Short abstract
Objective
Although nociceptive sensitisation is an important pathophysiological process in migraine and migraine chronification, its underlying mechanisms remain unclear. Toll-like receptor 4 (TLR4), a pattern-recognition molecule, has a critical role in both neuropathic pain and morphine tolerance. The present study examined whether elements of the TLR4 pathway contribute to hyperalgesia induced by dural inflammation in rats.
Methods
A rat model of migraine was established by infusing a dural inflammatory soup. A group pretreated with TAK-242 was used to inhibit the activation of TLR4. The protein levels of TLR4 and its downstream molecules in the trigeminal pathway were examined by Western blot and immunofluorescence. The expression of activated microglia and astrocytes was also analysed. Levels of interleukin-1 beta, tumour necrosis factor-alpha, and brain-derived neurotrophic factor were measured by enzyme-linked immunosorbent assay.
Results
Acute inflammatory soup infusion induced time-dependent facial mechanical hyperalgesia, which was blocked by TAK-242 pretreatment. The inflammatory soup stimulus increased the production of TLR4 downstream molecules and interleukin-1 beta. Higher levels of microglia activation and brain-derived neurotrophic factor release were observed following the administration of the inflammatory soup but were alleviated by TAK-242.
Conclusions
These data suggest that the TLR4 signalling pathway promotes hyperalgesia induced by acute inflammatory soup delivery by stimulating the production of proinflammatory cytokines and activating microglia.
Keywords: Migraine, toll-like receptor 4, neuroinflammation, hyperalgesia, microglia
Introduction
Migraine is a prevalent brain disorder with quite high disabling rates, but effective treatments are limited due to confusion regarding the pathogenesis of the disease.1,2 During an attack, migraine sufferers may experience hypersensitivity to external stimuli, such as sound, light, and movement.2 Many patients exhibit allodynia, the perception of pain in response to a normally nonpainful stimulus, even after the headache phase.3 Hyperalgesia has been associated with migraine pathology, such as peripheral and central sensitisation, which is attributed to neuroinflammation in the trigeminovascular system or the brain stem.4–6 However, a detailed understanding of the effect of innate immunity in this process is limited.
Toll-like receptor 4 (TLR4) is a pattern-recognition receptor of the innate immune system7 and is also sensitive to endogenous danger-associated molecular patterns released during tissue injury or stressful events.8 Numerous studies have shown that the activation of TLR4 plays an important role in promoting the expression of proinflammatory products by upregulating nuclear factor-kappa B (NF-κB) in the immune system as well as interleukin-1 beta (IL-1β), tumour necrosis factor-alpha (TNF-α), and inducible nitric oxide synthases.8–10 These molecules further promote the activation of glia and the production of inflammatory cytokines to act on the nociceptive pathway, resulting in the hyperalgesic state.11,12
Rodent studies have confirmed that the activation of the TLR4–NF–κB signalling pathway in the dorsal/trigeminal root ganglia or the spinal dorsal horn induces hyperalgesia in several animal models of inflammatory or neuropathic pain.13,14 It is also well accepted that an overdose of morphine activates TLR4 and increases the production of IL-1β, TNF-α, and IL-6 in activated glia.15 Blocking this pathway can effectively slow the development of morphine tolerance and exert an analgesic effect.16,17 Moreover, in our previous study, TLR4 was involved in the development of hyperalgesia, induced by repeated dural inflammatory stimulation in rats, as well as systematic rizatriptan overuse (unpublished results).
Based on this evidence, we hypothesised that the activation of the TLR4–NF–κB pathway promotes hyperalgesia in headache-related pain. Dural infusion of an inflammatory soup (IS), a mixture of inflammatory mediators, in awake rats has been widely used to study acute or chronic migraine, as this kind of animal model can not only simulate migraine-related behaviour but also effectively induce hyperalgesia.18–20 In the present study, an IS rat model was used to explore whether the TLR4–NF–κB signalling pathway in the trigeminal ganglion (TG) and trigeminocervical complex (TCC) participates in the development of cutaneous hypersensitivity. Moreover, a specific TLR4 inhibitor, TAK-242, was administered to analyse its possible role in regulating neuroinflammation.
Materials and methods
Animals
Twenty-seven male Sprague–Dawley rats (weight, 190–210 g) were housed individually in a temperature- and humidity-controlled environment with free access to food and water. A standard 12-/12-h light/dark cycle, with the lights turned on at 07:00 a.m., was provided. This study was approved by the Committee on Animal Use for Research and Education of the Laboratory Animals Centre at Chinese PLA General Hospital (Beijing, China), and it followed the ethical guidelines for the study of pain in conscious animals.21 Every effort was made to minimise any possible suffering by the animals.
