Abstract
Management of advanced urogenital malignancies has profoundly changed in recent years due to the development of novel targeted drugs that have significantly improved patient’s clinical outcomes. This process has been made possible mainly thanks to better knowledge of tumor genetic alterations and molecular altered pathways. Despite these remarkable results, several issues such as early detection of the disease as well as the research into early markers of recurrence or disease progression still remain an open challenge for clinical research. The detection of circulating tumor cells and circulating DNA appears an attractive option since it is a minimally invasive approach potentially able to allow clinicians an accurate diagnosis and maybe lead to more customized treatment strategies. This review focuses on the current techniques adopted for the detection and isolation of circulating tumor cells in genitourinary tumors highlighting their present and possible future application in clinical practice.
Keywords: biomarkers, circulating tumor cells, early diagnosis, prostate cancer, renal cell carcinoma, urothelial cancer
Introduction
In the last few years the management of advanced urogenital malignancies has enlisted several new drugs allowing the achievement of important results in terms of patient’s clinical outcomes. However, despite these improvements, early stages of urogenital tumors, especially urothelial carcinoma and renal cell carcinoma (RCC), still remain diseases absent of reliable biomarkers able to drive clinicians to an accurate diagnosis. Even in prostate cancer (PCa) where the detection of PCa serum prostate-specific antigen (PSA) assumes an important role from the preclinical stages, the lack of sensitive and specific markers in castration-resistant prostate cancer (CRPC) remains a critical issue.
Moreover, owing to the approval of different agents in the metastatic setting, research into serum markers helping clinicians to achieve more customized therapeutic decisions is urgently required.
The study of circulating tumor cells (CTCs) appears to be an attractive option potentially able to give information about the presence, persistence, or recurrence of the disease and, at the same time, to provide data related to molecular alterations of primary/metastatic tumors.
To date, CellSearch (described below) is the only FDA approved test and it is adopted in the early detection of metastatic PCa assuming a key role in tumor castration-resistant state due to the lack of PSA sensitivity and specificity in this stage.
Different strategies have been adopted in experimental studies to detect and isolate CTCs. In the following we discuss the current and possible application of CTCs in prostate, urothelial, and kidney cancer highlighting clinical and biological rational concerning the application of this approach and focusing on the principal detection techniques adopted for CTCs isolation and detection (Figure 1).
Figure 1.
Possible applications of the detection of circulating tumor cells (CTCs) in genitourinary tumors.
Prostate cancer
PCa is among the most common adult malignancies worldwide, and one of the major leading causes of cancer death in men in Western societies.1 As is well known, from the early stages, the blood concentration of PCa serum PSA serves as a useful biomarker for the detection of the disease as well as the early diagnosis of disease recurrence but it lost its sensitivity and specificity in CRPC.2
The detection of CTCs from the early stages could help clinicians to identify more aggressive tumors that could benefit from a close follow-up program and perhaps a more aggressive treatment at the time of clinical recurrence.
Owing to the lack of sensitivity and specificity of PSA detection in patients with metastatic castration-resistant prostate cancer (mCRPC), CTCs represent an interesting marker hypothetically able to lead to an early diagnosis of castration-resistant status and this could help clinicians to achieve more customized treatment planning, supervised tumor response, and treatment progression.
Thanks to these possible applications, the detection and identification of CTCs is a promising technique representing a possible answer to different challenges regarding clinical PCa detection and management.3
CTCs and PCa
The role of CTCs as a prognostic biomarker for PCa has been broadly investigated.
CellSearch test [the only test approved by the US Food and Drug Administration (FDA)] demonstrated to detect CTCs in patients with metastatic carcinoma (including PCa) with a wide range of frequencies (⩾2 CTCs in 57% of metastatic PCa blood samples, 14% of which contained ⩾50 CTCs) while these were extremely rare in healthy subjects.4
Based on these promising findings, several prospective studies have evaluated the prognostic utility of the CellSearch test in mCRPC patients.5–7
In a prospective analysis performed on 120 blood samples of mCRPC patients, baseline CTC levels were predictive of survival while there was a modest correlation between CTC counts and extent of disease (PSA value and bone scan index) suggesting that CTCs are independent from tumor burden.5
Subsequently, in a large prospective study (the IMMC-38 trial) carried out on 231 mCRPC patients, de Bono et al. divided patients in two main groups according to CTCs blood concentration.6 Unfavorable group (patients with five or more CTCs per 7.5 ml of blood) had a median overall survival nearly half than patients with favorable (<5 CTCs per 7.5 ml) CTC counts (11.5 versus 21.7 months; p < 0.0001). Of note, changes of CTC count during or after first-line chemotherapy were strictly related to overall survival (OS). Indeed, patients who switched from an unfavorable to favorable group showed a better OS whereas patients who showed higher CTC blood level compared with baseline had a worse survival. Owing to the higher accuracy of CTCs counts compared with PSA decrement algorithms in terms of OS predictions, the FDA approved the CTCs assay as a tool for independent prediction of OS in CRPC.6,8 The OS predictive role of CTCs was also confirmed on subsequent preplanned analyses of different phase III trials.7,9,10
Recently, a meta-analysis of 10 eligible studies enrolling 1206 patients demonstrated that CTC positivity indicated a poor prognosis in patients with CRPC, thus confirming the role of CTCs as an independent prognostic factor of survival rate in patients with CRPC in clinical practice.11
