Abstract
Accumulating evidence demonstrates that complement activation is involved in the pathogenesis of osteoarthritis (OA). However, the intimate complement regulation and cross talk with other signaling pathways in joint-associated tissues remain incompletely understood. Recent insights are summarized and discussed here, to put together a more comprehensive picture of complement involvement in OA pathogenesis. Complement is regulated by several catabolic and inflammatory mediators playing a key role in OA. It seems to be involved in many processes observed during OA development and progression, such as extracellular cartilage matrix (ECM) degradation, chondrocyte and synoviocyte inflammatory responses, cell lysis, synovitis, disbalanced bone remodeling, osteophyte formation, and stem cell recruitment, as well as cartilage angiogenesis. In reverse, complement can be activated by various ECM components and their cleavage products, which are released during OA-associated cartilage degradation. There are, however, some other cartilage ECM components that can inhibit complement, underlining the diverse effects of ECM on the complement activation. It is hypothesized that complement might also be directly activated by mechanical stress, thereby contributing to OA. The question arises whether keeping the complement activation in balance could represent a future therapeutic strategy in OA treatment and in the prevention of its progression.
Keywords: Osteoarthritis, chondrocyte, complement, exercise
Introduction
Osteoarthritis (OA), the most common joint disease can develop as a sequela of a joint cartilage trauma. It is a “whole joint disease,” meaning that all joint-associated tissues are affected by OA and contribute to its pathogenesis.1,2 In the case of OA of the knee (gonarthrosis), in addition to the joint cartilage and the subchondral bone, the synovial membrane, even including the outer fibrous layer of the joint capsule, ligaments—especially intra-articular ligaments, such as anterior cruciate ligament (ACL) and posterior cruciate ligament3—menisci,4 and the intra-articular fat pads, such as Hoffa fat pad, can be affected by the inflammatory processes. Synovitis, which used to be per definition primarily associated with rheumatoid arthritis (RA) is also defined as an important aspect of OA.2,5,6 A role of complement in the pathogenesis of OA was suggested 10 years ago7 and still remains a promising field for research.8 In a recent study, abnormally high complement expression was found in synovial fluid (SF) and membrane samples of human patients with OA.8 In the same study, significantly lower expression of inflammatory markers in mice deficient in the central complement component C5 could be shown compared with wild-type mice in an OA model.8
Furthermore, in a cartilage blunt trauma model in rabbits, the deposition of the terminal complement complex (TCC) on chondrocyte surface also known as membrane attack complex (MAC) could be demonstrated.9 Despite these novel results,8 the detailed pathogenetic pathway triggered in the joint by complement activation and its cross talk with other signaling routes contributing to OA still remains incompletely characterized.
Osteoarthritis
Traditionally, OA has usually been defined as a result of impulsive or repetitive overloading, leading to an excess of biomechanical stress of a joint. Also, excessive body weight or aberrations from the physiological axis of the articulating bones resulting in an overload of particular joint areas leading to degradation of the involved articular cartilage10 were identified as important causal factors. Even though repetitive physiological mechanical loading is an essential regulator for the metabolic activity of the chondrocytes,11 excessive burden to the joint triggers cartilage inflammation with subsequent degradation, mediated by the release of inflammatory and extracellular cartilage matrix (ECM)-degrading factors such as interleukin 1β (IL-1β), tumor necrosis factor α (TNF-α), and matrix metalloproteinase (MMP)-1, MMP-3, and MMP-9.12,13 In addition to overloading and joint instability, the modern concept of OA pathogenesis includes genetic, endocrinologic, metabolic, age-dependent, and other risk factors disturbing the homeostasis of the whole joint by the release of a multitude of factors from joint-associated tissues, which are catabolic, inflammatory, or impairing tissue integrity.2,14,15 Hence, the major contribution of IL-1β and TNF-α to OA pathogenesis, both representing key pro-inflammatory and catabolic cytokines, has been well accepted for the past decades.6,16–18 These factors initiate a low-grade inflammation and degradation of ECM components including collagen and proteoglycans (PG) by the induction of various MMPs through activated synovial macrophages, synovial fibroblasts, or the chondrocytes themselves.19,20 These cytokines directly facilitate persistent joint inflammation and joint cartilage destruction in OA. Obesity and diabetes mellitus, the last mentioned being one of the most common comorbidities in OA, are known to be associated with low-grade systemic inflammation.21,22 In addition, complement activation appears critical in OA pathogenesis and also affects the expression of inflammatory and degradative molecules in chondrocytes.8,14,23 Some complement components seem also to be dysregulated in obese and could contribute to insulin resistance.24,25 This possibly shared link could serve as a future potential therapeutic target for OA treatment. First, however, a better understanding of the interconnection between the complement system and various other inflammatory and noninflammatory factors in the OA scenario is necessary.
The Complement System
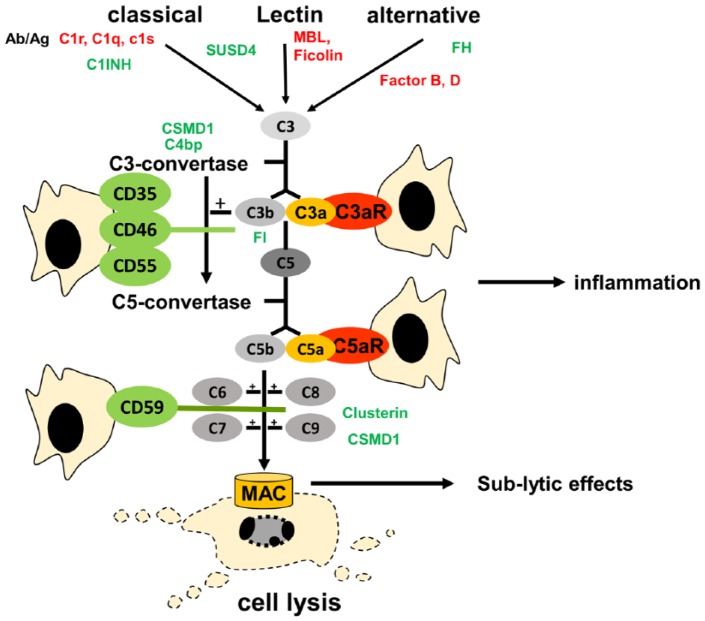
The complement system represents an important part of the innate immune system, which mediates multiple responses including the initiation of opsonization, phagocytosis of pathogens, inflammatory response, and terminating in cell lysis.26,27 Over the past decade, the complement system has been seen as a bridge between the innate and the acquired immunity.28 The 3 complement activation pathways (classical, alternative, and mannose-activated) have all been described as resulting in the cleavage of the C3 (complement component C3) component mediated by the C3 convertases27 and later in cleavage of the C5 molecule into C5a and C5b that subsequently forms the TCC (C5b-9) by recruiting downstream complement proteins29 (Figure 1). The TCC mediates lysis of affected or infected target cells, but sublytic TCC and soluble C5b-9 can also arise, which exerts a multitude of noncytolytic immune functions.29 Selection of examples of noncytolytic effects is summarized in Table 1, which suggests a cell type–dependent profile. Apparently, the complement system can be triggered in later stages of the cascade as a couple of other proteolytic factors, such as cathepsin D,45 thrombin,46 and plasmin,47 are known to activate the downstream part of the complement cascade. During complement activation, the inflammatory cleavage products, anaphylatoxins, C3a, C4a, and C5a, are released.48 They bind to their respective anaphylatoxin receptors, C3aR (C3a anaphylatoxin receptor), C4aR, and C5aR (C5a anaphylatoxin receptor),49 mediating various but mostly inflammatory effects such as increase in vascular permeability; induction of histamine release in mast cells; smooth muscle cell contraction; synthesis of angiogenetic factors by mesenchymal stromal cells (MSCs); release of chemoattractants by neutrophil, eosinophil, and basophil granulocytes; and regulation of apoptosis (Table 1). Various diseases have been associated with a disbalance in the activation or inhibition of the complement system, such as RA, systemic lupus erythematosus, hyperacute graft rejection after transplantation, and sepsis.50–53 To prevent damage of host cells, an efficient regulatory system is required to prevent excessive complement activation. The regulatory network includes soluble complement inhibitors, such as C1 inhibitor (C1-INH), C4b-binding protein (C4bp), factor H (FH), factor I (FI), and clusterin (Figure 1).23,54,55 In addition, cytoprotective complement regulatory proteins (CRPs) including CD35, CD46, CD55, and CD59 are localized within the cell membrane at the cell surface56 (Figure 1, Table 2). CD46, CD55, and CD59 have been detected on chondrocytes and found to be regulated by major inflammatory cytokines such as IL-1β and TNF-α.57,58 It has been demonstrated that the MAC inhibitory protein CD59 is most likely involved in protection against OA.8 Prominent expression of CD59 was seen on the synoviocytes, endothelial cells, and stromal cells in cases of OA.59 However, a thorough search for other CRPs in arthritic joints has yet to be undertaken.
Figure 1.
Simplified scheme of complement activation. Ab/Ag, antibody-antigen interaction; C1-INH, C1 inhibitor; C4bp, C4-binding protein; CSMD1, CUB and Sushi multiple domains 1; FH, factor H; FI, factor I; MBL, mannose-binding lectin; SUSD4, Sushi domain–containing protein 4.
Table 1.