Surgical procedure
A cannula was implanted in each rat to carry out the dural infusion, as described previously.19 Briefly, rats were anaesthetised to a deep surgical plane with 3% pentobarbital sodium (2 mL/kg, i.p.). A plastic cap with a stainless steel inner cannula (C = 1 mm; RWD Life Science Co., Ltd., Shenzhen, Guangdong Province, China) was implanted in a previously drilled cranial window aimed at the left frontal bone (1.0 mm in diameter, 1.5 mm beyond the transverse sinuses, and 1.5 mm left of the superior sagittal sinus) without touching the meningeal tissue. Two small screws were implanted with dental cement around each cannula to hold them securely in place. The cannula was sealed with an obturator cap (G = 0 mm; RWD Life Science) to prevent scar tissue from blocking the inner cannula. All rats recovered for at least three days before the experimental procedure to ensure that their sensory thresholds had returned to pre-surgical baselines.
Experimental procedure
All rats were divided into three groups randomly: the control (CON) group, the migraine model (IS) group, and the TLR4 inhibiting (TAK-242) group. To build the animal model of migraine, 10 μL of IS consisting of 2 mM histamine, 2 mM serotonin, 2 mM bradykinin, and 0.2 mM prostaglandin E2 in normal saline was applied to each rat via the implanted cannula. Rats in the CON group received 10 μL of normal saline. To inhibit the effects of TLR4, TAK-242, a specific TLR4 antagonist, was administered intraperitoneally 1.5 h prior to the dural IS infusion at a dose of 3 mg/kg (diluted in 1% dimethyl sulphoxide; Millipore, Bedford, MA, USA). The vehicle was given in the same way to the rats in the other two groups.
Two sensory tests were conducted to confirm the development of hyperalgesia. Baseline behavioural responses to facial probing were obtained from all rats prior to drug administration (baseline) and again after the dural infusion at 1-h intervals for 6 h. Behavioural responses to a hot plate were determined at baseline and at 1, 3, and 6 h after the dural infusion.
After the behavioural tests, all rats were humanely killed, and brain tissue samples were obtained immediately. Deeply anaesthetised with an intraperitoneal injection of 3% pentobarbital sodium (2.2 mL/kg), four rats from each group were transcardially perfused with 200 mL of cold fresh saline, followed by 400 mL of fresh fixative (0.1 M phosphate buffer containing 4% paraformaldehyde [pH 7.4]). The brain, including the TG, was removed, post-fixed, and stored until immunofluorescent staining. The other five rats from each group were perfused through the heart with 200 mL of cold fresh saline, and the caudal medulla and upper cervical spinal cord were quickly collected on ice. Using a cytoplasmic and nuclear protein extraction kit (P0027; Beyotime Institute of Biotechnology, Jiangsu China), the cytoplasmic and nuclear proteins were extracted from tissue samples immediately and stored at −80°C for Western blot analysis and enzyme-linked immunosorbent assay (ELISA).
Behavioural test
The facial mechanical withdraw threshold of rats was determined by applying a von Frey monofilament (North Coast Medical Co., Gilroy, CA, USA) to the periorbital region. Using an ‘up-down’ method,22 the nociceptive threshold was defined as the filament weight at which a rat exhibited a positive response at least three of five applications. However, if no positive response to the 15 g (peak weight) filament was observed, 15 g was assigned for the analysis. The hot-plate test was conducted to confirm whether the rats had thermal allodynia. Each rat was placed on the hot plate (Bioseb, Vitrolles, France) with the temperature adjusted to 52 ± 0.2°C, and the latency to the first paw licking or withdrawal was recorded. A cut-off time of 15 s was set to avoid injuring the rats.
ELISA analysis
IL-1β and TNF-α levels in cytoplasmic protein were determined with ELISA kits (R&D Systems, Inc., Minneapolis, MN, USA) according to the manufacturer’s instructions. In addition, the expression of brain-derived neurotrophic factor (BDNF), secreted by activated microglia was detected by ELISA (Westang, China). Each sample was run in duplicate, and mean concentrations were calculated and expressed as picograms of antigen per gram of protein.
Western blot analysis
To confirm the involvement of the TLR4 signalling pathway, the expression of TLR4, and its downstream molecules, including myeloid differentiation factor 88 (MyD88), toll interleukin-1 receptor domain containing adapter protein-inducing interferon beta (TRIF), inhibitory nuclear factor-kappa (IκB), and phosphorylated IκB (p-IκB) were detected by Western blot. NF-κB p65 subunit content in cytoplasmic and nuclear protein was examined. The expression of ionised calcium-binding adapter molecule 1 (Iba-1) and glial fibrillary acidic protein (GFAP) in cytoplasmic protein was measured to assess the activation of microglia and astrocytes, respectively. The protein content of the supernatant was estimated using a bicinchoninic acid protein assay kit (CW0014; Beyotime). Equal amounts of protein were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis for each assay, as described previously. The loading amount was 15 μg in the GFAP analysis and 40 μg for all others. After transfer and blocking, the membranes were incubated overnight at 4°C with diluted primary antibodies for anti-TLR4 (1:2000, sc-293072; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-MyD88 (1:1000, ab2064; Abcam, Cambridge, MA, USA), anti-TIR-domain-containing adapter-inducing interferon-β (TRIF) (1:400, ab13810; Abcam), anti- IκB (1:100, sc-1643; Santa Cruz Biotechnology), anti-p IκB (1:200, sc-8404; Santa Cruz Biotechnology), anti-p65 (1:100, sc-8008; Santa Cruz Biotechnology), anti-Iba-1 (1:200, sc-32725; Santa Cruz Biotechnology), anti-GFAP (1:100000, MAB360; Millipore, Bedford, MA, USA), anti-histone H3 (nuclear inner control, 1:2000, AF0009; Beyotime), and anti-beta-actin (inner control, 1:4000, AA128; Beyotime). After washing with TBST, the membranes were incubated with their respective horseradish peroxidase-conjugated secondary antibodies (1:5000, ZB-2301/ZB-2305; ZSGB-BIO) for 1 h at room temperature. The antibody-reactive bands were visualised using enhanced chemiluminescence detection reagents (P90719; Millipore) and a gel imaging system (Tanon-5200; YPH-BIO). The results were quantified with Image J software (National Institutes of Health, Bethesda, MD, USA).
