Short abstract
Background
Surgeries causing nerve injury can result in chronic neuropathic pain, which is clinically managed by using antidepressant or anticonvulsant drugs. Currently, there is a growing interest for investigating preemptive treatments that would prevent this long-term development of neuropathic pain. Our aim was to compare analgesic drugs using two distinct treatment modalities: either treatment onset at surgery time or following a couple of weeks of neuropathic pain.
Methods
In male C57BL/6J mice, neuropathic pain was induced by cuffing the sciatic nerve, and allodynia was assessed using von Frey filaments. We tested the effect of anticonvulsants (gabapentin 10 mg/kg and carbamazepine 40 mg/kg), antidepressants (desipramine 5 mg/kg, duloxetine 10 mg/kg, and fluoxetine 10 mg/kg), dexamethasone (2 mg/kg), and ketamine (15 mg/kg). Drugs were injected daily or twice a day, starting either at surgery time or on day 25 postsurgery (15 days of treatment for antidepressants and 10 days for other drugs).
Results
Ketamine was the only effective treatment during the early postsurgical period. Although early anticonvulsant treatment was not immediately effective, it prevented chronification of allodynia. When treatments started at day 25 postsurgery, desipramine, duloxetine, and anticonvulsants suppressed the mechanical allodynia.
Conclusions
Our data show that allodynia measured in experimental neuropathic pain model likely results from a combination of different processes (early vs. late allodynia) that display different sensitivity to treatments. We also propose that early anticonvulsant treatment with gabapentin or carbamazepine may have a prophylactic effect on the chronification of allodynia following nerve injury.
Keywords: anticonvulsants, antidepressants, chronic pain, dexamethasone, ketamine, mice, neuropathic pain
Introduction
The International Association for the Study of Pain defines neuropathic pain as a direct consequence of a lesion or disease affecting the somatosensory system.1 Neuropathic pain is often a chronic and disabling condition that is poorly responsive to usual analgesic drugs.2 The first-line treatments presently recommended against neuropathic pain are either anticonvulsant drugs, such as gabapentinoids, or antidepressant drugs, such as tricyclic antidepressants (TCAs) or more selective serotonin and noradrenaline reuptake inhibitors (SSNRIs).3
Surgery is a major cause of chronic neuropathic pain, with a prevalence estimated between 6% and 68% depending on the type of surgery.4 The concept of preventive analgesia aims at decreasing the occurrence of pain with an early treatment starting in the perisurgical period. The most frequent targets for preventive analgesia are the inflammatory mediators (nonsteroidal anti-inflammatory drugs), the µ-opioid receptors in the nociceptive pathway (opioid drugs), the N-methyl-D-aspartate (NMDA) receptor (ketamine), ion channels like sodium voltage-gated channels (nerve blocks with local anesthetics), and the α2-δ1 subunit of voltage-dependent calcium channels (gabapentinoids).5 Although such prevention usually concerns short-term postsurgical pain, the occurrence of longer term neuropathic pain is less often considered. There is however a clinical need for preventing such risk of long-term neuropathic pain following surgical procedures. Rodent models that reproduce peripheral nerve injuries, through section or compression of the sciatic nerve using a surgical procedure,6 offer the possibility to compare the long-term impact of perisurgical treatments (“early” treatment) and of treatments starting a couple of weeks after neuropathic allodynia appeared (“late” treatment).
In the present study, we addressed the effect of early or late treatments on neuropathic allodynia in a model of sciatic nerve compression. We tested various drugs, including antidepressants (desipramine, duloxetine, and fluoxetine), anticonvulsants (gabapentin and carbamazepine), ketamine, and dexamethasone. Treatments were delivered during 10 to 15 days starting either at surgery time or after a 25-day delay.
Materials and methods
Animals
Experiments were conducted in the animal facilities Chronobiotron UMS3415, which are registered for animal experimentation under the Animal House Agreement A67-2018-38. All animal protocols were carried out in accordance with European Communities Council Directive of 22 September 2010 (2010/63/UE), and approved by the Ethical Committee for Animal Experimentation of Strasbourg, France (CREMEAS, CEEA35; authorization no. Al/04/04/01/13). Experiments were performed using adult male C57BL/6J mice (n = 224 for all the studies, six weeks old upon arrival; Charles River, L’Arbresle, France). Mice were group-housed four to five per cage and maintained under a 12-h light/dark cycle (lights on at 06:00 a.m.), with food and water available ad libitum. Experiments started after two weeks of habituation to the animal facilities. All behavioral assays were performed during the light period.
Surgery
Neuropathic pain was induced by cuffing the common branch of the right sciatic nerve.7,8 Surgeries were done under aseptic conditions and ketamine/xylazine anesthesia (ketamine 68 mg/kg i.p., xylazine 10 mg/kg i.p.; Centravet, Taden, France). The common branch of the right sciatic nerve was exposed, and a 2-mm section of split PE-20 polyethylene tubing (Harvard Apparatus, Les Ulis, France) was placed around it (Cuff group). The shaved skin layer was closed using suture. Sham-operated mice underwent similar surgical procedure, without implantation of the cuff (Sham group).
Treatments
Desipramine (5 mg/kg i.p.) was used as TCA, duloxetine (10 mg/kg i.p.) as SSNRI, and fluoxetine (10 mg/kg i.p.) as selective serotonin reuptake inhibitor that is clinically considered as poorly effective against neuropathic pain, gabapentin (10 mg/kg i.p.) or carbamazepine (40 mg/kg s.c.) as anticonvulsants, ketamine (15 mg/kg i.p.) as NMDA receptor antagonist, and dexamethasone (2 mg/kg s.c) as steroidal anti-inflammatory drug. Preventive treatments began 1 h prior to surgical procedure (cuff implantation or sham operation), while curative treatments began 25 days after the surgery. Mice received two injections per day (morning and evening) of antidepressant drugs, anticonvulsant drugs, or ketamine. Dexamethasone was administered once a day. Antidepressant treatments lasted 15 consecutive days, based on previously published delayed therapeutic onset with these drugs in the cuff model of neuropathic pain.7 The other drugs were administered for 10 consecutive days. All drugs were purchased from Sigma-Aldrich (St. Quentin Fallavier, France). Besides carbamazepine, drugs were dissolved in 0.9% NaCl solution that was also used for control injections and were administered in 5 mL/kg volume. The addition of 1% of Tween 40 was required to prepare a carbamazepine suspension in 0.9% NaCl, which was injected subcutaneously to prevent the risk of peritoneal irritation with repeated administration.
