CRRT. Patients treated with AN69ST-CRRT had a significantly higher probability for open abdominal surgery, DIC, and sepsis diagnoses, and with a significantly greater degree of ventilator and vasopressor support. Crude ICU mortality was 22.4 vs. 26.9% (p = 0.26), and crude in-hospital mortality was 50.0 vs. 54.0% (p = 0.36) in the AN69ST-CRRT and standard-only-CRRT groups, respectively.
Table 1.
Characteristics of the study cohort, by type of CRRT
| Variable | AN69ST-CRRT | Standard-only-CRRT | p value |
|---|---|---|---|
| Total | 156 | 2,322 | |
| Age, years, mean (SD) | 70.4 (14.2) | 70.3 (14) | 0.88 |
| Gender, male, n (%) | 99 (63.5) | 1,544 (66.5) | 0.43 |
| Type of admission (emergency), n (%) | 119 (76.3) | 1,676 (72.2) | 0.31 |
| Type of surgery, n (%) | |||
| Noninvasive abdominal | 6 (3.9) | 68 (2.9) | 0.47 |
| Invasive abdominal | 51 (32.7) | 453 (19.5) | <0.0005 |
| Cardiac | 34 (21.8) | 681 (29.3) | 0.04 |
| CCI, score, mean (SD) | 2.13 (2.01) | 2.29 (2.05) | 0.25 |
| AKI, n (%) | 121 (77.6) | 1,814 (78.1) | 0.84 |
| DIC, n (%) | 99 (63.5) | 1,067 (46) | <0.0005 |
| Sepsis, n (%) | 100 (64.1) | 1,096 (47.2) | <0.0005 |
| Vasopressors number, mean (SD) | |||
| Infusions, n (%) | 2.0 (1.0) | 1.7 (1.2) | 0.01 |
| Plat/FFP | 87 (55.8) | 1,194 (51.4) | 0.32 |
| RBC | 89 (57.1) | 1,252 (53.9) | 0.46 |
| Mechanical ventilation, n (%) | |||
| Death, n (%) | 136 (87.2) | 1,740 (74.9) | <0.0005 |
| In ICU | 35 (22.4) | 624 (26.9) | 0.26 |
| In hospital | 78 (50.0) | 1,253 (54.0) | 0.36 |
| ICU LOS, days, median (IQR) | 7 (2–14) | 7 (3–14) | |
AKI, acute kidney injury; CCI, Charlson Comorbidity Index; CRRT, continuous renal replacement therapy; DIC, disseminated intravascular coagulation; FFP, fresh frozen plasma; ICU LOS, intensive care unit length of stay; RBC, red blood cell.
Main Results
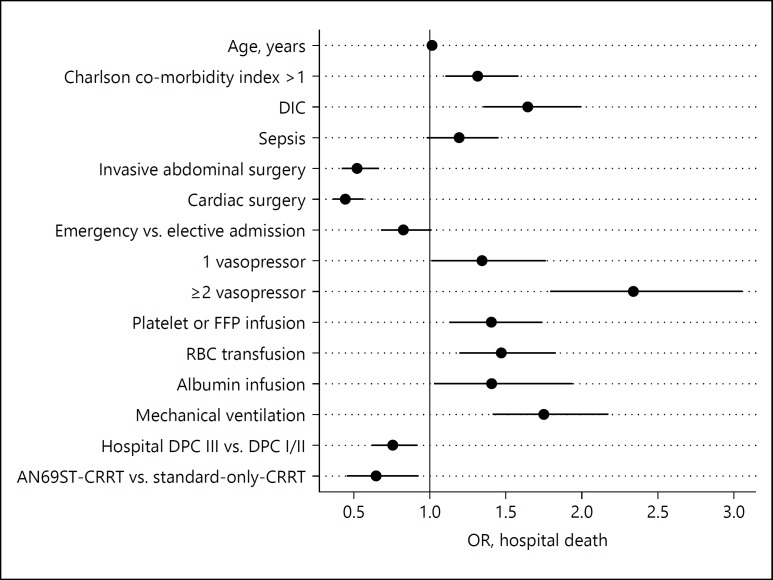
The fully adjusted main effects from the logistic regression model for hospital mortality are illustrated in Figure 1. The following variables were omitted from the model due to 2-tailed partial likelihood ratio test p values >0.2 when jointly adjusted for other covariates: AKI, emergency versus elective surgery, hospital size, year of admission, and gender. In the final mortality model, AN69ST-CRRT was associated with a statistically significant lower mortality risk than standard-only-CRRT (OR 95% CI 0.65 [0.45–0.93]), fully adjusted for the covariates (Table 1).