Immunofluorescence assays
The expression of Fos, TLR4, and NF-κB p65 in the TG and the TCC, which is composed of the trigeminal nucleus caudalis (TNC) and the upper cervical spinal cord (UCSC), was measured using an immunofluorescence assay. The levels of Iba-1 and GFAP in the TNC and UCSC were analysed. The fixed samples were embedded in Tissue-Tek OCT Compound (Sakura Finetek, Torrance, CA, USA) and cut into 20-μm-thick serial sections on a freezing microtome (CM1850; Leica, Wetzlar, Germany). The sections were permeabilised in 0.2% Triton X-100, blocked with 10% goat serum (ZLI-9005; ZSGB-BIO), and incubated overnight at 4°C with respective diluted primary antibodies for anti-Fos (1:200, ABE457; Millipore), anti-TLR4 (1:500, sc-293072; Santa Cruz Biotechnology), anti-p65 (1:50, sc-8008; Santa Cruz Biotechnology), anti-Iba-1 (1:400; Wako Pure Chemical Industries, Osaka, Japan), and anti-GFAP (1:5000, MAB360; Millipore). After washing with 0.01 M phosphate-buffered saline, the sections were incubated for 2 h in the dark at room temperature with respective secondary antibodies for anti-mouse-conjugated AlexaFluor594 (1:1000, A-11034; Thermo Fisher, Waltham, MA, USA) and anti-rabbit-conjugated fluorescein isothiocyanate (1:1000, A-21424; Thermo Fisher). According to the atlas by Paxinos and Watson,23 three randomly selected images at 20× magnification were obtained for each brain region per sample using a microscope (DP73; Olympus, Tokyo, Japan). The immunoreactive cells were recognised using Image J software without identity of the rat, and the average integrated density of three images was defined as the final result for each one.
Statistical analysis
SPSS 19.0 software (IBM Corp., Armonk, NY, USA) was used for the statistical analysis, and Origin 9.1 (OriginLab, Northampton, MA, USA) was used generate the graphs. Levene’s test for homogeneity was conducted to test the data distributions. Abnormally distributed data were analysed using the Kruskal–Wallis test to determine differences among the groups. All other data were analysed using analysis of variance (ANOVA) and the least significant difference (LSD) t-test (when the variance was regular) or Dunnett’s T3 test (when the variance was irregular) for comparisons between the groups. All data are expressed as mean ± standard deviation (SD), and a P value < 0.05 was considered significant.
Results
Acute dural inflammatory stimulus induces facial, but not hindpaw, allodynia, which was alleviated by pretreatment of TAK-242
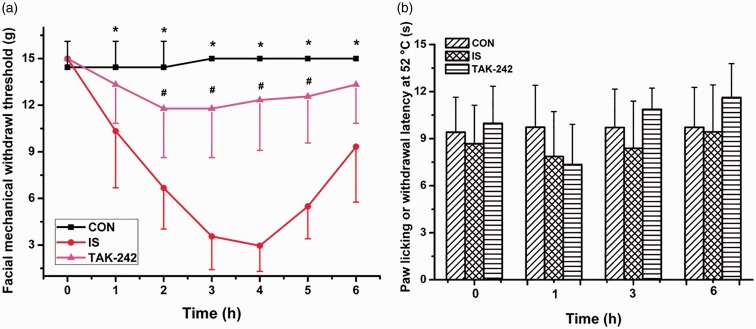
The Kruskal–Wallis test comparing the facial allodynia threshold showed a significant interaction between group and time (P < 0.05, Figure 1(a)). Post hoc tests showed that rats in the IS group exhibited significant declines in their periorbital mechanical withdrawal thresholds compared with those in the CON group beginning during the first hour after the dural infusion, with the maximum difference at hour 4. Furthermore, this decline was reversed by the TAK-242 treatment, as rats in the TAK-242 group had a higher withdrawal threshold than did those in the IS group from hours 2 to 5 (Figure 1(a)). However, no difference was found in paw licking or withdrawal latency to the hot plate among the three groups (two-way repeated-measures ANOVA, F0.05, (2,24) = 0.429, P = 0.857; Figure 1(b)).
