Abstract
Background: Antimicrobials are among the most frequently prescribed medications for pediatric patients. However, inappropriate use of them can increase morbidity, mortality, healthcare costs, and largely antimicrobial resistance. This study aims to assess the antimicrobial utilization pattern in the pediatric ward of Hiwot Fana Specialized University Hospital. Methods: Retrospective cross-sectional study was conducted to assess the antimicrobial utilization. In this study, 403 pediatric medical records selected by systematic random sampling were reviewed. Data were collected using structured data abstraction format. Results: Ceftriaxone (n = 176, 26.5%), gentamicin (n = 125, 18.82%), and ampicillin (n = 119, 17.9%) were the most frequently prescribed antimicrobials, whereas ampicillin and gentamicin combination took the largest percentage share (n = 91, 43.3%). The most common reasons for which antimicrobials prescribed were severe pneumonia (n = 93, 18.82%), severe acute malnutrition (n = 69, 13.97%), and meningitis (n = 67, 13.56%). On average, the highest number of antimicrobials per card was observed in neonates. The percentage of antimicrobials administered by parenteral route was found to be 84.33%. Less than half of antimicrobials (46.98%) were prescribed with dosage form. Besides, strength and duration were recorded in 20.03% and 4.21% of antimicrobial agents, respectively. Conclusion: Generally, there was an overuse of injectables despite the fact that oral formulations are safer alternatives. This result is too far from World Health Organization (WHO) standard (13.4%-21.1%). The degree of polypharmacy of antimicrobials falls within the WHO cutoff point (<2). There are no antibiogram tests conducted in the hospital. By and large, this study provides an impetus towards the establishment of antimicrobial stewardship programs.
Keywords: antimicrobials, pediatrics, medical records, Hiwot Fana Specialized University Hospital
Introduction
Antimicrobials are the key drugs for the treatment and prevention of infectious diseases and are among the most widely prescribed drugs in pediatric population.1 The inevitable consequence of the widespread use of these agents leads to the emergence of antimicrobial-resistant pathogens, urging an ever-increasing need for new drugs. The current global increase in antimicrobial resistance (AMR) and, simultaneously, the downward trend in the development of new antimicrobials have serious public health and economic implications. Therefore, rational and judicious selection of these agents for the therapy of infectious diseases requires clinical judgment and in-depth knowledge of microbiological and pharmacological factors.2-4
Antimicrobial agents have 3 general purposes: empirical therapy, definitive therapy, and prophylactic therapy. When used as empirical therapy, the antimicrobial agent should cover all the likely pathogens because the infecting organism has not yet been defined. Either combination therapy or, preferably, treatment with a single broad-spectrum agent may be employed. However, once the infecting microorganism is identified, definitive antimicrobial therapy should be instituted with a narrow-spectrum and safe agent to complete the course of treatment. Prescribing and consumption pattern should be studied, and control mechanisms must be in place to avoid drug resistance, treatment failure, adverse drug reactions, superinfections, and extra cost. However, such studies and antimicrobial use guidelines are not available in resource-limited settings.1-4 Responsible utilization of antimicrobials is very critical as their stock out or resistance can be life-threatening in certain conditions in health care settings.5 The worldwide emergence of AMR is a major public health problem that significantly impacts patient treatment outcomes.6,7
Specifically, pediatric populations are commonly affected by various infectious diseases in developing countries.8 For such conditions, antimicrobials are commonly prescribed as empirical therapy rather than prophylactic or definitive therapy. There have been fewer studies on antimicrobial use in pediatrics than in adult patients. Pediatrics represent a large part of the population in developing countries and are the most vulnerable population groups to contract illnesses and develop harmful effects of drugs due to differences in the pharmacodynamic and pharmacokinetic profiles.9 The use of antimicrobial agents has become a routine practice for the treatment of pediatric illnesses.10 Higher incidence of infection in pediatrics than adults leads to greater antimicrobials utilization and hence overwhelmed health care cost.11 This problem has been largely observed in developing countries through inappropriate prescribing habits, overinterested desires to treat every infection children are mostly suffering from. The fact that the fate of infants and children is decided by parents or any other third party can have a negative impact on the provision of health care for them. The roles of health care workers in the selection of antimicrobials for pediatric patients are crucial.12 The present study, therefore, aims to assess the antimicrobial utilization pattern in pediatric patients admitted to Hiwot Fana Specialized University Hospital (HFSUH) in 2016.
Methods
Study Setting, Design, and Period
The study was conducted in HFSUH, a tertiary care hospital located in Harar town, which is 526 km away from the capital of Ethiopia, Addis Ababa, to the east. Currently, it has become the major teaching and referral hospital in the eastern segment of the country. The hospital has different units including internal medicine, gynecology/obstetrics, surgery, dentistry, antenatal care, ophthalmology, hospital pharmacy, dermatology, and antiretroviral therapy clinic, among others. Cross-sectional study design was employed to assess the antimicrobial utilization pattern in the pediatric ward of this hospital from February 20 to March 20, 2017.
Population
Medical records of pediatric patients who were admitted at the pediatric ward of HFSUH and who took at least 1 antimicrobial agent from January 1, 2016, to December 31, 2016 were taken as a study population from which sampling units were drawn. Incomplete medical records were excluded from the study.
