Abstract
Study Objectives
Precision medicine for obstructive sleep apnea (OSA) requires noninvasive estimates of each patient’s pathophysiological “traits.” Here, we provide the first automated technique to quantify the respiratory arousal threshold—defined as the level of ventilatory drive triggering arousal from sleep—using diagnostic polysomnographic signals in patients with OSA.
Methods
Ventilatory drive preceding clinically scored arousals was estimated from polysomnographic studies by fitting a respiratory control model (Terrill et al.) to the pattern of ventilation during spontaneous respiratory events. Conceptually, the magnitude of the airflow signal immediately after arousal onset reveals information on the underlying ventilatory drive that triggered the arousal. Polysomnographic arousal threshold measures were compared with gold standard values taken from esophageal pressure and intraoesophageal diaphragm electromyography recorded simultaneously (N = 29). Comparisons were also made to arousal threshold measures using continuous positive airway pressure (CPAP) dial-downs (N = 28). The validity of using (linearized) nasal pressure rather than pneumotachograph ventilation was also assessed (N = 11).
Results
Polysomnographic arousal threshold values were correlated with those measured using esophageal pressure and diaphragm EMG (R = 0.79, p < .0001; R = 0.73, p = .0001), as well as CPAP manipulation (R = 0.73, p < .0001). Arousal threshold estimates were similar using nasal pressure and pneumotachograph ventilation (R = 0.96, p < .0001).
Conclusions
The arousal threshold in patients with OSA can be estimated using polysomnographic signals and may enable more personalized therapeutic interventions for patients with a low arousal threshold.
Keywords: arousability, pathophysiology, personalized medicine, endotype
Statement of Significance
A low respiratory arousal threshold (i.e., greater ease of arousal from sleep, or “sleep instability”) may prevent some patients with obstructive sleep apnea from achieving stable breathing. Gold standard arousal threshold assessment requires measurement of respiratory effort via esophageal catheters or strategic manipulation of continuous positive airway pressure (CPAP) by trained physiologists. Here, we present a novel noninvasive approach to estimate the arousal threshold using polysomnographic signals. The approach employs an uncalibrated ventilation signal (e.g., nasal pressure) and clinical scoring (arousals and respiratory events). Our arousal threshold estimates correlated well (R ~ 0.7) with gold standard measures (esophageal pressure, intraesophageal diaphragm EMG, and CPAP manipulation). Thus, arousal threshold estimation using respiratory signals can now be performed clinically.
INTRODUCTION
Currently, treatments for obstructive sleep apnea (OSA) are not prescribed with due consideration of the underlying pathophysiological mechanisms responsible. Such mechanisms can vary greatly across individuals and may affect therapeutic effectiveness.1–4
Approximately one-third of patients with moderate-to-severe OSA exhibit increased respiratory arousability—i.e., a low respiratory arousal threshold—defined as the occurrence of arousal from sleep with a small rise in ventilatory drive.1,3–9 In principle, this trait predisposes particular patients to OSA (see Supplementary Figure S1).10 Because increased ventilatory drive activates pharyngeal dilator muscles and improves pharyngeal patency in most patients,7,11–14 premature termination of sleep would prevent such muscle activation and reduce the likelihood of stable breathing.15 Thus, a lower arousal threshold in lighter versus deeper sleep is believed to contribute to sleep apnea.9,15–17 A lower arousal threshold also probably interacts with increased chemosensitivity to promote respiratory instability.18,19 Available evidence also suggests that increasing the arousal threshold with a hypnotic (eszopiclone) can improve OSA severity in patients with a low arousal threshold.3
To date, however, quantitative assessment of the arousal threshold has been invasive, specialized, and thereby restricted to physiological laboratories.6,7 One approach was to develop an arousal threshold predictive score based on regression and polysomnographic summary variables (apnea–hypopnea index [AHI], nadir oxygen saturation, and percentage of respiratory events that were hypopneas)20; although this approach is highly accessible, it does not leverage the rich respiratory data available in routine sleep studies. Recently, we developed an automated, noninvasive estimate of ventilatory drive, i.e., the “intended” ventilation that would be observed if the airway were patent,21 based on a polysomnographic ventilation signal. The method is based on the concept that the ventilatory drive that builds up during each obstructive respiratory event becomes revealed in the ventilation signal when the airway is reopened after an event (see Methods). Such an estimate of the ventilatory drive should enable polysomnographic estimation of the arousal threshold. Accordingly, we prospectively examined whether our ventilatory drive estimate of the arousal threshold compares favorably with values obtained using esophageal pressure and intraoesophageal diaphragm EMG as gold-standard measures of ventilatory drive.5,22 We also compared our method against results obtained via the continuous positive airway pressure (CPAP) drop technique (retrospective analysis; see Methods).
We emphasize that the objective was not to replace gold-standard methods; rather, we aimed to provide a means to estimate the arousal threshold contribution to OSA in the clinical setting.
METHODS
Participants
In this study, participants were recruited under three distinct protocols (details below). In each case, patients diagnosed with moderate-to-severe OSA (AHI ≥ 15 events/hour) were eligible. Exclusion criteria included use of respiratory stimulants or depressants (including opioids and benzodiazepines), heart failure or lung diseases, central sleep apnea, and pregnancy. Participants provided written informed consent and approval was granted by the Partners’ Institutional Review Board.