After the IMMC-38 trial results, a post-treatment change in CTC count emerged as a potential biomarker of response (or resistance) to treatment. However, the lack of an established predictive value of the CellSearch test that could help to drive clinician therapeutic decisions has remarkably limited its routine clinical use. In addition, demonstrating true surrogacy of CTCs as a biomarker for survival remains difficult and controversial, and requires evaluation in multiple prospective randomized clinical trials, powered on survival end points, with subsequent confirmation by meta-analytic analyses. In particular, to fulfill the criteria of surrogacy for OS, the post-treatment CTC enumeration has to be prognostic, needs to be influenced by an effective treatment, and must identify the proper consequence of the therapy on the outcome measure.12 Curiously, in the COU-301 trial, modification of a complex biomarker panel composed of CTC counts and lactate dehydrogenase (LDH) serum levels after 12 weeks of treatment was shown to be a surrogate for survival at the individual-patient level, allowing patients to be stratified into good (CTC <5 and LDH ⩽250, 2-year OS of 46%), intermediate (CTC <5 and LDH >250), and poor (CTC⩾ 5, 2-year OS of 2%) risk groups, but no correlation between CTCs counts modification and response to therapy has been emerged and so the role of CTCs as a predictive marker of response to treatment remain unclear.13
The potential and attractive role of CTC counts decline as predictor of PCa response has been also evaluated in different studies but to date clinical practice is still orphan of a validate method able to translate CTC levels modification in a means able to drive therapeutic decisions.13–15
Beyond a quantitative assay, detection of CTCs represents a unique approach able to provide several information related to molecular characterization, genetic assessment and protein markers of tumors. In other words, through a minimally invasive assay we could obtain an extensive genomic characterization of the disease that could help clinicians to customize treatment options, provide prognostic information, and monitor treatment efficacy by the early recognition of acquired resistance mechanisms.16 In line with this, the identification of key genetic alterations linked to important therapeutic implications such as loss of phosphatase and tensin homolog (PTEN) tumor suppressor, MYC amplification, point mutation of the androgen receptor (AR) gene, and chromosomal translocations resulting in TMPRSS2–ERG gene fusion has been investigated in different studies.17–21 Since no specific molecular markers have been validated as predictive marker of response to treatment as well as no effective therapies targeting specific genetic mutations have been approved for treatment of PCa, the molecular analysis of PCa-related CTCs has still limited clinical relevance. Nonetheless, different CTCs detection techniques have been designed for the isolation of CTCs expressing different protein markers such as Ki-67 and androgen receptor splice variant 7 (AR V7), which is a receptor variant associated with poor response to abiraterone and enzalutamide.20,22–24 In 2010, Stott et al. reported the results of a pilot analysis employing a quantitative automated imaging system for the analysis of prostate CTCs. In this study, tumor cells were separated from blood by a CTC-Chip that adopted the transmembrane protein EpCAM then a rabbit polyclonal antibody to PSA was flowed through the Chip followed by a fluorescent-tagged goat antibody to rabbit immunoglobulin. CTCs were therefore quantified through a complex image-processing algorithms. This integrated technique was developed for the isolation of also a small number of CTCs. Nonetheless isolation of CTCs was reached in 8 (42%) of 19 patients with localized disease and 23 (64%) of 36 patients with metastatic disease.20 Regarding the role of CTCs in early prostate tumor stages, Thalgott et al. performed a CTCs assay with CellSearch in different stages of PCa including locally advanced high-risk, metastatic resistant, and taxane-refractory patients. The result of this assay showed that CTC counts in metastatic taxane-refractory patients was significantly increased compared with patients with locally advanced high-risk disease. Moreover, patients with bone and visceral lesions showed a high median CTC count whereas patients with soft-tissue metastases presented CTC counts comparable with controls. CTCs counts revealed a positive correlation with alkaline phosphatase and LDH serum levels whereas a negative association emerged with hemoglobin and PSA doubling time.25 Taking together the results of these study seems to reduce the diagnostic role of CTCs in early stage of the disease.
It is interesting to observe that regarding PCa, the detection of CTCs assumes a critical role mainly in advanced stages of the disease. Indeed, differently to kidney and bladder cancer, management of early stages of PCa can take advantage of PSA, which is a validated and sensitive soluble biomarker exploited for the detection and monitoring of the disease until the development of castration-resistant state. For this reason, the assessment of CTCs has been further evaluated in advanced stages with a particular interest on its prognostic and prediction of response role.
Urothelial carcinoma
Urothelial cancer of the bladder, renal pelvis, ureter, and other urinary organs is the second most frequent genitourinary malignancy and the sixth most common malignancy in men.26 Despite its high incidence, no routine screening tests have been validated for early detection of the disease and, therefore, diagnosis of urothelial carcinoma is generally made at the time disease-related symptoms develop. In addition, when urothelial cancer is highly suspected no serum biomarkers could help clinicians to obtain a diagnosis and, as a consequence, invasive diagnostic techniques such as cystoscopy are generally required. Cystoscopy is a technique with high sensitivity and specificity for the detection of urothelial carcinoma of the bladder. Moreover, this endoscopic approach offers the possibility to remove small tumors and obtain samples for histological examination.27 Urinary cytology is a noninvasive technique with a high sensitivity rate for high-grade urothelial cancer (84%) and high overall specificity rate (84–100%) but with a low sensitivity for low-grade tumors (16%).28,29 Several noninvasive tests exploring different urinary biomarkers have been developed and commercially approved for the detection of early low-grade tumors, but their role in clinical practice remains unclear mainly due to the high number of false-positive results30,31 and low sensitivity in early stages.32 In advanced stages, there are no standardized serum markers able to monitor disease recurrence or predict disease progression. Moreover, urothelial carcinoma is a disease still absent of selective biomarkers able to predict the presence of micrometastasis after surgery helping to predict those patients that could benefit more from perioperative chemotherapy. In early stages, the detection of CTCs could help clinicians to identify the disease and to isolate patients who can benefit more from an endoscopic examination of the bladder or from a more aggressive surgery. After surgery, CTCs detection appears an attractive method able to provide early recognition of disease recurrence. Moreover, this method may offer the possibility to analyze the molecular profile of tumor cells allowing for more customized treatment strategies and the identification of patients who would benefit more from a perioperative approach.