Nociceptive testing
The mechanical threshold of hindpaw withdrawal was evaluated using the Semmes-Weinstein von Frey hairs (Touch test, Stoelting, Ireland), and results were expressed in grams.8 Mice were placed in clear Plexiglas boxes (7 × 9 × 7 cm) on an elevated mesh screen. Calibrated monofilaments were applied to the plantar surface of each hindpaw, in a series of ascending forces, until they just bend. Brisk withdrawal was considered as a positive response. In the absence of a response, a filament of next-greater force was applied. Each filament was tested five times per paw, and the threshold was defined as three or more withdrawals observed out of the five trials.
To study the chronic therapeutic effect, we tested the animals in the morning, on given days, prior to the injection of the drug. The tests were initiated prior to the surgical procedure (baselines) and pursued until day 50 or 60 postsurgery.
Statistical analysis
Data are expressed as mean ± SEM. Statistical analyses were performed with Statistica (Statsoft, Tulsa, OK), using analysis of variance. Surgical procedure (Sham vs. Cuff) and treatments (solution of 0.9% NaCl vs. drugs) were considered as between-group factors and time points as within-subject factor. When analysis of variance was significant (P < 0.05), multiple comparisons were realized by using the posttest of Duncan.
Results
Treatments with antidepressant drugs
The unilateral insertion of a cuff on the sciatic nerve induced a long-lasting ipsilateral mechanical allodynia (F13,156 = 5.76, P < 0.001; post hoc: cuff baseline > cuff postsurgery at P < 0.05 on postsurgery days 2–49). In the Sham group, postsurgical allodynia recovered in a few days (post hoc: sham baseline > sham postsurgery at P < 0.05 on postsurgery days 2–6).
We compared the preventive versus curative effects of three antidepressant drugs: desipramine, duloxetine, and fluoxetine. Preventive treatments starting 1 h prior to the surgery and lasting 15 days had no effect on the cuff-induced allodynia (surgery–treatment time interaction, desipramine: F13,286 = 0.93, P = 0.52; duloxetine: F13,273 = 1.53, P = 0.10; fluoxetine: F13,273 = 0.89, P = 0.55; Figure 1(a), (c), and (e)). However, curative treatment with desipramine and duloxetine, but not with fluoxetine, starting 25 days after the surgery fully suppressed mechanical allodynia (desipramine (Des): surgery–treatment time interaction, F13,286 = 2.01, P < 0.005; post hoc: cuffDes > cuffSal at P < 0.05 on postsurgery days 35–42; Figure 1(b)), (duloxetine (Dulox): F13,273 = 2.91, P < 0.001; post hoc: cuffDulox > cuffSal at P < 0.05 on postsurgery days 35–42; Figure 1(d)), and (fluoxetine (Fluox): F13,286 = 0.80, P = 0.65; Figure 1(f)). When desipramine and duloxetine curative treatments were interrupted, a relapse of mechanical allodynia was observed (cuffDes < shamDes at P < 0.01 on days 45–49; cuffDulox < shamDulox at P < 0.001 on days 45–49). Treatments had no effect either on the paw withdrawal threshold of Sham mice (Figure 1(b), (d), and (f)) or on the contralateral paw of Cuff mice (data not shown).
Figure 1.
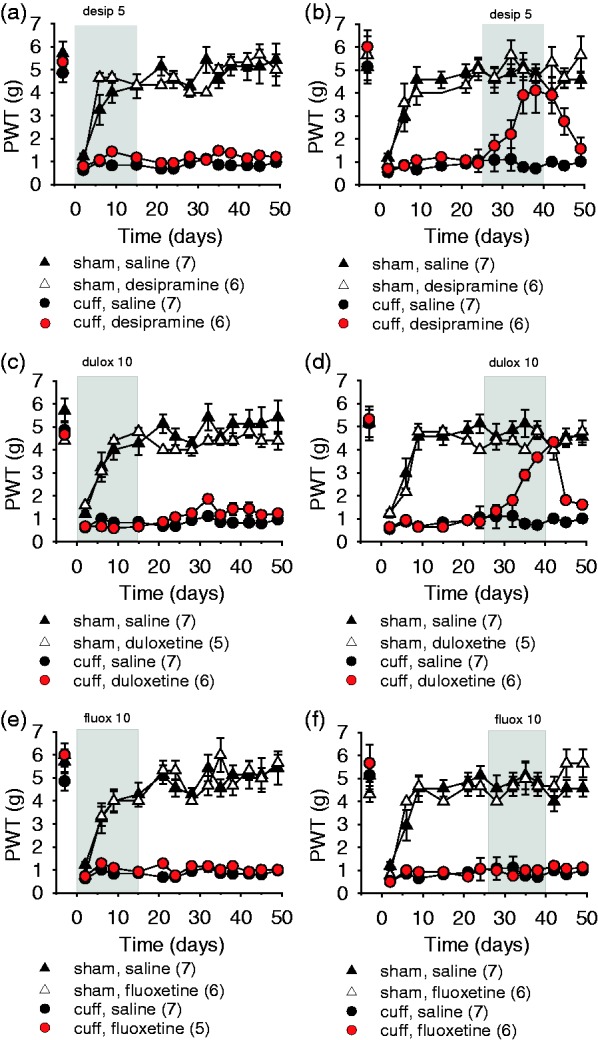
Early and late treatment with the antidepressants desipramine, duloxetine, and fluoxetine. The mechanical threshold was evaluated using von Frey hairs, data are represented as mean ± SEM, and n are expressed between brackets. Early 15-day treatments starting 1 h prior to the surgery with desipramine (desip 5 mg/kg i.p. twice a day) (a), duloxetine (dulox 10 mg/kg i.p. twice a day) (c), or fluoxetine (fluox 10 mg/kg i.p. twice a day) (e) had no effect on allodynia. Later treatments, starting 25 days after surgery, with desipramine (b) or duloxetine (d) relieved allodynia, and a relapse was observed after treatment interruption; while the treatment with fluoxetine (f) had no effect on cuff-induced allodynia. PWT: paw withdrawal threshold.
