Abstract
Background:
Minimally invasive surfactant therapy (MIST) is a new strategy to avoid mechanical ventilation (MV) in respiratory distress syndrome. The primary aim of this study was to test MIST as a means of avoiding MV exposure and pneumothorax occurrence in moderate and late preterm infants (32 to 36 weeks’ gestational age).
Methods:
This was a randomized controlled trial including three Canadian centres. Patients were randomized to standard management or to the intervention if they required nasal continuous positive airway pressure of 6 cm H2O and 35% FiO2 in the first 24 hours of life. Patients from the intervention group received MIST immediately after inclusion. The primary outcome was either need for MV or development of a pneumothorax requiring a chest tube. To ensure that clinicians were not biased toward delaying intubation in the intervention group, clinical failure criteria were also used as a primary outcome. The primary outcome was analyzed using bivariate and multivariate logistic regressions.
Results:
Among 45 randomized patients, 24 were assigned to MIST and 21 to standard management. Eight infants (33%) from the intervention group met the primary outcome criteria versus 19 (90%) in the control group (absolute risk reduction 0.57, 95% confidence interval 0.54 to 0.60). One patient in each group reached the primary outcome because of pneumothorax occurrence. The other patients were exposed to MV. None of the patients reached the clinical failure criteria.
Conclusion:
MIST for respiratory distress syndrome management in moderate and late preterm infants was associated with a significant reduction of MV exposure and pneumothorax occurrence.
Keywords: Hyaline membrane disease, Infant, Premature, Pulmonary surfactants, Respiratory distress syndrome, Spontaneous breathing.
Moderate and late preterm infants (320/7 to 366/7 weeks’ gestational age [GA]) account for the largest group of infants hospitalized in neonatal intensive care unit (NICU) (1). Respiratory distress syndrome (RDS) and pneumothorax cause significant morbidity in those infants (2–5). Up to 14% and 2.5% of all late preterm infants hospitalized in Canadian NICU presented with RDS and pneumothorax, respectively (2). No recommendation specifically addresses moderate and late preterm infants for ventilation strategies and surfactant administration. Noninvasive ventilation (NIV) is often the primary option for RDS management.
It is well established that invasive mechanical ventilation (MV), even for a short period, may cause or exacerbate pre-existing lung injuries (6,7). A growing literature supports that avoidance of MV in very preterm infants improves respiratory outcomes (8–11). Selective administration of surfactant after an initial period of nasal continuous positive airway pressure (NCPAP) is now recognized as an appropriate option for RDS management. It is thought that NCPAP provides more physiological air volume distribution than MV so it is safer (12,13). However, NCPAP without surfactant administration is associated with an increased risk of pneumothorax (8,14,15). On the other hand, early surfactant administration decreases the risk of pneumothorax but requires MV when using the standard mode of delivery (16).
Minimally invasive surfactant therapy (MIST) has been used as an alternative (17–24) and appears to potentially reduce the incidence of MV exposure (17–21). End-expiratory lung volume is improved after MIST and is associated with oxygenation improvement (25). In some studies, improvement of oxygen requirements and reduction of MV exposure were more impressive in older preterm infants (17,18,20,26). However, the efficacy of MIST in moderate and late preterm infants has not been studied and they were only included in limited numbers in previous cohorts studies (20,26,27).
This study aimed to evaluate the efficacy of MIST to reduce MV exposure and pneumothorax in a moderate and late preterm population with RDS.
METHODS
Trial design, setting and participants
This was a multicentre randomized control trial involving three Canadian level 3 NICUs conducted from January 6, 2014 to May 4, 2016. Preterm infants between 320/7 and 366/7 weeks GA presenting with RDS were eligible in their first 24 hours of life. Inclusion criteria were: a requirement of a fraction of inspired oxygen (FiO2) of 35% and NCPAP support of 6 cm of H2O to maintain saturation ≥90%. The 35% oxygen threshold was chosen based on a questionnaire completed by the neonatologists involved with the study and fits with the threshold used in previous studies (20,21,26). Exclusion criteria were lethal conditions or significant congenital malformations or intubation or pneumothorax prior to enrollment. Immediate extubation after surfactant administration (INSURE procedure) is not routinely performed in the three units involved. The trial was approved by the research ethics board from each participating centre and written parental consent was obtained for eligible infants after admission to the NICU.