Sample Size Determination and Sampling Technique
Sample size was calculated based on single population proportion formula considering a P value of .5, 95% confidence level (Z = 1.96), and margin of error (d) of 5%. Based on the formula, ni = Zα2pq / d2, the initial sample size was calculated to be 384 medical records. However, 1-year (2016) study population was found to be less than 10 000: 7840 medical records (traced from pediatric antimicrobial prescriptions). Therefore, further adjustment of the initial sample size was undertaken using the following formula, nf = ni / [1 + (ni / N)], where ni is the initial sample size calculated above, nf is the final sample size, and N is the number of study population. The final sample size was obtained as 366 records. With 10% contingency (37 records), the overall samples collected during the study were 403 pediatric medical records. With the aid of card number of prescriptions as a tracer, sampling units were collected using systematic random sampling by taking every 20th (7840/403) once the records had been arranged in chronological order and the first card had been randomly selected.
Data Collection Procedures
Data were collected retrospectively with the aid of data abstraction format which includes sociodemographic and clinical characteristics of pediatric patients as well as patterns of antimicrobial utilization during the study period. The data collection format contains key points that can address major drug use problems during antimicrobial utilization in a quantitative basis. The format includes degree of polypharmacy of all drugs and antimicrobials in particular, dosage regimen, route of administration, prevalence of single and combination antimicrobials, and prevalence of disease states to which antimicrobials were prescribed, among others. Every relevant datum was recorded in the format from the patient medication records.
Variables
Sociodemographic characteristics (age, sex, and address) and clinical characteristics (weight, diagnoses, and laboratory tests) were considered as explanatory variables, whereas utilization pattern of antimicrobials was considered as the dependent variable.
Data Quality Control
Pretest was conducted to check whether the data collection format was valid and reliable in Jugal hospital. The clarity and completeness of data collection format was checked prior to the actual data collection. Data clearing was also done on a daily basis.
Data Processing, Analysis, and Presentation
The collected data were processed and analyzed by using SPSS version 21 (SPSS Inc, Chicago, Illinois). Descriptive statistics followed by cross tabulation was employed to provide the frequency and percentage distributions of the variables included in the study. The result was presented using tables, figures, and pie charts.
Results
To assess the antimicrobial utilization pattern, a total of 403 pediatric medical records containing antimicrobial agents were reviewed and analyzed in the pediatric ward of HFSUH. From a total of pediatric medical records included, a larger proportion of male patients (n = 254, 63%) were admitted in the ward during the review period with a sex ratio of 1.7:1. The highest and lowest numbers of patients were from the age group of 1 month to 1 year (infants) and <1 month (neonates), respectively. Majority of the patients (n = 347, 86.1%) came from areas outside of Harar city. This study showed that the majority of infants (n = 97, 24.06%) and patients aged 1 to 5 years (n = 80, 19.85%) have a body weight of 9.9 kg (total, 43.9%), whereas 31 (7.69%) patients with the age group of 5 to 10 years and greater than 10 years, in each, have a body weight of greater than 20 kg (15.4%). What is more, neonates have a body weight of less than 4.9 kg (14.6%) (Table 1).
Table 1.
Sociodemographic and Clinical Characteristics of Pediatric Patients at the HFSUH Pediatric Ward from January 1, 2016, to December 31, 2016 (n = 403).
| Sociodemographic and clinical characteristics | Category | Frequency (%) |
|---|---|---|
| Age | <1 mo (neonates) | 15 (3.7) |
| 1 mo-1 y (infants) | 144 (35.7) | |
| 1-5 y | 132 (32.8) | |
| 5-10 y | 78 (19.4) | |
| 10-14 y | 34 (8.4) | |
| Sex | Male | 254 (63.0) |
| Female | 149 (37.0) | |
| Address | Harar city | 56 (13.9) |
| Outside of Harar | 347 (86.1) | |
| Weight, kg | <4.9 | 59 (14.6) |
| 5-9.9 | 177 (43.9) | |
| 10-14.9 | 70 (17.4) | |
| 15-19.9 | 35 (8.7) | |
| >20 | 62 (15.4) |
Note. HFSUH = Hiwot Fana Specialized University Hospital.
Coming to the degree of polypharmacy per age category of pediatric patients, on average, the highest number of antimicrobials per patient card was observed in neonates, mean (±standard error of the mean [±SEM]) (95% confidence interval [CI]) = 1.87 (±0.09) (1.67-2.06), followed by those with the age group of 10 to 14 years, 1.76 (±0.12) (1.52-2.01). From the total drugs, the maximum average number of drugs per patient was observed in pediatrics with 1 to 5 years, 2.79 (±0.12) (2.55-3.03), and 5 to 10 years, 2.73 (±0.16) (2.41-3.05) (Table 2).
Table 2.
Cross Tabulation of Age with Total Drugs and Antimicrobials Prescribed in HFSUH Pediatric Ward from January 1, 2016, to December 31, 2016.
| Age | From total drugs (%) | Mean (±SEM) (95% CI) | From antimicrobials (%) | Mean (±SEM) (95% CI) |
|---|---|---|---|---|
| <1 mo | 32 (3.08) | 2.13 (±0.165) (1.78-2.49) | 28 (4.22) | 1.87 (±0.09) (1.67-2.06) |
| 1 mo-1 y | 332 (32.01) | 2.31 (±0.10) (2.11-2.50) | 237 (35.69) | 1.65 (±0.06) (1.53-1.76) |
| 1-5 y | 368 (35.49) | 2.79 (±0.12) (2.55-3.03) | 214 (32.23) | 1.62 (±0.07) (1.48-1.76) |
| 5-10 y | 213 (20.54) | 2.73 (±0.16) (2.41-3.05) | 124 (18.67) | 1.59 (±0.08) (1.43-1.75) |
| 10-14 y | 92 (8.87) | 2.71 (±0.20) (2.31-3.10) | 61 (9.19) | 1.76 (±0.12) (1.52-2.01) |
| Total | 1037 | 2.57 (±0.06) (2.45-2.70) | 664 | 1.65 (±0.04) (1.57-1.72) |
Note. HFSUH = Hiwot Fana Specialized University Hospital; SEM = standard error of the mean within individual age group; CI = confidence interval.