Treatments with anticonvulsant drugs
Early treatments with gabapentin or carbamazepine began 1 h prior to the surgery and lasted 10 days. At the end of the 10-day treatment, there was no relief of allodynia. However, after the end of the treatments, a gradual improvement in the nociceptive thresholds was observed in the Cuff mice, leading to full recovery from allodynia and long-term maintenance of this recovery (gabapentin (Gaba): surgery–treatment time interaction, F15,375 = 2.90, P < 0.001; post hoc: cuffGaba > cuffSal at P < 0.05 on days 23–59; Figure 2(a); carbamazepine: surgery–treatment time interaction, F15,330 = 5.37, P < 0.001; post hoc: cuffCarba > cuffSal at P < 0.05 on days 23–59; Figure 2(c)). Early treatments thus prevented the allodynia chronification. The same treatments had no effect either on the paw withdrawal threshold of Sham mice or on the contralateral paw of Cuff mice (data not shown).
Figure 2.
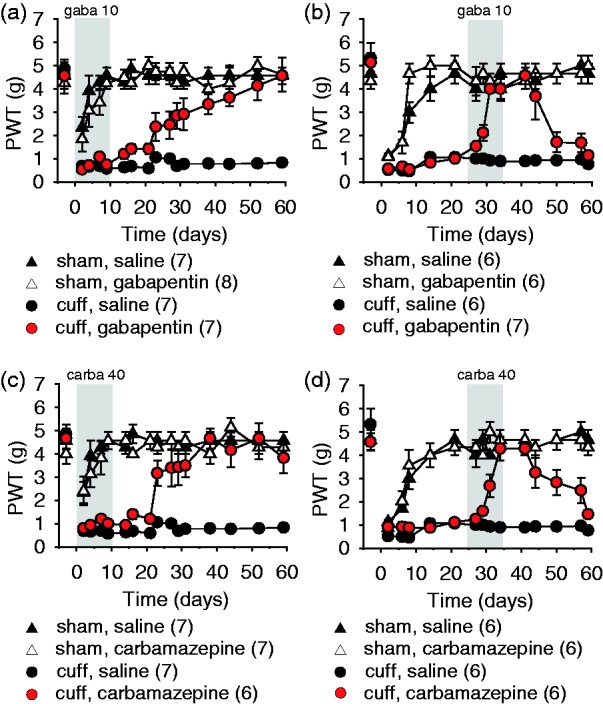
Early and late treatments with the anticonvulsants gabapentin and carbamazepine. The mechanical threshold was evaluated using von Frey hairs, data are represented as mean ± SEM, and n are expressed between brackets. Early 10-day treatments with gabapentin (gaba 10 mg/kg s.c. twice a day) (a) or carbamazepine (carba 40 mg/kg s.c. twice a day) (c) did not affect the mechanical thresholds after surgery. After the end of the treatments, a progressive recovery from allodynia was observed for both anticonvulsants. Later treatments, starting 25 days after surgery, with gabapentin (gaba 10 mg/kg s.c. twice a day) (b) or carbamazepine (carba 40 mg/kg s.c. twice a day) (d) relieved allodynia, and a relapse was observed after treatment interruption. PWT: paw withdrawal threshold.
When treatments started on day 25 postsurgery, i.e., on animals with a history of sustained neuropathic pain, gabapentin and carbamazepine alleviated neuropathic allodynia after a few days of treatment (gabapentin: surgery–treatment time interaction, F14,294 = 6.45, P < 0.001; post hoc: cuffGaba > cuffSal at P < 0.05 on days 29–44; Figure 2(b); carbamazepine: F14,294 = 3.54, P < 0.001; post hoc: cuffCarba > cuffSal at P < 0.05 on days 31–57; Figure 2(d)). After 10 days of treatment and total recovery, the treatments were stopped. A relapse of the neuropathic allodynia was observed in the two weeks following treatment interruption (gabapentin: cuffGaba < shamSal at P < 0.05 on days 50–59; Figure 2(b); carbamazepine: post hoc: cuffCarba > shamSal at P < 0.05 on days 50–59; Figure 2(d)). Treatments had no effect either on the paw withdrawal threshold of Sham mice or on the contralateral paw of Cuff mice (data not shown).
Treatments with ketamine
Early treatment with ketamine led to a progressive recovery from neuropathic allodynia in Cuff mice. After 10 days, the treatment with ketamine was interrupted and allodynia slowly reappeared over two months (surgery–treatment time interaction, F14,294 = 2.04, P < 0.01; post hoc: cuffKeta > cuffSal at P < 0.05 on days 7–44; Figure 3(a)). The late treatment, starting at day 25 postsurgery, led to a partial recovery from neuropathic allodynia. After interruption of the treatment, this partial recovery still remained present for over 10 days and was followed by a relapse of allodynia (F14,280 = 2.45, P = 0.002; post hoc: cuffKeta > cuffSal at P < 0.05 on days 31–50; Figure 3(b)).
Figure 3.