Figure 1.
The mechanical (a) or thermal (b) withdrawal thresholds of rats during the 6-hour experiment. Data are presented as the mean ± SD, n = 9. Compared with the CON group, rats in IS group showed a time-dependent and reversible decline in periorbital withdrawal thresholds to mechanical stimulus (*P < 0.05), which was alleviated by TAK-242 pretreatment (#P < 0.05). As for the paw licking or withdrawal latency at 52°C, no significant difference was found among the three groups.
IS induces higher Fos expression in the trigeminal nociceptive pathway
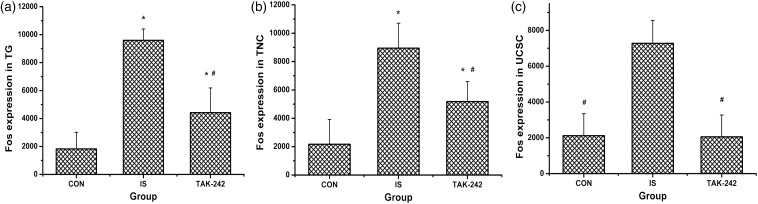
Fos, a neuronal activity marker, was examined by immunofluorescence in this study to explore the activation of the trigeminal pathway. Rats in both the IS and TAK-242 groups showed higher Fos expression in the TG than in the CON group, with the IS group showing the highest expression level (ANOVA, F0.05, (2,9) = 36.07, P < 0.001; Figure 2(a)). Similar results were obtained in the TNC (ANOVA, F0.05, (2,9) = 16.97, P = 0.001; Figure 2(b)). A significant increase in Fos level was observed in the UCSC for the IS group compared with the CON and TAK-242 groups, but no difference was found between the latter two groups (ANOVA, F0.05, (2,9) = 23.22, P < 0.001; Figure 2(c)).
Figure 2.
The level of Fos in trigeminal ganglion (a), trigeminal nucleus caudalis (b), and upper cervical spinal cord (c). All values given are the mean ± SD, n = 4. *P < 0.05 versus control group. #P < 0.05 versus IS group.
The TLR4-NF-κB pathway is involved in the rat model of migraine
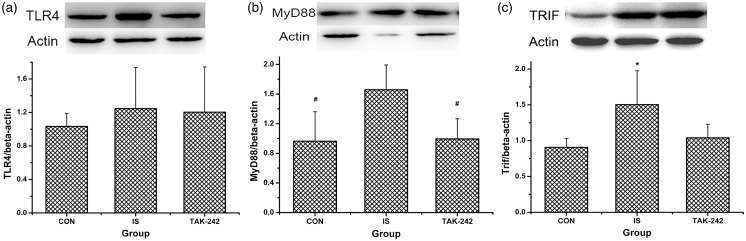
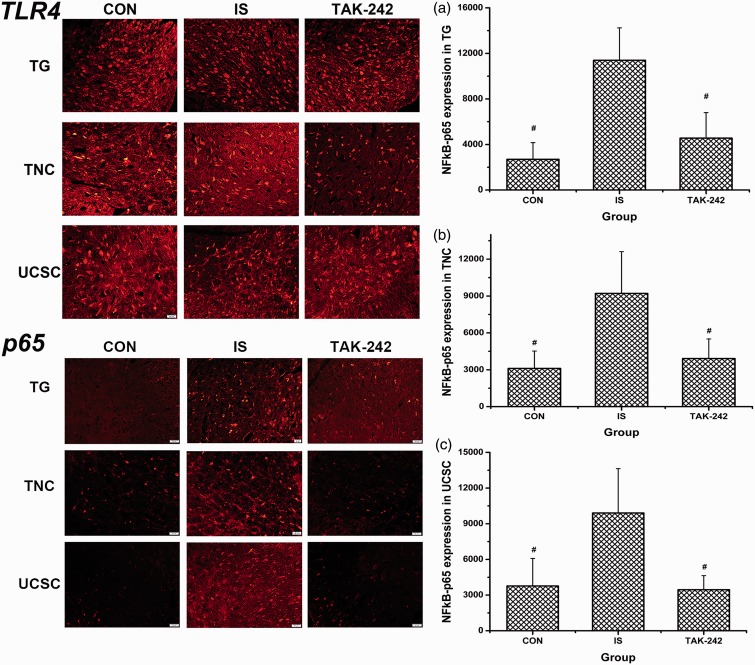
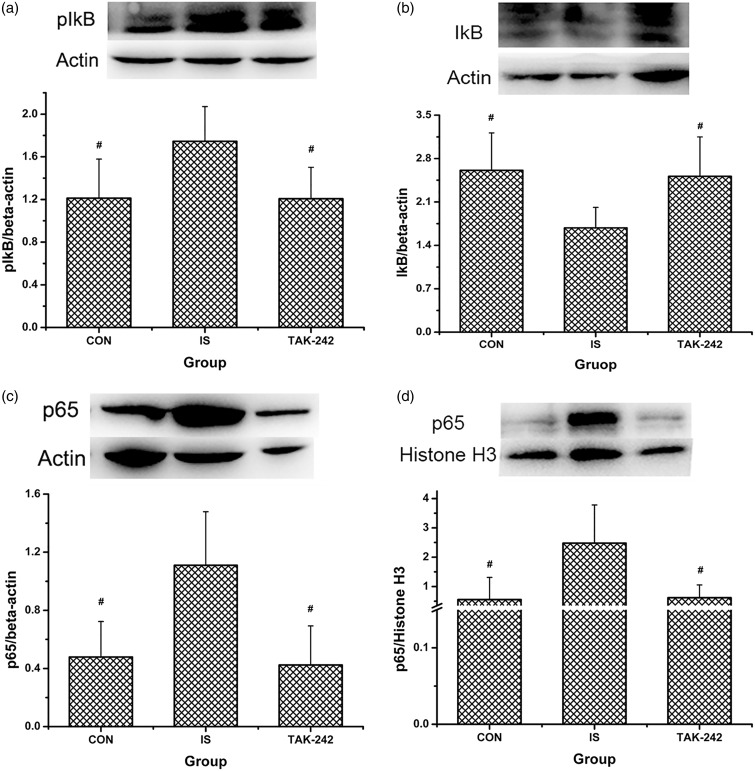
No significant difference in TLR4 expression was found among the three groups by Western blot (ANOVA, F0.05, (2,12) = 0.34, P = 0.718; Figure 3(a)) or by immunofluorescence (ANOVA, P > 0.05; data not shown, Figure 5 showed the typical immunofluorescence samples). In contrast, MyD88 and TRIF expression were significantly greater in the TCC of IS-treated rats compared with the controls (ANOVA, P < 0.05; Figure 3(b) and (c)), and the high MyD88 expression was blocked by TAK-242 treatment (LSD-t, P = 0.009). As shown in Figure 4(a) to (d), NF-κB p65 and p-IκB protein expression increased; accordingly, that of IκB decreased in rats in the IS group compared with the controls (P < 0.05). Moreover, all of these changes were blocked by TAK-242 (P < 0.05). The higher expression of NF-κB p65 in trigeminal pathway in IS-treated rats was also proved by immunofluorescence, also blocked by TAK-242 (ANOVA, P < 0.05; Figure 5).