Nonlytic functions of main complement components.
| Complement components | Functions | Involved tissue/cells | Source |
|---|---|---|---|
| C5b-9 | Release of pro-inflammatory cytokines such as IL-8 and chemokines | Umbilical vein endothelial cells | Kilgore et al30 |
| Cell proliferation | Renal mesangial cells | Brandt et al31 | |
| Aortic smooth muscle cells | Niculescu et al32 | ||
| Schwann cells | Dashiell et al33 | ||
| Expression of growth factors such as PDGF, bFGF | Endothelial cells | Benzaquen et al34 | |
| Induces apoptosis | Renal mesangial cells | Nauta et al35 | |
| Inhibits apoptosis | Oligodendrocytes and Schwann cells | Rus et al36 | |
| C5a | Inhibits apoptosis | Neutrophil granulocytes | Lee et al37 |
| Induces apoptosis | Neuronal cell line | Farkas et al38 | |
| Early increase and later suppression in gene expression of CD46, CD55, CD59 | Tenocytes | Own unpublished work | |
| Chemotaxis | MSCs | Schraufstatter et al39 | |
| Chemotaxis | Neutrophil granulocytes | Ehrengruber et al40 | |
| Secretion and activation of MMP-9 | Neutrophil and eosinophil granulocytes | DiScipio et al41 | |
| C3a | Inhibits apoptosis | Mesangial cells | van Beek et al42 |
| Production of angiogenic factors, such as VEGF, CXCL8/IL-8, and IL-6 | MSCs | DiScipio et al43 | |
| Hemotaxis | Eosinophil granulocytes | Daffern et al44 |
Abbreviations: bFGF, basic fibroblast growth factor; IL-6, interleukin 6; IL-8, interleukin 8; MMP, matrix metalloproteinase; MSCs, mesenchymal stromal cells; PDGF, platelet-derived growth factor; VEGF, vascular endothelial growth factor.
Table 2.
Complement regulators.
| Regulator | Interaction with complement |
|---|---|
| SUSD4 (Sushi domain–containing protein 4) | Classical and lectin pathway inhibited via binding to C1q |
| CSMD1 (CUB and Sushi multiple domains 1) | C3 convertase inhibited, MAC (C7 incorporation inhibited) |
| CD59 | (Cell membrane anchored) MAC (C7 incorporation inhibited) |
| CD55 (DAF, decay-accelerating factor) | (Cell membrane anchored but also in soluble form, eg, in synovial fluid) C3 convertase inhibited, accelerating the decay of C3/C5 convertases |
| CD46 (MCP, membrane cofactor protein) | (Cell membrane anchored) C3 convertase inhibited |
| CD35 (CR1, complement receptor 1) | (Cell membrane anchored) Acts on the C3b and C4b inactivation, immune complex processing and cleaning |
| C4b-binding protein (C4bp) | Inhibits the classical and the lectin pathways (C4) Can bind C3b, facilitates decay of C3 convertase Serves as cofactor for factor I which cleaves C4b and C3b |
| Properdin | (Soluble) Regulates alternative pathways by C3 convertase stabilization |
| Factor H (FH) | (Soluble) Inhibits alternative pathway |
| Factor I (FI) | Inactivation of C3b and C4b to iC3b and iC4b (CD35 acts as a cofactor) |
| C1 inhibitor (C1INH) | Can inactivate C1s, C1r (and kallikrein, plasmin, and coagulation factors XI, XII |
| Clusterin | Binds C7, C8, and C9b |
| Carboxypeptidase B | Inactivates C5a |
Abbreviations: C1r, complement C1r subcomponent; C1s, complement C1s subcomponent; C3, complement component C3; MAC, membrane attack complex.
Sources for Complement in the Joint
Diarthrotic joints contain various tissues, which serve as source for complement proteins. The SF is a known source for complement factors8,60,61 and can also distribute complement within the joint. It is well known, that the complement cascade is activated in the SF in RA joints.62 Stuglics et al60 showed that the SF in OA and RA joints, pyrophosphate arthritis, and acute knee injuries contains substantial amounts of complement factors, such as C4d, C5 convertase of the alternative pathway (C3bBbP), and soluble TCC (as soluble end products of C4b, properdin-containing C3 convertase, and TCC, respectively), in comparison with the reference group. It was also suggested that the complement factors in the SF might originate from synoviocytes and chondrocytes, although all the tissues in the formation of a joint could similarly contribute to it. Another consideration is that the complement load in an affected joint can be increased through an elevated systemic concentration as plasma filtrate63 as a consequence of systemic inflammation as in RA and OA.
Cartilage, synovial membrane, and chondrocytes are important sources for the protein expression of components of the classical complement pathways such as C1q, C1s, C4, and C2. These proteins were detected immunohistologically in chondrocytes in situ in normal human articular cartilage and macroscopically unaffected articular cartilage from the femoral heads of patients with OA.64 Corresponding with these observations, the gene expression of these components was shown in articular cartilage.64 Articular chondrocytes cultured in vitro expressed C1r, C1s, C4, C2, C3, and C1 inhibitor but not C1q, C4bp, or FI. Hence, complement factor and CRP expression differed between cartilage and freshly isolated and cultured chondrocytes.58,64
The complement components, C3aR and C5aR, and the CRPs, CD46, CD55, and CD59, were expressed not only in articular chondrocytes but also in nonarticular chondrocytes58 and in intervertebral disc–derived fibrochondrocytes.65 Interestingly, in synovial membrane, an extracellular deposition of CD55 produced by synovial fibroblasts and attached to the intimal collagen fiber network was shown.66 The expression of CD35 could not be demonstrated in cultured chondrocytes.58 As C4 is involved in the classical pathway, complement factor B (CFB) is represented only in the alternative pathway.67 Assirelli et al found expression of C3, C4, and CFB in osteoarthritic cartilage, synovial membrane, and cultured chondrocytes and synoviocytes. More CFB was expressed in chondrocytes and cartilage explants than C3 and C4, suggesting a substantial role for the alternative pathway in OA. There was a higher level of C5b-9 in supernatants of cartilage explant cultures compared with supernatants of synoviocyte cultures.67 In cultured chondrocytes, complement expression was regulated by the pro-inflammatory cytokines IL-1β, TNF-α, and IFN-γ.64 The complement component C1s (complement C1s subcomponent) was increased by TNF-α, which is a key factor in the OA.68 Both CFB and C3 were amplified by IL-1β stimulation in cultured chondrocytes and synoviocytes.67
However, further analysis of the expression profiles regarding zone and OA grade dependency is required.
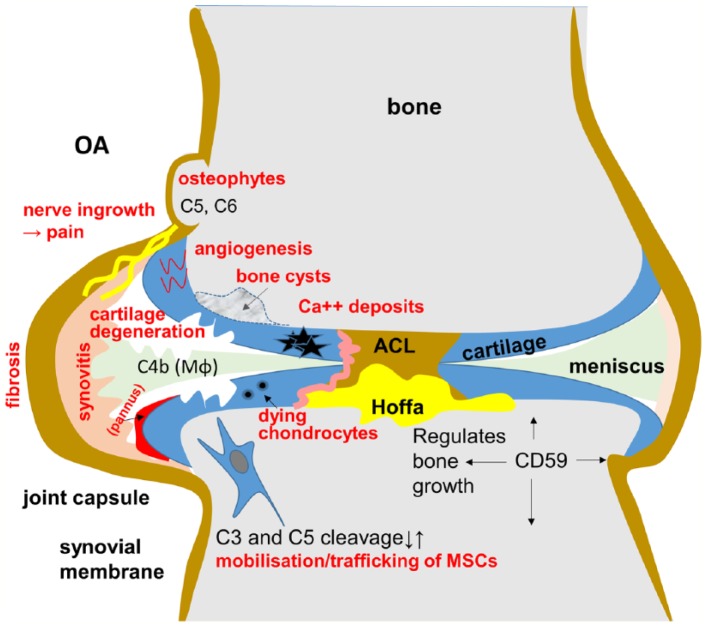
The osteoblasts in the subchondral bone express the central complement proteins, C3 and C5, and the anaphylatoxin receptors, C3aR and C5aR, respectively.69 Based on the results from experimentally induced OA in C5 and C6 knockout mice models, C5 and C6 proteins have been suggested to contribute—in addition to cartilage loss—also to the formation of osteophytes, which represent a typical macroscopical feature in OA joints.8 Overexpression of C5aR exclusively in osteoblasts led to impaired fracture healing in mice, and the C5aR was suggested to increase osteoclast activity called osteoclastogenesis.70,71 The CRP CD59a is possibly a sex-dependent regulator of bone growth maintaining bone density and bone stability, as it is observed exclusively in male CD59a knockout mice.72 The same authors also observed an increased osteoclastogenesis in vitro. During OA, multiple features of bone alterations can be observed, such as subchondral bone remodeling, sclerosis, and fractures, leading to cyst formation and osteophyte formations (Figure 2).15,73,74
Figure 2.
Simplified scheme depicting complement involvement in OA. OA indicates osteoarthritis.