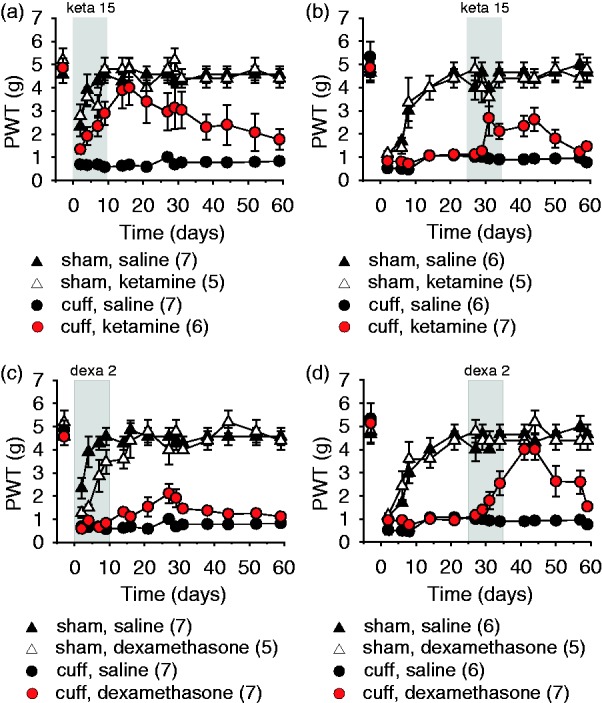
Early and late treatments with ketamine and dexamethasone. The mechanical threshold was evaluated using von Frey hairs, data are represented as mean ± SEM, and n are expressed between brackets. Early ketamine treatment (keta 15 mg/kg i.p. twice a day) progressively relieved neuropathic allodynia, with a relapse of allodynia slowly reappearing over two months (a). Late ketamine treatment led to a partial recovery, and a relapse was observed after treatment interruption (b). Early dexamethasone treatment (dexa 2 mg/kg s.c. once a day) did not significantly affect the mechanical thresholds after the surgery (c). Late dexamethasone treatment alleviated the allodynia, and a relapse was observed after treatment interruption (d). PWT: paw withdrawal threshold.
Treatments with dexamethasone
The early treatment with dexamethasone at high dose did not significantly modify nociceptive thresholds (surgery–treatment time interaction, F14,308 = 1.96, P = 0.02; post hoc: cuffDexa = cuffSal at P > 0.05; Figure 3(c)). The late treatment starting 25 days postsurgery induced a partial recovery of allodynia during the 10 days of treatment, which further developed into full recovery during the week following the interruption of the treatment, and was then followed by a slow relapse of neuropathic allodynia (F14,280 = 6.57, P < 0.001; post hoc: cuffDexa > cuffSal at P < 0.05 on days 29–50; Figure 3(d)).
Discussion
Here, we showed that an early and perioperative administration of anticonvulsant drugs, but not of antidepressant drugs, ketamine, or dexamethasone can prevent the chronification of neuropathic allodynia in an experimental model of nerve compression.
Our experiments were not designed to study acute analgesic action, but rather designed to detect sustained allodynia relief. This was done by testing the animals in the morning, before the first drug administration on the considered day, thus looking at the lasting effect of previous days of treatment.7 Our results showed that among all the drugs tested, ketamine was the only one with a rapid and long-lasting action on the cuff-induced allodynia in the early postsurgical period. Approved by the Food and Drug Administration since 1969 for a clinical use, ketamine is an analgesic and anesthetic agent, and its administration during anesthesia decreases postoperative pain intensity for up to 48 h as well as opioid drugs consumption.9–11 Clinically, perioperative ketamine is also beneficial on postsurgical inflammatory response.12 This action of ketamine on pain mechanisms has been mostly related to its noncompetitive antagonistic action on NMDA receptors.13
Although ketamine displayed a postsurgical antiallodynic effect that lasted one day to the next, such lasting action was not observed with a gabapentinoid, a corticosteroid or antidepressants when these drugs were given in the early postsurgical period. However, it should be noted that it does not preclude the possibility to obtain short-term transitory pain relief in the postsurgical period with these other drugs. Indeed, some of them are routinely used in the clinic. In human, perioperative gabapentinoids have been clinically well studied14 and are often used in multimodal analgesia, combining different analgesic drugs. Most studies, but not all of them, support the benefit of gabapentinoid use on postoperative pain and on the reduction of opioid consumption; conflicting results being likely associated with treatment doses and regimens or with the type of surgery.15–23 On the other hand, animal research is based on single perioperative drugs, and only few studies explored the benefit of perioperative gabapentinoids. Recently, Camara et al.24 reported an analgesic effect of daily oral gabapentin on mechanical allodynia at days 5 and 15 of sciatic nerve constriction in the rat; but this antiallodynic effect was assessed at high doses (30–60–120 mg/kg) and acutely (60 min after the last administration) (see Table 1). Together with our data, it suggests that gabapentinoid action on perisurgical pain might be related to a short-term transitory analgesia, while another effect of these drugs would impact pain chronification. Corticosteroid anti-inflammatory agents have also shown acute analgesic effects in both human and animal studies. In experimental models of sciatic nerve injury, early administration of corticosteroids can in some cases reduce the inflammatory processes and neuropathic pain behaviors in the days following surgery.25 Clinically, corticosteroids are sometimes used to reduce inflammatory response after the surgery26; indeed they can display analgesic and antihyperalgesic effects in acute experimental27 and postoperative pain.28 However, this action highly depends on the considered treatment and type of pain,29 and other clinical studies showed no benefit of perioperative corticosteroids on acute postoperative pain30 and no delayed benefit at six weeks or one year after a single perioperative dose (see Table 1).31 Concerning antidepressants, it has been shown that duloxetine at high dose can have a transitory analgesic action when delivered 24 h after paw incision in the rat,32 and that 10 days of venlafaxine at high dose can acutely reduce hypersensitivity in a model of sciatic nerve constriction.33 In both cases, these actions are however transitory and likely due to the recruitment of α2-adrenoceptors in descending controls of pain. This limited action of antidepressants may also be present clinically. Indeed, in a systematic review evaluating clinical trials of antidepressants for postsurgical pain,34 some beneficial actions of antidepressants on early postoperative pain were reported in 8 out of 15 trials (see Table 1). However, adverse effects of these drugs together with the high dose that may be required to observe a perisurgical benefit are limiting the interest in using antidepressants in this context. Therefore, they remain more appropriate against sustained neuropathic pain for which few therapeutic alternatives are present.
Table 1.