Randomization and masking
All infants were initially managed with NCPAP. Different NIV modes, including synchronized and nonsynchronized nasal intermittent positive pressure ventilation, could be used after randomization. If those NIV modes were used prior to randomization, patients were switched back to NCPAP following enrollment. The randomization sequence was created by an independent statistician with SAS 9.3 (SAS Institute Inc., Cary, NC). Participants were randomized immediately after inclusion using sealed opaque envelopes (prepared by a nurse not involved in the study), in a 1:1 ratio by blocks of four stratified for GA to standard management or to the intervention. In the situation of multiple births, all infants were allocated to the same group.
Intervention
In the standard management group (control group), surfactant was only given after intubation based on the judgment of the attending physician. In the intervention group, infants received surfactant (Beractant, 4 mL/kg, 25 mg of phospholipids/mL) immediately after randomization. Atropine (20 mcg/kg) and fentanyl (1 mcg/kg) were administered prior to the procedure. A laryngoscopy was performed while patients were supported on NCPAP and surfactant was administered after tracheal insertion of a 5 French sterile and flexible gavage tube with Magill forceps. If desaturation or bradycardia occurred, the procedure was temporarily interrupted. Repeating MIST was allowed if the FiO2 rose to or remained above 30% as per Canadian recommendations (28).
Outcomes
The primary outcome was either a need for MV or the occurrence of a pneumothorax requiring chest tube insertion in the first 3 days on the study. Because the study was not blinded, to ensure that clinicians were not biased toward delaying intubation in the intervention group, two additional criteria for respiratory failure were used as primary outcomes for the patients in the intervention group: (i) development of respiratory acidosis confirmed by two pH results lower than 7.20 associated with partial pressure of carbon dioxide (pCO2) greater than 70 (mmHg) or (ii) lack of improvement of oxygen requirements in the 4 hours following MIST. Patients who met failure criteria were regarded as having reached the primary outcome in the analysis even if MV was not initiated. Those criteria were not applied to the control group to prevent overestimation of the intervention efficacy.
Secondary outcomes were the number of laryngoscopy attempts and the adverse events documented during MIST procedures (surfactant reflux and desaturations), or for intubation procedures (desaturations). The presence or absence of surfactant reflux was observed during laryngoscopy by the person performing the MIST procedure. Desaturations were classified as moderate (between 80% and 60%) or severe (below 60%).
Statistical analysis and sample size
Unpublished data from a previous study in one of the involved centres (CHU de Québec, 2013) revealed a combined rate of MV and pneumothorax of 60% in late preterm infants who required FiO2 level ≥35% in the first day of life. Because moderate preterm infants were also included in the present trial, we expected the primary outcome to occur in more than 60% of patients in the control group. Based on previous studies in which intubation rate after MIST in 28 to 34 weeks GA preterm infants ranged between 0% and 22% in the 72 hours after MIST, we hypothesized a primary event rate lower than 15% in the intervention group (17,20). Therefore, 44 participants would be required for a power of 80% and a type 1 error of 5% using the Fisher Exact Test.
The analysis was done according to the intention-to-treat principle. Patients' characteristics and respiratory outcomes were compared between groups using an exact-chi square test or Wilcoxon Mann–Whitney test, for qualitative and quantitative variables, respectively. The primary dichotomous outcome variables were analyzed using bivariate and multivariate logistic regressions with a Firth correction for bias when appropriate. Two sets of variables were considered and chosen a priori for the multivariate analysis. A first model (model 1) was adjusted for GA, antenatal corticosteroids administration, sex and inborn status. A second model (model 2) was adjusted for the previous variables as well as the following: age at oxygen introduction (< or ≥2 hours of life), age at surfactant administration (< or ≥12 hours of life), pH (< or ≥7.25) and pCO2 level (< or ≥65 mmHg) previous to surfactant administration. Those variables and their value were chosen a priori based on the average value for each parameter presented by the intubated patients in our previous cohort study. Absolute risk reduction, adjusted odds ratio (AOR) and number needed to treat were calculated with their 95% confidence intervals (95% CIs). The analyses were made with SAS 9.3 (SAS Institute Inc., Cary, NC) by an independent statistician.