Mesenchymal stromal cells, eg, residing in the subchondral bone marrow contribute to remodeling and repair. The MSCs can produce complement inhibitory factor FH which could play a protective role in the joint against the complement activity.75
There are no data available in the published literature concerning joint-associated fatty tissues and complement activation. Adipocytes can be found in the subintima of the synovial membrane or in Hoffa fat pad in the middle of the knee joint attached to the anterior part of the joint capsule. However, the Hoffa fat pad is directly covered by the synovial membrane and serves as a source of various mediators involved in OA.76,77 Complement plays an important role in fatty tissue, adipocytes produce complement proteins,78 and complement activation can lead to low-grade inflammation in adipose tissue.21 Metabolic disbalances in fat tissue are observed in metabolic diseases, such as diabetes mellitus, which represent a predisposition for OA.79 Moreover, C3aR and C5aR are involved in the development of adipocytes’ insulin resistance through macrophage infiltration and the activation of adipose tissue.21 Taken together, fatty tissue synthesizes and regulates complement components, which present a source for the release of the anaphylatoxins C3a and C5a.80
Ligaments and tendons express complement factors.81,82 The complement split fragment C3a, which is produced during the early phase of inflammation, induces gene expression of the anaphylatoxin receptors, C3aR and C5aR, as well as expression of the pro-inflammatory cytokines TNF-α and IL-1β. Furthermore, it impairs the gene expression of the cytoprotective CRPs, CD46 and CD55, in human tenocytes in vitro.81 The expression of C3aR and C5aR was also regulated (induced and suppressed) time dependently by mechanical cell injury in vitro, whereas the CRPs, CD55 and CD46, were induced under these conditions.82 Of note, it is well known that intra-articular ligaments, such as the ACL, are prone to OA and show various features of degeneration in osteoarthritic knee joints.3
Even in the fibrocartilaginous meniscus tissue, C4d deposits have been detected immunohistochemically in the ECM areas of mucoid degeneration or fibrillation after meniscoectomy.83 In some of the samples, an association with the macrophage marker CD68 was found.83 However, the degeneration of the meniscus may not only go along with the onset of gonarthrosis but also might actively contribute to it.84
Complement Regulation and Activation in Cartilage and Chondrocytes
Complement activation and OA
Complement activation has been described to be critical for the development of OA.8 Wang et al stated that complement activation results in the formation of TCC on chondrocytes, which either leads to cell death or initiates them to produce matrix-degrading enzymes (such as MMPs), inflammatory mediators (eg, macrophage colony-stimulating factor, cyclooxygenases, C-C motif ligands 2 and 5), and further complement effectors—all of which promote joint pathology. The C5aR is expressed in cartilage of normal patients and patients with OA and RA and is induced by IL-1β in chondrocytes.85 The cell surface glycoproteins, CD35, CD46, CD55, and CD59, are widely distributed on normal tissue cells, protecting them from complement-mediated cell lysis.86 These CRPs are upregulated in arthritic joint diseases87; however, this mechanism appears to fail and cannot protect from tissue damage. Another role of complement activation in OA is indicated by its effect on pain, a characteristic clinical feature of OA. The anaphylatoxins, C5a and C3a, play a vital role in the onset of pain by activation and sensitization of nociceptors in vivo and in vitro. Inhibitors or antagonists of C5aR receptors might be key candidates for pain therapy in OA as well.88,89
Even though C5a and C3a are generally well known for their catabolic function, they also have a vital role in mobilizing, trafficking, and homing of bone marrow–derived hematopoietic stem cells and MSCs, which can contribute to OA-associated bone remodeling in vitro.90–95 This in return can promote the repair of the cartilage tissue as well (Figure 2). It indicates that the inhibition of C3aR and C5aR could likewise prevent the chemotactic response to MSC.39 Moreover, the interaction of C5a/C5aR has been implicated in osteogenic differentiation of MSCs96 which is important for subchondral bone remodeling.
Activation of complement by ECM components and fragments
Endogenous cartilage ECM components have been shown to be involved in OA pathogenesis.97 In OA, the damaged cartilage releases ECM components such as degradation products of collagen type II, fibromodulin, fibronectin, and hyaluronan (HA) into the SF. Other fragments and motifs of ECM proteins, the so-called damage-associated molecular patterns (DAMPs), which are exposed in OA cartilage by the dysregulated activity of various proteases, including MMPs, ADAM-TS, and others, can activate pro-inflammatory pathways.6,98 Many of these pathways feature interactions with complement factors (Table 2).
Complement activation can be mediated not only by various ECM components, such as fibromodulin,8,98 aggrecan,8,99 and cartilage oligomeric matrix protein (COMP)100 (Table 3), as described in detail below, but also by hydroxyapatite and calcium pyrophosphate dihydrate crystals, by apoptotic cells and the resulting cell debris.18
Table 3.
Cartilage ECM components and chondrocyte cell surface proteins interacting with complement factors.
| ECM component | Normal function | Interaction with complement | Reference |
|---|---|---|---|
| Type II collagen | Specific and major collagen type in cartilage ECM | (+) Formation of collagen-antibody immune complexes in cartilage and subsequent complement activation via classical pathway | Koobkokkruad et al118 |
| Type IX collagen | Specific collagen type in cartilage ECM | (−) NC4 domain of collagen IX inhibits complement directly due to attenuation of MAC formation and indirectly through binding and enhancing activity of complement inhibitors, C4B-binding protein, and factor H | Kalchishkova et al119 |
| Aggrecan | Specific and major proteoglycan in cartilage ECM | (+) The C-type lectin of the aggrecan G3 domain activates complement | Wang et al8, Furst et al99 |
| GAGs, eg, CS | Major component of aggrecan | (+) Factor H provides binding sites for GAGs (−) Complement factors, such as CFB, C1s, C3, and C1r, were decreased by CS |
Li et al140, Clark et al117, Calamia et al107 |
| Hyaluronan | Attached to aggrecan | (−) A case was reported where an induction of anaphylatoxin C5a and TCC led to joint inflammation in response to multiple intra-articular injections of hylan G-F 20 | Sofat97, Dragomir et al105 |
| COMP | Mediates collagen fibrillogenesis | (+) COMP induces activation and deposition of C3b and C9 via the alternative pathway in RA (−) COMP inhibits the classical and the lectin pathways due to direct interaction with the stalk region of C1q and mannose-binding lectin in RA Both could not be shown in OA by Happonen et al100 |
Blom23, Happonen et al100,141 |
| Fibronectin and its fragments | Fibronectin regulates cell differentiation, adhesion, and migration | (Effects not shown) binding to the C1q component of complement | Barilla et al142, Casons et al143 |
| Biglycan and decorin (SLRP) | Decorin: limits collagen fiber formation, regulates TGF-β functions | (−) Decorin and biglycan: bind to C1q, can inhibit classical pathway, biglycan: inhibits also lectin pathway | Groeneweld et al110, Krumdieck et al111 |
| Fibromodulin (SLRP) | Keratan sulfate PG, bound to collagen fiber surface: limits collagen fiber formation | (+) Activates complement by binding to C1q, interaction with factor H | Wang et al8, Sofat97, Sjoberg et al98 |
| Chondroadherin and osteoadherin (SLRP) | Cell-matrix interaction binds to collagens and α2β1 integrin | (+) Activates complement by binding to C1q, interaction with factor H | Sjoberg et al112 |
| DAMP, damage-associated molecular patterns | Cartilage ECM or cellular fragments arising during OA-associated tissue disintegration | (+) A subgroup of DAMPs acts as neoantigens and exerts complement activation | Liu-Bryan6, Land144 |
| Integrins and TLR | Integrins: important cell-ECM receptors regulating diverse cellular processes TLR: besides recognizing patterns of microbial origin and activating innate immunity, they can also bind to various DAMPs, fibronectin, hyaluronan, and biglycan fragments |
(Effect not shown) α2β1 integrin Interacted with C1q Direct interaction between TLR and C5a/C5aR signaling, shown in immune cells |
Hajishengallis and Lambris145, Holst et al146, Sillat et al147, Zutter and Edelson148 |
Abbreviations: COMP, cartilage oligomeric protein; CS, chondroitin sulfate; DAMP, damage-associated molecular pattern; ECM, extracellular cartilage matrix; GAGs, glycosaminoglycans; OA, osteoarthritis; RA, rheumatoid arthritis; SLRP, small leucine-rich repeat protein; TGF-β, transforming growth factor β; TLR, toll-like receptor.
Hyaluronan is a major component of the ECM in cartilage and in various tissues. This ubiquitously found glycosaminoglycan (GAG) has a high turnover rate by natural degradation through hyaluronidase. Not only due to its viscoelastic property but also due to its anti-inflammatory functions,101 intra-articular HA injection shows pain-relieving and chondroprotective influence in clinical practice.102,103 However, there are reports suggesting that the chronic increment of HA fragments (<500 KDa) turns the benign inflammation into a chronic pro-inflammatory effect.104 One case reports a patient who received multiple intra-articular HA (Hylan G-F 20) injections which lead to complement activation, detectable by increased C5a and TCC release and accompanied by an induction of interleukin 6 (IL-6) and IL-1β.105 Possibly, this HA formulation could contain HA complexes or residual animal-derived antigens that induce immunologic responses in sensitive individuals. Therefore, it has to be considered that a chronic exposition of the joint cartilage to particular HA fragments in SF or joint-associated tissues could promote complement activation, which may contribute to development or progression of OA.