Figure 3.
The protein levels of TLR4 and its downstream molecules in rats’ medullary and upper cervical spinal cord. Protein levels of TLR4 (a), MyD88 (b), and TRIF (c) were analysed by Western blot. Typical Western blot strips obtained were exhibited at the top of each image, and for all strips, each blot from left to right means sample from rat in group CON, IS, and TAK-242, respectively. All values given are the mean ± SD, n = 5. *P < 0.05 versus CON group. #P < 0.05 versus IS group.
Figure 5.
Immunofluorescence of TLR4 and NF-κB p65 expression in the trigeminal pathway. The typical immunofluorescence samples were presented at the left part of the image, with the scale bar equalling to 50 μm. The right part showed the statistical results of NF-κB p65 expression in TG (a), TNC (b), and UCSC (c), respectively. All values given are the mean ± SD, n = 4. #P < 0.05 versus IS group. TG: trigeminal ganglion; TNC: trigeminal nucleus caudalis; UCSC: upper cervical spinal cord.
Figure 4.
The protein levels of NF-κB p65 and related molecules in rats’ medullary and upper cervical spinal cord. Protein levels of IκB (a), p-IκB (b), NF-κB p65 (c), and nuclear NF-κB p65 (d) were analysed by Western blot. Typical Western blot strips obtained were exhibited at the top of each image, and for all strips, each blot from left to right means sample from rat in group CON, IS, and TAK-242, respectively. All values given are the mean ± SD, n = 5. #P < 0.05 versus IS group.
IS enhances expression of IL-1β and BDNF, but not TNF-α
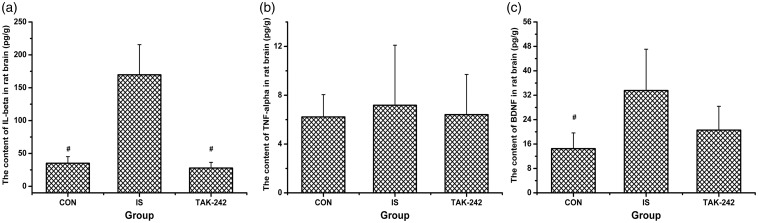
Brain concentrations of IL-1β and BDNF increased after the dural IS infusion (ANOVA, P < 0.05; Figure 6(a) and (c)). Moreover, TAK-242 blocked the high expression level of IL-1β induced by IS (Dunnett’s T3, P = 0.005; Figure 6(a)). In contrast, no obvious change in TNF-α level was detected among the three groups (ANOVA, F0.05,(2,12) = 0.097, P = 0.908; Figure 6(b)).