Fig. 1.
Fully adjusted main effects from the logistic regression model for hospital mortality. DIC, disseminated intravascular coagulation; FFP, fresh frozen plasma; RBC, red blood cell; DPC, diagnosis procedure combination; CRRT, continuous renal replacement therapy; OR, odds ratio.
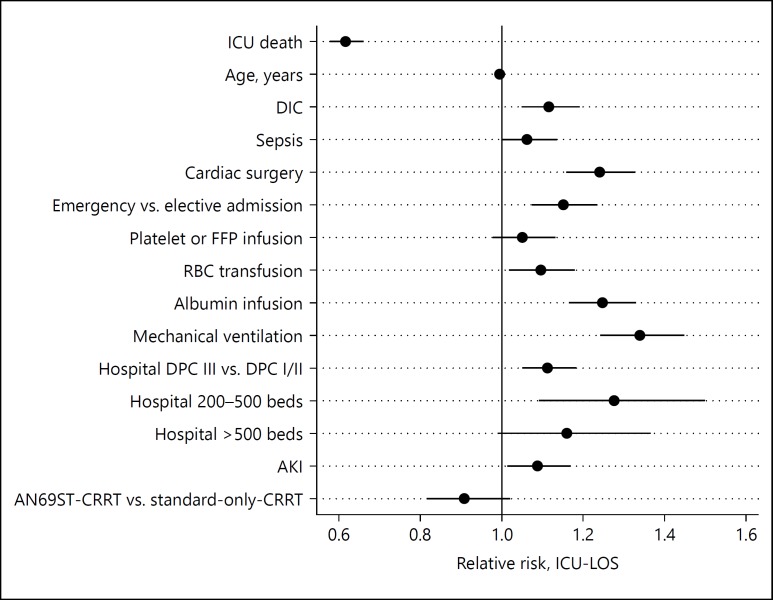
The fully adjusted main effects from the generalized linear model for ICU-LOS log link are illustrated in Figure 2. The following variables were omitted from the model due to 2-tailed partial likelihood ratio test p values >0.2 when jointly adjusted for other covariates: invasive and noninvasive abdominal surgery; emergency versus elective surgery, CCI, number of vasopressors, year of admission, and gender. AN69ST-CRRT was associated with a statistically nonsignificant trend to shorter ICU-LOS than standard-only-CRRT with a relative risk (RR) of 0.91 (0.81–1.02). However, ICU death was a strong and significant predictor of shorter ICU-LOS with an RR of 0.62 (0.58–0.66), suggesting that ICU-LOS is better modeled as 2 competing risks: “ICU discharge without death”, vs. “death at ICU discharge”.
Fig. 2.
Fully adjusted main effects from the generalized linear model for intensive care unit length of stay (ICU-LOS). DIC, disseminated intravascular coagulation; AKI, acute kidney injury; FFP, fresh frozen plasma; RBC, red blood cell; DPC, diagnosis procedure combination; CRRT, continuous renal replacement therapy.
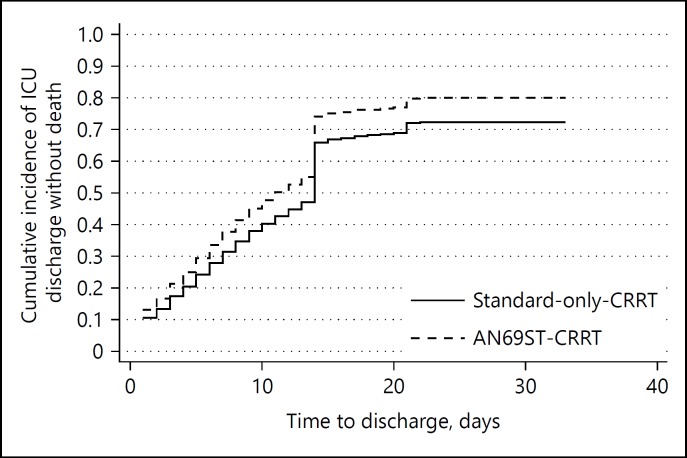
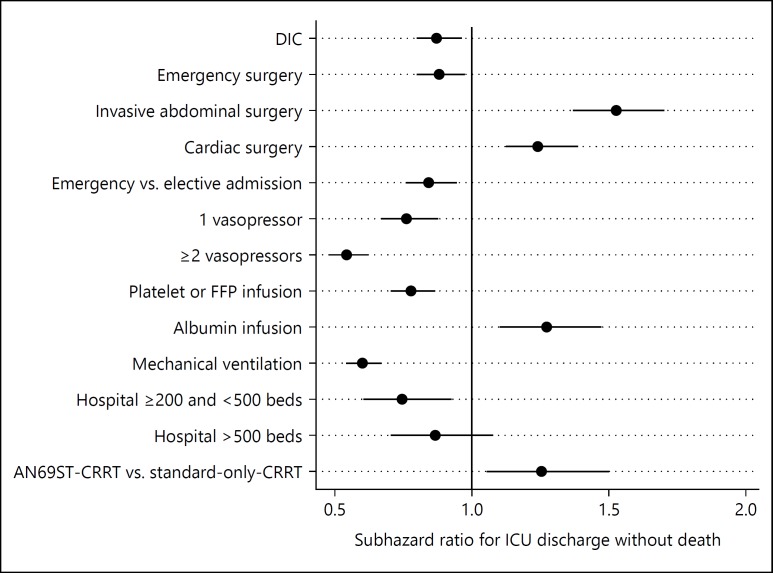
The cumulative incidence of ICU discharge without death is shown in Figure 3. We observed a step-up of cumulative incidence of ICU discharge at day 14. This is probably because of the Japanese reimbursement system in which ICU management fee is covered up to 14 days. The fully adjusted main effects from the competing risks model for “ICU discharge without death” are illustrated in Figure 4. The following variables were omitted from the model due to 2-tailed partial likelihood ratio test p values >0.2 when jointly adjusted for other covariates: sepsis, age, albumin infusion, CCI, cardiac surgery, red blood cell infusion, year of admission, and