Mainly sulfated GAGs, such as chondroitin sulfate and keratan sulfate, with small portions of dermatan sulfate, bound to a core protein, build the cartilage-specific large PG aggrecan. Fragments of aggrecan, the most abundant large cartilage-specific PG, released in damaged cartilage can activate the complement system.99 The aggrecan C-type lectin domain (CLD), a part of the G3 domain, is seen released in articular diseases. They can activate the classical but to a lesser extent also the alternative complement pathway, via binding of C1q and C3, respectively. However, the observed complement activation is attenuated due to binding of complement inhibitor FH to CLD and CRP domains.99 This study therefore suggested aggrecan CLD as one factor involved in the sustained inflammation of the joint. GAG are generally described to have anti-complementary effects.106 Accordingly, chondroitin sulfate reduced inflammation directly by decreasing the expression of several complement components such as CFB, C1s, C3, and C1r (complement C1r subcomponent).107 Apart from the large aggregating PG aggrecan, small PGs, such as decorin, biglycan, and fibromodulin, are important components in articular cartilage.108 The homeostasis of these components may be altered in case of chronic inflammation.
Small leucine-rich repeat PGs (SLRPs), such as fibromodulin, were shown to bind directly to C1q and activate the classical pathway of complement.98 Other members of this family, namely, biglycan and decorin can also bind to C1q because they share significant sequence homology with fibromodulin,109 but the binding sites differ (stalk of the C1q molecule versus head) and both exert an inhibitory function on the classical pathway110,111 and do not activate complement.112 Although activators of C1q bind also the inhibitor FH, nonactivators, such as biglycan and decorin, do not interact with FH.112 This observation underlines regulatory interactions at multiple points of the cascade.
Other ECM components, such as osteoadherin in bones and chondroadherin in cartilage, tendon, bony growth plates, skeletal, and cardiac muscle, also tend to interact with C1q and activate the classical complement pathway. However, only moderate activation of the terminal pathway can be observed here.
Although with lower affinity than the above-mentioned SLRPs, lumican interacts with C1q component as well. Lumican is a glycoprotein localized in the ECM of many connective tissues, including cartilage. Similar to fibromodulin, it binds to fibrillar collagens and limits their growth.113 The authors distinguished 2 different SLRP-binding sites on C1q, at the head and stalk of the molecule, respectively; the binding site to the head could activate the complement system.112 In contrast to the hypothesis of complement activation by ECM components, Struglics et al60 found that none of the complement factors (C4d, C3bBbP, and soluble TCC) measured after knee injury in the SF correlated with proteolytic fragments of aggrecan or COMP, a noncollagenous protein also called thrombospondin 5. However, 0 to 12 weeks after knee injury, the concentrations of C4d, C3bBbP, and soluble TCC in the SF correlated positively with levels of IL-1β, IL-6, and TNF-α (rs range: 0.232-0.547). Although C3bBbP and soluble TCC went down to reference levels after 3 to 12 weeks, C4d was still elevated several years after injury.60 Nevertheless, COMP has been reported as a biomarker in serum that correlates with the severity of OA.114–116 The cleavage of COMP by various proteases can result in a couple of neoepitopes.116 In another report, it was shown that the patients with OA had significantly higher COMP-C3b complex concentration in SF than in serum.100 How could COMP influence the complement activity in the cartilage turnover process? In the same report, it was shown that COMP inhibits the classical and the lectin pathways as it interacts with the stalk region of C1q and mannose-binding lectin. However, the complement activation still progresses via alternative complement pathway associated with a release and activation of the split fragments C3b and C9 mediated by an interaction of COMP and properdin100 suggesting no protective effect of COMP in OA. Whether COMP contributes to OA complement activation as reported for RA remains questionable.100
Clark et al117 concluded that the glycomatrix in cartilage, which is prone to age-dependent or disease-dependent changes can recruit diverse positive and negative regulators of the complement system thereby possibly contributing to OA. In contrast to collagen type II which binds antibodies thereby facilitating complement activation via the classical pathway,118 a direct and indirect inhibition of complement was induced by the cartilage-specific collagen type IX (NC4 domain).119 The small number of chondrocytes residing in cartilage is surrounded by a dense matrix of the PG aggrecan bound to HA, which are embedded in a network of collagen fibrils stabilizing the ECM and providing protection for the cells. In the immediate pericellular environment of chondrocytes, particularly, type IX collagen exerts a protective role: the NC4 domain of the cartilage-specific collagen type IX binds both C4bp and FH indirectly inhibiting the complement activation and preventing directly the C9 polymerization and MAC formation.119 This result underlines the protective function of the cartilage-specific collagen type IX mediated by interacting with complement in the cartilage. Degradation or any congenital defect of this collagen type could facilitate the progression of OA increasing the vulnerability of the chondrocytes against the complement action.
Complement and angiogenesis and anti-angiogenesis
During OA, vascularization of the naturally avascular articular cartilage can be observed.120,121 The expression of vascular endothelial growth factor (VEGF) has been strongly implicated in this process.122 The anaphylatoxins, C5a and C3a, have been identified as pro-angiogenetic factors inducing the expression of VEGF in the chorion tissue.123 The maintenance of cartilage avascularity through control of angiogenesis in cartilage could function against cartilage deterioration in arthritis. As mentioned earlier, aspartate protease cathepsin D is able to cleave C5 in vitro, resulting in the generation of C5a.45 At the same time, cathepsin D is able to cleave prolactin (PRL) to generate vasoinhibins, a family of antiangiogenic peptides which can also inhibit vasopermeability and vasodilation and can have pro-inflammatory effects.124 Prolactin is present in SF.125 In addition, PRL and vasoinhibins are produced in joint tissues, including cartilage,126 synoviocytes,127 vascular endothelial cells,128,129 and immune cells.127 Prolactin promotes cartilage survival and attenuates inflammation in inflammatory arthritis.130 Moreover, PRL and vasoinhibins have been suggested to play a role in inhibition of angiogenesis in RA,131 which may also be the case in OA. The relevance of the generation of C5a and vasoinhibins by cathepsin D in OA is yet unknown, but their partly antagonistic and partly synergistic profile of biological effects in terms of stimulating (C5a) or inhibiting (vasoinhibins) angiogenesis and promoting inflammation, both indicates that investigating their relative contribution to OA is justified and may provide novel insights into OA etiopathology.
However, in OA, cathepsin D levels were impaired in blood serum compared with healthy individuals.132
Complement and exercise
High levels of exercise can induce complement activity detectable by C5a release as already shown in 1990.133,134 This observation might be explained by some tissue micro-damage due to exercise. In agreement with this assumption, activation of C3 was correlated with an increase in creatinine kinase. It might be possible, therefore, to relate muscle damage and complement activation after strenuous exercise.135 In response to high-intensity exercise, the expression of cathepsin D, a protease, which can cleave complement C5 component resulting in the generation of C5a, was significantly downregulated in the deep cartilage zone in horses and its expression differed also regionally in the joint, reflecting biomechanical differences.136 On the contrary, it is known that OA-associated pain and disability can be inhibited by moderate exercise.137 Exercise augmented the effect of medical therapy on OA, in general, showing a pain limitation and an improvement of mobility in the joint function in comparison with the control group.138 A meta-analysis showed that the strengthening exercises combined with other modalities, such as stretching and aerobic exercises, provide benefit in OA.139 However, the interrelation of direct mechanical stress or different degrees of mechanical strain and complement activity has not been described on the joint level and should be addressed in future.
Inhibitors of complement in the joint
To prevent uncontrolled complement activation and subsequent cell lysis, complement is controlled by several soluble and membrane-bound natural inhibitors23 (Table 2). CD59 is one of the membrane-bound proteins that inhibits the assembly of the downstream components, C5b-9, resulting in failure of TCC formation149 (Figure 1). The direct inhibitory role of CD59 in the development of OA has been proven in a mice model.8
Sushi domain–containing protein 4 (SUSD4) inhibits the formation of the classical C3 convertase and can thereby interrupt the early phase of complement activation.150 However, its effects have not been shown in cartilage yet.
Synovial expression of complement inhibitors, such as the C5a-inactivating enzyme carboxypeptidase B (CPB), is suppressed in OA.18,151,152 Moreover, CPB also serves as a protective mediator against the development of OA by inhibiting the formation of TCC.151 Likewise, regulation of TCC formation was also observed through supplementation of frequently found GAGs in cartilage tissues and chondroitin sulfates, more specifically, low-molecular-weight chondroitin sulfates, which have been developed as therapeutic complement inhibitors, and revealed attenuation of OA in a mouse OA model.140 Furthermore, antiangiogenic, anti-inflammatory, and anti-catabolic properties of chondroitin sulfate decreasing the expression of several complement components, such as CFB, C1s, C3, and C1r,107 add to its OA attenuating potential.
As mentioned earlier, the cartilage-specific collagen type IX inhibits complement directly due to attenuation of TCC formation and indirectly through binding and enhancing activity of complement inhibitors C4bp and FH.119 CR2-fH is a synthetically assembled fusion protein that regulates the activation of the alternative pathway of the complement system inhibiting the activation of C3 and C5 in vitro and attenuating the development of collagen antibody–induced OA in a mouse in vivo model.8,153
There are very few therapeutics so far, such as ezulizumab, a C1-INH which has been approved for clinical use. However, various therapeutics and still plenty of remedies focusing on the complement systems are on trials.