Figure 6.
The protein levels of IL-1β (a), TNF-α (b), and BDNF (c) in rat brain by enzyme-linked immunosorbent assay. All values given are the mean ± SD, n = 5. #P < 0.05 versus IS group.
Microglia, but not astrocytes, are activated by the acute IS infusion
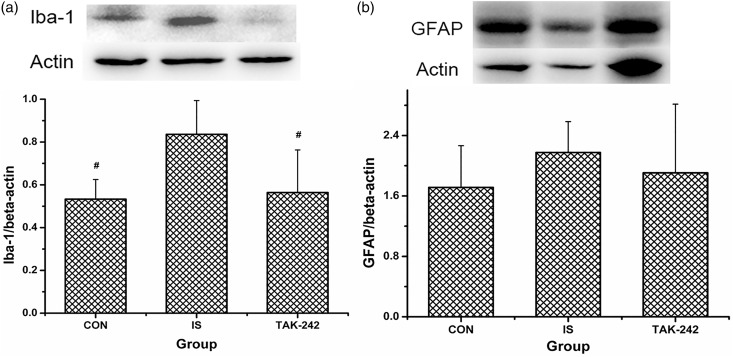
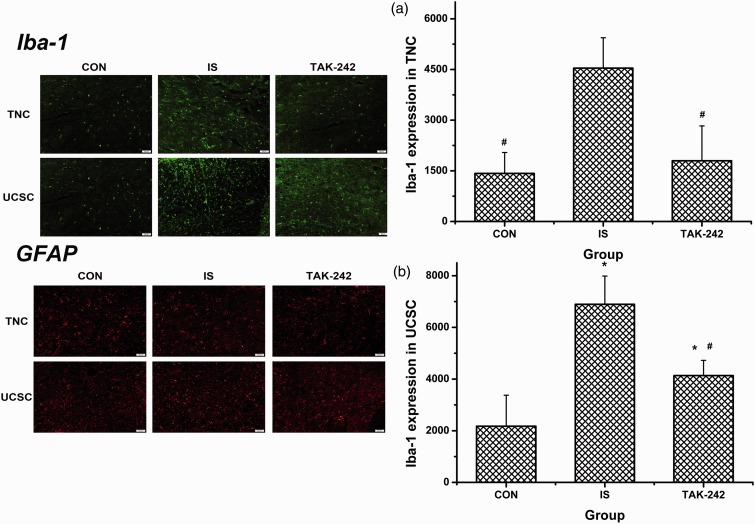
IS-treated rats expressed significantly more Iba-1 in TCC compared with the controls, which was reversed by TAK-242 (ANOVA, F0.05,(2,12) = 5.725, P = 0.018; Figure 7(a)). Similar data were obtained by immunofluorescence analysis, as the density of Iba-1 in both TNC and UCSC was upregulated among rats in the IS group, and this upregulation was fully or partially blocked by TAK-242 (ANOVA, P < 0.05; Figure 8). No significant difference in GFAP expression was found by either Western blot (ANOVA, F0.05,(2,12) = 0.554, P = 0.621; Figure 7(b)) or immunofluorescence (ANOVA, P > 0.05; data not shown, Figure 8 showed the typical immunofluorescence samples).
Figure 7.
Expression of microglia (a) and astrocytes (b) activating marker in rats’ medullary and upper cervical spinal cord. Protein levels of Iba-1 (ionised calcium-binding adapter molecule 1) and GFAP (glia fibrillary acidic protein) were analysed by Western blot. Typical Western blot strips obtained were exhibited at the top of each image, and for all strips, each blot from left to right means sample from rat in group CON, IS, and TAK-242, respectively. All values given are the mean ± SD, n = 5. #P < 0.05 versus IS group.
Figure 8.
Immunofluorescence of Iba-1 and GFAP expression in the trigeminal pathway. The typical immunofluorescence samples were presented at the left part of the image, with the scale bar equalling to 50 μm. The right part showed the statistical results of Iba-1 expression in TNC (a) and UCSC (b), respectively. All values given are the mean ± SD, n = 4. #P < 0.05 versus IS group. TNC: trigeminal nucleus caudalis; UCSC: upper cervical spinal cord.
Discussion
The present study tested the hypothesis that the activation of the TLR4–NF–κB signalling pathway in the trigeminal system contributes to the development of hyperalgesia in migraine. Our data provide evidence that the activation of the TLR4–NF–κB signalling pathway and subsequent cytokine release in the medulla and the UCSC play a crucial role in the development of cutaneous hypersensitivity in IS-treated rats. Moreover, the activation of microglia induced by dural inflammation was regulated by TLR4.