gender. AN69ST-CRRT was associated with a statistically significant greater probability of “ICU discharge without death,” with a SHR of 1.23 (1.03–1.50). Accounting for the competing risk of death at ICU discharge, the median ICU-LOS can be estimated from the cumulative incidence function, and was 11 days for the AN69ST-CRRT group and 13 days for the standard-only-CRRT group (median ICU LOS ratio 1.18).
Fig. 3.
Cumulative incidence plot of ICU discharge without death. ICU, intensive care unit.
Fig. 4.
Fully adjusted main effects from the competing risk model for intensive care unit length of stay (ICU-LOS), accounting for competing risk of ICU death. DIC, disseminated intravascular coagulation; AKI, acute kidney injury; FFP, fresh frozen plasma; DPC, diagnosis procedure combination; CRRT, continuous renal replacement therapy.
Other Results
Although no effect modification by AKI diagnosis was observed in the mortality model (p = 0.132), there was statistically significant effect modification by sepsis diagnosis (p = 0.012), with a slightly lower point estimate for AN69ST-CRRT versus standard-only-CRRT in those without sepsis (OR 0.53 [0.28–1.02]) compared to those with sepsis (0.73 [0.46–1.16]). There was significant effect modification by AKI diagnosis in the generalized linear model of ICU-LOS (p = 0.037), with a slightly lower point estimate for AN69ST-CRRT versus standard-only-CRRT in those with AKI (RR 0.84 [0.66–1.06]) compared to those without AKI (0.93 [0.82–1.06]). There was no significant effect modification by AKI or sepsis diagnosis in the competing risks ICU-LOS model. In all interaction models, CIs are wide as the result of the halved sample sizes.
Table 2 summarizes the results of our sensitivity analyses for unmeasured confounding. Only adjustment for a strong confounder (e.g., one that produces a >3–10 fold decrease in risk of death) resulted in the attenuation of the association between type of CRRT and mortality risk. Adjustment for more moderate confounding produced little change in the results. These results suggest that the associations for AN69ST-CRRT versus standard-only-CRRT are unlikely to have been due to confounding only.
Table 2.