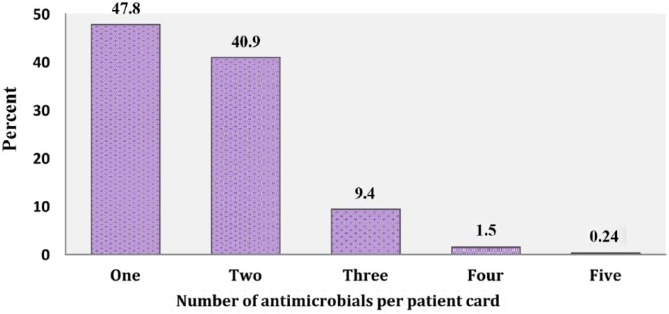
Considering the overall degree of polypharmacy, 1 antimicrobial agent was administered in 193 (47.8%) patients, 2 antimicrobials in 165 (40.9%), 3 antimicrobials in 38 (9.4%), 4 antimicrobials in 6 (1.5%), and 5 antimicrobials in only 1 patient, among all cases. The average number of antimicrobials per patient was found to be 1.65 (±0.73) (Figure 1). Besides, the total number of drugs prescribed (including other agents) was found to be 1037, and the average number of drugs per patient card was 2.57 (±1.29) (Figure 2).
Figure 1.
Percentage distribution of antimicrobials per patient card in the pediatric ward of HFSUH from January 1, 2016, to December 31, 2016.
Note. HFSUH = Hiwot Fana Specialized University Hospital.
Figure 2.
Percentage distribution of number of total drugs per patient card in the pediatric ward of HFSUH from January 1, 2016, to December 31, 2016.
Note. HFSUH = Hiwot Fana Specialized University Hospital.
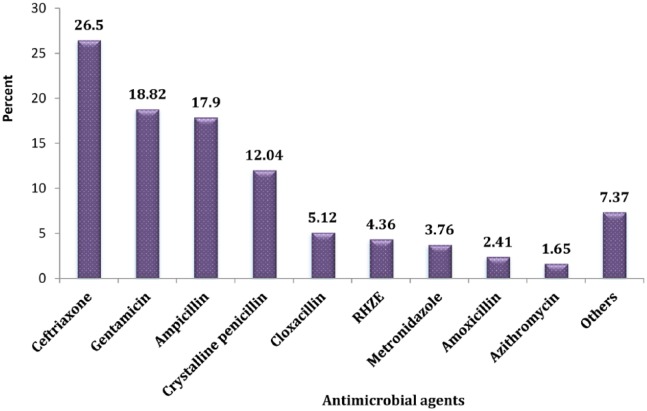
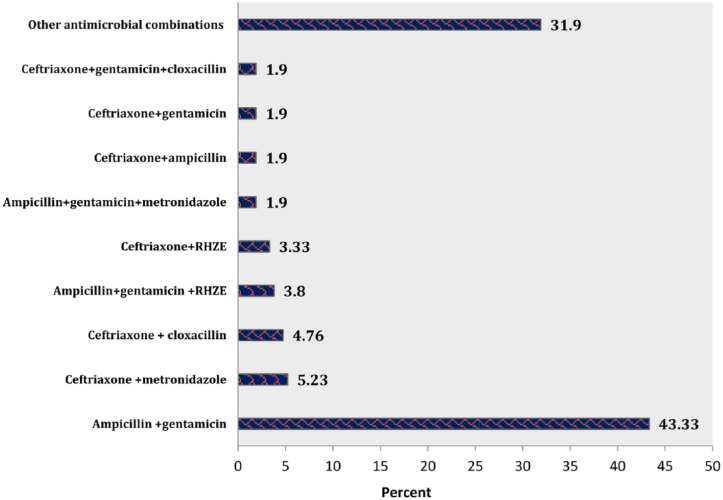
Among all pediatric medical records containing antimicrobials, ceftriaxone (n = 176, 26.5%) was the most commonly used antimicrobial agent, followed by gentamicin (n = 125, 18.82%), ampicillin (n = 119, 17.9%), and crystalline penicillin (n = 80, 12.04%) (Figure 3). Coming to the combination of antimicrobial agents, ampicillin and gentamicin combination took the largest percentage share (n = 91, 43.3%), followed by ceftriaxone + metronidazole (n = 11, 5.23%), ceftriaxone +cloxacillin (n = 10, 4.76%), and ampicillin + gentamicin + [rifampin + isoniazid + pyrazinamide + ethambutol (RHZE)] (n = 8, 3.8%) (Figure 4).
Figure 3.
Distribution of individual antimicrobial drugs at HFSUH pediatric ward from January 1, 2016, to December 31, 2016 (n = 664).
Note. Others: miconazole, tetracycline, quinine, cotrimoxazole, vancomycin, coartum, benzylpenicillin, ceftazidime; RHZE, rifampin, isoniazid, pyrazinamide, and ethambutol. HFSUH = Hiwot Fana Specialized University Hospital.
Figure 4.
Percentage distribution of antimicrobial combinations in pediatric patients at HFSUH pediatric ward from January 1, 2016, to December 31, 2016.
Note. HFSUH = Hiwot Fana Specialized University Hospital.