In this study, time-dependent and reversible facial mechanical hyperalgesia due to a dural IS was successfully established. These responses occurred over several hours following IS, consistent with previous studies and clinical observations of cutaneous allodynia seen in patients with migraine.18,24 Pretreatment with TAK-242 effectively blocked this hyperalgesia, indicating a crucial role of TLR4 in the process. In contrast, no significant difference in response to the hot plate was found after IS delivery, suggesting that hindpaw allodynia had not developed in this acute dural stimulation model. This is reasonable, as extracephalic allodynia usually occurs when central sensitisation extends to third-order trigeminal neurons in the thalamus, which is mostly seen in frequent or chronic migraine.25
Sensitisation has always been the key point in the pathophysiology of migraine, as well as other chronic painful conditions.26 In this study, Fos, an indicator of neuronal excitability, was detected to examine the activation state of the trigeminal nociceptive pathway. Chemically irritating the dura activated and sensitised the first-order trigeminovascular neurons of the TG. Additionally, IS-induced increased expression of Fos was confirmed in second-order neurons and an important relay station to subcortical structures in nociceptive modulation in the TCC of rats, indicating the development of central sensitisation.27 Sensitisation is a physiological process underlying periorbital cutaneous allodynia, as neurons located in the medullary dorsal horn receive convergent input from the dura and the cranial skin.4 Moreover, TAK-242 abolished this IS-induced high Fos expression in both the TG and TCC, suggesting the involvement of TLR4 in peripheral/central sensitisation.
TLR4 is an important mediator of the inflammatory response through recruitment of NF-κB and downstream gene transcription, including the production of a series of proinflammatory cytokines such as IL-1β and TNF-α.28 Prior studies have demonstrated that the expression levels of NF-κB, IL-1β, and TNF-α increase in the spinal cords of rats with complete Freund’s adjuvant-induced pain29 or chronic neuropathic pain.30 In addition, blocking TLR4 inhibited expression of its downstream signalling components in the TG alleviated nociceptive behaviour in a rat acute pulpitis model.14 In the present study, despite the subtle change in TLR4 expression, the activation of its signalling pathway was confirmed. Protein levels of MyD88, TRIF, and NF-κB were upregulated in the cytoplasm of the IS-treated brain. On the other hand, we also confirmed increased IκB phosphorylation/degradation and enhanced expression of NF-κB in the nucleus following IS, promoting release of IL-1β. All of these data support the claim that the activation of the TLR4 signalling pathway promotes the development of IS-induced hyperalgesia.
TLR4 is expressed primarily on microglia and to a lesser degree on astrocytes.28 Inhibiting TLR4 may decrease the activation of glia and thereby relieve neuropathic pain or morphine tolerance.30,31 In the present study, we also found an increased level of microglia in the TCC following IS and, subsequently, higher production of BDNF. These data suggest that microglia are also involved in the process of IS-induced central sensitisation. In contrast, no significant change in astrocyte activation was found, but this does not exclude the possibility of its role in central sensitisation, as this kind of glia usually participates in the late phase of the immune response.
TAK-242, a specific inhibitor of TLR4 that can pass through the brain–blood barrier, exerts analgesic effects in several painful conditions.29,30 In this study, pretreatment with TAK-242 not only inhibited IS-induced upregulation of molecules downstream of TLR4, including MyD88, TRIF, and NF-κB, but also decreased the expression of IL-1β. Additionally, TAK-242 effectively abolished IS-induced microglia activation and subsequent release of BDNF. These data further support the role of TLR4 in the hyperalgesia of migraine-related pain and also indicate that TLR4 may be a possible target of migraine therapy.
In summary, IS-treated rats provided a valid model for migraine with facial mechanical hypersensitivity. The TLR4-NF-κB signalling pathway has a substantial role in the development of hyperalgesia following dural inflammation. Besides neuronal modulation, TLR4 also affects IS-induced microglia activation in the TCC. In addition, inhibition of TLR4 with TAK-242 pretreatment effectively alleviated IS-induced hyperalgesia and glia activation. Nonetheless, further investigation is necessary because of several limitations, such as the limited sample size and the lack of an assessment of the chronic process by repeated IS delivery.
Author contributions
All authors read and approved the final manuscript. Min Su performed experiments, analysed data, prepared figures, and drafted the manuscript. Ye Ran and Zizi He performed part of experiments and analysed data. Mingjie Zhang and Guanqun Hu analysed data. Wenjing Tang and Dengfa Zhao provided technical supports during the experiments. Shengyuan Yu designed this work and also edited the manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China (grants 81471147, 81671077, and 81600952), Beijing Science and Technology Project (grant 161100002616013), and Beijing Natural Science Foundation (grant 7162178).