CTCs in urothelial carcinoma
Of note, differently from kidney cancer and PCa, in urothelial carcinoma the research of CTCs and DNA has been also performed in urinary samples with interesting results.
Urine DNA can be classified as two types that are easily separated by centrifugation or filtration: genomic DNA in exfoliated cells (the cell pellet) and cell-free DNA (cfDNA) in the supernatant/filtrate. Two studies have directly compared the utility of urinary cell pellet DNA and urinary cfDNA for detecting bladder cancer using microsatellite analysis or copy number changes.33,34 Both found a higher proportion of tumor DNA (tDNA) relative to nontumor DNA in the supernatant than in the cell pellet. In one study, urinary cfDNA and cellular DNA were analyzed and compared to matched formalin-fixed paraffin embedded (FFPE) tDNA in 23 patients. Urinary DNA was highly representative of DNA derived from tumors. cfDNA from urine had a higher tumor genome burden and allowed greater detection (90%) of clinically actionable genomic aberrations than cellular DNA from urine (61%).33
More recently, thanks to the application of new techniques of enrichment, detection, and isolation of CTCs, the focus has shifted to the detection and analysis of CTCs in patients’ blood. The increasing interest related to this approach is partly due to the observation that CTCs were detected in approximately 50% of patients with metastatic urothelial cancer and that CTCs could assume a prognostic role in this phase.35,36 In 2007, Naoe et al. evaluated the CellSearch assay in patients with nonmetastatic and metastatic urothelial carcinoma.37 Of note, they carried out a preclinical evaluation of CellSearch assay in samples made of peripheral blood mononuclear cells of healthy volunteers mixed with known concentration of bladder cancer cells marked with antibodies. Through this process, Naoe et al. demonstrated that the CellSearch assay was a sensitive and reliable method for the identification of urothelial cancer cells (defined as nucleated cells lacking CD45 and expressing cytokeratin 27 and 28) and so explored this technique in 12 and 14 patients with nonmetastatic and metastatic urothelial carcinoma. CellSearch assay was able to identify CTCs from blood of eight patients with metastatic disease while no CTCs could be detected in patients with nonmetastatic tumors.
Controversial results were obtained when investigating CTCs in patients with clinically localized bladder cancer, mainly due to the small sample size of the studies, heterogeneity of patients for stage, and short follow up.35–40 To date, the largest prospective study aimed to clarify the significance of CTCs in patients with clinically nonmetastatic bladder cancer has identified preoperative CTCs by CellSearch in 23 of 100 patients undergoing radical cystectomy and demonstrated that the presence of even a single CTC conferred a worse prognosis in terms of disease recurrence, cancer-specific mortality, and overall mortality.39 Conversely, in another study, CTCs were detected by CellSearch in 21% of 43 patients prior to undergoing radical cystectomy, and did not correlate with more advanced pathological extravesical or node-positive disease.40 A large and homogeneous population of high-risk non-muscle-invasive bladder cancer (NMIBC) patients (T1G3) demonstrated that the presence of at least one CTC is a strong predictor of decreased time to first recurrence and time to progression, defined as an increase in T stage to T2 or greater or lymph node (N+) disease or distant metastasis (M1).41 Thus, CTCs may be useful even in NMIBC to identify those patients with a high risk of recurrence or progression who could potentially benefit from early systemic therapy. In an unselected population of patients with histopathological diagnosis of NMIBC (Ta, T1, G1–3), CTCs were detectable in 8/44 patients (18%). The presence of CTCs was found significantly associated with shorter time to first recurrence in 24 months follow up.42
A meta-analysis investigated the diagnostic accuracy of CTC detection in early and advanced urothelial carcinoma. The overall sensitivity of CTC detection was 35.1%; overall specificity was 89.4%, LR+ 3.77 (95% CI, 1.95–7.30), and LR- 0.72 (95% CI, 0.64–0.81), suggesting that urothelial cancer CTC detection assays have limited diagnostic sensitivity because they fail to identify approximately two thirds of patients. On the other hand, CTC detection demonstrated high specificity for diagnosis of bladder and other urothelial cancers. Therefore, CTC detection may have limited value as first-line screening or diagnostic test but may be useful in confirming the cancer diagnosis. The same meta-analysis showed that CTC-positive patients were significantly more likely to have advanced (stage III–IV) disease compared with CTC-negative patients (OR, 5.05; 95% CI, 2.49–10.26). This association indicates that CTC assessment can be used to identify patients who are more likely to be classified as stage III–IV cancer despite initial clinical classification into locally confined (stage ⩽II) disease and who may be more likely to benefit from neoadjuvant chemotherapy.43
Though no standardized method of circulating or urinary CTCs has been involved in clinical practice, it is highly likely that in the next few years this emerging approach will offer a more accurate biomarker for the noninvasive detection of urothelial bladder cancer. Thus, it could help clinicians to reduce the number of cystoscopies performed, as they are burdensome for patients and expensive for healthcare providers. In addition, by using CTCs as prognostic (or even predictive) biomarkers could better guide patient management, especially for those patients with high-risk non-muscle-invasive bladder cancer who represent a treatment challenge.