Definition
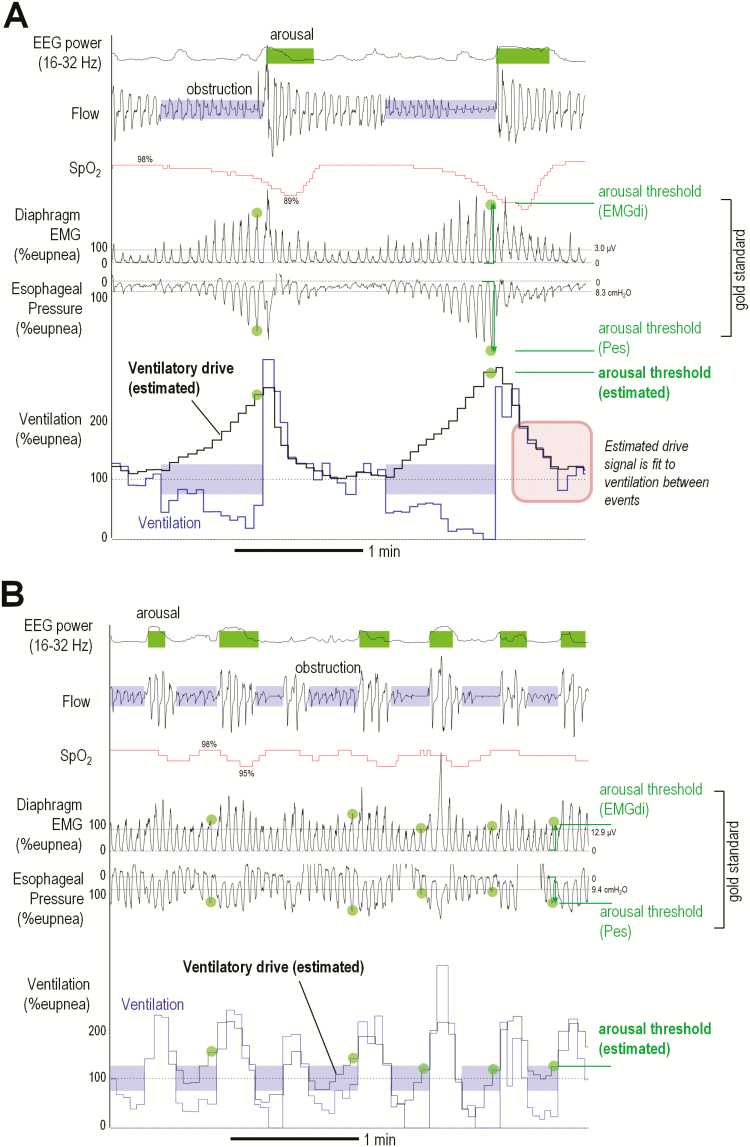
We use the term “arousal threshold” in reference to the respiratory neuromechanical drive or pressure that is present immediately prior to a clinically defined electroencephalography (EEG) (cortical) arousal (see Figure 1, green arrows indicate respiratory drive and pressure prior to arousal). In each analysis, we present the percentage increase in ventilatory drive above baseline “eupneic” levels to describe the arousal threshold (see below for details), thereby facilitating comparison of individuals with substantially different eupneic ventilatory drive. Presenting the arousal threshold in this way also makes it possible to quantify the arousal threshold without the need for a calibrated ventilation signal, a major goal of our study.
Figure 1.
Example illustration of arousal threshold estimation in patients with (A) high and (B) low arousal thresholds. Green circles illustrate values of the arousal threshold, i.e., the value of ventilatory drive that precedes (triggers) EEG arousal. Staircase traces illustrate breath-by-breath values of ventilation (blue) and model-based estimation of ventilatory drive (black); the model is best fit to ventilation between events (shaded blue). Note that the time course of the estimated ventilatory drive trace matches that of esophageal pressure and diaphragm EMG. SpO2 = pulse oxygen saturation. EMGdi = diaphragm EMG (integrated). Pes = Esophageal pressure. High frequency EEG power is shown to corroborate clinically scored EEG arousals (shaded green; sigmoid transformed, arbitrary units). Eupneic ventilation (dashed line) is estimated from the mean ventilation in the window. Both examples are from supine non-REM sleep.
Polysomnographic Setup
Polysomnographic studies in the three protocols included EEG, electrooculography, electrocardiography, thoracoabdominal movements, and pulse oximetry. Sleep, arousals, and respiratory events were scored according to standard criteria (hypopneas: 30% reduction in flow with ≥3% desaturation or arousal). EEG arousals were defined by an abrupt shift in EEG frequency (θ, α, or β power, but not spindles) for ≥3 seconds23. Patients were encouraged to sleep supine for the duration of the night. Analyses were limited to supine nonrapid eye movement (non-REM) sleep as REM durations are typically brief in our physiological studies4–7 (Table 1).
Table 1.
Patient Characteristics.
| Characteristics | Study 1 | Study 2 | Study 3 |
|---|---|---|---|
| Comparison to esophageal pressure and diaphragm EMG (N = 29) | Comparison to CPAP drops (N = 28)a | Nasal pressure vs. pneumotach (N = 11)b | |
| Demographics | |||
| Age (yr) | 57 ± 9 | 48 ± 10 | 57 ± 8 |
| Sex (M:F) | 21:8 | 19:9 | 9:2 |
| Body mass index (kg/m2) | 32 ± 6 | 35 ± 6 | 30 ± 6 |
| Currently treated (CPAP:oral appliance:untreated) | 11:2:16 | 28:0 | 2:1:8 |
| Polysomnography | |||
| OSA severity (mild:moderate:severe) | 9:4:16 | 0:9:19 | 4:1:6 |
| Apnea–hypopnea index, total (events/h) | 40.9 ± 28.0 | 44.4 ± 26.3 | 41.6 ± 38.3 |
| Apnea–hypopnea index, non-REM (events/h)c | 40.7 ± 28.5 | 46.1 ± 26.5 | 41.7 ± 38.8 |
| Central events, non-REM (% respiratory events)c | 0.0 ± 0.0 | 0.9 ± 2.2 | 2.5 ± 8.2 |
| Hypopneas, non-REM (% respiratory events)c | 57.6 ± 30.9 | 82.4 ± 19.8 | 64.7 ± 36.4 |
| Arousal index, non-REM (events/h)c | 55.3 ± 25.6 | 44.1.21.9 | 50.3 ± 26.4 |
| Total sleep time (min) | 221 ± 100 | 321 ± 51 | 254 ± 102 |
| Diagnostic sleep time off CPAP (min)d | 124 ± 86 | 321 ± 51 | 210 ± 126 |
| Non-REM 1 (% total sleep time) | 38 ± 19 | 43 ± 17 | 30 ± 14 |
| Non-REM 2 (% total sleep time) | 49 ± 15 | 46 ± 14 | 54 ± 14 |
| Non-REM 3 (% total sleep time) | 5 ± 5 | 1 ± 3 | 8 ± 13 |
| REM (% total sleep time) | 8 ± 8 | 10 ± 5 | 7 ± 6 |
Values are mean ± SD.