Sensitivity of HRs for the association between AN69ST-CRRT and mortality risk (relative to standard-only-CRRT), adjusted for unmeasured confounding
| p1 | p0 | HRcd |
||||
|---|---|---|---|---|---|---|
| 0.9 | 0.7 | 0.5 | 0.3 | 0.1 | ||
| 0.65 | 0.2 | 0.81 | 0.76 | 0.87 | 1.03 | 1.28 |
| 0.6 | 0.25 | 0.77 | 0.73 | 0.81 | 0.92 | 1.10 |
| 0.55 | 0.3 | 0.73 | 0.71 | 0.76 | 0.83 | 0.94 |
| 0.5 | 0.35 | 0.70 | 0.68 | 0.72 | 0.76 | 0.81 |
| 0.45 | 0.4 | 0.67 | 0.66 | 0.67 | 0.68 | 0.70 |
| 0.4 | 0.45 | 0.63 | 0.64 | 0.63 | 0.62 | 0.60 |
| 0.35 | 0.5 | 0.60 | 0.62 | 0.59 | 0.56 | 0.52 |
| 0.3 | 0.55 | 0.58 | 0.60 | 0.55 | 0.51 | 0.45 |
| 0.25 | 0.6 | 0.55 | 0.58 | 0.52 | 0.46 | 0.39 |
| 0.2 | 0.65 | 0.52 | 0.56 | 0.49 | 0.41 | 0.33 |
HRs are computed as point estimates only. The base model assumes an HR of 0.65, adjusted for known confounders, when unmeasured confounders are present but unaccounted for. When an unmeasured confounder of varying strength and prevalence is introduced into the models, the hazard ratios are only sensitive to strong unmeasured confounding, with an effect that remains after adjustment for only weak-moderate confounding.
HRs, hazard ratios; CRRT, continuous renal replacement therapy; HRCD, hazard ratio for the association between unmeasured confounder and mortality risk; p1, prevalence of unmeasured confounder among those on AN69ST-CRRT; p0, prevalence of unmeasured confounder among those on to standard-only-CRRT.
Discussion
Severe AKI that requires CRRT, especially complicated with sepsis, shows unacceptably high mortality rates [39]. In our study, the ICU and hospital mortality of our patients were 22.4 and 26.9%, respectively. These mortality rates are somewhat lower than the previous reports from other countries possibly because of region-specific reasons (e.g., proportion of respiratory support in ICU patients is lower in Japan than other countries). Of note, the mortality rates in this study are similar to other reports on septic ICU patients treated in Japan [40].
Although CRRT with any currently available membrane can control uremia sufficiently, additive therapeutic effects beyond renal replacement might improve the outcomes of these severely ill patients. Several membranes that have high capability of adsorption including AN69ST are expected to remove the middle-size molecules of inflammatory humoral mediators. In this study, with an anonymized large claim-based database, the use of AN69ST membrane was significantly associated with lower in-hospital mortality (adjusted OR of 0.65, 95% CI 0.45–0.93) and shorter ICU-LOS among patients receiving CRRT in Japanese ICUs.
Hypercytokinemia is observed in a variety of serious conditions in ICU patients, including sepsis, trauma, pancreatitis, and burns, and plays a key role in the inflammation cascade [41]. In particular, plasma cytokine levels such as IL-6 and high-mobility group protein B1 are increased with impaired kidney function [42, 43, 44, 45]. Therefore, removing cytokines in addition to renal replacement seems a plausible intervention to reduce the maladaptive inflammation cascade in sepsis. AN69ST membrane has been shown to have a high capacity to adsorb cytokines (tumor necrosis factor-α, IL-1β, IL-6, and IL1ra) and HMGM1 in vitro experiments [46, 47].
However, only one clinical study by Shiga et al. [48], which evaluated AN69ST-CRRT has been reported so far. A prospective multicenter study demonstrated that the 28-day survival of 34 patients receiving AN69ST-CRRT was 73.5%, which was higher than the predicted 28-day survival of 20.3% based on their severity at ICU admission (Acute Physiology and Chronic Health Evaluation II score 32.7 ± 9.8). Because the study by Shiga et al. [48] was a single-arm study, there was no control group using other CRRT membranes. AN69ST membrane was approved by the National Health Insurance System in Japan since 2014. Therefore, analyzing the claim data for AN69ST use in our study enables us to examine the real-world effectiveness of AN69ST-CRRT in comparison with other CRRT membranes.
The information obtained from the Japanese claim database revealed that the patients in the AN69ST-CRRT group were more likely to receive larger number of vasopressors and mechanical ventilator support than those in the standard-only-CRRT group, suggesting that AN69ST membrane was preferentially used for patients in a more severe condition. After adjusting for differences including severity, the use of AN69ST was significantly associated with a lower in-hospital mortality with adjusted OR of 0.65 (95% CI 0.45–0.93). Although no data regarding blood cytokine concentrations were available in this study, better control of humoral mediators achieved by AN69ST might contribute to the favorable use of this membrane for higher survival rate.