Renal cell carcinoma
Kidney cancer has shown an increasing rate of incidence in the last 20 years, but at present this rate is showing a plateau/decreasing phase. Each year 62,700 new diagnoses of RCC are estimated worldwide with 5% mortality.1
RCC involves different types of tumors. Clear cell RCC (ccRCC) is certainly the most common histology detectable (70%) followed by papillary RCC (pRCC) and chromophobe RCC (chRCC) that present with incidence rates of 10% and 5%, respectively.44
Early stages of RCC are not generally associated with clinical manifestation and so the early detection of the disease remains an open challenge.45
Since early and accurate diagnosis avoids inadequate treatments and improves patient’s clinical outcomes, research into diagnostic biomarkers remains a critical issue. Moreover, an ideal biomarker could help clinicians to differentiate malignant RCC from benign tumor after the incidental detection of a small kidney mass. After surgery, the use of specific markers could also be useful to isolate patients with higher risk of recurrence who could benefit more from a closer follow-up program and an adjuvant treatment. Other possible applications of specific biomarkers involve the early detection of synchronous metastasis not demonstrable by imaging as well as the prediction of prognosis after diagnosis. Regarding this last point, prognosis of patients with metastatic disease is estimated through clinical score such as Inter-national Metastatic Renal Cell Carcinoma Database Consortium and Memorial Sloan-Kattering Cancer Center scores45–49 and none of them includes genetic assessment of the disease as well as plasmatic, serum, or pathological biomarkers.
Finally, it is also important to remember that in the last few years several treatments have been developed for the management of metastatic RCC (mRCC). Even if different treatments for mRCC patients are now available, no response predictive markers have been included in clinical practice and so the choice between these different drugs is generally made by considering the clinical outcome perused, patient’s preference, and toxicity profile of each agent.
Several biomarkers have been investigated in RCC in different setting such as early detection, diagnosis, prediction of prognosis, and response to treatment but, to date, none of them have been included in clinical practice.
The assessment of circulating RCC tumor cells represents a promising approach for the early detection of primary tumors and metastases, but like other biomarkers it remains only an experimental technique.
CTCs in RCC
In recent years, several studies have focused their attention on the genomic assessment of RCC.50–52 In particular, The Cancer Genome Atlas (TCGA) network recognized 19 significantly mutated genes, with VHL, PBRM1, SETD2, KDM5C, PTEN, BAP1, MTOR and TP53 representing the 8 most extreme members. More recently, Rini et al. validated a 16-gene assay on resected primary tumor that is strictly related to recurrence rate and patient’s clinical outcomes.53 These studies represented an important step for the understanding of the mechanisms involved in the development and progression of RCC. Furthermore, these demonstrated a close relationship between genetic arrangement of the disease and clinical outcomes of patients. It is important to observe that these important results coming from genomic assessment have been performed mainly on tissue from primary tumor and/or metastasis and so the opportunity to obtain this information by peripheral analysis of CTCs appears an attractive option.
Regarding the detection of CTCs, different techniques have been tested with different results. In 2003, Shimazui et al. identified cadherin-6 mRNA as a marker of circulating RCC tumor cells since it was detectable by reverse transcriptase polymerase chain reaction (RT-PCR) only in patients with mRCC compared with healthy volunteer’s controls population.54 Two years later, Blümke et al. collected blood samples of 214 RCC patients before and after surgery or during adjuvant immune treatment. After density gradient centrifugation they isolated a CD45 negative cell population through a semi-automated immunomagnetic depletion procedure, then they stained these cells for cytokeratin and performed a pathologic examination. Even if the detection of ccRCCtc was possible only in 37% of patients with a median cell number of five, the presence of ccRCCtc was associated with progressive disease and development of metastases.55 Subsequently, a large study carried out on 964 patients with metastatic disease (breast, PCa, colon, RCC, etc.), 199 patients with nonmalignant disease and 145 healthy donors leads to the approval of CellSearch system® by FDA for the detection of circulating tumor cells.4 The few patients with mRCC enrolled in this study as well as the lack of epithelial adhesion molecule such as EpCam and cytokeratin mainly due to the mesenchymal–epithelial transition receptor/pathways (MET) often expressed by mRCC tumor cells has led to the hypothesis that the CellSearch system could underestimate the number of cRCCtc and so other approach have been tested to increase the sensibility of cRCCtc detection. Indeed, it is possible that the lack of cytokeratin expression by CTCs could partially explain the low sensitivity of CellSearch assay in patients with RCC. Since circulating cells with no clear cytokeratin staining are not recognized as CTC but defined as ‘suspicious objects’, different techniques have been adopted to evaluate the expression profile of these atypical cells but to date none of these assays have shown to increase the detection sensibility of CTCs from patients with RCC.56
From the discussion of these studies it appears clear that the detection and analysis of CTCs could be an interesting and valid option for RCC early detection and treatment strategies planning. Unfortunately, none of the techniques explored have been adopted in clinical practice mainly due to a low detection sensibility.