The most common reasons for which antimicrobials prescribed were severe pneumonia (n = 93, 18.82%), severe acute malnutrition (SAM) (n = 69, 13.97%), meningitis (n = 67, 13.56%), pneumonia (n = 57, 11.53%), and late-onset neonatal sepsis (LONS) (n = 38, 7.69%) (Table 3). Regarding specific indication, the most common reasons for which ceftriaxone was prescribed include meningitis (n = 40, 9.92%), pneumonia (n = 21, 5.21%), and severe pneumonia (n = 18, 4.46%). Besides, about 46 (11.41%) pediatric patients with severe pneumonia took crystalline penicillin, whereas patients with LONS (n = 33, 8.18%) and SAM (n = 32, 7.94%) were given ampicillin + gentamicin combination (Table 4).
Table 3.
Top 10 Diagnoses in Pediatric Patients in HFSUH Pediatric Ward from January 1, 2016, to December 31, 2016.
| Diagnosis | Frequency (%) |
|---|---|
| Severe pneumonia | 93 (18.82) |
| SAM | 69 (13.97) |
| Meningitis | 67 (13.56) |
| Pneumonia | 57 (11.53) |
| LONS | 38 (7.69) |
| GI onset sepsis | 24 (4.86) |
| SCAP | 18 (3.64) |
| PTB | 15 (3.03) |
| Malaria | 14 (2.83) |
| Amebiasis | 12 (2.43) |
| Othersa | 87 (17.61) |
| Total | 494 (100) |
Note. HFSUH = Hiwot Fana Specialized University Hospital; SAM = severe acute malnutrition; LONS = late-onset neonatal sepsis; SCAP = severe community-acquired pneumonia; PTB = pulmonary tuberculosis; GI = gastrointestinal.
Others: oral thrush, bacterial conjunctivitis, early-onset neonatal sepsis, and disseminated tuberculosis.
Table 4.
Frequency and Percentage Distribution of Prevailing Disease Conditions for Which Antimicrobial Agents are Prescribed in Pediatric Patients in HFSUH from January 1, 2016, to December 31, 2016, 2016.
| Drug | Indication |
||||
|---|---|---|---|---|---|
| Pneumonia | Severe pneumonia | Meningitis | LONS | SAM | |
| Ceftriaxone | 21 (5.21%) | 18 (4.46%) | 40 (9.92%) | — | — |
| Crystalline penicillin | — | 46 (11.41%) | — | — | — |
| Ampicillin + gentamicin | — | — | — | 33 (8.18%) | 32 (7.94%) |
Note. LONS = late-onset neonatal sepsis; SAM = severe acute malnutrition.
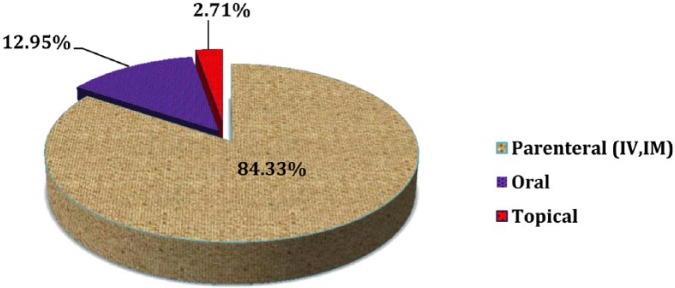
Considering the route of drug administration, out of the 664 antimicrobial drugs, parenteral route contributed the highest prevalence (n = 560, 84.33%), followed by oral route (n = 86, 12.95%) (Figure 5).
Figure 5.
Route of drug administration in pediatric patients at HFSUH pediatric ward from January 1, 2016, to December 31, 2016.
Note. HFSUH = Hiwot Fana Specialized University Hospital; IV = intravenous; IM = intramuscular.
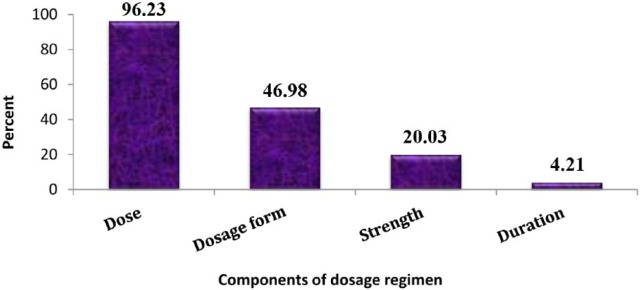
This study showed that 639 (96.23%) antimicrobial drugs were prescribed with dose. Less than half of antimicrobials (46.98%) were prescribed with dosage form. Besides, strength and duration were recorded in 20.03% and 4.21% of antimicrobial agents, respectively (Figure 6).
Figure 6.
Percentage distribution of antimicrobials prescribed with dose, dosage form, strength, and duration in pediatric patients at HFSUH pediatric ward from January 1, 2016, to December 31, 2016.
Note. HFSUH = Hiwot Fana Specialized University Hospital.
According to this study, ampicillin + gentamicin combination was prescribed to all age groups less than 5 years: 47 (11.66%), 28 (6.94%), and 12 (2.97%) for pediatrics with 1 month to 1 year, 1 to 5 years, and <1 month, respectively. Besides, ceftriaxone was equally prescribed for both infants and age group of 1 to 5 years (n = 34, 8.43%), and the same is true for crystalline penicillin (n = 29, 7.19%) (Table 5).
Table 5.
Distribution of Antimicrobial Agents by Age in Pediatric Patients at HFSUH Pediatric Ward from January 1, 2016, to December 31, 2016 (n = 403).
| Age | Commonly prescribed antimicrobials |
||
|---|---|---|---|
| Ampicillin + gentamicin | Ceftriaxone | Crystalline penicillin | |
| <1 mo | 12 (2.97%) | — | — |
| 1 mo-1 y | 47 (11.66%) | 34 (8.43%) | 29 (7.19%) |
| 1-5 y | 28 (6.94%) | 34 (8.43%) | 29 (7.19%) |
| 5-10 y | — | 20 (4.96%) | — |
| 10-14 y | — | 8 (1.98%) | — |
Note. HFSUH = Hiwot Fana Specialized University Hospital.