Therapeutically, HA injection has shown promising effects on the attenuation of pain in patients with OA. Treatment of the affected joint with autologous platelet-rich plasma (PRP) injection,154 which usually contains the entire complement of plasma complement factors, has been compared with the synthetic HA injection, which in summary showed comparable beneficial effects, although with less risk of side effects. Platelet-rich plasma can inhibit the nuclear factor κB activation,155 which explains the absence of TNF-α explaining its anti-inflammatory character.156 However, the complement activation has also been shown through activated PRPs, which rather contradict this anti-inflammation.157 Even though the complement activation has been proven, the increased expression of CRPs, such as CD55 and CD59, in the platelet cell membrane158,159 could provide protective effects against the complement attack. In addition, PRP generates via platelet stimulation thrombin activating the coagulation pathway. In fact, thrombin has recently been found to act directly as a C5 convertase and promotes terminal complement pathway activation46 underlining that the cross talk between the coagulation and complement pathways is intimate. Overall, we can conclude that there is an influence of the complement system in the PRP therapy, which has to be further defined in relation to OA.
Conclusions
Accumulated evidence demonstrates that complement activation contributes to OA pathogenesis and progression. Complement activation either via the 3 recognized pathways or direct activations through proteolytic enzymes could be involved in main features of OA, such as disbalanced bone remodeling, osteophyte formation, stem cell trafficking, synovitis, joint and meniscal cartilage-derived chondrocyte inflammatory response, and cell death and cartilage vascularization as well as ligament degeneration. Several intact or cleaved ECM components, released from degraded cartilage during OA, could present neoepitopes or DAMPs suspected to initiate and regulate complement activity. This so-called glycomatrix in the joint117 is not only affected by OA, initiating complement activation, but could also be modified by predisposing conditions for OA such as aging and underlying diseases including diabetes or joint calcinosis thereby facilitating OA progression. Understanding the role of the complement system in the joint and the involved tissues could present a future strategy for the treatment of OA.
Acknowledgments
The authors would like to thank Ms Lynne-Brennan for proofreading.
Footnotes
Funding:The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study is supported by the B. Braun Foundation (EMID: c6c8a1ca067517d0).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: SS and GS-T wrote the first draft of the manuscript. JT and TB contributed to the writing of the manuscript.
References
- 1. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruschke K, Meier C, Ullah M, et al. Bone morphogenetic protein 2/SMAD signalling in human ligamentocytes of degenerated and aged anterior cruciate ligaments. Osteoarthritis Cartilage. 2016;24:1816–1825. [DOI] [PubMed] [Google Scholar]
- 4. Pauli C, Grogan SP, Patil S, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthritis Cartilage. 2011;19:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scanzello CR, Goldring SR. The role of synovitis in osteoarthritis pathogenesis. Bone. 2012;51:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu-Bryan R. Synovium and the innate inflammatory network in osteoarthritis progression. Curr Rheumatol Rep. 2013;15:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. John T, Stahel PF, Morgan SJ, Schulze-Tanzil G. Impact of the complement cascade on posttraumatic cartilage inflammation and degradation. Histol Histopathol. 2007;22:781–790. [DOI] [PubMed] [Google Scholar]
- 8. Wang Q, Rozelle AL, Lepus CM, et al. Identification of a central role for complement in osteoarthritis. Nat Med. 2011;17:1674–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joos H, Leucht F, Riegger J, et al. Differential interactive effects of cartilage traumatization and blood exposure in vitro and in vivo. Am J Sports Med. 2015;43:2822–2832. [DOI] [PubMed] [Google Scholar]
- 10. Radin EL, Paul IL, Rose RM. Role of mechanical factors in pathogenesis of primary osteoarthritis. Lancet. 1972;1:519–522. [DOI] [PubMed] [Google Scholar]
- 11. Urban JP. The chondrocyte: a cell under pressure. Br J Rheumatol. 1994;33:901–908. [DOI] [PubMed] [Google Scholar]
- 12. Honda K, Ohno S, Tanimoto K, et al. The effects of high magnitude cyclic tensile load on cartilage matrix metabolism in cultured chondrocytes. Eur J Cell Biol. 2000;79:601–609. [DOI] [PubMed] [Google Scholar]
- 13. Fujisawa T, Hattori T, Takahashi K, Kuboki T, Yamashita A, Takigawa M. Cyclic mechanical stress induces extracellular matrix degradation in cultured chondrocytes via gene expression of matrix metalloproteinases and interleukin-1. J Biochem. 1999;125:966–975. [DOI] [PubMed] [Google Scholar]
- 14. Malemud CJ. Biologic basis of osteoarthritis: state of the evidence. Curr Opin Rheumatol. 2015;27:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12:632–644. [DOI] [PubMed] [Google Scholar]
- 16. Riyazi N, Slagboom E, de Craen AJ, et al. Association of the risk of osteoarthritis with high innate production of interleukin-1beta and low innate production of interleukin-10 ex vivo, upon lipopolysaccharide stimulation. Arthritis Rheum. 2005;52:1443–1450. [DOI] [PubMed] [Google Scholar]
- 17. Pelletier JP, Roughley PJ, DiBattista JA, McCollum R, Martel-Pelletier J. Are cytokines involved in osteoarthritic pathophysiology? Semin Arthritis Rheum. 1991;20:12–25. [DOI] [PubMed] [Google Scholar]
- 18. Liu-Bryan R, Terkeltaub R. Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol. 2015;11:35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;3:237–246. [PubMed] [Google Scholar]
- 20. Mrosewski I, Jork N, Gorte K, et al. Regulation of osteoarthritis-associated key mediators by TNFα and IL-10: effects of IL-10 over expression in human synovial fibroblasts and a synovial cell line. Cell Tissue Res. 2014;357:207–223. [DOI] [PubMed] [Google Scholar]
- 21. Vlaicu SI, Tatomir A, Boodhoo D, Vesa S, Mircea PA, Rus H. The role of complement system in adipose tissue-related inflammation. Immunol Res. 2016;64:653–664. [DOI] [PubMed] [Google Scholar]
- 22. Schwarz S, Mrosewski I, Silawal S, Schulze-Tanzil G. The interrelation of osteoarthritis and diabetes mellitus: considering the potential role of interleukin-10 and in vitro models for further analysis [published online ahead of print December 1, 2017]. Inflamm Res. doi: 10.1007/s00011-017-1121-8. [DOI] [PubMed] [Google Scholar]
- 23. Blom AM. The role of complement inhibitors beyond controlling inflammation. J Intern Med. 2017;282:116–128. [DOI] [PubMed] [Google Scholar]
- 24. Moreno-Navarrete JM, Fernandez-Real JM. The complement system is dysfunctional in metabolic disease: evidences in plasma and adipose tissue from obese and insulin resistant subjects [published online ahead of print October 26, 2017]. Semin Cell Dev Biol. doi: 10.1016/j.semcdb.2017.10.025. [DOI] [PubMed] [Google Scholar]
- 25. Ghosh P, Sahoo R, Vaidya A, Chorev M, Halperin JA. Role of complement and complement regulatory proteins in the complications of diabetes. Endocr Rev. 2015;36:272–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Neher MD, Weckbach S, Flierl MA, Huber-Lang MS, Stahel PF. Molecular mechanisms of inflammation and tissue injury after major trauma—is complement the “bad guy.” J Biomed Sci. 2011;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Carroll MV, Sim RB. Complement in health and disease. Adv Drug Deliv Rev. 2011;63:965–975. [DOI] [PubMed] [Google Scholar]
- 28. Wills-Karp M. Complement activation pathways: a bridge between innate and adaptive immune responses in asthma. Proc Am Thorac Soc. 2007;4:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Woodruff TM, Nandakumar KS, Tedesco F. Inhibiting the C5-C5a receptor axis. Mol Immunol. 2011;48:1631–1642. [DOI] [PubMed] [Google Scholar]
- 30. Kilgore KS, Schmid E, Shanley TP, et al. Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-kappa B activation. Am J Pathol. 1997;150:2019–2031. [PMC free article] [PubMed] [Google Scholar]
- 31. Brandt J, Pippin J, Schulze M, et al. Role of the complement membrane attack complex (C5b-9) in mediating experimental mesangioproliferative glomerulonephritis. Kidney Int. 1996;49:335–343. [DOI] [PubMed] [Google Scholar]
- 32. Niculescu F, Badea T, Rus H. Sublytic C5b-9 induces proliferation of human aortic smooth muscle cells: role of mitogen activated protein kinase and phosphatidylinositol 3-kinase. Atherosclerosis. 1999;142:47–56. [DOI] [PubMed] [Google Scholar]