Techniques adopted for the detection, isolation, and enrichment of CTCs
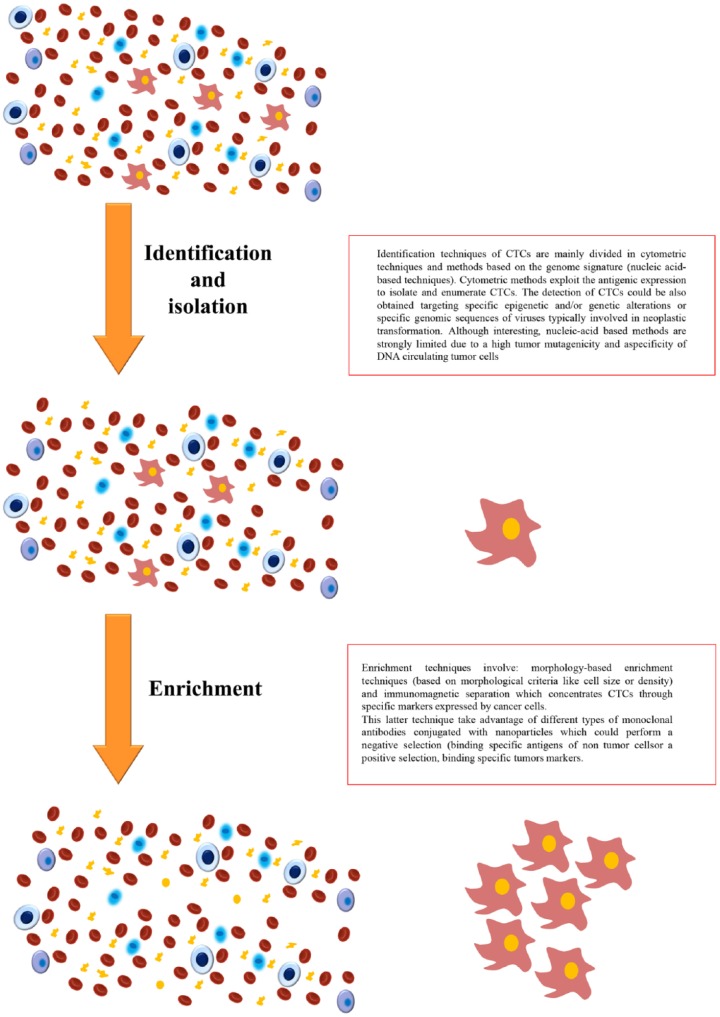
In general, detection of CTCs requires specific techniques able to overcome problems related to identification and isolation of tumors cells from blood (Figure 2).
Figure 2.
Detection, isolation, and enrichment of circulating tumor cells.
Indeed, there is no a specific marker that allows to uniquely distinguish a CTC from other blood cells, since tumors with different histological and molecular features express diverse patterns of markers, and even a single histological tumor type can present heterogeneous markers. Moreover, given the considerably small number of CTCs possibly present in peripheral blood when compared with the other blood circulating cells, enrichment techniques are necessary to increase the sensitivity to an acceptable level.
Identification and enrichment techniques
Regarding enrichment techniques these involve morphology-based enrichment techniques and immunomagnetic separation that concentrates CTCs through specific markers expressed by cancer cells.57 In morphology-based enrichment techniques tumor cells are isolated from blood thanks to their larger size (compared with leukocytes) or thanks to their different density gradients. Immunomagnetic separation takes advantage of different types of monoclonal antibodies conjugated with nanoparticles that could perform a negative selection (anti-CD45 and anti-CD61 bind leukocytes and megakaryocytes/platelets sequestering them from blood samples) or a positive selection, binding specific tumors markers such as EpCAM or Her 2 (MACS-Miltenyi Biotec GmbH, Bergisch Gladbach, Germany). Available kits for the enrichment of CTCs include AdnaTest, which combines two anti-MUC1 antibodies and an anti-EpCAM antibody adsorbed on magnetic microspheres [the standard AdnaTest includes primers against PSA, prostate-specific membrane antigen (PSMA), and the epidermal growth factor receptor (EGFR)], and RARE (StemCell Technologies, Vancouver, Canada), which is a technique combining a separation system based on a densitometric gradient with a phase of antibody-mediated enrichment.58
Techniques performed to identify and number the CTCs are mainly divided into cytometric techniques and methods based on the genome signature (nucleic acid-based techniques). Both these systems usually include an enrichment and detection phases.
Cytometric methods exploit the antigenic expression to isolate and enumerate CTCs. Through this technique CTCs are not lysed and could be subjected to further characterization and assays. The main limit is the current lack of a tumor-specific antigen. The detection of CTCs could also be obtained targeting specific epigenetic and/or genetic alterations or specific genomic sequences of viruses typically involved in neoplastic transformation.59
Since different assays such as AdnaTest and CellSearch (see below) adopted antibodies against EpCAM, CTCs that do not express this epithelial marker, for example those who have undergone an EMT could not be detected by these techniques and so other strategies have been explored to overcome this limit. The Epic Sciences test (San Diego, CA) is an alternative technique in which peripheral sample after an initial phase of red blood cells lysis and removal is pelleted and spread onto custom glass slides covered in a proprietary adhesive coating and stained for DAPI, CD45, and cytokeratins. Stained slides are scanned using fiberoptic array scanning technology (FAST) and acquired images are then analyzed by image-processing software that identifies CTCs. With this technology, different CTC phenotypes are detected including traditional CTCs, CTC clusters, small CTCs, cytokeratin-negative CTCs, and apoptotic CTCs.60
In the AccuCyte CyteFinder system, commercialized by RareCyte (Seattle, WA), nucleated blood cells are isolated from red blood cells using a density-based separation method. The isolated cell fraction is then smeared onto glass slides and stained with fluorescently labeled antibodies of the investigator’s choosing. High-throughput imaging is employed to identify candidate CTCs with a scanning microscope and image analysis tools. A unique feature of the system is the integration of a single-cell micromanipulator called the CytePicker. Using this retrieval device, single cells can be retrieved from stained glass slides for downstream molecular analysis, including DNA sequencing and array comparative genomic hybridization. The ability of the RareCyte system to identify CTCs with low EpCAM levels confers a greater sensibility in detecting CTCs compared with the CellSearch system.60 Another approach for CTCs isolation consisted on the application of microfluidic devices. Through microvortices directing CTCs to anti-EpCAM antibodies the herringbone chip (HB-chip) binds CTCs to chip’s surface. The CTC-iChip performed a positive or negative selection of CTCs through a three-step process. First, separation of nucleated cells from red blood cells and platelets using deterministic lateral displacement; second, alignment of nucleated cells within a microfluidic channel using inertial focusing; and, third, deflection of magnetically labeled cells into a collection channel.60,61
Devices combining enrichment and identification techniques
Different devices adopted a combined enrichment and identification techniques. The CellSearch test (Janssen diagnostics, USA) is the only FDA-approved assay which includes a combined enrichment and identification technique. This is an in vitro diagnostic, semiautomatic method that allows the counting of CTCs of epithelial origin.