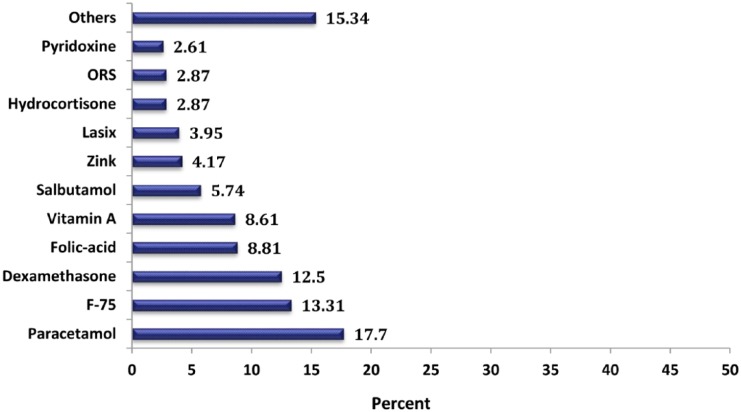
The most frequently coadministered drugs with antimicrobials were paracetamol (n = 68, 17.7%), F-75 (n = 51, 13.31%), dexamethasone (n = 48, 12.5%), folic acid (n = 34, 8.81%), and vitamin A (n = 33, 8.61%) (Figure 7).
Figure 7.
Frequently coadministered drugs with antimicrobial drugs in pediatrics ward of HFSUH from January 1, 2016, to December 31, 2016.
Note. Others: spironolactone, tramadol, diazepam, and Haem Up syrup. ORS, oral rehydration salt; lasix, furosemide; F-75, starter formula in infant milk.
This study showed that the most common laboratory tests performed for pediatric patients were white blood cell (WBC) count (n = 354, 92.4%), lymphocyte count (n = 323, 84.3%), neutrocyte count (n = 318, 83.0%), hemoglobin (n = 293, 76.5%), blood film (n = 152, 39.6%), and blood grouping (n = 108, 28.2%) (Table 6).
Table 6.
Frequency and Percentage Distribution of Laboratory Tests in Pediatric Patients in HFSUH Pediatric Ward from January 1, 2016, to December 31, 2016.
| Type of laboratory tests | Frequency (%) |
|---|---|
| White blood cell count | 354 (92.4) |
| Absolute lymphocyte count | 323 (84.3) |
| Absolute neutrocyte count | 318 (83.0) |
| Hemoglobin | 293 (76.5) |
| Peripheral blood film | 152 (39.6) |
| Blood grouping | 108 (28.2) |
| Hematocrit | 60 (15.6) |
| Monocyte count | 59 (15.4) |
| FBS/RBS | 57 (14.8) |
| MCV | 45 (11.7) |
| MCH | 45 (11.7) |
| Red blood cell count | 44 (11.5) |
| Platelet | 40 (10.4) |
| ESR | 39 (10.2) |
| Othersa | 199 (51.9) |
Note. FBS = fasting blood sugar; RBS = random blood sugar; MCV = mean corpuscular volume; MCH = mean corpuscular hemoglobin; ESR = erythrocyte sedimentation rate.
Others: mean corpuscular hemoglobin concentration (MCHC), basophil count, serum creatinine, red cell distribution width (RDW), blood urea nitrogen (BUN)/urea, ova/parasite.
Discussion
In this study, the majority of pediatric patients were male (n = 254, 63%), infants (n = 144, 35.7%), and 1 to 5 years old (n = 132, 32.8%); had a body weight of 5 to 9.9 kg (n = 177, 43.9%); and came from rural area of Harar town (n = 347, 86.1%). When compared with another study done in a tertiary care hospital in Erode, Tamil Nadu, India, more 147 (53.8%) male patients were admitted in the pediatric ward.11 The same is true for pediatric hospital of Kathmandu Valley,13 Hawassa University Teaching Referral Hospital (HUTRH),2 and Nekemte Referral Hospital.12 This result is also concordant with another study done in Jimma University Specialized Hospital (JUSH), Southwest Ethiopia, where male patients, rural residents, and infants and those with age between 1 and 5 years were the predominant hospital attendees in the pediatric ward.14 What is more, in Ajman, the United Arab Emirates, male to female ratio was found to be 1.24 and majority of children (n = 225, 46%) were also between 1 and 5 years of age in the pediatric ward.15
Coming to the degree of polypharmacy of prescribed drugs in general and antimicrobials in particular across all age groups of pediatrics, on average, the highest number of antimicrobials per patient card was observed in neonates, 1.87 (±0.09) (1.67-2.06), followed by those with an age range of 10 to 14 years, 1.76 (±0.12) (1.52-2.01), whereas from all drugs, the maximum average number of drugs per patient card was observed in children with an age range of 1 to 5 years, 2.79 (±0.12) (2.55-3.03), followed by those with an age range of 5 to 10 years, 2.73 (±0.16) (2.41-3.05). This study showed that majority (47.89%) of the patients took a single drug, whereas 40.9% of patients took combination of 2 drugs and the maximum number of antimicrobial drugs prescribed for these patients was found to be 5. The overall average number of drugs and specifically antimicrobials per patient card was 2.57 and 1.65, respectively. The same is true for the study conducted in JUSH where the maximum average number of antimicrobials was observed in neonates 2.3 (2.1 ± 2.5, SD = 0.72). Similarly, the maximum count of medicines per prescription was 8 for all types of drugs in general, and 5 for antimicrobials in particular.14 Bergicho et al reported that the average number of drugs per encounter was 1.22 which is lower than the present finding.16 Similarly, in the pediatric ward of a tertiary care facility in Southern Nigeria, the average number of antibiotics prescribed per patient was 1.38 ± 0.66.17 Higher than the present finding was also reported from in Ajman, the United Arab Emirates, where the degree of polypharmacy was found to be 3.86.15 Such findings can necessitate that there is a need to build capacity for health care provision and to develop strict antimicrobial policies and guidelines as well as stewardship programs.