References
- 1.Steiner TJ Stovner LJ andVos T.. GBD 2015: migraine is the third cause of disability in under 50s. J Headache Pain 2016; 17: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goadsby PJ, Holland PR, Martinsoliveira M, et al. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017; 97: 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathew NT Kailasam J andSeifert T.. Clinical recognition of allodynia in migraine. Neurology 2004; 63: 848–852. [DOI] [PubMed] [Google Scholar]
- 4.Noseda R and Burstein R.. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain 2013; 154 Suppl 1 (6): S44-S53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moskowitz MA. Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology 1993; 43: 16–20. [PubMed] [Google Scholar]
- 6.Malhotra R. Understanding migraine: Potential role of neurogenic inflammation. Ann Indian Acad Neurol 2016; 19: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeuchi O andAkira S.. Pattern recognition receptors and inflammation. Cell 2010; 140: 805. [DOI] [PubMed] [Google Scholar]
- 8.Gay NJ andGangloff M.. Structure and function of Toll receptors and their ligands. Annu Rev Biochem 2007; 76:141. [DOI] [PubMed] [Google Scholar]
- 9.Chen YL Law PY andLoh HH.. Nuclear factor kappaB signaling in opioid functions and receptor gene expression. J Neuroimmune Pharm 2006; 1: 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jack CS, Arbour N, Manusow J, et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J Immunol 2005; 175: 4320–4330. [DOI] [PubMed] [Google Scholar]
- 11.Chen ZY, Zhang XW, Yu L, et al. Spinal toll-like receptor 4-mediated signalling pathway contributes to visceral hypersensitivity induced by neonatal colonic irritation in rats. EJP 2015; 19: 176–186. [DOI] [PubMed] [Google Scholar]
- 12.Zhu H-T, Bian C, Yuan J-C, et al. Curcumin attenuates acute inflammatory injury by inhibiting the TLR4/MyD88/NF-κB signaling pathway in experimental traumatic brain injury. J Neuroinflammation. 2014; 11: 59-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agalave NM, Larsson M, Abdelmoaty S, et al. Spinal HMGB1 induces TLR4-mediated long-lasting hypersensitivity and glial activation and regulates pain-like behavior in experimental arthritis. Pain 2014; 155: 1802–1813. [DOI] [PubMed] [Google Scholar]
- 14.Lin J-J, Du Y, Cai W-K, et al. Toll-like receptor 4 signaling in neurons of trigeminal ganglion contributes to nociception induced by acute pulpitis in rats. Sci Rep 2015; 5: 12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eidson LN andMurphy AZ.. Blockade of Toll-like receptor 4 attenuates morphine tolerance and facilitates the pain relieving properties of morphine. J Neurosci 2013; 33: 15952–15963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bettoni I, Comelli F, Rossini C, et al. Glial TLR4 receptor as new target to treat neuropathic pain: efficacy of a new receptor antagonist in a model of peripheral nerve injury in mice. Glia 2010; 56: 1312–1319. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Xin Y, Gao J, et al. Analgesic effect of TAK-242 on neuropathic pain in rats. Int J Clin Exp Med 2015; 8: 11202. [PMC free article] [PubMed] [Google Scholar]
- 18.Edelmayer RM, Vanderah TW, Majuta L, et al. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol 2009; 65: 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su M, Ran Y, Han X, et al. Rizatriptan overuse promotes hyperalgesia induced by dural inflammatory stimulation in rats by modulation of the serotonin system. Eur J Neurosci 2016; 44: 2129–2138. [DOI] [PubMed] [Google Scholar]
- 20.Melocarrillo A andLopezavila A.. A chronic animal model of migraine, induced by repeated meningeal nociception, characterized by a behavioral and pharmacological approach. Cephalalgia Int J Headache 2013; 33: 1096. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109. [DOI] [PubMed] [Google Scholar]
- 22.Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 1994; 53: 55–63. [DOI] [PubMed] [Google Scholar]
- 23.Paxinos G andWatson C.. The rat brain in stereotaxic coordinates. Rat Brain Stereotaxic Coordinates 2007; 3: 6. [Google Scholar]
- 24.Landy SH Rn MG andMa MD.. Clarification of developing and established clinical allodynia and pain-free outcomes. HeadacheJ Head Face Pain 2007; 47: 247–252. [DOI] [PubMed] [Google Scholar]
- 25.Louter MA, Bosker JE, van Oosterhout WP, et al. Cutaneous allodynia as a predictor of migraine chronification. Brain 2013; 136: 3489–3496. [DOI] [PubMed] [Google Scholar]
- 26.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain 2011; 152: S2-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aguggia M, Saracco MG, Cavallini M, et al. Sensitization and pain. Neurol Sci 2013; 34 Suppl 1: S37–S40. [DOI] [PubMed] [Google Scholar]
- 28.Akira S andTakeda K.. Toll-like receptor signalling. Nat Rev Immunol 2004; 4: 499. [DOI] [PubMed] [Google Scholar]
- 29.Woller SA, Ravula SB, Tucci FC, et al. Systemic TAK-242 prevents intrathecal LPS evoked hyperalgesia in male, but not female mice and prevents delayed allodynia following intraplantar formalin in both male and female mice: the role of TLR4 in the evolution of a persistent pain state. Brain Behav Immun 2016; 56: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, Xin Y, Gao J, et al. Analgesic effect of TAK-242 on neuropathic pain in rats. Int J Clin Exp Med 2015; 8: 11202–11207. [PMC free article] [PubMed] [Google Scholar]
- 31.Watkins LR, Hutchinson MR, Rice KC, et al. The “toll” of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol Sci 2009; 30: 581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]