The CellSearch CTC kit contains a reagent for the selection, consisting of iron-fluid, and immunofluorescent reagents (Table 1). The iron-fluid reagent consists of nanoparticles with a magnetic core surrounded by a polymer layer covered with antibodies directed towards the epithelial cell adhesion molecule-1 (EpCAM) antigen.4 Blood sample, incubated with the ferroparticles coated in anti-EpCAM antibodies, is processed through a magnetic field for retaining the EpCAM-positive cells, while the EpCAM-negative cells, mainly of hematopoietic origin, are eliminated. After immunomagnetic selection and enrichment, the EpCAM-positive enriched cells react with fluorescent reagents, including the 4ʹ,6‑diamidino-2‑phenylindole (DAPI) dye, useful for staining the cell nucleus; monoclonal antibodies specific for leukocytes (anti-CD45 antibodies conjugated with allophycocyanin); phycoerythrin conjugated antibodies, anti-cytokeratins 8, 18 and 19 (anti-CK-PE) to identify intracellular cytokeratins (CK) of epithelial cells. After staining, CTCs are enumerated using the semiautomated Celltracks Analyzer II System, a semiautomated fluorescence microscope, which allows a computer reconstruction of cell images. A cell is then classified as CTC when its morphology is consistent with that of a cancer cell (oval or round shape), and simultaneously shows an EpCAM + phenotype, does not have specific antigens of leukocyte line (CD45), exhibit cytoplasmic expression of cytokeratin, and contains a nucleus that binds DAPI.
Table 1.
Applications of the CellSearch system in urogenital malignancies.
| Prostate cancer |
| - CTCs detected in patients with metastatic prostate cancer: ⩾2 CTCs in 57% of metastatic PCa blood samples, 14% of which contained ⩾50 CTCs whereas these were extremely rare in healthy subjects6 - Independent correlation between CTCs blood concentration detected by CellSearch system and OS.10–16 - No correlation between CTCs levels and response to treatment18–20 |
| Urothelial carcinoma |
| - CellSearch system has shown to be a sensible and reliable technique for the identification of CTCs in patients with metastatic urothelial carcinoma but it has not been validated in this setting.43–45
- Several studies have explored the prognostic role of CTCs detected with CellSearch in localized bladder tumor with controversial results. Although the identification of at least one CTC could be a strong predictor of disease recurrence after surgery, this method has not been validated and included in clinical practice.43–50 - In a meta-analysis investigating the diagnostic accuracy of CTC detection in early and advanced urothelial carcinoma the overall sensitivity of CTC detection was only 35.1% suggesting that this method has a limited value as first-line screening or diagnostic test.51 |
| Renal cell carcinoma |
| - CellSearch system showed a very low detection rate in patients with localized and metastatic RCC.6 The expression of MET by RCC cancer cells resulting in a lack of epithelial adhesion molecules could partially explain the failure of this method. |
CTC, circulating tumor cell; MET, mesenchymal–epithelial transition receptor/pathways; OS, overall survival; PCa, prostate cancer; RCC, renal cell carcinoma.
Conclusion
Although the detection of CTCs represents an interesting noninvasive approach, potentially able to achieve an early diagnosis of genitourinary tumors, to date the only validated assay approved by FDA for the detection of CTCs in PCa is the CellSearch system.
Despite this, CTC detection is an approach rarely adopted in clinical practice. Nevertheless, thanks to their multiple applications, CTC detection techniques should be investigated in larger clinical trials especially in urothelial cancer and RCC, which are diseases still lacking reliable and noninvasive markers.
Beyond this, it is highly likely that trials evaluating other different methods such as circulating DNA and mRNA will soon produce interesting results. Therefore, the future development of combined techniques able to detect both circulating tumor DNA/mRNA and CTCs represents a promising approach that could be explored in future preclinical and clinical studies.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Contributor Information
Francesco Massari, Division of Oncology, S.Orsola-Malpighi Hospital, Via Albertoni n.15, Bologna, 40138, Italy.
Vincenzo Di Nunno, Division of Oncology, S.Orsola-Malpighi Hospital, Bologna, Italy.
Francesca Comito, Division of Oncology, S.Orsola-Malpighi Hospital, Bologna, Italy.
Marta Cubelli, Division of Oncology, S.Orsola-Malpighi Hospital, Bologna, Italy.
Chiara Ciccarese, Department of Medical Oncology, Azienda Ospedaliera Universitaria Integrata (AOUI), Verona, Italy.
Roberto Iacovelli, Department of Medical Oncology, Azienda Ospedaliera Universitaria Integrata (AOUI), Verona, Italy.
Michelangelo Fiorentino, Pathology department, S.Orsola-Malpighi Hospital, Bologna, Italy.
Rodolfo Montironi, Section of Pathological Anatomy, Polytechnic University of the Marche Region, School of Medicine, United Hospitals, Ancona, Italy.