RESULTS
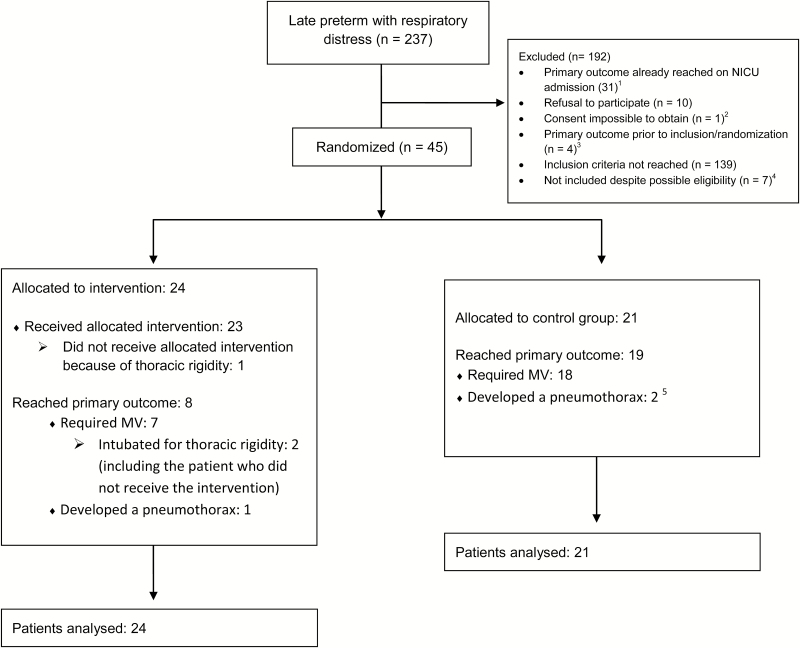
There were 237 infants screened (Figure 1). Forty-five were enrolled and randomized. All participants were analyzed according to their allocated group. Clinical characteristics of both groups are presented in Table 1. Among the 24 patients of the intervention group, 3 had repeated MIST. One of those three patients and six additional patients were intubated to receive extra doses of surfactant. Among the 21 patients of the control group, 19 were intubated and received surfactant (2 doses for five infants). Additional information concerning respiratory outcomes is presented in Table 2.
Figure 1.
Flow diagram. 1Among these, 27 were outborn patients and 4 patients were intubated in delivery room. 2One patient presented with severe RDS and was intubated because parents were not available to consent to the study. Clinicians decided not to delay surfactant administration. 3Four eligible patients developed a pneumothorax prior to inclusion and randomization. 4Three patients were not included by involuntary omission of the attending physician to recruit them. Three patients were intubated soon after NICU admission because the clinician judged that attempting MIST was not appropriate as the infants had a poor respiratory drive. One patient was intubated because the RDS diagnosis was not obvious. 5One patient developed a pneumothorax after he was intubated. MIST Minimally invasive surfactant therapy; MV Mechanical ventilation; N Number; NICU Neonatal intensive care unit; RDS Respiratory distress syndrome.
Table 1.