We also compared ICU-LOS between the AN69ST-CRRT and standard-only-CRRT groups, as early discharge from ICU is an indirect marker of patients' improved conditions. This outcome is also important in terms of cost effectiveness and hospital resource utilization. Because crude ICU mortality was lower in the AN69ST-CRRT group than the standard-only-CRRT group (22.4 vs. 26.9%), we took into account the competing risk (i.e., early death leads to early discharge from ICU) in our statistical analysis. As a result, the use of the AN69ST membrane was also independently associated with shorter ICU-LOS. This result may suggest that AN69ST-CRRT not only improved survival rate but also enhanced early recovery from a severe condition.
There are several limitations in our study. First, there may be a selection bias of participating hospitals because the Medical Data Vision's database consists of hospitals voluntarily contributing to the company, and there is no detailed information on how different these hospitals and other Japanese hospitals are in terms of patient characteristics and medical care including CRRT management. However, we acknowledge at least that demographics and crude in-hospital mortality of patients in the current study were similar to those of CRRT-treated patients (median age 72, male 66.0%, and in-hospital mortality of 50.6%) in a Japanese nationwide database consisting of around 1,000 hospitals [39]. Second, although we adjusted for a number of potential confounding factors in regression analyses, residual confounding cannot be fully removed in observational studies. Unmeasured confounding factors mainly include vital signs and laboratory results. However, we were able to adjust for the use of vasopressors, mechanical ventilation, blood products and albumin, which are expected to reflect patients' severity. Moreover, our sensitivity analysis suggested that current findings are unlikely to be explained only by confounding, unless the level of confounding is very strong (Table 2).
Third, we used recorded diagnoses (ICD-10 and DPC codes) of sepsis, DIC, and AKI for confounding adjustment and test for interaction (effect modification). However, recorded diagnoses of sepsis and DIC are known to have high specificity but low sensitivity in the Japanese claims system [49]. This was similar in a US validation study of AKI diagnosis [50], although there has been no validation study of AKI diagnosis in Japan. Misclassification of disease diagnoses may make the interpretation of our interaction test difficult. More accurate classification of sepsis, DIC, and AKI status based on vital signs and laboratory results would be necessary for identifying the best target group of this treatment in future studies. Finally, we were dependent on the classification of filters in the Japanese claims data, which distinguish only AN69ST membranes from other filters by virtue of a different reimbursement code. Our standard group included patients treated with polysulfone, cellulose, and PMMA membranes. Although evidence suggests that AN69ST might have slightly greater adsorption of HMGB-1 [47], AN69ST and PMMA are both classified as adsorptive membranes. Unfortunately, we cannot differentiate PMMA from other membranes in the standard group, since the reimbursement is the same for all membranes other than AN6ST, and our study is based on claim records. We cannot estimate the proportion of patients treated with PMMA in the standard group, although the inclusion of PMMA membranes in this group would potentially reduce the observed and modeled benefit of AN69ST in our study, if adsorption were truly effective. Therefore, the benefit of AN69ST observed in this study might be underestimated.
In conclusion, this retrospective observational study suggests that the use of AN69ST membrane might be associated with improved outcomes including in-hospital mortality and ICU-LOS among patients receiving CRRT in ICU. Further prospective studies including randomized control trials are warranted to confirm this finding and to better elucidate the clinical significance of cytokine removal in patients admitted to ICUs.
Disclosure Statement
M.R.M. is a Director of Medical Affairs, Therapeutic Area, Baxter Healthcare (Asia) Pte. Ltd, and E.Y. was an employee of Baxter Japan Ltd at the time of her contribution to the study. The remaining authors declare that they have no competing interests.
Supplementary Material
Supplementary data
Acknowledgment
Dr. Masao Iwagami is funded by the Honjo International Scholarship Foundation. The funders had no role in the execution of this study or interpretation of results.