Andrea Ardizzoni, Division of Oncology, S.Orsola-Malpighi Hospital, Bologna, Italy.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin 2016; 66: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Scher HI, Morris MJ, Larson S, et al. Validation and clinical utility of prostate cancer biomarkers. Nat Rev Clin Oncol 2013; 10: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ciccarese C, Montironi R, Fiorentino M, et al. Circulating tumor cells: a reliable biomarker for prostate cancer treatment assessment? Curr Drug Meta. Epub ahead of print 18 May 2017. DOI: 10.2174/1389200218666170518163549. [DOI] [PubMed] [Google Scholar]
- 4. Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004; 10: 6897–6904. [DOI] [PubMed] [Google Scholar]
- 5. Danila DC, Heller G, Gignac GA, et al. Circulating tumor cell number and prognosis in progressive castration-resistant prostate cancer. Clin Cancer Res 2007; 13: 7053–7058. [DOI] [PubMed] [Google Scholar]
- 6. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008; 14: 6302–6309. [DOI] [PubMed] [Google Scholar]
- 7. Goldkorn A, Ely B, Quinn DI, et al. Circulating tumor cell counts are prognostic of overall survival in SWOG S0421: a phase III trial of docetaxel with or without atrasentan for metastatic castration-resistant prostate cancer. J Clin Oncol 2014; 32: 1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scher HI, Jia X, de Bono JS, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol 2009; 10: 233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Scher HI, Heller G, Molina A, et al. Circulating tumor cell biomarker panel as an individual-level surrogate for survival in metastatic castration-resistant prostate cancer. J Clin Oncol 2015; 33: 1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fleisher M, Danila DC, Fizazi K, et al. Circulating tumor cell (CTC) enumeration in men with metastatic castration-resistant prostate cancer (mCRPC) treated with enzalutamide post-chemotherapy (phase 3 AFFIRM study). J Clin Oncol 2015; (Suppl.): abstract 5035. [Google Scholar]
- 11. Zheng Y, Zhang C, Wu J, et al. Prognostic value of circulating tumor cells in castration resistant prostate cancer: a meta-analysis. Urol J 2016; 11: 2881–2888. [PubMed] [Google Scholar]
- 12. Mehra N, Zafeiriou Z, Lorente D, et al. CCR 20th anniversary commentary: circulating tumor cells in prostate cancer. Clin Cancer Res 2015; 21: 4992–4995. [DOI] [PubMed] [Google Scholar]
- 13. Olmos D, Arkenau HT, Ang JE, et al. Circulating tumour cell (CTC) counts as intermediate end points in castration-resistant prostate cancer (CRPC): a single-centre experience. Ann Oncol 2009; 20: 27–33. [DOI] [PubMed] [Google Scholar]
- 14. Lorente D, Olmos D, Mateo J, et al. EARLY CTC decline as a biomarker of response to treatment in castration-resistant prostate cancer (CRPC): analysis of the COU-AA-301 and IMMC38 trials. J Clin Oncol 2015; (Suppl.): abstract 5014. [Google Scholar]
- 15. Ciccarese C, Massari F, Iacovelli R, et al. Prostate cancer heterogeneity: discovering novel molecular targets for therapy. Cancer Treat Rev 2017; 54: 68–73. [DOI] [PubMed] [Google Scholar]
- 16. Miyamoto DT, Sequist LV, Lee RJ. Circulating tumour cells-monitoring treatment response in prostate cancer. Nat Rev Clin Oncol 2014; 11: 401–412. [DOI] [PubMed] [Google Scholar]
- 17. Attard G, Swennenhuis JF, Olmos D, et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res 2009; 69: 2912–2918. [DOI] [PubMed] [Google Scholar]
- 18. Leversha MA, Han J, Asgari Z, et al. Fluorescence in situ hybridization analysis of circulating tumor cells in metastatic prostate cancer. Clin Cancer Res 2009; 15: 2091–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang Y, Palma JF, Agus DB, et al. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin Chem 2010; 56: 1492–1495. [DOI] [PubMed] [Google Scholar]
- 20. Stott SL, Lee RJ, Nagrath S, et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci Transl Med 2010; 2: 25ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danila DC, Anand A, Sung CC, et al. TMPRSS2-ERG status in circulating tumor cells as a predictive biomarker of sensitivity in castration-resistant prostate cancer patients treated with abiraterone acetate. Eur Urol 2011; 60: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Antonarakis ES, Lu C, Wang H, et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med 2014; 371: 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steinestel J, Luedeke M, Arndt A, et al. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. Oncotarget. Epub ahead of print April 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakazawa M, Lu C, Chen Y, et al. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann Oncol 2015; 26: 1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thalgott M, Rack B, Maurer T, et al. Detection of circulating tumor cells in different stages of prostate cancer. J Cancer Res Clin Oncol 2013; 139: 755–763. [DOI] [PubMed] [Google Scholar]
- 26. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136: 359–386. [DOI] [PubMed] [Google Scholar]
- 27. Van der Poel HG, Debruyne FM. Can biological markers replace cystoscopy? An update. Curr Opin Urol 2001; 11: 503–509. [DOI] [PubMed] [Google Scholar]
- 28. Yafi FA, Brimo F, Steinberg J, et al. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol Oncol 2015; 33: 25–31. [DOI] [PubMed] [Google Scholar]
- 29. Lokeshwar VB, Habuchi T, Grossman HB, et al. Bladder tumor markers beyond cytology: international consensus panel on bladder tumor markers. Urology 2005; 66(Suppl. 1): 35–63. [DOI] [PubMed] [Google Scholar]
- 30. Smith ZL, Guzzo TJ. Urinary markers for bladder cancer. F1000Prime Rep 2013; 5: 5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Huber S, Schwentner C, Taeger D, et al. Nuclear matrix protein-22: prospective evaluation in a population at risk for bladder cancer. Results from the UroScreen study. BJU Int 2012; 110: 699–708. [DOI] [PubMed] [Google Scholar]
