Abstract
Dofetilide’s hepatotoxicity is not well described. In this case report, we describe acute hepatocellular jaundice related to dofetilide use in a 33-year-old male being treated for atrial fibrillation. Both viral and ischemic causes of hepatocellular damage were ruled out as unlikely in this case. This case report outlines a rare yet probable report of idiosyncratic dofetilide-induced liver injury.
Keywords: adverse drug reactions, adverse drug reactions reporting/monitoring, cardiac agents, cardiovascular, gastrointestinal disorders
Introduction
Dofetilide is a class III antiarrhythmic medication that blocks the rapid delayed potassium rectifier current (IKr) resulting in prolongation of the repolarization phase of the cardiac myocyte.1 While adverse events of dofetilide such as Torsades de Pointes (TdP) have been well described as an intrinsic class effect of class III antiarrhythmics, drug-induced liver injury (DILI) has not. The purpose of this case report is to describe potential DILI with dofetilide as the causative agent.
A MEDLINE search using the terms (“Drug-Induced Liver Injury” AND “dofetilide”) as well as (“dofetilide” AND “Hepatitis”) did not yield results to describe dofetilide’s ability to cause DILI. Dofetilide has been described to cause liver damage in ≤2% of its overall use and numerically more frequent than placebo per manufacturer data.2
Patient Case Presentation
A 33-year-old, 60-kg Caucasian male coming from home presents with a chief complaint of worsening shortness of breath. For 4 months prior to admission (PTA), the patient experienced dyspnea walking upstairs and lying flat. These symptoms became exacerbated during the 2 weeks PTA. The patient was subsequently admitted with a diagnosis of acute hypoxic respiratory failure secondary to congestive heart failure.
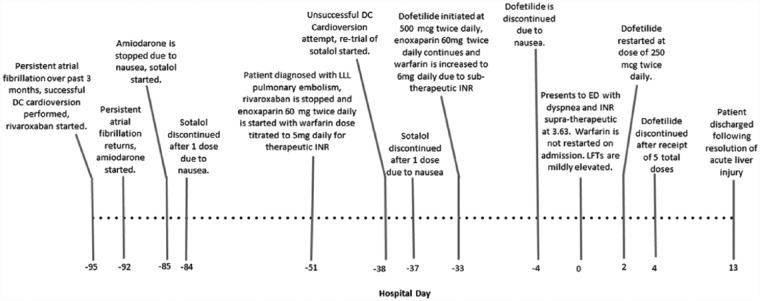
The patient’s medical history was significant for congenital transposition of the great arteries status post right and left aortopulmonary shunt placement in adolescence as well as persistent atrial fibrillation for 7 months PTA. Further details of this patient’s clinical course are outlined in Figure 2. Approximately 1 month PTA, the patient was successfully cardioverted and initiated on dofetilide 500 µg every 12 hours. Dofetilide use continued until 4 days PTA when the patient reported nausea and dofetilide was discontinued. At the time of admission, the patient who is an accurate historian self-assessed good medication adherence and stated he was taking furosemide 60 mg daily, levothyroxine 50 µg daily before breakfast, loratadine 10 mg daily, and warfarin 6 mg daily. The patient’s family and social history are unremarkable and negative for alcohol or illicit drug use.
Figure 2.
A timeline of patient events leading up to and throughout hospital admission.
DC = Direct Current; ED = Emergency Department; INR = International Normalized Ratio; LFTs = Liver Function Tests; LLL = Left Lower Lobe.
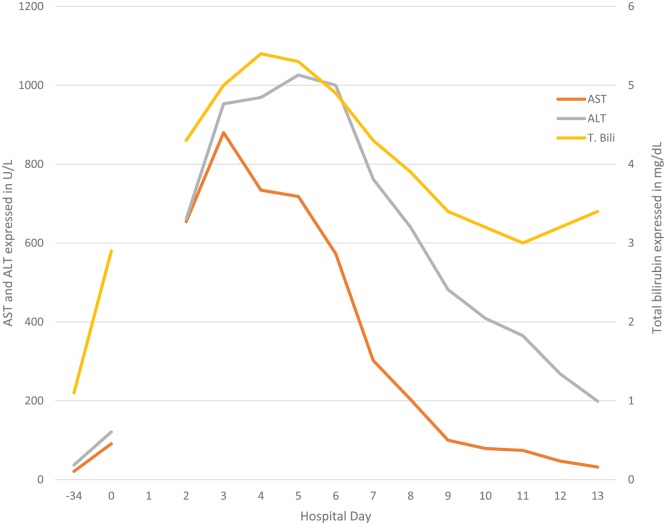
Upon admission, a supratherapeutic INR likely secondary to warfarin, mildly elevated hepatic transaminases, and elevated total bilirubin were noted, and these values continued to rise until hospital day 5 (Figure 1). Albumin and alkaline phosphatase values were within normal limits on and throughout admission. The patient was subsequently diagnosed with acute liver failure. Dofetilide was restarted at 250 µg every 12 hours on hospital day 2 after morning labs were taken and all anticoagulation was held throughout the hospital stay. Other medications the patient received during his hospital course were an oral acetylcysteine 72-hour protocol beginning on hospital day 4, albuterol, bumetanide, furosemide, ipratropium-albuterol, levothyroxine, lorazepam, methylprednisolone, oxazepam, phytonadione, spironolactone, and thiamine.