- 33. Dashiell SM, Rus H, Koski CL. Terminal complement complexes concomitantly stimulate proliferation and rescue of Schwann cells from apoptosis. Glia. 2000;30:187–198. [DOI] [PubMed] [Google Scholar]
- 34. Benzaquen LR, Nicholson-Weller A, Halperin JA. Terminal complement proteins C5b-9 release basic fibroblast growth factor and platelet-derived growth factor from endothelial cells. J Exp Med. 1994;179:985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nauta AJ, Daha MR, Tijsma O, van de, Water B, Tedesco F, Roos A. The membrane attack complex of complement induces caspase activation and apoptosis. Eur J Immunol. 2002;32:783–792. [DOI] [PubMed] [Google Scholar]
- 36. Rus HG, Niculescu F, Shin ML. Sublytic complement attack induces cell cycle in oligodendrocytes. J Immunol. 1996;156:4892–4900. [PubMed] [Google Scholar]
- 37. Lee A, Whyte MK, Haslett C. Inhibition of apoptosis and prolongation of neutrophil functional longevity by inflammatory mediators. J Leukoc Biol. 1993;54:283–288. [PubMed] [Google Scholar]
- 38. Farkas I, Baranyi L, Liposits ZS, Yamamoto T, Okada H. Complement C5a anaphylatoxin fragment causes apoptosis in TGW neuroblastoma cells. Neuroscience. 1998;86:903–911. [DOI] [PubMed] [Google Scholar]
- 39. Schraufstatter IU, Discipio RG, Zhao M, Khaldoyanidi SK. C3a and C5a are chemotactic factors for human mesenchymal stem cells, which cause prolonged ERK1/2 phosphorylation. J Immunol. 2009;182:3827–3836. [DOI] [PubMed] [Google Scholar]
- 40. Ehrengruber MU, Geiser T, Deranleau DA. Activation of human neutrophils by C3a and C5A. Comparison of the effects on shape changes, chemotaxis, secretion, and respiratory burst. FEBS Lett. 1994;346:181–184. [DOI] [PubMed] [Google Scholar]
- 41. DiScipio RG, Schraufstatter IU, Sikora L, Zuraw BL, Sriramarao P. C5a mediates secretion and activation of matrix metalloproteinase 9 from human eosinophils and neutrophils. Int Immunopharmacol. 2006;6:1109–1118. [DOI] [PubMed] [Google Scholar]
- 42. van Beek J, Nicole O, Ali C, et al. Complement anaphylatoxin C3a is selectively protective against NMDA-induced neuronal cell death. Neuroreport. 2001;12:289–293. [DOI] [PubMed] [Google Scholar]
- 43. DiScipio RG, Khaldoyanidi SK, Moya-Castro R, Schraufstatter IU. Complement C3a signalling mediates production of angiogenetic factors in mesenchymal stem cells. J Biomed Sci Eng. 2013;6:1–13. [Google Scholar]
- 44. Daffern PJ, Pfeifer PH, Ember JA. Hugli TE. C3a is a chemotaxin for human eosinophils but not for neutrophils. I. C3a stimulation of neutrophils is secondary to eosinophil activation. J Exp Med. 1995;181:2119–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huber-Lang M, Denk S, Fulda S, et al. Cathepsin D is released after severe tissue trauma in vivo and is capable of generating C5a in vitro. Mol Immunol. 2012;50:60–65. [DOI] [PubMed] [Google Scholar]
- 46. Krisinger MJ, Goebeler V, Lu Z, et al. Thrombin generates previously unidentified C5 products that support the terminal complement activation pathway. Blood. 2012;120:1717–1725. [DOI] [PubMed] [Google Scholar]
- 47. Leung LL, Morser J. Plasmin as a complement C5 convertase. EBioMedicine. 2016;5:20–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barnum SR. C4a: an anaphylatoxin in name only. J Innate Immun. 2015;7:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Baldwin WM, 3rd, Pruitt SK, Brauer RB, Daha MR, Sanfilippo F. Complement in organ transplantation. Contributions to inflammation, injury, and rejection. Transplantation. 1995;59:797–808. [PubMed] [Google Scholar]
- 51. Belmont HM, Hopkins P, Edelson HS, et al. Complement activation during systemic lupus erythematosus. C3a and C5a anaphylatoxins circulate during exacerbations of disease. Arthritis Rheum. 1986;29:1085–1089. [DOI] [PubMed] [Google Scholar]
- 52. Kemp PA, Spragg JH, Brown JC, Morgan BP, Gunn CA, Taylor PW. Immunohistochemical determination of complement activation in joint tissues of patients with rheumatoid arthritis and osteoarthritis using neoantigen-specific monoclonal antibodies. J Clin Lab Immunol. 1992;37:147–162. [PubMed] [Google Scholar]
- 53. Gardinali M, Padalino P, Vesconi S, et al. Complement activation and polymorphonuclear neutrophil leukocyte elastase in sepsis. Correlation with severity of disease. Arch Surg. 1992;127:1219–1224. [DOI] [PubMed] [Google Scholar]
- 54. Morgan BP. The complement system: an overview. Methods Mol Biol. 2000;150:1–13. [DOI] [PubMed] [Google Scholar]
- 55. Gasque P. Complement: a unique innate immune sensor for danger signals. Mol Immunol. 2004;41:1089–1098. [DOI] [PubMed] [Google Scholar]
- 56. Piccoli AK, Alegretti AP, Schneider L, Lora PS, Xavier RM. Expression of complement regulatory proteins CD55, CD59, CD35, and CD46 in rheumatoid arthritis. Rev Bras Reumatol. 2011;51:503–510. [PubMed] [Google Scholar]
- 57. Hyc A, Osiecka-Iwan A, Strzelczyk P, Moskalewski S. Effect of IL-1beta, TNF-alpha and IL-4 on complement regulatory protein mRNA expression in human articular chondrocytes. Int J Mol Med. 2003;11:91–94. [PubMed] [Google Scholar]
- 58. Schulze-Tanzil G, Kohl B, El Sayed K, et al. Anaphylatoxin receptors and complement regulatory proteins in human articular and non-articular chondrocytes: interrelation with cytokines. Cell Tissue Res. 2012;350:465–475. [DOI] [PubMed] [Google Scholar]
- 59. Konttinen YT, Ceponis A, Meri S, et al. Complement in acute and chronic arthritides: assessment of C3c, C9, and protectin (CD59) in synovial membrane. Ann Rheum Dis. 1996;55:888–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Struglics A, Okroj M, Sward P, et al. The complement system is activated in synovial fluid from subjects with knee injury and from patients with osteoarthritis. Arthritis Res Ther. 2016;18:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Peffers MJ, McDermott B, Clegg PD, Riggs CM. Comprehensive protein profiling of synovial fluid in osteoarthritis following protein equalization. Osteoarthritis Cartilage. 2015;23:1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Brodeur JP, Ruddy S, Schwartz LB, Moxley G. Synovial fluid levels of complement SC5b-9 and fragment Bb are elevated in patients with rheumatoid arthritis. Arthritis Rheum. 1991;34:1531–1537. [DOI] [PubMed] [Google Scholar]
- 63. Swaak AJ, Van Rooyen A, Planten O, Han H, Hattink O, Hack E. An analysis of the levels of complement components in the synovial fluid in rheumatic diseases. Clin Rheumatol. 1987;6:350–357. [DOI] [PubMed] [Google Scholar]
- 64. Bradley K, North J, Saunders D, et al. Synthesis of classical pathway complement components by chondrocytes. Immunology. 1996;88:648–656. [PMC free article] [PubMed] [Google Scholar]
- 65. Grönblad M, Habtemariam A, Virri J, Seitsalo S, Vanharanta H, Guyer RD. Complement membrane attack complexes in pathologic disc tissues. Spine (Phila Pa 1976). 2003;28:114–118. [DOI] [PubMed] [Google Scholar]
- 66. Karpus ON, Kiener HP, Niederreiter B, et al. CD55 deposited on synovial collagen fibers protects from immune complex-mediated arthritis. Arthritis Res Ther. 2015;17:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Assirelli E PL, Addimanda O, Lisignoli G, Mariani E, Meloconi R. Complement factor expression in osteoarthritis joint compartments. Osteoarthritis Cartilage. 2016;24:S383–S384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nakagawa K, Sakiyama H, Tsuchida T, et al. Complement C1s activation in degenerating articular cartilage of rheumatoid arthritis patients: immunohistochemical studies with an active form specific antibody. Ann Rheum Dis. 1999;58:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ignatius A, Schoengraf P, Kreja L, et al. Complement C3a and C5a modulate osteoclast formation and inflammatory response of osteoblasts in synergism with IL-1β. J Cell Biochem. 2011;112:2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bergdolt S, Kovtun A, Hagele Y, et al. Osteoblast-specific overexpression of complement receptor C5aR1 impairs fracture healing. PLoS ONE. 2017;12:e0179512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Huber-Lang M, Kovtun A, Ignatius A. The role of complement in trauma and fracture healing. Semin Immunol. 2013;25:73–78. [DOI] [PubMed] [Google Scholar]
- 72. Bloom AC, Collins FL, Van’t Hof RJ, et al. Deletion of the membrane complement inhibitor CD59a drives age and gender-dependent alterations to bone phenotype in mice. Bone. 2016;84:253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hugle T, Geurts J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology (Oxford). 2016;56:1461–1471. [DOI] [PubMed] [Google Scholar]
- 74. Findlay DM, Kuliwaba JS. Bone-cartilage crosstalk: a conversation for understanding osteoarthritis. Bone Res. 2016;4:16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Tu Z, Li Q , Bu H, Lin F. Mesenchymal stem cells inhibit complement activation by secreting factor H. Stem Cells Dev. 2010;19:1803–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther. 2013;15:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Clockaerts S, Bastiaansen-Jenniskens YM, Runhaar J, et al. The infrapatellar fat pad should be considered as an active osteoarthritic joint tissue: a narrative review. Osteoarthritis Cartilage. 2010;18:876–882. [DOI] [PubMed] [Google Scholar]