- 32. Hajdinjak T. UroVysion FISH test for detecting urothelial cancers: meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol Oncol 2008: 26: 646–651. [DOI] [PubMed] [Google Scholar]
- 33. Togneri FS, Ward DG, Foster JM, et al. Genomic complexity of urothelial bladder cancer revealed in urinary cfDNA. Eur J Hum Genet 2016; 24: 1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Szarvas T, Kovalszky I, Bedi K, et al. Deletion analysis of tumor and urinary DNA to detect bladder cancer: urine supernatant versus urine sediment. Oncol Rep 2007; 18: 405–409. [PubMed] [Google Scholar]
- 35. Gallagher DJ, Milowsky MI, Ishill N, et al. Detection of circulating tumor cells in patients with urothelial cancer. Ann Oncol 2009; 20: 305–308. [DOI] [PubMed] [Google Scholar]
- 36. Flaig TW, Wilson S, van Bokhoven A, et al. Detection of circulating tumor cells in metastatic and clinically localized urothelial carcinoma. Urology 2011; 78: 863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Naoe M, Ogawa Y, Morita J, et al. Detection of circulating urothelial cancer cells in the blood using the CellSearch System. Cancer 2007; 109: 1439–1445. [DOI] [PubMed] [Google Scholar]
- 38. Rink M, Chun FK, Minner S, et al. Detection of circulating tumor cells in peripheral blood of patients with advanced non-metastatic bladder cancer. BJU Int 2011; 107: 1668–1675. [DOI] [PubMed] [Google Scholar]
- 39. Rink M, Chun FK, Dahlem R, et al. Prognostic role and HER2 expression of circulating tumor cells in peripheral blood of patients prior to radical cystectomy: a prospective study. Eur Urol 2012; 61: 810–817. [DOI] [PubMed] [Google Scholar]
- 40. Guzzo TJ, McNeil BK, Bivalacqua TJ, et al. The presence of circulating tumor cells does not predict extra-vesical disease in bladder cancer patients prior to radical cystectomy. Urol Oncol 2012; 30: 44–48. [DOI] [PubMed] [Google Scholar]
- 41. Gazzaniga P, de Berardinis E, Raimondi C, et al. Circulating tumor cells detection has independent prognostic impact in high-risk non-muscle invasive bladder cancer. Int J Cancer 2014; 135: 1978–1982. [DOI] [PubMed] [Google Scholar]
- 42. Gazzaniga P, Gradilone A, Berardinis ED, et al. Prognostic value of circulating tumor cells in non-muscle invasive bladder cancer: a CellSearch analysis. Ann Oncol 2012; 23: 2352–2356. [DOI] [PubMed] [Google Scholar]
- 43. Msaouel P, Koutsilieris M. Diagnostic value of circulating tumor cell detection in bladder and urothelial cancer: systematic review and meta-analysis. BMC Cancer 2011; 11: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol 2016; 70: 93–105. [DOI] [PubMed] [Google Scholar]
- 45. Gospodarowicz MK, Miller D, Groome PA, et al. The process for continuous improvement of the TNM classification. Cancer 2004; 100: 1–5. [DOI] [PubMed] [Google Scholar]
- 46. Motzer RJ, Mazumdar M, Bacik J, et al. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol 1999; 17: 2530–2540. [DOI] [PubMed] [Google Scholar]
- 47. Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005; 23: 832–841. [DOI] [PubMed] [Google Scholar]
- 48. Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009; 27: 5794–5799. [DOI] [PubMed] [Google Scholar]
- 49. Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol 2013; 14: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 2013; 499: 43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cancer Genome Atlas Research Network. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Eng J Med 2016; 374: 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fiorentino M, Gruppioni E, Massari F, et al. Wide spectrum mutational analysis of metastatic renal cell cancer: a retrospective next generation sequencing approach. Oncotarget 2017; 8: 7328–7335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rini B, Goddard A, Knezevic D, et al. A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: development and validation studies. Lancet Oncol 2015; 16: 676–685. [DOI] [PubMed] [Google Scholar]
- 54. Shimazui T, Yoshikawa K, Uemura H, et al. Detection of cadherin-6 mRNA by nested RT-PCR as a potential marker for circulating cancer cells in renal cell carcinoma. Int J Oncol 2003; 23: 1049–1054. [PubMed] [Google Scholar]
- 55. Blümke K, Bilkenroth U, Schmidt U, et al. Detection of circulating tumor cells from renal carcinoma patients: experiences of a two-center study. Oncol Rep 2005; 14: 895–899. [PubMed] [Google Scholar]
- 56. Gradilone A, Iacovelli R, Cortesi E, et al. Circulating tumor cells and “suspicious objects” evaluated through CellSearch® in metastatic renal cell carcinoma. Anticancer Res 2011; 31: 4219–4221. [PubMed] [Google Scholar]
- 57. Mostert B, Sleijfer S, Foekens JA, et al. Circulating tumor cells (CTCs): detection methods and their clinical relevance in breast cancer. Cancer Treat Rev 2009; 35: 463–474. [DOI] [PubMed] [Google Scholar]
- 58. Todenhöfer T, Hennenlotter J, Feyerabend S, et al. Preliminary experience on the use of the Adnatest® system for detection of circulating tumor cells in prostate cancer patients. Anticancer Res 2012; 32: 3507–3513. [PubMed] [Google Scholar]
- 59. Lambrechts AC, Bosma AJ, Klaver SG, et al. Comparison of immunocytochemistry, reverse transcriptase polymerase chain reaction, and nucleic acid sequence-based amplification for the detection of circulating breast cancer cells. Breast Cancer Res Treat 1999; 56: 219–231. [DOI] [PubMed] [Google Scholar]
- 60. Gorin MA, Verdone JE, van der Toom E, et al. Circulating tumour cells as biomarkers of prostate, bladder, and kidney cancer. Nat Rev Urol 2017; 14: 90–97. [DOI] [PubMed] [Google Scholar]
- 61. Karabacak NM, Spuhler PS, Fachin F, et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat Protoc 2014; 9: 694–710. [DOI] [PMC free article] [PubMed] [Google Scholar]