According to this study, the most frequently prescribed individual antimicrobial agents were ceftriaxone, gentamicin, ampicillin, and crystalline penicillin. In concordant with the present finding, study done in the pediatric ward of Ayder Referral Hospital (ARH), Tigray Region, Northern Ethiopia, also explored that ceftriaxone, gentamicin, and ampicillin were the most commonly prescribed individual antimicrobial agents.6 The pattern of antimicrobial utilization in this setting is somewhat different from other studies done in Ethiopia and abroad. In Indonesia, records from pediatric wards indicated that cefotaxime, amoxicillin, and ampicillin were the most frequently prescribed antibiotics.18 Study done in a pediatric hospital of Erode, Tamil Nadu, India, indicated that the most frequently prescribed individual antimicrobial drugs were cephalosporins, namely, cefotaxime, ceftriaxone, cefpodoxime, and cefaclor.11 Coming to our country, Ethiopia, the most frequently prescribed individual antimicrobials were penicillin G crystalline (n = 146, 28.4%) in HUTRH,2 and cotrimoxazole and amoxicillin in Jimma and Addis Ababa tertiary care hospitals.16,19 A similar study done in Dessie Referral Hospital (DRH), Northeast Ethiopia, explored that the most commonly prescribed single antibiotic was crystalline penicillin, followed by ceftriaxone, amoxicillin, and cotrimoxazole.3 The plausible justification for these differences could be partly attributable to variations in the prevalence rate of infectious diseases, sociocultural factors, awareness of AMR among health care professionals, poor prescribing policy and guidelines, and physicians’ skill to diagnose common communicable diseases.14
Regarding the antimicrobial combinations, commonly prescribed antimicrobial combinations were ampicillin + gentamicin, followed by ceftriaxone + metronidazole, ceftriaxone + cloxacillin, and ampicillin + gentamicin + RHZE. Related to the present finding, the study conducted at HUTRH by Woldu et al showed that the most commonly prescribed multiple antibiotic prescription was ampicillin + gentamicin (n = 113, 27%), followed by chloramphenicol + cloxacillin (n = 60, 14.4%).2 In neonatal intensive care unit of Bishoftu General Hospital, the most frequently prescribed antibiotic was the combination of ampicillin and gentamicin (n = 67, 21.9%).20 Besides, supporting to the present finding, a study done in another part of Ethiopia, DRH, explored that the most commonly prescribed multiple drugs were ampicillin + gentamicin, followed by cloxacillin + chloramphenicol and ceftriaxone + gentamicin.3 Hospital-based data suggest alarming rates of resistance to ampicillin and gentamicin, the first-line antimicrobial agents recommended by WHO for the treatment of serious infections in young infants.21 One study showed that, among the 3 major pathogens studied (Escherichia coli, Staphylococcus aureus, and Klebsiella species), a high proportion of E coli were ampicillin (72%) and cotrimoxazole (78%) resistant; 19% were resistant to third-generation cephalosporins. Among Klebsiella species, almost all were resistant to ampicillin, 45% to cotrimoxazole, and 66% to third-generation cephalosporins. Resistance to gentamicin was low among E coli (13%) but much higher among Klebsiella species (60%).21
Besides, a study done in the pediatric ward of Cipto Mangunkusumo Hospital, Indonesia, explored that the most frequently prescribed multiple antimicrobials were ampicillin + chloramphenicol, cefotaxime + amikacin, cefotaxime + cotrimoxazole, and cefotaxime + RHZE.18 Regarding specific age groups, this study showed that ampicillin and gentamicin were commonly prescribed for children below 5 years and had been the predominant antimicrobial therapy in neonates, whereas ceftriaxone and crystalline penicillin were prescribed predominately to infants and children with the age group of 1 to 5 years. This result supports a study carried out in ARH where ampicillin and gentamicin were frequently prescribed to the age group of less than 1 year; however, ceftriaxone was primarily prescribed to children with the age group of greater than 5 years.6 This study was also comparable with the study done in Indonesian pediatric wards where the greatest user of antibiotics was the age group of 1 to 5 years old.18 Apart from the present finding, Warrier et al also reported that ampicillin and cefotaxime had the highest exposure rates in neonatal intensive care units.22 For patients receiving ampicillin, the concurrent use of cefotaxime during the first 3 days after birth either is a surrogate for an unrecognized factor or is itself associated with an increased risk of death, compared with the concurrent use of gentamicin.23
Concerning to the clinical indication, the study showed that the most common reason for which antimicrobials prescribed was severe pneumonia, followed by SAM, meningitis, pneumonia, LONS, and gastrointestinal onset sepsis. This study was almost similar to the study done in pediatric wards of ARH where the most common diagnosis was pneumonia, followed by SAM and acute gastroenteritis (AGE).6 What is more, in line with the present finding, a similar study done in the pediatric ward of JUSH indicated that the most frequent clinical indication for which antibiotics prescribed was severe pneumonia, followed by LONS with meningitis.9 Kebede et al also reported that pneumonia, sepsis, and meningitis were the main reasons for antimicrobial use in the pediatric ward of JUSH.14 This might be due to a similar prevalence of infectious diseases and empirical prescribing habits of health care professionals without any microbiological evidence. This is also further supported by a study from DRH where the most common diagnosis was severe pneumonia.3 In Bishoftu hospital pediatric wards, the major disorders for which antibiotics were prescribed include pneumonia (56.3%) and AGE (9.40%). The most commonly used antibiotics were ceftriaxone and gentamicin which accounted for 43.5% and 25.6%, respectively.24