Figure 1.
Liver function tests and total bilirubin throughout hospital admission.
Note. Hospital day 0 = day of admission; AST = aspartate aminotransferase; ALT = alanine aminotransferase; T. Bili = total bilirubin.
The patient’s renal function was normal, and no existing hepatic dysfunction could be noted at baseline (Figure 1). No medication allergies were reported other than intolerances to amiodarone and sotalol as outlined in Figure 2. Physical findings on admission were presence of jugular venous distension and rales heard on pulmonary exam. The patient’s abdomen was soft, nondistended, and without tenderness.
Several diagnostic tests to evaluate hepatic failure etiology were performed during this admission. An abdominal ultrasound was performed on hospital day 3 which revealed all veins to be patent with a small amount of right upper quadrant ascites and borderline hepatomegaly. The gallbladder was found to be thickened but no presence of stones or sludge. Alpha-1 antitrypsin testing was negative. Normal viral hepatitis serology tests were performed and negative.
On hospital day 6, hepatic transaminases began to improve and total bilirubin continued to trend downward which continued until discharge. On hospital day 11, the patient noticed a diffuse maculopapular rash on his chest and back which may have been an immune-related reaction. On hospital day 13, the patient was discharged following resolution of his dyspnea and acute liver injury.
Discussion
This case report is unique in that DILI from dofetilide has been previously poorly characterized. While the potential for hepatotoxicity from dofetilide has been found in manufacturer studies, it is unclear as to the exact nature of how these reactions have occurred.2 Chemically similar sotalol has been described in 1 case as a potential causative agent for chronic hepatits.3 The Roussel Uclaf Causality Assessment Method (RUCAM) is one of the scoring tools used to assess the likelihood of DILI.4 An assessment of the RUCAM score specific to this case is described above (Table 1). All of these factors add up to a RUCAM score of 9 which may be interpreted as dofetilide being a “highly probable” cause of this patient’s hepatocellular injury.
Table 1.
RUCAM Causality Assessment Tool Assessment for Hepatocellular Injury (R Ratio = 33).
| Assessment | Descriptor | Score |
|---|---|---|
| Time of onset from cessation of the drug | ≤15 d | +1 |
| Course after stopping drug (decrease in ALT between peak value and ULN) | Decrease by ≥50% within 8 d | +3 |
| Risk factors (alcohol and age ≥55) | Patient denies alcohol use and is <55 | 0 |
| Concomitant drugs | Both amiodarone and sotalol stopped >15 d prior to event. Warfarin use has suggestive time of onset but is typically associated with cholestatic injury. | 0 |
| Exclusion of other causes of liver injury | Group I causes: Hepatitis A, B, and C; biliary obstruction, alcoholism, and recent history of hypotension/shock/ischemia ruled out. Group II causes: Complications of underlying autoimmune hepatitis, sepsis, chronic HBV or HCV, primary biliary cirrhosis, sclerosing cholangitis, CMV, EBV, and HSV ruled out. All causes in groups I and II ruled out |
+2 |
| Previous information on hepatotoxicity of the drug | Reaction labeled in product characteristics | +2 |
| Response to readministration | Compatible, dofetilide readministered on day 2 following labs being taken for that day. ALT proceeds to increase and peak on day 5. | +1 |
| Total score: 9 | Interpretation: Scores of 9 or greater imply dofetilide is a “highly probable” cause of liver injury. | |
Note. The Roussel Uclaf Causality Assessment Method (RUCAM) for drug-induced liver injury (DILI). R ratio calculated from alanine aminotransferase (ALT) and alkaline phosphatase (ALK P) values on hospital day 5. Calculation: R ratio = (ALT / ALT ULN) ÷ (ALK P / ALK P ULN), where ALT ULN = 40 and ALK P ULN = 130. CMV = cytomegalovirus; EBV = Epstein-Barr virus; HBV = Hepatitis B Virus; HCV = Hepatitis C Virus; HSV = herpes simplex virus; ULN = Upper Limit of Normal.