- 78. Arend WP, Mehta G, Antonioli AH, et al. Roles of adipocytes and fibroblasts in activation of the alternative pathway of complement in inflammatory arthritis in mice. J Immunol. 2013;190:6423–6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Le Clanche S, Bonnefont-Rousselot D, Sari-Ali E, Rannou F, Borderie D. Inter-relations between osteoarthritis and metabolic syndrome: a common link? Biochimie. 2016;121:238–252. [DOI] [PubMed] [Google Scholar]
- 80. Barbu A, Hamad OA, Lind L, Ekdahl KN, Nilsson B. The role of complement factor C3 in lipid metabolism. Mol Immunol. 2015;67:101–107. [DOI] [PubMed] [Google Scholar]
- 81. Busch C, Girke G, Kohl B, et al. Complement gene expression is regulated by pro-inflammatory cytokines and the anaphylatoxin C3a in human tenocytes. Mol Immunol. 2013;53:363–373. [DOI] [PubMed] [Google Scholar]
- 82. Girke G, Kohl B, Busch C, et al. Tenocyte activation and regulation of complement factors in response to in vitro cell injury. Mol Immunol. 2014;60:14–22. [DOI] [PubMed] [Google Scholar]
- 83. Dankof A, Krenn V. C4d deposits mark sites of meniscal tissue disintegration. Virchows Arch. 2006;449:230–233. [DOI] [PubMed] [Google Scholar]
- 84. Sun Y, Mauerhan DR, Honeycutt PR, et al. Analysis of meniscal degeneration and meniscal gene expression. BMC Musculoskelet Disord. 2010;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Onuma H, Masuko-Hongo K, Yuan G, et al. Expression of the anaphylatoxin receptor C5aR (CD88) by human articular chondrocytes. Rheumatol Int. 2002;22:52–55. [DOI] [PubMed] [Google Scholar]
- 86. Kim DD, Song WC. Membrane complement regulatory proteins. Clin Immunol. 2006;118:127–136. [DOI] [PubMed] [Google Scholar]
- 87. Davies ME, Horner A, Loveland BE, McKenzie IF. Upregulation of complement regulators MCP (CD46), DAF (CD55) and protectin (CD59) in arthritic joint disease. Scand J Rheumatol. 1994;23:316–321. [DOI] [PubMed] [Google Scholar]
- 88. Quadros AU, Cunha TM. C5a and pain development: an old molecule, a new target. Pharmacol Res. 2016;112:58–67. [DOI] [PubMed] [Google Scholar]
- 89. Jang JH, Clark JD, Li X, Yorek MS, Usachev YM, Brennan TJ. Nociceptive sensitization by complement C5a and C3a in mouse. Pain. 2010;148:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ratajczak MZ, Reca R, Wysoczynski M, Yan J, Ratajczak J. Modulation of the SDF-1-CXCR4 axis by the third complement component (C3)—implications for trafficking of CXCR4+ stem cells. Exp Hematol. 2006;34:986–995. [DOI] [PubMed] [Google Scholar]
- 91. Ratajczak MZ, Kim C, Ratajczak J, Janowska-Wieczorek A. Innate immunity as orchestrator of bone marrow homing for hematopoietic stem/progenitor cells. Adv Exp Med Biol. 2013;735:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lee H, Ratajczak MZ. Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells. Arch Immunol Ther Exp (Warsz). 2009;57:269–278. [DOI] [PubMed] [Google Scholar]
- 93. Schraufstatter IU, Khaldoyanidi SK, DiScipio RG. Complement activation in the context of stem cells and tissue repair. World J Stem Cells. 2015;7:1090–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Diekman BO, Rowland CR, Lennon DP, Caplan AI, Guilak F. Chondrogenesis of adult stem cells from adipose tissue and bone marrow: induction by growth factors and cartilage-derived matrix. Tissue Eng Part A. 2010;16:523–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hengartner NE, Fiedler J, Schrezenmeier H, Huber-Lang M, Brenner RE. Crucial role of IL1beta and C3a in the in vitro-response of multipotent mesenchymal stromal cells to inflammatory mediators of polytrauma. PLoS ONE. 2015;10:e0116772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Huber-Lang M, Wiegner R, Lampl L, Brenner RE. Mesenchymal stem cells after polytrauma: actor and target. Stem Cells Int. 2016;2016:6289825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sofat N. Analysing the role of endogenous matrix molecules in the development of osteoarthritis. Int J Exp Pathol. 2009;90:463–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Sjoberg A, Onnerfjord P, Morgelin M, Heinegard D, Blom AM. The extracellular matrix and inflammation: fibromodulin activates the classical pathway of complement by directly binding C1q. J Biol Chem. 2005;280:32301–32308. [DOI] [PubMed] [Google Scholar]
- 99. Fürst CM, Mörgelin M, Vadstrup K, Heinegard D, Aspberg A, Blom AM. The C-type lectin of the aggrecan G3 domain activates complement. PLoS ONE. 2013;8:e61407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Happonen KE, Saxne T, Aspberg A, Morgelin M, Heinegard D, Blom AM. Regulation of complement by cartilage oligomeric matrix protein allows for a novel molecular diagnostic principle in rheumatoid arthritis. Arthritis Rheum. 2010;62:3574–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Feinberg RN, Beebe DC. Hyaluronate in vasculogenesis. Science. 1983;220:1177–1179. [DOI] [PubMed] [Google Scholar]
- 102. Bhandari M, Bannuru RR, Babins EM, et al. Intra-articular hyaluronic acid in the treatment of knee osteoarthritis: a Canadian evidence-based perspective. Ther Adv Musculoskelet Dis. 2017;9:231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang CT, Lin J, Chang CJ, Lin YT, Hou SM. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee: a meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2004;86-A:538–545. [DOI] [PubMed] [Google Scholar]
- 104. Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Dragomir CL, Scott JL, Perino G, Adler R, Fealy S, Goldring MB. Acute inflammation with induction of anaphylatoxin C5a and terminal complement complex C5b-9 associated with multiple intra-articular injections of hylan G-F 20: a case report. Osteoarthritis Cartilage. 2012;20:791–795. [DOI] [PubMed] [Google Scholar]
- 106. Li L, Li Y, Ijaz M, Shahbaz M, Lian Q , Wang F. Review on complement analysis method and the roles of glycosaminoglycans in the complement system. Carbohydr Polym. 2015;134:590–597. [DOI] [PubMed] [Google Scholar]
- 107. Calamia V, Lourido L, Fernandez-Puente P, et al. Secretome analysis of 94chondroitin sulfate-treated chondrocytes reveals anti-angiogenic, anti-inflammatory and anti-catabolic properties. Arthritis Res Ther. 2012;14:R202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cs-Szabo G, Roughley PJ, Plaas AH, Glant TT. Large and small proteoglycans of osteoarthritic and rheumatoid articular cartilage. Arthritis Rheum. 1995;38:660–668. [DOI] [PubMed] [Google Scholar]
- 109. Antonsson P, Heinegard D, Oldberg A. Structure and deduced amino acid sequence of the human fibromodulin gene. Biochim Biophys Acta. 1993;1174:204–206. [DOI] [PubMed] [Google Scholar]
- 110. Groeneveld TW, Oroszlan M, Owens RT, et al. Interactions of the extracellular matrix proteoglycans decorin and biglycan with C1q and collectins. J Immunol. 2005;175:4715–4723. [DOI] [PubMed] [Google Scholar]
- 111. Krumdieck R, Hook M, Rosenberg LC, Volanakis JE. The proteoglycan decorin binds C1q and inhibits the activity of the C1 complex. J Immunol. 1992;149:3695–3701. [PubMed] [Google Scholar]
- 112. Sjoberg AP, Manderson GA, Morgelin M, Day AJ, Heinegard D, Blom AM. Short leucine-rich glycoproteins of the extracellular matrix display diverse patterns of complement interaction and activation. Mol Immunol. 2009;46:830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kafienah W, Cheung FL, Sims T, et al. Lumican inhibits collagen deposition in tissue engineered cartilage. Matrix Biol. 2008;27:526–534. [DOI] [PubMed] [Google Scholar]
- 114. Clark AG, Jordan JM, Vilim V, et al. Serum cartilage oligomeric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42:2356–2364. [DOI] [PubMed] [Google Scholar]
- 115. Zivanovic S, Rackov LP, Zivanovic A, Jevtic M, Nikolic S, Kocic S. Cartilage oligomeric matrix protein: inflammation biomarker in knee osteoarthritis. Bosn J Basic Med Sci. 2011;11:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ahrman E, Lorenzo P, Holmgren K, et al. Novel cartilage oligomeric matrix protein (COMP) neoepitopes identified in synovial fluids from patients with joint diseases using affinity chromatography and mass spectrometry. J Biol Chem. 2014;289:20908–20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Clark SJ, Bishop PN, Day AJ. The proteoglycan glycomatrix: a sugar microenvironment essential for complement regulation. Front Immunol. 2013;4:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Koobkokkruad T, Kadotani T, Hutamekalin P, Mizutani N, Yoshino S. Arthrogenicity of type II collagen monoclonal antibodies associated with complement activation and antigen affinity. J Inflamm (Lond). 2011;8:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kalchishkova N, Furst CM, Heinegard D, Blom AM. NC4 domain of cartilage-specific collagen IX inhibits complement directly due to attenuation of membrane attack formation and indirectly through binding and enhancing activity of complement inhibitors C4B-binding protein and factor H. J Biol Chem. 2011;286:27915–27926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–398. [DOI] [PubMed] [Google Scholar]