Selected studies on the perisurgical preventive treatment of pain.
| Ref. | Species | Pain disorder | Treatment | Duration | Efficacy |
|---|---|---|---|---|---|
| Amr 2010 15 | Human | Breast cancer surgery | Gabapentin 300mg | Before and 10 days postop | No at month 6 |
| Brogly 2008 16 | Human | Thyroidectomy | Gabapentin 1200mg | 2h before | Yes at month 6 |
| Clarke 2009 17 | Human | Hip arthroplasty | Gabapentin 600mg | 2h before | No at month 6 |
| Fassoulaki 2002 18 | Human | Breast cancer surgery | Gabapentin 1200mg | Before and 10 days postop | No at month 3 |
| Fassoulaki 2005 19 | Human | Breast cancer surgery | Gabapentin 1600mg + local anesthetics | Before and 8 days postop | Yes at month 3 |
| No at month 6 | |||||
| Moore 2011 49 | Human | Caesarian delivery | Gabapentin 600mg | 2h before | No at month 3 |
| Sen 2009 20 | Human | Hysterectomy | Gabapentin 1200mg | 1h before | Yes at month1,3 and 6 |
| Sen 2009 21 | Human | Inguinal herniorraphy | Gabapentin 1200mg | 1h before | Yes at month1,3 and 6 |
| Camara 2015 24 | Rat | Chronic constriction sciatic nerve | Gabapentin oral 30, 60, 120 mg/kg | 1h before and 15 days along | Yes at 5 and 15 days (60min after administration) |
| Burke 2010 22 | Human | Lumbar discectomy | Pregabalin 300mg | Before and 150mg at 12 and 24h postop | Yes at 3 months |
| Buvanendran 2010 52 | Human | Knee arthroplasty | Pregabalin 300mg | Before and 50-150mg 14 days postop | Yes at 6 months |
| Pesonen 2011 23 | Human | Cardiac surgery | Pregabalin 150mg | Before and 5 days postop | Yes at 3 months |
| Matsutani 2015 51 | Human | Thoracotomy | Pregabalin 75mg | Before and 150mg 14 days postop | Yes at 1, 2 and 3 months |
| Li 2007 25 | Rat | Sciatic nerve ligation | Corticosteroid triamcinolone s.c. 1.5mg/kg | 1h before and 3 days | Yes 7 days after surgery |
| Bergeron 2009 31 | Human | Hip arthroplasty | Corticosteroid dexamethasone IV 40mg | One preoperative dose | No at 6 weeks and 1 year |
| Romundstad 2004 28 | Human | Orthopaedic surgery | Corticosteroid methylprednisolone IV 125mg | 1 day after surgery | Yes at 24h after infusion |
| De Kock 2001 10 | Human | Resection of rectal cancer | Ketamine IV 0.5mk/kg bolus and infusion 0.25mg/kg/h | 30min before and during surgery | Yes at 2 weeks, 1 and 6 months, 1year |
| Loftus 2010 11 | Human | Major lumbar spine surgery | Ketamine IV 0.5mk/kg bolus and 10µg/kg/min | Before and during surgery | Yes at 6 weeks |
| Sun 2014 32 | Rat | Plantar incision | Duloxetine IP 20 or 40mg/kg | 24h after incision | Yes 120min after administration |
| Hajhashemi 2014 33 | Rat | Chronic constriction injury (CCI) | Venlafaxine IP 10 and 20mg/kg | Day 1 after CCI and daily until day 14 | Yes at days 7, 10, 14 after CCI |
| Venlafaxine IP 20mg/kg | Day 10 after CCI until day 21 | No | |||
| Ho 2010 38 | Human | Knee replacement | Duloxetine 60mg orally | 2h before and day 1 | No at months 3 and 6 |
| Chocron 2013 39 | Human | Coronary artery bypass grafting | Escitalopram 10mg orally | 2-3 weeks before and 6 months postop | No at months 1, 3 and 6 |
| Amr 2010 15 | Human | Breast cancer surgery | Venlafaxine 37.5mg orally | before and 10 days postop | Yes at 6 months on burning and stabbing pain No on pain at rest |
Some antidepressants are among first-line drugs for neuropathic pain relief.3 TCAs as well as selective SSNRIs are indeed effective in relieving neuropathic pain in several animal models and human neuropathic conditions.35 Accordingly, a 15-day treatment with the TCA desipramine or the SSNRI duloxetine, starting three weeks after the surgery, decreased the cuff-induced mechanical allodynia. In both cases, a relapse of allodynia was observed after the cessation of the treatments, which is also frequently observed clinically. Furthermore, in our study, the selective serotonin reuptake inhibitor fluoxetine was ineffective, which is consistent with several experimental and clinical studies.36 The most common hypotheses for the analgesic action of antidepressant drugs35 implicate either the central recruitment of noradrenergic descending pathways that inhibit nociceptive responses or the peripheral recruitment of noradrenergic fibers that sprout into the dorsal root ganglia following peripheral nerve injury.37 Although desipramine and duloxetine displayed antiallodynic action on well-installed neuropathic pain, an early administration of these drugs in the perioperative period was ineffective in preventing neuropathic pain chronification. Clinically, three trials addressed chronic postsurgical pain prevention with antidepressants, using the SSNRIs duloxetine38 and venlafaxine15 or the selective serotonin reuptake inhibitor escitalopram.39 Only venlafaxine, with a 10-day perioperative treatment, was superior to placebo in reducing burning and stabbing pain six months after the surgery.15 However, these last results were obtained in a context of breast surgery, whose chronification is tightly linked to the emotional profile of the patients,40 which may be impacted by the antidepressant. Results concerning prevention of pain chronification with antidepressants must be interpreted with caution and may depend on the considered surgical act.