DILI has been described to occur by a variety of mechanisms. The most apparent mechanism of hepatotoxicity is the production of reactive metabolites typically through phase I metabolism via the cytochrome P450 (CYP450) pathway.5 Another mechanism of hepatotoxicity is parent drug or metabolite interaction with the bile salt export pump (BSEP) leading to cholelithiasis and hepatocellular damage. Finally, idiosyncratic mechanisms have been proposed for immune-mediated reactions with parent drug or metabolite acting as a hapten and binding to a hepatic protein such as a CYP450 isoenzyme eliciting an adaptive immune response leading to cytotoxic T-cell activation and hepatocellular death. This idiosyncratic mechanism allows for the explanation of hepatotoxicity that occurs without other apparent cause such as production of reactive oxygen species.
Dofetilide is chemically classified as a methanesulfonanilide that is primarily excreted unchanged by renal elimination with hepatic metabolism accounting for 20% to 30% of total drug disposition.1,6 Of the hepatic metabolites, dofetilide is primarily metabolized by CYP3A4 via N-demethylation to N-desethyl dofetilide. Hepatic metabolites of dofetilide have shown 20-fold decreased potency to the parent compound and are not relevant in the pharmacologic action of dofetilide given at therapeutic doses.6 It is not known whether these metabolites are reactive oxygen species; however, this may be a theoretical cause of DILI from dofetilide.
Other common causes of hepatocellular liver injury were ruled out in this case. Viral hepatitis was extensively ruled out by negative viral serology.4 Ischemic hepatitis would be of concern with this patient as he did present with an exacerbation of heart failure on admission; however, abdominal ultrasound showed patent blood flow and the patient was not noted to be hemodynamically unstable throughout admission making ischemic hepatitis unlikely. Alpha-1 antitrypsin deficiency was ruled out as well by negative lab test. This patient had no reported history of alcohol use or cirrhosis. Other less common differential diagnoses including autoimmune hepatitis, Wilson disease, and Budd-Chiari syndrome were not evaluated to be ruled out in this case as there was low clinical suspicion that this patient’s liver injury would be related to these causes.4 However, it is important to be aware of these possible causes as the RUCAM assessment for dofetilide causality was “highly probable” and not definitive.
There were several limitations to this case report. On the day of hospital admission (day 0), transaminases were only mildly elevated and proceeded to increase prior to the reinitiation of dofetilide on hospital day 2, making an assessment of hepatic injury recurrence on rechallenge difficult. It is also unclear the degree of baseline hepatic dysfunction as the supratherapeutic INR upon admission was at least in part contributed by the patient being anticoagulated on warfarin. Warfarin use was an additional confounder in this case report as it has been associated with DILI as well although cholestatic liver injury has been typically described with its use.7
Conclusion
This case report correlates the use of dofetilide as a highly probable causative agent of DILI in this patient. Given that other possible causes of acute hepatocellular injury were ruled out in this case and both a temporal and causal relationship of DILI related to dofetilide use were established, dofetilide was the likely factor causing liver injury. As idiosyncratic DILI is an immune-mediated reaction, it is difficult to predict which patients may be affected by this reaction. This case helps to affirm the importance in evaluating medication use as a potential cause of liver injury.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Mounsey JP, DiMarco JP. Dofetilide. Circulation. 2000;102(21):2665-2670. [DOI] [PubMed] [Google Scholar]
- 2. Pfizer Inc. Tikosyn (dofetilide capsule). In: DailyMed. New York, NY: US National Library of Medicine; https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=02438044-d6a3-49e9-a1ac-3aad21ef2c8c. Accessed October 10, 2017. [Google Scholar]
- 3. Kootte AM, Janssens AR, Ouwehand DK, van Leeuwen AM. [Chronic hepatitis ascribed to the use of sotalol]. Ned Tijdschr Geneeskd. 2001;145(48):2340-2343. [PubMed] [Google Scholar]
- 4. Chalasani NP, Hayashi PH, Bonkovsky HL, et al. ACG clinical guideline: the diagnosis and management of idiosyncratic drug-induced liver injury. Am J Gastroenterol. 2014;109(7):950-966; quiz 967. [DOI] [PubMed] [Google Scholar]
- 5. Yuan L, Kaplowitz N. Mechanisms of drug induced liver injury. Clin Liver Dis. 2013;17(4):507-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walker DK, Alabaster CT, Congrave GS, et al. Significance of metabolism in the disposition and action of the antidysrhythmic drug, dofetilide. In vitro studies and correlation with in vivo data. Drug Metab Dispos. 1996;24(4):447-455. [PubMed] [Google Scholar]
- 7. Zimmerman HJ. Heparin. Drugs used in cardiovascular disease. In: Zimmerman HJ, ed. Hepatotoxicity: the Adverse Effects of Drugs and Other Chemicals on the Liver. 2nd ed. Philadelphia, PA: Lippincott; 1999:639-412. [Google Scholar]