REM = rapid eye movement sleep.
aPatients in Study 2 were a separate group who were retrospectively examined as part of a larger published study;1,6,25 the 28-patient subgroup has been described previously.21
b N = 7/11 patients in Study 3 also participated in Study 1.
cRespiratory event data in non-REM reflect the supine position only.
dA portion of the nights were used for CPAP manipulation leaving a reduced duration available for analysis.
Quantifying the Arousal Threshold Using Polysomnography
Polysomnographic arousal threshold values were estimated during spontaneous breathing (i.e., off CPAP). First, 7-minute windows containing non-REM sleep were automatically identified.21 For each window, the instantaneous flow signal was integrated to yield a breath-to-breath ventilation time series (tidal volume × respiratory rate); ventilation time series was normalized by dividing each value by the “eupneic ventilation” estimated using the mean ventilation in each window (see Supplementary Figure S2). Ventilatory drive—i.e., the level of ventilation that would be observed if the pharyngeal airway was suddenly made patent—was estimated using a chemoreflex feedback control model (gain, response time, delay) and the ventilation signal21 (Figure 1). For each window, the feedback model converted ventilatory fluctuations into an opposing ventilatory drive signal that is best fit to the ventilation signal when the airway is considered to be patent (i.e., the ventilation between scored obstructive apneas and hypopneas, see red square in Figure 1A). Automated fine-tuning of start/end times of clinically scored apneas/hypopneas was employed,21 see Supplementary Material. The ventilatory response to arousal, reflecting the average additional increase in ventilatory drive caused by arousal, was accounted for via a fourth parameter.21 For each window, the ventilatory drive immediately prior to the start of each scored EEG arousal (e.g., at the termination of a respiratory event) was identified, and the arousal threshold calculated as the mean value of these ventilatory drive values.6 Median values across all windows were taken to yield a single value of the arousal threshold for each patient. Analysis was fully automated using in-house software (Matlab, Mathworks, Natick, MA, USA).
Study 1—Esophageal Pressure and Diaphragm EMG
Procedures
Thirty-one patients attended a prospective overnight physiological study aimed at evaluating our method in comparison to esophageal pressure and diaphragm EMG measures of the arousal threshold. One patient was unable to tolerate esophageal catheter placement and one individual no longer exhibited OSA (AHI <5 events/hour) on the study night despite their diagnosis and was excluded, leaving N = 29 available for analysis. Ventilatory flow was assessed via a pneumotachograph (Hans Rudolph, Shawnee, KS, USA; Validyne Engineering, Northridge, CA, USA) attached to a sealed mask; the uncalibrated flow signal was used for our polysomnographic arousal threshold measure. An esophageal catheter (Millar, Inc., Houston, TX, USA) was placed via a port in the mask through a lidocaine-anesthetized nostril such that it lay in the lower third of the esophagus. An intraoesophageal diaphragm EMG catheter was placed via the same nostril (Servo-i Ventilator, Maquet Getinge Group, Wayne, NJ) such that the center of its electrode array lay at the level of the crural diaphragm.
Quantifying Arousal Threshold Using Esophageal Pressure
Pressure swings were quantified based on the nadir pressure minus the level at the start of inspiration. Quantitative arousal inclusion or exclusion criteria were modified from prior physiological studies20,24: breaths immediately prior to arousal onset were identified. Candidate breaths were included if they were >3 breaths after sleep onset to ensure sleep was established. Pressure swings rose ≥5% above eupneic levels; arousals failing this criterion were considered spontaneous arousals and excluded. The median value was reported for each patient.
To describe the arousal threshold using the percent increase above baseline (eupneic) levels, we estimated eupneic esophageal pressure swings for each patient during sleep by measuring (1) wakefulness respiratory mechanics using the ratio Y = ventilation/[pressure swing] and (2) eupneic ventilation based on mean ventilation (sleep and arousals) across all windows of the analyzed non-REM sleep (7.0 ± 1.7 L/minute, mean ± SD; see Supplementary Figure S2). Eupneic pressure swings were then calculated using [eupneic ventilation]/Y (mean 13.2 ± 5.7 cm H2O); see Supplementary Material.
Quantifying the Arousal Threshold Using Diaphragm EMG
The analysis described for esophageal pressure was repeated for diaphragm EMG. The integrated EMG (root-mean-squared, smoothed [160 milliseconds]) was used for analysis. Diaphragm EMG swings on each breath prior to arousal were calculated based on the peak integrated diaphragm EMG minus the start-inspiratory (nadir) level. Values were presented as a percentage of eupneic diaphragm EMG (as described for esophageal pressure).
Study 2—CPAP Drops
Procedures
We retrospectively analyzed data from 28 participants who took part in a larger OSA phenotyping protocol where arousal threshold was assessed using CPAP manipulation.1,6,21,25 Patients were selected based on polysomnographic data availability and successful completion of the parent study.21 Patients completed a clinical polysomnographic study and, ~1–2 weeks later, completed a separate CPAP drop study to measure the arousal threshold (model-based ventilatory drive values).6 To provide a polysomnographic arousal threshold estimate, the nasal pressure signal from the clinical study was used as a surrogate of ventilatory flow (Alice Sleepware, Philips Respironics, Murrysville, PA, USA; linearized using a square-root transform21,26).
Quantifying the Arousal Threshold Using CPAP Manipulation
Gold standard values of the ventilatory drive preceding arousal were measured during the CPAP drop studies as follows: In brief, CPAP was lowered abruptly from a therapeutic level to various subtherapeutic levels to reduce ventilation (measured via pneumotachograph and sealed mask) and raise ventilatory drive. After 3 minutes, CPAP was abruptly returned to therapeutic levels to characterize the reflex ventilatory drive response to reduced ventilation6,21,27; this response was used to estimate the chemical drive preceding arousals during the drops.6,27
Study 3—Nasal Pressure Versus Pneumotachograph Ventilation
Eleven patients completed a prospective validation study to assess the validity of using nasal pressure as a clinical surrogate of ventilatory flow in our method. To simultaneously measure nasal pressure (nasal cannula) and ventilatory flow (pneumotachograph with oronasal mask), a modified cannula (cut to fit under the mask) provided a nasal pressure signal that was referenced to mask pressure to reflect the pressure signal available clinically. For each patient, the arousal threshold was estimated using nasal pressure (square-root transformed) and then separately quantified using pneumotachograph flow for comparison.