In the present model of experimental neuropathic pain, anticonvulsants were the only drugs having a main impact on allodynia chronification. Gabapentinoid anticonvulsants, such as gabapentin or pregabalin, are among first-line treatments for neuropathic pain.3 These drugs bind the α2-δ1 subunit of voltage-dependent calcium channels,41 thus interfering with channels’ function and trafficking.42 By decreasing the hyperexcitability of dorsal horn neurons, gabapentinoids limit the hyperalgesia and central sensitization induced by tissue damage and nerve injury.35 Carbamazepine is an older drug43 which remains a first-line therapy against trigeminal neuralgia3,44 and also displayed efficacy against painful diabetic neuropathy and postherpetic neuralgia.45 It is a blocker of voltage-dependent sodium channels,46 but it can also affect calcium channels.47 In mice, we observed that both carbamazepine and gabapentin display an antiallodynic effect when administered in a state of chronic neuropathic pain, and that both drugs given for 10 days in the perioperative period prevented the chronification of neuropathic allodynia. Some patients do not tolerate carbamazepine, due to frequent sensations of dizziness, nausea, and somnolence. It also induces cytochrome isoenzymes and can influence the serum levels of other drugs. Because of this poor tolerability and pharmacokinetic interactions, the use of carbamazepine and its analog oxcarbamazepine is mostly limited to the treatment of trigeminal neuralgia,48 and no clinical trial has studied perioperative administrations. However, some trials addressed the prophylactic action of perioperative gabapentinoids on chronic postsurgical pain (see Table 1). A meta-analysis of 11 perioperative gabapentinoids clinical trials was conducted by Clarke et al.14 It suggested that 600 mg gabapentin, given once before surgery, fails to reduce the incidence of chronic pain,17,49 whereas the use of 1200 mg may reduce this incidence.16,20,21 Accordingly, our previous clinical work on thoracotomy-induced chronic pain showed no correlation between a single preoperative dose of 600 mg gabapentin and the incidence of chronic postsurgical pain.50 When gabapentin administration is prolonged to the postoperative period, trials’ data on pain reduction after two months postsurgery are conflicting, but it has been suggested that the high variability of gabapentin’s bioavailability in patients may interfere and require controlling for gabapentin plasma level.50 Clinically, pregabalin has a better absorption profile than gabapentin, and it has been shown in patients that, when given before surgery and continued for two weeks after the surgery, pregabalin reduced the incidence of chronic postsurgical pain following thoracotomy51 or knee arthroplasty.52 However, these clinical data require confirmation through more refined trials, taking also into account the neuropathic and nociceptive components of pain. Together with present results in an experimental model, these findings suggest that the clinical potential of anticonvulsants in the prevention of long-term neuropathic pain following surgery merits further exploration, in order to confirm or not the benefit of such treatment, and to optimize the dose and the most appropriate duration of treatment in a perioperative context.
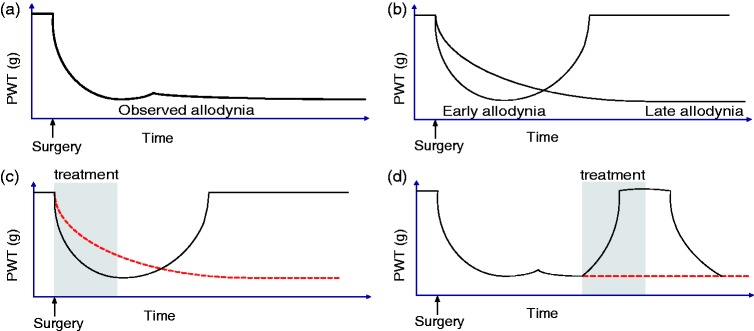
As expected, administration of treatments at various stages of nerve injury has a different impact. Drugs recommended to treat neuropathic pain (i.e., anticonvulsants, TCA, SSNRI), as well as a high dose of corticosteroid, alleviate chronic neuropathic allodynia. However, these drugs had no rapid effect on allodynia when they were administered in the perisurgical period. Interestingly, the early treatment with gabapentin or carbamazepine is prophylactic on the chronification of neuropathic allodynia. Mechanical allodynia is not a unitary process, but it may combine different mechanisms that follow different time-courses. In this context, we might hypothesize that the long lasting decrease in paw withdrawal thresholds results from at least two distinct sequences of allodynia (Figure 4(a) and (b)). First, an early, or postsurgical, allodynia is present, which can be related to transitory phenomena following the surgical lesion and acute local nerve inflammation. This allodynia may disappear spontaneously in days or weeks after the surgery and initial nerve compression, and it is sensitive to ketamine. However, at behavioral level, spontaneous relief of this early and reversible allodynia is not detected because of the occurrence of later allodynic mechanisms. This second type of allodynia results from sensitization processes and underlies pain chronification, with a slow onset during the days following surgery. Thus, perioperative anticonvulsants may block the onset of these chronification mechanisms during their development, while having no impact per se on the early allodynia (Figure 4(c)). If these mechanisms of chronification are not blocked at an early stage, gabapentinoids and carbamazepine would then exert a symptomatic and reversible effect (Figure 4(d)), which reflects their present clinical use.
Figure 4.
Conceptual illustration of early and late treatment impact on mechanical allodynia. The allodynia measured following sciatic nerve compression (a) may be the result of two distinct allodynia mechanisms (b). Early treatments preventing allodynia chronification may block the induction of late allodynia without affecting the early allodynia and have long-lasting consequences (c). Classical treatments in a more chronic stage of neuropathic pain would temporarily alleviate late allodynia (d).
Here, we used an animal model in which the sustained neuropathic allodynia is relieved with the same classes of drugs (gabapentin, antidepressants) as in the clinic, and with a similar pattern of response (delayed relief, relapse after the cessation of the treatment). Present results highlight the importance of a long-term follow up of treatment assessment in experimental models. Indeed, delayed prophylactic action of drugs on neuropathic pain may not be detected during the treatment itself. Surgery is an important cause of lasting neuropathic pain, and while preventive analgesia often addresses short-term postsurgical pain, the risk of neuropathic pain chronification is less often studied, despite the clinical need for preventing such long-term risks.
Our main finding is the presence of a delayed preemptive and long-lasting antiallodynic action with a 10-day early anticonvulsant drug treatment, leading to a persisting absence of allodynia two months after the surgery. These results support the interest for further clinical assessment of preventive prolonged anticonvulsant treatment with gabapentinoids for reducing the long-term risk of postsurgical neuropathic pain.
Acknowledgments
The authors thank the Chronobiotron facilities (UMS3415 CNRS), Stéphane Doridot and Edouard Gottschalk for animal care, and Elisabeth Waltisperger for assistance during surgeries.