References
- 1.Doi K, Rabb H. Impact of acute kidney injury on distant organ function: recent findings and potential therapeutic targets. Kidney Int. 2016;89:555–564. doi: 10.1016/j.kint.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Clark E, Molnar AO, Joannes-Boyau O, Honoré PM, Sikora L, Bagshaw SM. High-volume hemofiltration for septic acute kidney injury: a systematic review and meta-analysis. Crit Care. 2014;18:R7. doi: 10.1186/cc13184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park JT, Lee H, Kee YK, Park S, Oh HJ, Han SH, Joo KW, Lim CS, Kim YS, Kang SW, Yoo TH, Kim DK. High-dose versus conventional-dose continuous venovenous hemodiafiltration and patient and kidney survival and cytokine removal in sepsis-associated acute kidney injury: a randomized controlled trial. Am J Kidney Dis. 2016;68:599–608. doi: 10.1053/j.ajkd.2016.02.049. [DOI] [PubMed] [Google Scholar]
- 4.Villa G, Zaragoza JJ, Sharma A, Neri M, De Gaudio AR, Ronco C. Cytokine removal with high cut-off membrane: review of literature. Blood Purif. 2014;38:167–173. doi: 10.1159/000369155. [DOI] [PubMed] [Google Scholar]
- 5.Fujimori A, Naito H, Miyazaki T. Adsorption of complement, cytokines, and proteins by different dialysis membrane materials: evaluation by confocal laser scanning fluorescence microscopy. Artif Organs. 1998;22:1014–1017. doi: 10.1046/j.1525-1594.1998.06083.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakada TA, Oda S, Matsuda K, Sadahiro T, Nakamura M, Abe R, Hirasawa H. Continuous hemodiafiltration with PMMA hemofilter in the treatment of patients with septic shock. Mol Med. 2008;14:257–263. doi: 10.2119/2007-00108.Nakada. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole L, Bellomo R, Davenport P, Tipping P, Ronco C. Cytokine removal during continuous renal replacement therapy: an ex vivo comparison of convection and diffusion. Int J Artif Organs. 2004;27:388–397. doi: 10.1177/039139880402700507. [DOI] [PubMed] [Google Scholar]
- 8.Vonesh E, Schaubel D, Hao W, Collins A. Statistical methods for comparing mortality among ESRD pateints: examples of regional/international variations. Kidney Int. 2000;57:S19–S27. [Google Scholar]
- 9.Urushihara H, Taketsuna M, Liu Y, Oda E, Nakamura M, Nishiuma S, Maeda R. Increased risk of acute pancreatitis in patients with type 2 diabetes: an observational study using a Japanese hospital database. PLoS One. 2012;7:e53224. doi: 10.1371/journal.pone.0053224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hashikata H, Harada KH, Kagimura T, Nakamura M, Koizumi A. Usefulness of a large automated health records database in pharmacoepidemiology. Environ Health Prev Med. 2011;16:313–319. doi: 10.1007/s12199-010-0201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang CH, Kusama M, Ono S, Sugiyama Y, Orii T, Akazawa M. Assessment of statin-associated muscle toxicity in Japan: a cohort study conducted using claims database and laboratory information. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002040. pii:e002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanatani T, Sai K, Tohkin M, Segawa K, Saito Y. Impact of Japanese regulatory action on metformin-associated lactic acidosis in type II diabetes patients. Int J Clin Pharm. 2015;37:537–545. doi: 10.1007/s11096-015-0097-0. [DOI] [PubMed] [Google Scholar]
- 13.Hori K, Kobayashi N, Atsumi H, Nagayama A, Kondoh M, Noge I, Kimura M, Utsugi H, Iwasaki T, Nakamura M, Kimura T. Changes in compliance with Japanese antiemetic guideline for chemotherapy-induced nausea and vomiting: a nationwide survey using a distributed research network. Support Care Cancer. 2014;22:969–977. doi: 10.1007/s00520-013-2048-4. [DOI] [PubMed] [Google Scholar]
- 14.Ueyama H, Hinotsu S, Tanaka S, Urushihara H, Nakamura M, Nakamura Y, Kawakami K. Application of a self-controlled case series study to a database study in children. Drug Saf. 2014;37:259–268. doi: 10.1007/s40264-014-0148-9. [DOI] [PubMed] [Google Scholar]