Early diagnosis of sepsis is difficult because signs are subtle and nonspecific. Criteria for therapy vary in different settings, but most physicians usually administer antibiotics to newborns at the least suspicion of sepsis because of the difficulty of clinical diagnosis and the high mortality.25 Moreover, more than 99% of neonatal deaths occur in the developing countries, and a quarter of these deaths are attributed to neonatal sepsis. Insufficient knowledge about the appropriate antibiotic choice and the emerging resistance to commonly prescribed antimicrobials in neonatal sepsis will hamper the ability to successfully treat this condition in the developing countries. Based on current available evidence, the combination of ampicillin and gentamicin is an appropriate choice for empirical therapy of early-onset sepsis in neonates.26 Despite the variety of complications and nature of suspected infectious pathogens, the addition of antibiotics to therapeutic regimens for uncomplicated SAM was associated with a significant improvement in recovery and mortality rates.27
Regarding the route of administration, the majority (n = 560, 84.33%) of antimicrobials were prescribed by parenteral formulations, followed by 86 (12.95%) and 18 (2.71%) for oral and topical formulations, respectively. In line with this result, among antimicrobials, 85.9% were in the form of injectables in JUSH.14 This report was higher than a study conducted in ARH where 71.8% of the antimicrobials were administered through parenteral route.6 This finding is lower than the study conducted in JUSH which indicated the most common route of antibiotic administration was found to be parenteral that accounted for 90.17%, followed by oral (5.08%).9 WHO recommends only 13.4% to 24.1% of injectables from the total prescriptions for health care settings, and the result is by far higher than this recommendation. Several factors could explain this discrepancy, and some of these observations including differences in the availability of antimicrobials in other forms, the gap in skills among health care providers, and the availability of standard treatment guidelines can be responsible for this deviation.14,28
Conclusion
This study gives an overview of the pattern of antimicrobials use in the HFSUH. Ceftriaxone, gentamicin, ampicillin, and crystalline penicillin were the most frequently prescribed individual antimicrobials. Ampicillin + gentamicin and ceftriaxone + metronidazole were the most frequently prescribed antimicrobial combinations. Severe pneumonia was the most prevalent disease, followed by SAM, meningitis, and pneumonia were found to be the primary causes for hospitalization among pediatric patients. Infants and age with 1 to 5 years were age groups for which antimicrobials were commonly prescribed. The percentage of injectable drugs was very high. More than 95% of antimicrobials were prescribed with dose, and less than half of antimicrobials were prescribed with dosage form. Moreover, only 20.03% and 4.21% of antimicrobials were prescribed with strength and duration, respectively. This indicates that there is poor recording practice of drug-related information on patient card. Antibiotic selection should be based on culture and sensitivity test results. Cost of long-term use of parenteral medications should be taken into account. Changing to safer oral alternatives should be considered whenever possible. All in all, the study provides a drive toward the establishment of antimicrobial stewardship programs.
Acknowledgments
The authors provide their deepest gratitude for Hiwot Fana Specialized University Hospital (HFSUH) staffs for their kind support during data collection.
Footnotes
Author Contributions: SG and MS conceived the original idea and helped to draft the proposal. MS, GM, FA, and DE also supervised the overall study. All authors were involved in data acquisition, analysis, interpretation, and write-up. MS also drafted the manuscript and prepared it for publication. All authors read and approved the final version of the manuscript.
Ethical Approval and Consent to Participate: Approval and permission were sought from Institutional Health Research Ethics Review Committee (IHRERC), College of Health and Medical Sciences, Haramaya University, and the approval letter was then successfully received from the committee. A permission letter was also received from HFSUH administrator for the study. Informed consent was not sought because the study did not directly involve patients; however, the confidentiality of the patient information in patient medical records was maintained throughout the study period. To ensure confidentiality, the names of patients were not included in the checklist.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors thank Haramaya University for providing fund to this research work.