Author Contributions
ES, IY, AM, and MB designed the study. ES and IY conducted the experiments and analyzed the data. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Centre National de la Recherche Scientifique (CNRS, contract UPR3212) and the Université de Strasbourg, France.
References
- 1.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008; 70: 1630–1635. [DOI] [PubMed] [Google Scholar]
- 2.Attal N, Cruccu G, Haanpaa M, et al. EFNS guidelines on pharmacological treatment of neuropathic pain. Eur J Neurol 2006; 13: 1153–1169. [DOI] [PubMed] [Google Scholar]
- 3.Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol 2015; 14: 162–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martinez V Baudic S andFletcher D.. Chronic postsurgical pain. Ann Fr Anesth Reanim 2013; 32: 422–435. [DOI] [PubMed] [Google Scholar]
- 5.Dahl JB andKehlet H.. Preventive analgesia. Curr Opin Anaesthesiol 2011; 24: 331–338. [DOI] [PubMed] [Google Scholar]
- 6.Barrot M. Tests and models of nociception and pain in rodents. Neuroscience 2012; 211: 39–50. [DOI] [PubMed] [Google Scholar]
- 7.Benbouzid M, Pallage V, Rajalu M, et al. Sciatic nerve cuffing in mice: a model of sustained neuropathic pain. Eur J Pain 2008; 12: 591–599. [DOI] [PubMed] [Google Scholar]
- 8.Yalcin I, Megat S, Barthas F, et al. The sciatic nerve cuffing model of neuropathic pain in mice. J Vis Exp 2014; 89. doi: 10.3791/51608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elia N andTramer MR.. Ketamine and postoperative pain – a quantitative systematic review of randomised trials. Pain 2005; 113: 61–70. [DOI] [PubMed] [Google Scholar]
- 10.De Kock M Lavand’homme P andWaterloos H. ‘ Balanced analgesia’ in the perioperative period: is there a place for ketamine? Pain 2001; 92: 373–380. [DOI] [PubMed] [Google Scholar]
- 11.Loftus RW, Yeager MP, Clark JA, et al. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology 2010; 113: 639–646. [DOI] [PubMed] [Google Scholar]
- 12.Dale O, Somogyi AA, Li Y, et al. Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth Analg 2012; 115: 934–943. [DOI] [PubMed] [Google Scholar]
- 13.Weinbroum AA. Non-opioid IV adjuvants in the perioperative period: pharmacological and clinical aspects of ketamine and gabapentinoids. Pharmacol Res 2012; 65: 411–429. [DOI] [PubMed] [Google Scholar]
- 14.Clarke H, Bonin RP, Orser BA, et al. The prevention of chronic postsurgical pain using gabapentin and pregabalin: a combined systematic review and meta-analysis. Anesth Analg 2012; 115: 428–442. [DOI] [PubMed] [Google Scholar]
- 15.Amr YM andYousef AA.. Evaluation of efficacy of the perioperative administration of Venlafaxine or gabapentin on acute and chronic postmastectomy pain. Clin J Pain 2010; 26: 381–385. [DOI] [PubMed] [Google Scholar]
- 16.Brogly N, Wattier JM, Andrieu G, et al. Gabapentin attenuates late but not early postoperative pain after thyroidectomy with superficial cervical plexus block. Anesth Analg 2008; 107: 1720–1725. [DOI] [PubMed] [Google Scholar]
- 17.Clarke H, Pereira S, Kennedy D, et al. Adding gabapentin to a multimodal regimen does not reduce acute pain, opioid consumption or chronic pain after total hip arthroplasty. Acta Anaesthesiol Scand 2009; 53: 1073–1083. [DOI] [PubMed] [Google Scholar]
- 18.Fassoulaki A, Patris K, Sarantopoulos C, et al. The analgesic effect of gabapentin and mexiletine after breast surgery for cancer. Anesth Analg. 2002; 95: 985–991. [DOI] [PubMed] [Google Scholar]
- 19.Fassoulaki A, Triga A, Melemeni A, et al. Multimodal analgesia with gabapentin and local anesthetics prevents acute and chronic pain after breast surgery for cancer. Anesth Analg. 2005; 101: 1427–1432. [DOI] [PubMed] [Google Scholar]
- 20.Sen H, Sizlan A, Yanarates O, et al. A comparison of gabapentin and ketamine in acute and chronic pain after hysterectomy. Anesth Analg 2009; 109: 1645–1650. [DOI] [PubMed] [Google Scholar]
- 21.Sen H, Sizlan A, Yanarates O, et al. The effects of gabapentin on acute and chronic pain after inguinal herniorrhaphy. Eur J Anaesthesiol 2009; 26: 772–776. [DOI] [PubMed] [Google Scholar]
- 22.Burke SM andShorten GD.. Perioperative pregabalin improves pain and functional outcomes 3 months after lumbar discectomy. Anesth Analg 2010; 110: 1180–1185. [DOI] [PubMed] [Google Scholar]
- 23.Pesonen A, Suojaranta-Ylinen R, Hammaren E, et al. Pregabalin has an opioid-sparing effect in elderly patients after cardiac surgery: a randomized placebo-controlled trial. Br J Anaesth 2011; 106: 873–881. [DOI] [PubMed] [Google Scholar]
- 24.Camara CC, Araujo CV, de Sousa KK, et al. Gabapentin attenuates neuropathic pain and improves nerve myelination after chronic sciatic constriction in rats. Neurosci Lett 2015; 607: 52–58. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Xie W, Strong JA, et al. Systemic antiinflammatory corticosteroid reduces mechanical pain behavior, sympathetic sprouting, and elevation of proinflammatory cytokines in a rat model of neuropathic pain. Anesthesiology 2007; 107: 469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holte K andKehlet H.. Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J Am Coll Surg 2002; 195: 694–712. [DOI] [PubMed] [Google Scholar]