Statistical Analysis
Arousal threshold values obtained from the polysomnographic method were correlated with gold standards. Correlations were chosen because the clinical utility of our method was considered to depend on whether our results correlated strongly with gold standard measures, rather than exactly measuring them (i.e., y = x); we also considered that a good correlation was essential (rather than agreement) if the method was to have value when implemented in future regression models to predict differences in outcomes. Correlation coefficients ≥0.7 were considered strong. Limits of agreement was assessed based on the SD of the regression (prediction interval) as recommended by Bland and Altman.28 Multiple linear regression was used to determine whether our polysomnographic arousal threshold values were associated with the primary gold standards (esophageal pressure, diaphragm EMG) independently of known clinical predictors (AHI, nadir oxygen saturation, the proportion of respiratory events that are hypopneas, and median apnea or hypopnea duration20), i.e., that our method provided unique information that was not otherwise available. Multiple regression was also employed to explore potential bias due to age, sex, or body mass index. Data are presented as mean ± SD, unless described otherwise. Significance was accepted at p < .05.
RESULTS
Baseline characteristics for the three study populations are detailed in Table 1. Illustrative examples of OSA patients with higher versus lower arousal thresholds are shown (Figure 1).
Comparison to Gold Standards
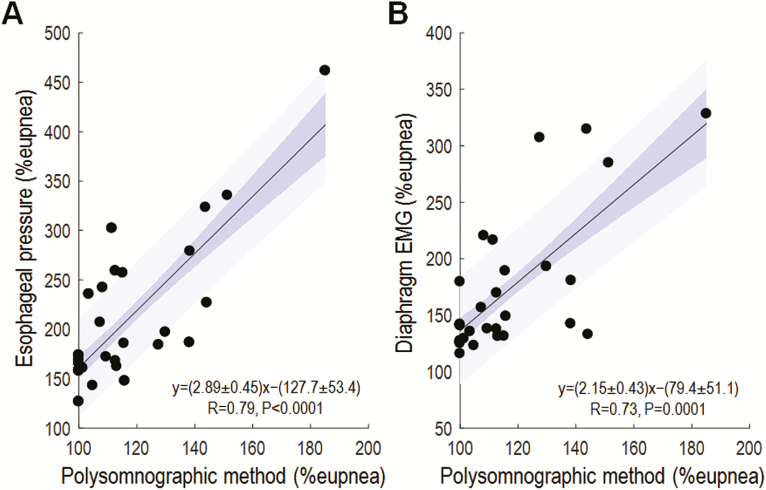
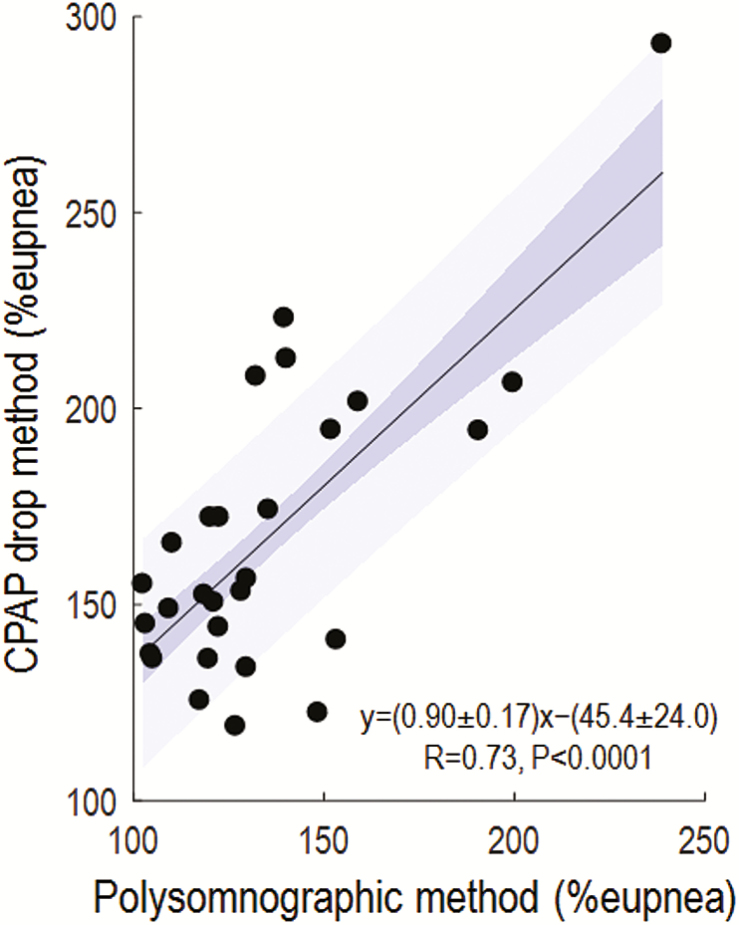
Our novel polysomnographic measures of the arousal threshold correlated strongly with values taken from esophageal pressure (Figure 2A), intra-esophageal diaphragm EMG (Figure 2B), and CPAP manipulation (Figure 3).
Figure 2.
Our novel measure of arousal threshold correlates favorably with measures using esophageal pressure (A) and intraoesophageal diaphragm EMG (B) (N = 29). Here, our polysomnographic method was implemented using pneumotach-measured ventilation. Blue shading shows the standard error (dark) and SD (light) of the regression. Note that y-axis scales are broader than the x-axis scales indicating that the polysomnographic values are smaller than the gold standards. The width of the prediction interval (1.96 × SD) indicates agreement (95% confidence); 1.96 × SD of the difference between the regression prediction and the gold standard is (A) ±89% eupnea and (B) ±86% eupnea.