- 15.Kamae I, Hashimoto Y, Koretsune Y, Tanahashi N, Murata T, Phatak H, Liu LZ, Tang AC, Feng Wang P, Okumura K. Cost-effectiveness analysis of apixaban against warfarin for stroke prevention in patients with nonvalvular atrial fibrillation in Japan. Clin Ther. 2015;37:2837–2851. doi: 10.1016/j.clinthera.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka K, Hamada K, Nakayama T, Matsuda S, Atsumi A, Shimura T, Nemoto M. Risk for cardiovascular disease in Japanese patients with rheumatoid arthritis: a large-scale epidemiological study using a healthcare database. Springerplus. 2016;5:1111. doi: 10.1186/s40064-016-2800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanabe M, Motonaga R, Terawaki Y, Nomiyama T, Yanase T. Prescription of oral hypoglycemic agents for patients with type 2 diabetes mellitus: a retrospective cohort study using a Japanese hospital database. J Diabetes Investig. 2016;8:227–234. doi: 10.1111/jdi.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teramoto T, Uno K, Miyoshi I, Khan I, Gorcyca K, Sanchez RJ, Yoshida S, Mawatari K, Masaki T, Arai H, Yamashita S. Low-density lipoprotein cholesterol levels and lipid-modifying therapy prescription patterns in the real world: an analysis of more than 33,000 high cardiovascular risk patients in Japan. Atherosclerosis. 2016;251:248–254. doi: 10.1016/j.atherosclerosis.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Hanatani T, Sai K, Tohkin M, Segawa K, Kimura M, Hori K, Kawakami J, Saito Y. A detection algorithm for drug-induced liver injury in medical information databases using the Japanese diagnostic scale and its comparison with the Council for International Organizations of Medical Sciences/the Roussel Uclaf Causality Assessment Method scale. Pharmacoepidemiol Drug Saf. 2014;23:984–988. doi: 10.1002/pds.3603. [DOI] [PubMed] [Google Scholar]
- 20.Bennett D, Davé S, Sakaguchi M, Chang CH, Dolin P. Association between therapy with dipeptidyl peptidase-4 (DPP-4) inhibitors and risk of ileus: a cohort study. Diabet Int. 2016;7:375–383. doi: 10.1007/s13340-016-0261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sugitani Y, Udagawa Y, Matsuda S, Yamada K, Miyawaki N, Konishi I. Postmarketing benefit-risk assessment for erythropoiesis-stimulating agents using a health care database. Ther Innov Regul Sci. 2016;50:823–832. doi: 10.1177/2168479016656029. [DOI] [PubMed] [Google Scholar]
- 22.Verburg IW, de Keizer NF, de Jonge E, Peek N. Comparison of regression methods for modeling intensive care length of stay. PLoS One. 2014;9:e109684. doi: 10.1371/journal.pone.0109684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zimmerman JE, Kramer AA, McNair DS, Malila FM, Shaffer VL. Intensive care unit length of stay: benchmarking based on acute physiology and chronic health evaluation (APACHE) IV. Crit Care Med. 2006;34:2517–2529. doi: 10.1097/01.CCM.0000240233.01711.D9. [DOI] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 26.Quan H, Parsons GA, Ghali WA. Validity of information on comorbidity derived rom ICD-9-CCM administrative data. Med Care. 2002;40:675–685. doi: 10.1097/00005650-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd ed. New Jersey: Wiley-Interscience, USA; 2000. [Google Scholar]
- 28.Verburg IW, de Keizer NF, de Jonge E, Peek N. Comparison of regression methods for modeling intensive care length of stay. PLoS One. 2014;9:e109684. doi: 10.1371/journal.pone.0109684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Straney L, Clements A, Alexander J, Slater A, ANZICS Paediatric Study Group A two-compartment mixed-effects gamma regression model for quantifying between-unit variability in length of stay among children admitted to intensive care. Health Serv Res. 2012;47:2190–2203. doi: 10.1111/j.1475-6773.2012.01421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moran JL, Solomon PJ. A review of statistical estimators for risk-adjusted length of stay: analysis of the Australian and New Zealand intensive care adult patient data-base, 2008–2009. BMC Med Res Methodol. 2012;12:68. doi: 10.1186/1471-2288-12-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 32.Grunkemeier GL, Jin R, Eijkemans MJ, Takkenberg JJ. Actual and actuarial probabilities of competing risks: apples and lemons. Ann Thorac Surg. 2007;83:1586–1592. doi: 10.1016/j.athoracsur.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 33.Bagshaw SM, Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Kellum JA, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol. 2007;2:431–439. doi: 10.2215/CJN.03681106. [DOI] [PubMed] [Google Scholar]
- 34.Uchino S, Bellomo R, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Doig GS, Oudemans van Straaten H, Ronco C, Kellum JA, Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. Kidney) Investigators External validation of severity scoring systems for acute renal failure using a multinational database. Crit Care Med. 2005;33:1961–1967. doi: 10.1097/01.ccm.0000172279.66229.07. [DOI] [PubMed] [Google Scholar]