- 27.Stubhaug A, Romundstad L, Kaasa T, et al. Methylprednisolone and ketorolac rapidly reduce hyperalgesia around a skin burn injury and increase pressure pain thresholds. Acta Anaesthesiol Scand 2007; 51: 1138–1146. [DOI] [PubMed] [Google Scholar]
- 28.Romundstad L, Breivik H, Niemi G, et al. Methylprednisolone intravenously 1 day after surgery has sustained analgesic and opioid-sparing effects. Acta Anaesthesiol Scand 2004; 48: 1223–1231. [DOI] [PubMed] [Google Scholar]
- 29.Romundstad L andStubhaug A.. Glucocorticoids for acute and persistent postoperative neuropathic pain: what is the evidence? Anesthesiology 2007; 107: 371–373. [DOI] [PubMed] [Google Scholar]
- 30.Dionne RA, Gordon SM, Rowan J, et al. Dexamethasone suppresses peripheral prostanoid levels without analgesia in a clinical model of acute inflammation. J Oral Maxillofac Surg 2003; 61: 997–1003. [DOI] [PubMed] [Google Scholar]
- 31.Bergeron SG, Kardash KJ, Huk OL, et al. Perioperative dexamethasone does not affect functional outcome in total hip arthroplasty. Clin Orthop Relat Res 2009; 467: 1463–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun YH, Li HS, Zhu C, et al. The analgesia effect of duloxetine on post-operative pain via intrathecal or intraperitoneal administration. Neurosci Lett 2014; 568: 6–11. [DOI] [PubMed] [Google Scholar]
- 33.Hajhashemi V, Banafshe HR, Minaiyan M, et al. Antinociceptive effects of venlafaxine in a rat model of peripheral neuropathy: role of alpha2-adrenergic receptors. Eur J Pharmacol 2014; 738: 230–236. [DOI] [PubMed] [Google Scholar]
- 34.Wong K, Phelan R, Kalso E, et al. Antidepressant drugs for prevention of acute and chronic postsurgical pain: early evidence and recommended future directions. Anesthesiology 2014; 121: 591–608. [DOI] [PubMed] [Google Scholar]
- 35.Kremer M, Salvat E, Muller A, et al. Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Neuroscience 2016; 338: 183–206. [DOI] [PubMed] [Google Scholar]
- 36.Dharmshaktu P Tayal V andKalra BS.. Efficacy of antidepressants as analgesics: a review. J Clin Pharmacol 2012; 52: 6–17. [DOI] [PubMed] [Google Scholar]
- 37.Mico JA, Ardid D, Berrocoso E, et al. Antidepressants and pain. Trends Pharmacol Sci 2006; 27: 348–354. [DOI] [PubMed] [Google Scholar]
- 38.Ho KY, Tay W, Yeo MC, et al. Duloxetine reduces morphine requirements after knee replacement surgery. Br J Anaesth 2010; 105: 371–376. [DOI] [PubMed] [Google Scholar]
- 39.Chocron S, Vandel P, Durst C, et al. Antidepressant therapy in patients undergoing coronary artery bypass grafting: the MOTIV-CABG trial. Ann Thorac Surg 2013; 95: 1609–1618. [DOI] [PubMed] [Google Scholar]
- 40.Baudic S, Jayr C, Albi-Feldzer A, et al. Effect of alexithymia and emotional repression on postsurgical pain in women with breast cancer: a prospective longitudinal 12-month study. J Pain 2016; 17: 90–100. [DOI] [PubMed] [Google Scholar]
- 41.Bian F, Li Z, Offord J, et al. Calcium channel alpha2-delta type 1 subunit is the major binding protein for pregabalin in neocortex, hippocampus, amygdala, and spinal cord: an ex vivo autoradiographic study in alpha2-delta type 1 genetically modified mice. Brain Res 2006; 1075: 68–80. [DOI] [PubMed] [Google Scholar]
- 42.Bauer CS, Nieto-Rostro M, Rahman W, et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J Neurosci 2009; 29: 4076–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blom S. Trigeminal neuralgia: its treatment with a new anticonvulsant drug (G-32883). Lancet 1962; 1: 839–840. [DOI] [PubMed] [Google Scholar]
- 44.McQuay H, Carroll D, Jadad AR, et al. Anticonvulsant drugs for management of pain: a systematic review. Bmj 1995; 311: 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Backonja MM. Anticonvulsants (antineuropathics) for neuropathic pain syndromes. Clin J Pain 2000; 16: S67–S72. [DOI] [PubMed] [Google Scholar]
- 46.Brau ME, Dreimann M, Olschewski A, et al. Effect of drugs used for neuropathic pain management on tetrodotoxin-resistant Na(+) currents in rat sensory neurons. Anesthesiology 2001; 94: 137–144. [DOI] [PubMed] [Google Scholar]
- 47.Ambrosio AF, Silva AP, Malva JO, et al. Carbamazepine inhibits L-type Ca2+ channels in cultured rat hippocampal neurons stimulated with glutamate receptor agonists. Neuropharmacology 1999; 38: 1349–1359. [DOI] [PubMed] [Google Scholar]
- 48.Attal N, Cruccu G, Baron R, et al. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol 2010; 17: 1113–e88. [DOI] [PubMed] [Google Scholar]
- 49.Moore A, Costello J, Wieczorek P, et al. Gabapentin improves postcesarean delivery pain management: a randomized, placebo-controlled trial. Anesth Analg 2011; 112: 167–173. [DOI] [PubMed] [Google Scholar]
- 50.Salvat E, Schweitzer B, Massard G, et al. Effects of beta2 agonists on post-thoracotomy pain incidence. Eur J Pain 2015; 19: 1428–1436. [DOI] [PubMed] [Google Scholar]
- 51.Matsutani N, Dejima H, Takahashi Y, et al. Pregabalin reduces post-surgical pain after thoracotomy: a prospective, randomized, controlled trial. Surg Today 2015; 45: 1411–1416. [DOI] [PubMed] [Google Scholar]
- 52.Buvanendran A, Kroin JS, Della Valle CJ, et al. Perioperative oral pregabalin reduces chronic pain after total knee arthroplasty: a prospective, randomized, controlled trial. Anesth Analg 2010; 110: 199–207. [DOI] [PubMed] [Google Scholar]