Patients characteristics
| Intervention group (N=24) | Standard management (N=21) | P value* | |
|---|---|---|---|
| Gestational age, mean (SD), weeks | 340/7 (1.4) | 336/7 (1.5) | 0.99 |
| 32 weeks | 7 | 8 | |
| 33 weeks | 4 | 3 | |
| 34 weeks | 7 | 5 | |
| 35 weeks | 3 | 2 | |
| 36 weeks | 3 | 3 | |
| Birthweight, mean (SD), grams | 2157 (487) | 2277 (658) | 0.56 |
| Apgar score† | |||
| 1 min | 8.0 (5.5–9.0) | 6.0 (5.0–8.0) | 0.48 |
| 5 min | 9.0 (7.0–9.0) | 7.0 (6.0–9.0) | 0.13 |
| Males (%) | 10 (42%) | 15 (71%) | 0.07 |
| Multiple births (%) | 3 (13%) | 6 (29%) | 0.19 |
| Antenatal steroids (%) | 16 (67%) | 11 (52%) | 0.37 |
| Caesarean section (%) | 18 (75%) | 18 (86%) | 0.38 |
| Outborn (%) | 4 (17%) | 6 (29%) | 0.48 |
| Age at oxygen initiation, hours† | 1.9 (1.2–4.3) | 0.5 (0.2–3.1) | 0.06 |
| Blood pH before surfactant† | 7.28 (7.24–7.29) | 7.23 (7.20–7.30) | 0.15 |
| pCO2 before surfactant† | 57 (52–60) | 63 (48–68) | 0.16 |
pCO 2 Partial pressure of carbon dioxide; SD Standard deviation.*Based on an exact Pearson chi-square test or Wilcoxon Mann–Whitney test. †Results presented as median with interquartile range.
Table 2.
Respiratory outcomes
| Intervention group (N=24) | Standard management (N=21) | P value* | |
|---|---|---|---|
| Age at surfactant administration, hours† | 15.4 (6.0–20.4) | 7.2 (4.8–17.5) | 0.26 |
| Number of surfactant doses per patient, mean (SD) | 1.5 (1.2–1.9) | 1.2 (0,88–1.40) | 0.11 |
| One surfactant dose, N (%) | 15 (62.5%) | 14 (66.7%) | 1.00 |
| Two or more surfactant doses, N (%) | 9 (37.5%) | 5 (23.8%) | 0.35 |
| Duration of NIV, days† | 4.2 (2.8–5.4) | 3.6 (2.3–4.9) | 0.56 |
| Duration of MV, days†,‡ | 2.9 (1.2–4.0) | 1.9 (0.8–3.2) | 0.27 |
| Duration of ventilation (NIV + MV), days† | 4.5 (3.4–6.7) | 6.3 (3.7–7.1) | 0.26 |
| Duration of oxygen administration, days† | 2.4 (1.9–2.8) | 2.3 (0.3–3.6) | 0.63 |
| Duration of NICU hospitalization, days† | 12.8 (7.0–20.1) | 16.2 (7.6–34.3) | 0.18 |
N Numbers; NICU Neonatal intensive care unit; NIV Noninvasive ventilation; MV Mechanical ventilation; SD Standard deviation.*Based on an exact Pearson chi-square test or Wilcoxon Mann–Whitney test. †Results presented as median with interquartile range. ‡Included 7 patients exposed to MV in the intervention group and 18 patients in the control group.
Primary outcome
All the patients in the intervention group but one received MIST (Figure 1). Eight (33%) met the primary outcome; seven patients required MV and one developed a pneumothorax. None met the criteria for respiratory failure during the 3 days on study. Among patients requiring MV, two were intubated because they had thoracic rigidity after fentanyl administration. One of those two patients had previously received MIST and was receiving premedication for a second MIST while the second had not yet received MIST. The five remaining patients did not improve after MIST so they were ultimately intubated. Among patients of the control group, 19 (90%) met one of the primary outcomes; 18 were intubated for surfactant administration and 1 developed pneumothorax. The mean FiO2 at intubation was 39% in the control group.
The incidence of the primary outcome was significantly lower in the intervention group (odds ratio 0.05, 95% CI 0.01 to 0.28; absolute risk reduction 0.57, 95% CI 0.54 to 0.60, number needed to treat 1.75, 95% CI 1.67 to 1.85; P<0.001). The same association was found using logistic regression (model 1: AOR 0.02, 95% CI 0.002 to 0.239, P<0.01 and model 2: AOR 0.05, 95% CI 0.0001 to 0.2634, P<0.01). Sensitivity analyses showed no difference in any outcomes (data not shown) after removing the second twin in the control group (for all other multiple births, only one infant was randomized).