- 35.Arah OA, Chiba Y, Greenland S. Bias formulas for external adjustment and sensitivity analysis of unmeasured confounders. Ann Epidemiol. 2008;18:637–646. doi: 10.1016/j.annepidem.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Lash TL, Fox CS, Fink AK. Applying Quantitative Bias Analysis to Epidemiologic Data. New York: Springer; 2009. [Google Scholar]
- 37.Sudan M, Kheifets L, Arah OA, Olsen J. Cell phone exposures and hearing loss in children in the Danish National Birth Cohort. Paediatr Perinat Epidemiol. 2013;27:247–257. doi: 10.1111/ppe.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vanderweele TJ, Arah OA. Bias formulas for sensitivity analysis of unmeasured confounding for general outcomes, treatments, and confounders. Epidemiology. 2011;22:42–52. doi: 10.1097/EDE.0b013e3181f74493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iwagami M, Yasunaga H, Noiri E, Horiguchi H, Fushimi K, Matsubara T, Yahagi N, Nangaku M, Doi K. Current state of continuous renal replacement therapy for acute kidney injury in Japanese intensive care units in 2011: analysis of a national administrative database. Nephrol Dial Transplant. 2015;30:988–995. doi: 10.1093/ndt/gfv069. [DOI] [PubMed] [Google Scholar]
- 40.Fujishima S, Gando S, Saitoh D, Mayumi T, Kushimoto S, Shiraishi S, Ogura H, Takuma K, Kotani J, Ikeda H, Yamashita N, Suzuki K, Tsuruta R, Takeyama N, Araki T, Suzuki Y, Miki Y, Yamaguchi Y, Aikawa N, Japanese Association for Acute Medicine Sepsis Registry (JAAM SR) Study Group A multicenter, prospective evaluation of quality of care and mortality in Japan based on the Surviving Sepsis Campaign guidelines. J Infect Chemother. 2014;20:115–120. doi: 10.1016/j.jiac.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Hirasawa H, Oda S, Nakamura M, Watanabe E, Shiga H, Matsuda K. Continuous hemodiafiltration with a cytokine-adsorbing hemofilter for sepsis. Blood Purif. 2012;34:164–170. doi: 10.1159/000342379. [DOI] [PubMed] [Google Scholar]
- 42.Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, Tao Y, Dursun B, Voelkel NF, Edelstein CL, Faubel S. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18:155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 43.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008;74:901–909. doi: 10.1038/ki.2008.314. [DOI] [PubMed] [Google Scholar]
- 44.Andres-Hernando A, Dursun B, Altmann C, Ahuja N, He Z, Bhargava R, Edelstein CE, Jani A, Hoke TS, Klein C, Faubel S. Cytokine production increases and cytokine clearance decreases in mice with bilateral nephrectomy. Nephrol Dial Transplant. 2012;27:4339–4347. doi: 10.1093/ndt/gfs256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doi K, Ishizu T, Tsukamoto-Sumida M, Hiruma T, Yamashita T, Ogasawara E, Hamasaki Y, Yahagi N, Nangaku M, Noiri E. The high-mobility group protein B1-Toll-like receptor 4 pathway contributes to the acute lung injury induced by bilateral nephrectomy. Kidney Int. 2014;86:316–326. doi: 10.1038/ki.2014.62. [DOI] [PubMed] [Google Scholar]
- 46.Rimmelé T, Assadi A, Cattenoz M, Desebbe O, Lambert C, Boselli E, Goudable J, Etienne J, Chassard D, Bricca G, Allaouchiche B. High-volume haemofiltration with a new haemofiltration membrane having enhanced adsorption properties in septic pigs. Nephrol Dial Transplant. 2009;24:421–427. doi: 10.1093/ndt/gfn518. [DOI] [PubMed] [Google Scholar]
- 47.Yumoto M, Nishida O, Moriyama K, Shimomura Y, Nakamura T, Kuriyama N, Hara Y, Yamada S. In vitro evaluation of high mobility group box 1 protein removal with various membranes for continuous hemofiltration. Ther Apher Dial. 2011;15:385–393. doi: 10.1111/j.1744-9987.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 48.Shiga H, Hirasawa H, Nishida O, Oda S, Nakamura M, Mashiko K, Matsuda K, Kitamura N, Kikuchi Y, Fuke N. Continuous hemodiafiltration with a cytokine-adsorbing hemofilter in patients with septic shock: a preliminary report. Blood Purif. 2014;38:211–218. doi: 10.1159/000369377. [DOI] [PubMed] [Google Scholar]
- 49.Yamana H, Horiguchi H, Fushimi K, Yasunaga H. Comparison of procedure-based and diagnosis-based identifications of severe sepsis and disseminated intravascular coagulation in administrative data. J Epidemiol. 2016;26:530–537. doi: 10.2188/jea.JE20150286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9:682–689. doi: 10.2215/CJN.07650713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data