References
- 1. Alakhali KM, Shaik Mohammad A. Prescribing pattern of antibiotics in pediatric patients in the Jazan Region, Kingdom of Saudi Arabia. RGUHS J Pharm Sci. 2014;4:120-124. [Google Scholar]
- 2. Woldu MA, Suleman S, Workneh N, Berhane H. Retrospective study of the pattern of antibiotic use in Hawassa University Referral Hospital Pediatric Ward, Southern Ethiopia. J Appl Pharm Sci. 2013;3:93-98. [Google Scholar]
- 3. Alemnew G, Atnafie SA. Assessment of the pattern of antibiotics use in pediatrics ward of Dessie Referral Hospital, North East Ethiopia. Int J Med Med Sci. 2015;7:1-7. [Google Scholar]
- 4. Admassie E, Hailu W. The need for antimicrobial stewardship: prospective study on antimicrobial utilization pattern at Gondar Referral Hospital, North West Ethiopia. Brit J Pharm Res. 2015;5:82-89. [Google Scholar]
- 5. Haadi A, Abdullah S, Faqruddin M, Khan MN, Khan MA, Omer M. A systemic review on antibiotic use evaluation in paediatrics. World J Pharm Sci. 2014;2:243-246. [Google Scholar]
- 6. Mezgebe HB, Tadesse B, Legesse B. Antibiotics prescribing pattern in pediatric unit of Ayder Referral Hospital, Tigray region, Northern Ethiopia. J Sci Inn Res. 2015;4:57-60. [Google Scholar]
- 7. Choudhury D, Bezbaruah B. Antibiotic prescriptions pattern in Paediatric In-Patient Department Gauhati Medical College and Hospital, Guwahati. J Appl Pharm Sci. 2013;3:144-148. [Google Scholar]
- 8. Shivaleela, Jagadees K, Revankar S, et al. A study of prescription pattern of antibiotics in pediatric in-patients of Mc-Gann Teaching Hospital Shivamogga Institute of Medical Sciences (SIMS), Shivamogga, Karnataka. IOSR-JDMS. 2014;13:67-71. [Google Scholar]
- 9. Achalu TS, Yimam B, Kebede TM. Antibiotics utilization pattern in pediatric ward: the case from Tertiary Teaching Hospital, South West Ethiopia. Int J Adv Multidis Res. 2015;2:54-61. [Google Scholar]
- 10. Maheshwari P, Ravichandiran V, Hemanth Bhaskar KK, Vydehi SSK, Baig TS, Shahel SN. Prescribing patterns of antibiotics in paediatrics for respiratory tract infections/disorders in Tertiary Care Hospital. Asian J Pharm Clin Res. 2015;8:259-261. [Google Scholar]
- 11. Shamshy K, Begum IM, Perumal P. Drug utilization of antimicrobial drug in pediatrics population in a tertiary care hospital in Erode, Tamilnadu, India. Int J Pharmtech Res. 2011;3:1530-1536. [Google Scholar]
- 12. Asefa L, Bayissa G, Abera Z. Antibiotics use evaluation for pediatrics at Nekemte Referral Hospital, East Wollega Zone, Oromia region, West Ethiopia. World J Med Sci. 2016;13:17-26. [Google Scholar]
- 13. Palikhe N. Prescribing pattern of antibiotics in paediatric hospital of Kathmandu Valley. Kathmandu Univ Med J (KUMJ). 2004;2:6-12. [PubMed] [Google Scholar]
- 14. Kebede HK, Gesesew HA, Woldehaimanot TE, Goro KK. Antimicrobial use in paediatric patients in a teaching hospital in Ethiopia. PLoS One. 2017;12:e0173290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hassan M, John L. 902 antimicrobial utilization pattern in respiratory tract infections among pediatric population in Ajman, UAE. Arch Dis Child. 2012;97:A258-12A9. [Google Scholar]
- 16. Bergicho M, Mohammed MA, Wabe NT. Assessment of the pattern of drug prescribing in pediatrics ward in tertiary setting hospital in Addis Ababa, Ethiopia. Gaziantep Med J. 2012;18:61-65. [Google Scholar]
- 17. Arute J, Adigom D, Erah P, Eichie F, Eniojukan J. Antibiotic prescription pattern in the paediatric ward of a tertiary health care facility in southern Nigeria. J Pharmaceut Allied Sci. 2011;8:1411-1420. [Google Scholar]
- 18. Husni A, Abdoerrachman H, Akib A. Antibiotic profile in pediatric wards, Department of Child Health, Cipto Mangunkusumo Hospital. Paediatrica Indonesiana. 2016;44:46-50. [Google Scholar]
- 19. Agalu A, Mekonnen H. Drug prescribing practice in a pediatrics ward in Ethiopian. Int Res J Pharm Pharmacol. 2012;2:132-138. [Google Scholar]
- 20. Woldu MA, Guta MB, Lenjisa JL, Tegegne GT, Tesafye G, Dinsa H. Assessment of the incidence of neonatal sepsis, its risk factors, antimicrobials use and clinical outcomes in Bishoftu General Hospital, neonatal intensive care unit, Debrezeit-Ethiopia. Int J Contemp Pediatr. 2017;1:135-141. [Google Scholar]
- 21. Thaver D, Ali SA, Zaidi AK. Antimicrobial resistance among neonatal pathogens in developing countries. Pediatr Infect Dis J. 2009;28:S19-S21. [DOI] [PubMed] [Google Scholar]
- 22. Warrier I, Du W, Natarajan G, Salari V, Aranda J. Patterns of drug utilization in a neonatal intensive care unit. J Clin Pharmacol. 2006;46:449-455. [DOI] [PubMed] [Google Scholar]
- 23. Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117:67-74. [DOI] [PubMed] [Google Scholar]
- 24. Feleke M, Yenet W, Lenjisa JL. Prescribing pattern of antibiotics in pediatric wards of Bishoftu Hospital, East Ethiopia. Int J Basic Clin Pharmacol. 2013;2:718-722. [Google Scholar]
- 25. Hammerschlag MR, Klein JO, Herschel M, Chen FC, Fermin R. Patterns of use of antibiotics in two newborn nurseries. N Engl J Med. 1977;296:1268-1269. [DOI] [PubMed] [Google Scholar]
- 26. Sivanandan S, Soraisham AS, Swarnam K. Choice and duration of antimicrobial therapy for neonatal sepsis and meningitis. Int J Pediatr. 2011;2011:712150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trehan I, Goldbach HS, LaGrone LN, et al. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med. 2013;368:425-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. WHO. How to Investigate Drug Use in Health Facilities: Selected Drug Indicators, action program on essential drugs (DAP). Geneva, Switzerland; 1993. http://apps.who.int/medicinedocs/en/d/Js2289e/. [Google Scholar]