Secondary outcomes
The average number of laryngoscopy attempts per patient was 2.3 (SD 1.2) in the intervention group and 2.3 (SD 1.9) in the control group (P=0.77). Surfactant reflux during MIST was documented in 16 patients (66%). The rates of moderate desaturations were 58% and 16% in the intervention and control group, respectively (P<0.01). The rates of severe desaturations were 42% and 58% in the intervention and control group, respectively (P=0.3).
DISCUSSION
This is the first study to assess MIST in moderate and late preterm infants. Systematic use of MIST allowed a significant reduction of the primary outcome compared with conventional management. The efficacy of this simple technique is quite remarkable considering its simplicity and lack of major complications. The rate of MV exposure in the control group is high but the study was specifically designed to select the most susceptible patients and obtain the best perspective on MIST efficacy.
Standardized management prior to study enrollment is a strength of this study, because it has promoted the selection of a uniform population and avoided delay in intervention. Early and systematic administration of surfactant in the intervention group was chosen in order to prevent as many complications as possible. Because pneumothorax occurred after 24 hours life in 15 patients who did not meet the study inclusion criteria as they did not require 35% FiO2 in the first 24 hours of life, it seems that administration of MIST at a lower FiO2 or after 24 hours of life may be useful. Repeating MIST cannot be recommended from our results because this was performed in only three patients, one of whom was ultimately intubated. Indeed, using a relatively low FiO2 threshold for MIST has the advantage of allowing repeating surfactant administration after intubation within an appropriate timeframe if required by the patient’s condition.
One limitation is that no specific criteria for surfactant administration were established in the control group. Nonetheless, avoiding MV where possible is the usual practice in the study centres. No patients in the intervention group met the criteria for respiratory failure so we do not think that clinicians delayed MV in the MIST group. It is possible that by chance patients in the control group had more severe RDS as they required both oxygen and surfactant administration sooner than did those in the MIST group. Those characteristics were taken into account in the multivariate regression model and did not impact the conclusions. However, this limitation must be considered carefully before generalization of the study results to populations of late preterm affected by severe RDS.
The MIST procedure was associated with a high rate of surfactant reflux, indicating that surfactant delivery was probably not optimal for all patients. For the purpose of the study, surfactant was administered in a bolus under direct vision during laryngoscopy. The Cologne method, which involves closing the mouth during surfactant administration, would probably have helped to reduce surfactant reflux (29). Furthermore, maintaining positive airway pressure, which is lost during laryngoscopy because of mouth opening, would allow more gradual surfactant administration without compromising patients’ respiratory stability (23,29). However, we worried that because they are more active than extremely preterm infants, moderate and late preterm patients could have displaced the gavage tube during the procedure, resulting in esophageal surfactant administration. A solution would be to use a more rigid catheter for the procedure, such as the Angiocath used in the Hobart method (20). Using a lower volume surfactant formula, such as Curosurf, would be another option to decrease surfactant reflux. The MIST procedure was also associated with a higher rate of moderate desaturations than the intubation procedure. Once again we believed that closing patients’ mouth during the procedure and using a lower volume surfactant formula could be part of the solution. Nonetheless, severe desaturations rates were not different between both interventions.
Finally, sedation remained an issue because two out of eight patients had to be intubated before MIST could be attempted because of thoracic rigidity after fentanyl administration. Very limited data are available about optimal sedation for MIST. Propofol has been used in one study, but it was associated with more respiratory complications and double the rate of MV exposure compared with patients receiving no sedation (30). Even though there is probably a risk of increasing failure of the intervention with sedation, we believe that it should not be withheld for patients receiving MIST.
The decision to intubate moderate or late preterm neonates affected by RDS is sometimes difficult, mainly when they are in an ‘in between’ condition or present with a moderate RDS. It is challenging to find a good balance between a safe and optimum management within the window for surfactant efficacy without being too aggressive. MIST seems a promising alternative to reduce MV exposure and RDS complications, making the decision to administer surfactant easier. Future improvements in the technique could optimize the benefits.