Figure 3.
Our novel measure of arousal threshold compares favorably with that estimated using the CPAP drop technique (N = 28). Here, our polysomnographic method was implemented using nasal pressure (linearized, see Methods). Blue shading shows the standard error (dark) and SD (light) of the regression. Agreement (1.96 × SD) is given by ±52% eupnea.
Effect of Normalization
Our arousal threshold measures were not correlated with arousal threshold values from absolute esophageal pressure swings in cmH2O (i.e., not presented as percentage of eupneic levels; R = 0.3, p = .12; see Supplementary Material Results section). There was a modest correlation with the arousal threshold values measured via CPAP drops in L/minute (R = 0.46, p = .01).
Within-Subject Variability
The within-subject SD of the polysomnographic arousal threshold (across windows) was 21 ± 10% of the median in the esophageal pressure study and 21 ± 9% of median in the CPAP drop study.
Low Versus High Arousal Threshold
Our measure also identified patients with a low versus high arousal threshold based on various gold standard definitions (receiver operating characteristic area under curve range 0.67–0.90; Supplementary Table S1). Values of the gold standard arousal threshold were significantly lower in patient subgroups defined based on a low versus high polysomnographic arousal threshold (Supplementary Table S2).
Use of Nasal Pressure as a Ventilation Surrogate
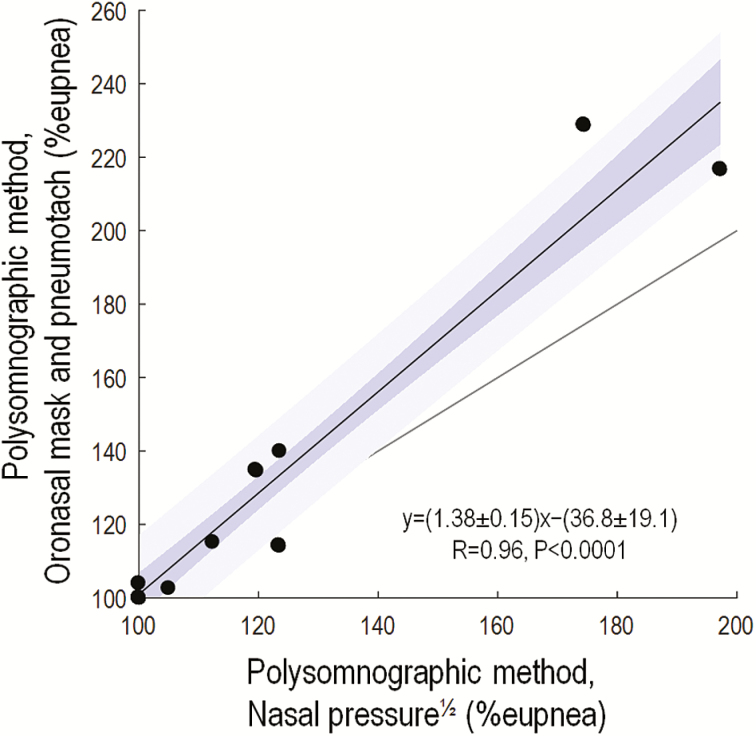
Remarkably similar values of the arousal threshold were obtained when using nasal pressure compared with pneumotachograph ventilation (Figure 4).
Figure 4.
Comparisons of our novel measures of arousal threshold taken using nasal pressure—a clinical surrogate of ventilation—and gold standard oronasal ventilation measured via a pneumotachograph (N = 11). Note the excellent correlations observed. Blue shading shows the standard error (dark) and SD (light) of the regression. Grey line shows the line of identity. Agreement (1.96 × SD) is given by ±24% eupnea. Note that two patients have data at x = 100, y = 100.
Multivariate Analyses
The polysomnographic arousal threshold remained significantly correlated with esophageal pressure values after adjusting for known clinical predictors20 of the arousal threshold (p < .001), namely, AHI (p = .17), nadir oxygen saturation (minSpO2, p = .7), the fraction of respiratory events that were hypopneas (Fhypopneas, p = .5), and respiratory event duration (p > .9). Similarly, the polysomnographic arousal threshold remained significantly correlated with diaphragm EMG values after adjusting for AHI (p = .27), minSpO2 (p = .78), Fhypopneas (p = .4), and event duration (p = .28). The polysomnographic arousal threshold values were associated with each of these clinical predictors (AHI: R = 0.53, p = .003; minSpO2: R = 0.44, p = .02; Fhypopneas: R = 0.49, p = .007; event duration: R = 0.43, p = .02). Diaphragm EMG values of the arousal threshold were associated with minSpO2, Fhypopneas, and event duration (minSpO2: R = 0.37, p = .05; Fhypopneas: R = 0.45, p = .015; event duration: R = 0.44, p = .017), but not the AHI (R = 0.27, p = .16). Esophageal pressure values (% eupnea) were not significantly associated with these variables (R = 0.27, 0.31, 0.31, 0.34, respectively; p = .07–.14). See Supplementary Material Results section for additional analyses and regression equations to predict arousal threshold from clinical variables.
The polysomnographic arousal threshold also remained significantly correlated with the esophageal pressure values (and with diaphragm EMG values) after adjusting for demographic variables (age, sex, and BMI; p < .0001 for both). Moreover, demographic variables did not contribute significantly to systematic bias.
DISCUSSION
Our study showed that the arousal threshold contribution to OSA is evident from the pattern of breathing and arousals during sleep. We also illustrated that nasal pressure, as a clinical surrogate of ventilation, provides the information necessary to estimate the arousal threshold using routine polysomnography. In combination with our measures of loop gain, and new measures of collapsibility,29–31 approaches to estimate the primary mechanisms underlying OSA in individual patients are rapidly emerging. Our hope is that clinical implementation of these techniques will lead the way for therapeutic interventions based on underlying pathophysiology.