- 121. Ashraf S, Walsh DA. Angiogenesis in osteoarthritis. Curr Opin Rheumatol. 2008;20:573–580. [DOI] [PubMed] [Google Scholar]
- 122. Murata M, Yudoh K, Masuko K. The potential role of vascular endothelial growth factor (VEGF) in cartilage: how the angiogenic factor could be involved in the pathogenesis of osteoarthritis? Osteoarthritis Cartilage. 2008;16:279–286. [DOI] [PubMed] [Google Scholar]
- 123. Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci U S A. 2006;103:2328–2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Clapp C, Thebault S, Macotela Y, Moreno-Carranza B, Triebel J, de la Escalera GM. Regulation of blood vessels by prolactin and vasoinhibins. Adv Exp Med Biol. 2015;846:83–95. [DOI] [PubMed] [Google Scholar]
- 125. Ogueta S, Munoz J, Obregon E, Delgado-Baeza E, Garcia-Ruiz JP. Prolactin is a component of the human synovial liquid and modulates the growth and chondrogenic differentiation of bone marrow-derived mesenchymal stem cells. Mol Cell Endocrinol. 2002;190:51–63. [DOI] [PubMed] [Google Scholar]
- 126. Macotela Y, Aguilar MB, Guzman-Morales J, et al. Matrix metalloproteases from chondrocytes generate an antiangiogenic 16 kDa prolactin. J Cell Sci. 2006;119:1790–1800. [DOI] [PubMed] [Google Scholar]
- 127. Nagafuchi H, Suzuki N, Kaneko A, Asai T, Sakane T. Prolactin locally produced by synovium infiltrating T lymphocytes induces excessive synovial cell functions in patients with rheumatoid arthritis. J Rheumatol. 1999;26:1890–1900. [PubMed] [Google Scholar]
- 128. Corbacho AM, Nava G, Eiserich JP, et al. Proteolytic cleavage confers nitric oxide synthase inducing activity upon prolactin. J Biol Chem 2000;275:13183–13186. [DOI] [PubMed] [Google Scholar]
- 129. Clapp C, Lopez-Gomez FJ, Nava G, et al. Expression of prolactin mRNA and of prolactin-like proteins in endothelial cells: evidence for autocrine effects. J Endocrinol. 1998;158:137–144. [DOI] [PubMed] [Google Scholar]
- 130. Adan N, Guzman-Morales J, Ledesma-Colunga MG, et al. Prolactin promotes cartilage survival and attenuates inflammation in inflammatory arthritis. J Clin Invest. 2013;123:3902–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Clapp C, Adan N, Ledesma-Colunga MG, Solis-Gutierrez M, Triebel J, de la Escalera GM. The role of the prolactin/vasoinhibin axis in rheumatoid arthritis: an integrative overview. Cell Mol Life Sci. 2016;73:2929–2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xia H, Huang J, Mao F, Peng K, Hu X. Activity of cathepsin D and alpha-1 antitrypsin in patients with hip or knee osteoarthritis. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2014;39:1151–1156. [DOI] [PubMed] [Google Scholar]
- 133. Smith JK, Chi DS, Krish G, Reynolds S, Cambron G. Effect of exercise on complement activity. Ann Allergy. 1990;65:304–310. [PubMed] [Google Scholar]
- 134. Camus G, Duchateau J, Deby-Dupont G, et al. Anaphylatoxin C5a production during short-term submaximal dynamic exercise in man. Int J Sports Med. 1994;15:32–35. [DOI] [PubMed] [Google Scholar]
- 135. Navarro Sanz A, Jesús E, Francioni B, et al. Effect of exhaustive exercise on the immune system, measured through complement activation and C-reactive protein. Arch Med Deporte. 2013;30:348–353. [Google Scholar]
- 136. Bowe EA, Murray RC, Jeffcott LB, Davies ME. Do the matrix degrading enzymes cathepsins B and D increase following a high intensity exercise regime? Osteoarthritis Cartilage. 2007;15:343–349. [DOI] [PubMed] [Google Scholar]
- 137. Juhl C, Christensen R, Roos EM, Zhang W, Lund H. Impact of exercise type and dose on pain and disability in knee osteoarthritis: a systematic review and meta-regression analysis of randomized controlled trials. Arthritis Rheumatol. 2014;66:622–636. [DOI] [PubMed] [Google Scholar]
- 138. Nejati P, Farzinmehr A, Moradi-Lakeh M. The effect of exercise therapy on knee osteoarthritis: a randomized clinical trial. Med J Islam Repub Iran. 2015;29:186. [PMC free article] [PubMed] [Google Scholar]
- 139. Pelland L, Brosseau L, Wells G, et al. Efficacy of strengthening exercises for osteoarthritis (part I): a meta-analysis. Phys Ther Rev. 2004;9:77–108. [Google Scholar]
- 140. Li L, Li Y, Feng D, et al. Preparation of low molecular weight chondroitin sulfates, screening of a high anti-complement capacity of low molecular weight chondroitin sulfate and its biological activity studies in attenuating osteoarthritis. Int J Mol Sci. 2016;17:E1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Happonen KE, Heinegard D, Saxne T, Blom AM. Interactions of the complement system with molecules of extracellular matrix: relevance for joint diseases. Immunobiology. 2012;217:1088–1096. [DOI] [PubMed] [Google Scholar]
- 142. Barilla ML, Carsons SE. Fibronectin fragments and their role in inflammatory arthritis. Semin Arthritis Rheum. 2000;29:252–265. [DOI] [PubMed] [Google Scholar]
- 143. Carsons SE, Schwartzman S, Diamond HS, Berkowitz E. Interaction between fibronectin and C1q in rheumatoid synovial fluid and normal plasma. Clin Exp Immunol. 1988;72:37–42. [PMC free article] [PubMed] [Google Scholar]
- 144. Land WG. Emerging role of innate immunity in organ transplantation part II: potential of damage-associated molecular patterns to generate immunostimulatory dendritic cells. Transplant Rev (Orlando). 2012;26:73–87. [DOI] [PubMed] [Google Scholar]
- 145. Hajishengallis G, Lambris JD. More than complementing Tolls: complement-Toll-like receptor synergy and crosstalk in innate immunity and inflammation. Immunol Rev. 2016;274:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Holst B, Raby AC, Hall JE, Labeta MO. Complement takes its toll: an inflammatory crosstalk between Toll-like receptors and the receptors for the complement anaphylatoxin C5a. Anaesthesia. 2012;67:60–64. [DOI] [PubMed] [Google Scholar]
- 147. Sillat T, Barreto G, Clarijs P, et al. Toll-like receptors in human chondrocytes and osteoarthritic cartilage. Acta Orthop. 2013;84:585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Zutter MM, Edelson BT. The alpha2beta1 integrin: a novel collectin/C1q receptor. Immunobiology. 2007;212:343–353. [DOI] [PubMed] [Google Scholar]
- 149. Farkas I, Baranyi L, Ishikawa Y, et al. CD59 blocks not only the insertion of C9 into MAC but inhibits ion channel formation by homologous C5b-8 as well as C5b-9. J Physiol. 2002;539:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Holmquist E, Okroj M, Nodin B, Jirstrom K, Blom AM. Sushi domain-containing protein 4 (SUSD4) inhibits complement by disrupting the formation of the classical C3 convertase. FASEB J. 2013;27:2355–2366. [DOI] [PubMed] [Google Scholar]
- 151. Lepus CM, Song JJ, Wang Q, et al. Brief report: carboxypeptidase B serves as a protective mediator in osteoarthritis. Arthritis Rheumatol. 2014;66:101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Woodman I. Osteoarthritis: carboxypeptidase B inhibits complement and cartilage loss. Nat Rev Rheumatol. 2013;9:697. [DOI] [PubMed] [Google Scholar]
- 153. Banda NK, Levitt B, Glogowska MJ, et al. Targeted inhibition of the complement alternative pathway with complement receptor 2 and factor H attenuates collagen antibody-induced arthritis in mice. J Immunol. 2009;183:5928–5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Cole BJ, Karas V, Hussey K, Pilz K, Fortier LA. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am J Sports Med. 2017;45:339–346. [DOI] [PubMed] [Google Scholar]
- 155. Bendinelli P, Matteucci E, Dogliotti G, et al. Molecular basis of anti-inflammatory action of platelet-rich plasma on human chondrocytes: mechanisms of NF-κB inhibition via HGF. J Cell Physiol. 2010;225:757–766. [DOI] [PubMed] [Google Scholar]
- 156. Cavallo C, Roffi A, Grigolo B, et al. Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. Biomed Res Int. 2016;2016:6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Del Conde I, Cruz MA, Zhang H, Lopez JA, Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J Exp Med. 2005;201:871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Yu GH, Holers VM, Seya T, Ballard L, Atkinson JP. Identification of a third component of complement-binding glycoprotein of human platelets. J Clin Invest. 1986;78:494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Morgan BP. Isolation and characterization of the complement-inhibiting protein CD59 antigen from platelet membranes. Biochem J. 1992;282:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]