Acknowledgements
On behalf of the entire team, I thank all the following collaborators who made the achievement of this project possible: Mylène Leblanc1, research nurse at the Centre hospitalier universitaire de Québec for her contribution to the randomization organization; Dr. Alyssa Morin1, Dr. Audrey Hébert1, Dr. Christine Drolet1, Dr. Geneviève Piuze1, Dr. Geneviève Tremblay1, Dr. Lyne Frenette1, Dr. Marc Beltempo1, Dr. Marianne Deschênes1 and Dr. Roger Giroux1 for their contribution in the recruitment of this trial; Dr. Romain Mandel2 for his contribution to the implementation and achievement of the project at Hôpital Maisonneuve-Rosemont; Émilie Beaulieu3 for her contribution to the implementation and achievement of the project at the CHU de Sherbrooke. Collaborators affiliations: 1Department of Pediatrics, CHU de Québec - Université Laval, 2705 boulevard Laurier, Québec, Québec G1V 4G2; 2Department of Pediatrics, Université de Montréal, Hôpital Maisonneuve-Rosemont, 5415, boul. l’Assomption, Montréal, Québec H1T 2M4; 3Department of Pediatrics, Université de Sherbrooke, Hôpital Fleurimont, 3001 12e Avenue N, Sherbrooke, Québec J1H 5H3. Funding sources: No funding was obtained for this study.
References
- 1. Hamilton BE, Martin JA, Ventura SJ. Births: Preliminary data for 2006. Natl Vital Stat Rep 2007;56:1–18. [PubMed] [Google Scholar]
- 2. Bassil KL, Shah PS, Shah V, Ye XY, Lee SK, Jefferies AL; Canadian Neonatal Network Impact of late preterm and early term infants on Canadian neonatal intensive care units. Am J Perinatol 2014;31:269–78. [DOI] [PubMed] [Google Scholar]
- 3. Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol 2011;205:374.e1–9. [DOI] [PubMed] [Google Scholar]
- 4. Consortium on Safe Labor , Hibbard JU, Wilkins I, Sun L, et al. Respiratory morbidity in late preterm births. JAMA 2010;304:419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Colin AA, McEvoy C, Castile RG. Respiratory morbidity and lung function in preterm infants of 32 to 36 weeks’ gestational age. Pediatrics 2010;126:115–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Attar MA, Donn SM. Mechanisms of ventilator-induced lung injury in premature infants. Semin Neonatol 2002;7:353–60. [DOI] [PubMed] [Google Scholar]
- 7. Bohlin K. RDS–CPAP or surfactant or both. Acta Paediatr 2012;101:24–8. [DOI] [PubMed] [Google Scholar]
- 8. Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB; COIN Trial Investigators Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med 2008;358:700–8. [DOI] [PubMed] [Google Scholar]
- 9. SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network , Finer NN, Carlo WA, Walsh MC, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med 2010;362:1970–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stevens TP, Finer NN, Carlo WA, et al. ; SUPPORT Study Group of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Respiratory outcomes of the Surfactant Positive Pressure and Oximetry Randomized Trial (SUPPORT). J Pediatr 2014;165:240–9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunn MS, Kaempf J, de Klerk A, et al. ; Vermont Oxford Network DRM Study Group. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011;128:e1069–76. [DOI] [PubMed] [Google Scholar]
- 12. Miedema M, van der Burg PS, Beuger S, de Jongh FH, Frerichs I, van Kaam AH. Effect of nasal continuous and biphasic positive airway pressure on lung volume in preterm infants. J Pediatr 2013;162:691–7. [DOI] [PubMed] [Google Scholar]