Consistency With Available Literature
Our finding that the arousal threshold can be quantified from the ventilatory pattern during sleep in patients with OSA is novel, but also consistent with available literature. Our technique relies on the intuitive concept that a greater arousal threshold manifests as a greater level of ventilation immediately after event termination, a concept also recently validated outside our laboratory.17 Our method also accounts for individual variability in the ventilatory response to arousal3,5,8,10; that is, simply measuring ventilation rather than estimating the ventilatory drive prior to arousal weakened the correlations observed (see Supplementary Material). Polysomnographic values were also related to known clinical correlates of the arousal threshold (event length, apneas versus hypopneas, desaturation): Respiratory events tend to become longer overnight as a manifestation of an increased arousal threshold.32 Nadir oxygen saturation is lower and respiratory events are more likely to be apneas rather than hypopneas in patients with a higher arousal threshold (more negative epiglottic pressure20). Interestingly, the gold standard measures of arousal threshold could be predicted, albeit to a lesser extent, using these clinical correlates (see Supplementary Material); whether our method offers sufficiently superior predictive values (over the more accessible use of clinical correlates) will ultimately be determined by utility for phenotyping and predicting responses to therapies. Overall, available evidence indicates that the arousal threshold can be assessed through the advanced analysis of polysomnographic signals.
Role of Arousal in OSA Pathogenesis
We recognize that the role of arousals in the pathogenesis of OSA is complex and not completely understood (see the work of Jordan et al. for a detailed review10). On one hand, arousals generally fail to promote a hypocapnia-related dilator muscle relaxation,33 a key postulated mechanism by which arousals might promote OSA. On the other hand, it is established that increased ventilatory drive during sleep raises pharyngeal muscle activity11–13,34 that greater pharyngeal muscle activity improves collapsibility,12,35,36 and by definition, a greater threshold for arousal enables greater ventilatory drive without fragmented sleep.3 It is also clear that some individuals have a higher arousal threshold as an adaptive phenomenon,37,38 given its reduction following treatment with CPAP. Nonetheless around one-third of patients with untreated moderate-to-severe OSA still manifest a low arousal threshold.20 Thus, it is clear that arousal-related OSA pathophysiology differs greatly between patients and that future clinical translation of findings related to arousability will require a noninvasive measure of the arousal threshold.10
Clinical Implications
Our study provides proof-of-principle that the arousability phenotype of OSA can be identified using signals available from a polysomnographic sleep study. Our polysomnographic method requires no esophageal catheters or CPAP manipulation and is computer-automated (except for routine clinical scoring). Consequently, our approach has strong potential for clinical implementation where nasal pressure signals are measured carefully alongside EEG assessment. We propose that such an estimate—when combined with other pathophysiological traits—is likely to provide insight into the likelihood of whether patients will respond to non-CPAP therapies.10 For example, a hypnotic therapy may be effective in a patient with a low arousal threshold (who also has effective pharyngeal dilator muscles), but it is highly unlikely to help in patients whose arousal threshold is already high.3 Interestingly, evidence already indicates that a lower polysomnographic arousal threshold helps us to predict a response to supplemental oxygen.39 Ultimately, further studies are required to test whether our technique helps us to select patients that will respond to medications increasing the arousal threshold and other non-CPAP therapies.
Methodological Considerations
The goal of our study was to provide a tool that, despite a loss of precision over invasive gold standards, provides a substantial increase in clinical applicability. Our values correlated with gold standard values with a correlation coefficient of ~0.7, thus explaining ~50% of the variance; this correlation was similar in strength to that between two gold standards (esophageal pressure versus diaphragm EMG measures, R = 0.75; see Supplementary Figure S4) and as strong as other clinical estimates of phenotypic traits.21,30 We believe that these correlations are sufficiently strong to have utility when used, in univariate or multivariate models with other physiologic traits, to define subgroups that have high versus low likelihoods of a favorable response to interventions. For example, our method demonstrates a strong capacity to predict high versus low arousal thresholds, e.g., a polysomnographic arousal threshold below the cutoff of 110% predicts lower versus higher esophageal pressure arousal thresholds with ~80% accuracy; 168 ± 9 vs. 241 ± 22 % eupnea, p < .01, see Figure 2A and Supplementary Tables S1 and S2).
We recognize that our method systematically underestimated gold standard values. Thus, care must be exercised when using our approach to make inferences about the magnitudes of the arousal threshold (see regression equations in Figures 2 and 3). Possible explanations include overestimation of the ventilatory response to arousal or incomplete airway reopening at arousal onset (see Supplementary Material for additional analysis).
The established gold standard measures used in our study—namely, esophageal pressure, diaphragm EMG, and the CPAP drop technique—also have their strengths and limitations. Esophageal pressure swings are viewed as the gold standard, largely based on findings that progressive hypoxia, hypercapnia, and inspiratory loads trigger arousals at similar levels of esophageal pressure.5 However, esophageal pressure swings are flow-dependent, in that pressure swings can increase substantially between open and obstructed conditions without changes in neural inspiratory drive.22,40 Intraoesophageal diaphragm EMG as a measure of efferent ventilatory drive may be less confounded by airflow,22 but relies on the assumptions that the local EMG at the crural diaphragm represents the efferent inspiratory drive to the whole diaphragm and accessory inspiratory muscles, and that the EMG is linearly related to ventilatory output when the airway is patent. The CPAP drop approach uses the same model-based technique as our polysomnographic method, but with the advantage of being under controlled experimental conditions without the potential confounders of cyclic arousals and reliance on clinically scored obstructive events; nonetheless, the measure is an indirect estimate. The finding that our measures correlate with each of the gold standards therefore lends credence to our approach.
Due to the invasive nature of our validation study, just six of our subjects exhibited >20 minutes of sleep in REM to compare methods in this state. Thus, for now, our method has been examined exclusively in non-REM sleep. Future studies will be needed to validate our approach in REM.
Several decisions were made regarding study design that are important for interpreting our results: (1) The primary study (Study 1) measured the arousal threshold using gold standard ventilation assessed via a sealed mask; thus, our results could be superior to those obtained using nasal pressure in the clinic. However, we showed a strong correlation (R = 0.96) between measures obtained using nasal pressure versus gold standard (pneumotachograph) ventilation (Study 3); we note, however, that care was taken to avoid common causes of reduced nasal pressure signal integrity in the clinical setting (clipping; baseline drift). Importantly, our validation against CPAP manipulation arousal threshold was conducted using nasal pressure (Figure 3) where such care was not possible. (2) We normalized ventilation and ventilatory drive data to present the arousal threshold as a proportion of eupneic drive, as used in prior physiological studies.2,36 Such normalization is critical to allow uncalibrated ventilation signals to be employed, but may be suboptimal in some cases (e.g., severe hypoventilation, see Supplementary Material Methods section). (3) We recognize that arousals with greater intensity (greater changes in EEG power and heart rate) produce a greater ventilatory response,41–43 but arousal intensity was not incorporated into our technique. Our method estimates and subtracts the mean ventilatory response to arousal within each window to reveal the mean arousal threshold. Future incorporation of graded arousal intensity metrics into our approach41–43 could potentially improve the accuracy of our method.
CONCLUSIONS
The current study provides the first method to estimate the respiratory arousal threshold directly from diagnostic polysomnographic signals without invasive instrumentation or specialized interventions. Combined with our measures of loop gain and collapsibility,21,29,30 we now have a clinically applicable approach to estimating the key traits causing OSA in individual patients. Further research is needed to determine the phenotypic characteristics of patients who are most responsive to a range of available OSA treatment options. We envisage that polysomnographic trait measurement will enable OSA patients to be matched to the most appropriate therapies.
SUPPLEMENTARY MATERIAL
Supplementary material is available at SLEEP online.
FUNDING
This work was supported financially by the National Institute of Health (5R01HL048531-16, R01HL102321, 1R01HL090897-01A2, 1K24HL093218-01A1, and 1P01HL095491), American Heart Association (15SDG25890059), National Health and Medical Research Council of Australia (1053201, 1035115, and 1064163), Menzies Foundation, American Thoracic Society Foundation, and Heart Foundation of Australia (101167). This work also supported by Harvard Catalyst (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Award UL1TR001102).
AUTHOR CONTRIBUTIONS
Conception: SS, PT, and AW. Study design: SS, BE, PT, SL, JB, DW, and AW. Data collection and analysis: SS, PT, CM, LT, and AW. All authors interpreted data, edited the manuscript for important intellectual content, and approved the final draft.
DISCLOSURE STATEMENT
PIT, BAE, AA, MM, CMM, SHL, and JPB declare no conflicts of interest. SAS, LTM, and AW served as consultants for Cambridge Sound Management. LTM served as a consultant for Novion Pharmaceuticals, Inc. DPW is the Chief Scientific Officer for Philips Respironics, serves as consultant for Night Balance, and was the Chief Medical Officer for Apnicure until December 2016. AW receives research support from Philips Respironics.
Supplementary Material
ACKNOWLEDGMENTS
The authors are grateful to Maquet Getinge Group for the loan of the Servo-i ventilator to measure intraoesophageal diaphragm EMG, and they are grateful for the technical assistance from Lauren Hess.
REFERENCES
- 1. Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013; 188(8): 996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edwards BA, Andara C, Landry S et al. . Upper-airway collapsibility and loop gain predict the response to oral appliance therapy in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2016; 194(11): 1413–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eckert DJ, Owens RL, Kehlmann GB et al. . Eszopiclone increases the respiratory arousal threshold and lowers the apnoea/hypopnoea index in obstructive sleep apnoea patients with a low arousal threshold. Clin Sci (Lond). 2011; 120(12): 505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edwards BA, Sands SA, Owens RL et al. . The combination of supplemental oxygen and a hypnotic markedly improves obstructive sleep apnea in patients with a mild to moderate upper airway collapsibility. Sleep. 2016; 39(11): 1973–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gleeson K, Zwillich CW, White DP. The influence of increasing ventilatory effort on arousal from sleep. Am Rev Respir Dis. 1990; 142(2): 295–300. [DOI] [PubMed] [Google Scholar]
- 6. Wellman A, Eckert DJ, Jordan AS et al. . A method for measuring and modeling the physiological traits causing obstructive sleep apnea. J Appl Physiol (1985). 2011; 110(6): 1627–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wellman A, Edwards BA, Sands SA et al. . A simplified method for determining phenotypic traits in patients with obstructive sleep apnea. J Appl Physiol (1985). 2013; 114(7): 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004; 169(5): 623–633. [DOI] [PubMed] [Google Scholar]
- 9. Ratnavadivel R, Stadler D, Windler S et al. . Upper airway function and arousability to ventilatory challenge in slow wave versus stage 2 sleep in obstructive sleep apnoea. Thorax. 2010; 65(2): 107–112. [DOI] [PubMed] [Google Scholar]
- 10. Jordan AS, O’Donoghue FJ, Cori JM, Trinder J. Physiology of arousal in OSA and potential impacts for sedative treatment. Am J Respir Crit Care Med. 2017. [DOI] [PubMed] [Google Scholar]
- 11. Malhotra A, Pillar G, Fogel RB et al. . Pharyngeal pressure and flow effects on genioglossus activation in normal subjects. Am J Respir Crit Care Med. 2002; 165(1): 71–77. [DOI] [PubMed] [Google Scholar]
- 12. Jordan AS, White DP, Owens RL et al. . The effect of increased genioglossus activity and end-expiratory lung volume on pharyngeal collapse. J Appl Physiol (1985). 2010;109(2):469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Younes M, Loewen AH, Ostrowski M, Laprairie J, Maturino F, Hanly PJ. Genioglossus activity available via non-arousal mechanisms vs. that required for opening the airway in obstructive apnea patients. J Appl Physiol (1985). 2012; 112(2): 249–258. [DOI] [PubMed] [Google Scholar]