- 13. Carvalho CG, Silveira RC, Procianoy RS. Ventilator-induced lung injury in preterm infants. Rev Bras Ter Intensiva 2013;25:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmölzer GM, Kumar M, Pichler G, Aziz K, O’Reilly M, Cheung PY. Non-invasive versus invasive respiratory support in preterm infants at birth: Systematic review and meta-analysis. BMJ 2013;347:f5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Village EG. Respiratory support in preterm infants at birth. Pediatrics 2013;133:171–4. [DOI] [PubMed] [Google Scholar]
- 16. Suresh GK, Soll RF. Overview of surfactant replacement trials. J Perinatol 2005;25(Suppl 2):S40–4. [DOI] [PubMed] [Google Scholar]
- 17. Göpel W, Kribs A, Ziegler A, et al. ; German Neonatal Network. Avoidance of mechanical ventilation by surfactant treatment of spontaneously breathing preterm infants (AMV): An open-label, randomised, controlled trial. Lancet 2011;378:1627–34. [DOI] [PubMed] [Google Scholar]
- 18. Kribs A, Roll C, Göpel W, et al. ; NINSAPP Trial Investigators. Nonintubated surfactant application vs conventional therapy in extremely preterm infants: A randomized clinical trial. JAMA Pediatr 2015;169:723–30. [DOI] [PubMed] [Google Scholar]
- 19. Kanmaz HG, Erdeve O, Canpolat FE, Mutlu B, Dilmen U. Surfactant administration via thin catheter during spontaneous breathing: Randomized controlled trial. Pediatrics 2013;131:e502–9. [DOI] [PubMed] [Google Scholar]
- 20. Dargaville PA, Aiyappan A, Cornelius A, Williams C, De Paoli AG. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed 2011;96:F243–8. [DOI] [PubMed] [Google Scholar]
- 21. Porath M, Korp L, Wendrich D, et al. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Arch Dis Child Fetal Neonatal Ed 2014;378:1607–8. [DOI] [PubMed] [Google Scholar]
- 22. More K, Sakhuja P, Shah PS. Minimally invasive surfactant administration in preterm infants: A meta-narrative review. JAMA Pediatr 2014;168:901–8. [DOI] [PubMed] [Google Scholar]
- 23. Aguar M, Vento M, Dargaville PA. Minimally invasive surfactant therapy: An update. Neoreviews 2014;15:e275–85. [Google Scholar]
- 24. Isayama T, Iwami H, McDonald S, Beyene J. Association of noninvasive ventilation strategies with mortality and bronchopulmonary dysplasia among preterm infants: A systematic review and meta-analysis. JAMA 2016;316:611–24. [DOI] [PubMed] [Google Scholar]
- 25. van der Burg PS, de Jongh FH, Miedema M, Frerichs I, van Kaam AH. Effect of minimally invasive surfactant therapy on lung volume and ventilation in preterm infants. J Pediatr 2016;170:67–72. [DOI] [PubMed] [Google Scholar]
- 26. Al Ethawi Y. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. J Clin Neonatol 2012;1:66–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aguar M, Cernada M, Brugada M, Gimeno A, Gutierrez A, Vento M. Minimally invasive surfactant therapy with a gastric tube is as effective as the intubation, surfactant, and extubation technique in preterm babies. Acta Paediatr 2014;103:e229–33. [DOI] [PubMed] [Google Scholar]
- 28. Davis DJ, Barrington KJ, Society CP. Recommendations for neonatal surfactant therapy. Paediatr Child Health (Oxford) 2012;17;109–16. [PMC free article] [PubMed] [Google Scholar]
- 29. Kribs A, Pillekamp F, Hünseler C, Vierzig A, Roth B. Early administration of surfactant in spontaneous breathing with nCPAP: Feasibility and outcome in extremely premature infants (postmenstrual age </=27 weeks). Paediatr Anaesth 2007;17:364–9. [DOI] [PubMed] [Google Scholar]
- 30. Dekker J, Lopriore E, Rijken M, Rijntjes-Jacobs E, Smits-Wintjens V, Te Pas A. Sedation during minimal invasive surfactant therapy in preterm infants. Neonatology 2016;109:308–13. [DOI] [PubMed] [Google Scholar]