- 14. Hicks A, Cori JM, Jordan AS et al. . Mechanisms of the deep slow-wave-sleep-related increase of upper airway muscle tone in healthy humans. J Appl Physiol (1985). 2017:122(5):1304–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jordan AS, White DP, Lo YL et al. . Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009; 32(3): 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ratnavadivel R, Chau N, Stadler D, Yeo A, McEvoy RD, Catcheside PG. Marked reduction in obstructive sleep apnea severity in slow wave sleep. J Clin Sleep Med. 2009; 5(6): 519–524. [PMC free article] [PubMed] [Google Scholar]
- 17. Wains SA, El-Chami M, Lin HS, Mateika JH. Impact of arousal threshold and respiratory effort on the duration of breathing events across sleep stage and time of night. Respir Physiol Neurobiol. 2017; 237: 35–41. [DOI] [PubMed] [Google Scholar]
- 18. Dunai J, Kleiman J, Trinder J. Ventilatory instability during sleep onset in individuals with high peripheral chemosensitivity. J Appl Physiol (1985). 1999; 87(2): 661–672. [DOI] [PubMed] [Google Scholar]
- 19. Quadri S, Drake C, Hudgel DW. Improvement of idiopathic central sleep apnea with zolpidem. J Clin Sleep Med. 2009; 5(2): 122–129. [PMC free article] [PubMed] [Google Scholar]
- 20. Edwards BA, Eckert DJ, McSharry DG et al. . Clinical predictors of the respiratory arousal threshold in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2014;190(11):1293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Terrill PI, Edwards BA, Nemati S et al. . Quantifying the ventilatory control contribution to sleep apnoea using polysomnography. Eur Respir J. 2015; 45(2): 408–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Luo YM, Wu HD, Tang J et al. . Neural respiratory drive during apnoeic events in obstructive sleep apnoea. Eur Respir J. 2008; 31(3): 650–657. [DOI] [PubMed] [Google Scholar]
- 23. Berry RB, Budhiraja R, Gottlieb DJ et al. ; American Academy of Sleep Medicine Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012; 8(5): 597–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sforza E, Krieger J, Petiau C. Arousal threshold to respiratory stimuli in OSA patients: evidence for a sleep-dependent temporal rhythm. Sleep. 1999; 22(1): 69–75. [PubMed] [Google Scholar]
- 25. Owens RL, Edwards BA, Eckert DJ et al. . An integrative model of physiological traits can be used to predict obstructive sleep apnea and response to non-positive airway pressure therapy. Sleep. 2015; 38(6): 961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thurnheer R, Xie X, Bloch KE. Accuracy of nasal cannula pressure recordings for assessment of ventilation during sleep. Am J Respir Crit Care Med. 2001; 164(10 Pt 1): 1914–1919. [DOI] [PubMed] [Google Scholar]
- 27. Edwards BA, Sands SA, Eckert DJ et al. . Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012; 590(5): 1199–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bland JM, Altman DG. Applying the right statistics: analyses of measurement studies. Ultrasound Obstet Gynecol. 2003;22(1):85–93. [DOI] [PubMed] [Google Scholar]
- 29. Sands SA, Edwards BA, Terrill PI et al. . Phenotyping sleep apnea using polysomnography: upper airway collapsibility and responsiveness [Abstract]. Am J Respir Crit Care Med. 2016;193:A6378. [Google Scholar]
- 30. Azarbarzin A, Sands SA, Taranto-Montemurro L et al. . Estimation of pharyngeal collapsibility during sleep by peak inspiratory airflow. Sleep. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Landry SA, Joosten SA, Eckert DJ et al. . Therapeutic CPAP level predicts upper airway collapsibility in patients with obstructive sleep apnea. Sleep 2017; 40(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sforza E, Krieger J, Petiau C. Nocturnal evolution of respiratory effort in obstructive sleep apnoea syndrome: influence on arousal threshold. Eur Respir J. 1998; 12(6): 1257–1263. [DOI] [PubMed] [Google Scholar]
- 33. Jordan AS, Cori JM, Dawson A et al. . Arousal from sleep does not lead to reduced dilator muscle activity or elevated upper airway resistance on return to sleep in healthy individuals. Sleep. 2015; 38(1): 53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stanchina ML, Malhotra A, Fogel RB et al. . Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med. 2002; 165(7): 945–949. [DOI] [PubMed] [Google Scholar]
- 35. McGinley BM, Schwartz AR, Schneider H, Kirkness JP, Smith PL, Patil SP. Upper airway neuromuscular compensation during sleep is defective in obstructive sleep apnea. J Appl Physiol (1985). 2008; 105(1): 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sands SA, Eckert DJ, Jordan AS et al. . Enhanced upper-airway muscle responsiveness is a distinct feature of overweight/obese individuals without sleep apnea. Am J Respir Crit Care Med. 2014: 190(8):930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Haba-Rubio J, Sforza E, Weiss T, Schröder C, Krieger J. Effect of CPAP treatment on inspiratory arousal threshold during NREM sleep in OSAS. Sleep Breath. 2005; 9(1): 12–19. [DOI] [PubMed] [Google Scholar]
- 38. Loewen A, Ostrowski M, Laprairie J et al. . Determinants of ventilatory instability in obstructive sleep apnea: inherent or acquired?Sleep. 2009; 32(10): 1355–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sands SA, Edwards BA, Terrill PI et al. . Phenotyping from polysomnography predicts obstructive sleep apnea responses to supplemental oxygen therapy [Abstract]. Am J Respir Crit Care Med. 2017;195: A2931. [Google Scholar]
- 40. Younes M, Riddle W, Polacheck J. A model for the relation between respiratory neural and mechanical outputs. III. Validation. J Appl Physiol Respir Environ Exerc Physiol. 1981; 51(4): 990–1001. [DOI] [PubMed] [Google Scholar]
- 41. Sforza E, Jouny C, Ibanez V. Cardiac activation during arousal in humans: further evidence for hierarchy in the arousal response. Clin Neurophysiol. 2000; 111(9): 1611–1619. [DOI] [PubMed] [Google Scholar]
- 42. Azarbarzin A, Ostrowski M, Hanly P, Younes M. Relationship between arousal intensity and heart rate response to arousal. Sleep. 2014; 37(4): 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amatoury J, Azarbarzin A, Younes M, Jordan AS, Wellman A, Eckert DJ. Arousal intensity is a distinct pathophysiological trait in obstructive sleep apnea. Sleep. 2016; 39(12): 2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.