Abstract.
Antiretroviral treatment (ART) interruptions increase the risk of severe morbidity/mortality in human immunodeficiency virus (HIV)-infected individuals from subSaharan Africa. We aimed to determine whether the risk is further increased among HIV–hepatitis B virus (HBV) co-infected patients in this setting. In this sub-analysis of a randomized-control trial, 632 participants from Côte d’Ivoire randomized to receive continuous-ART (C-ART), structured ART interruptions of 2-months off, 4-months on (2/4-ART), and CD4-guided ART interruptions (CD4GT, interruption at 350/mm3 and reintroduction at 250/mm3) were analyzed. Incidence rates (IR) of serious HIV- and non-HIV-related morbidity were compared between patients stratified on hepatitis B surface antigen (HBsAg) status. Overall, 65 (10.3%) were HBsAg-positive, 29 (44.6%) of whom had HBV-DNA levels > 10,000 copies/mL. After a median 2.0 year (range = 0.2–3.1) follow-up, ≥ 1 serious HIV-related events occurred in 101 HIV mono-infected and 15 HIV–HBV co-infected patients (IR = 10.0 versus 13.2/100 person/years, respectively, P = 0.3), whereas the highest incidence was observed in co-infected patients with baseline HBV-replication > 10,000 copies/mL (IR = 24.0/100 person/years, P versus HIV mono-infected = 0.002). Incidence of bacterial infections was also highest in the co-infected group with HBV-replication > 10,000 copies/mL (IR = 12.9 versus 3.3/100 person/years in HIV mono-infected patients, P = 0.001). The relative effect of CD4GT or 2/4-ART versus C-ART was not different between infection groups (P for interaction = 0.4). No increase in the incidence of non-HIV-related morbidity was observed for co-infected patients (P = 0.5), even at HBV-replication levels > 10,000 copies/mL (P = 0.7). In conclusion, co-infected patients with elevated HBV-replication at ART-initiation are more susceptible to HIV-related morbidity, especially invasive bacterial diseases, during treatment interruption.
INTRODUCTION
Interrupting antiretroviral therapy (ART) is widely known to have detrimental consequences to individuals infected with the human immunodeficiency virus (HIV). In several large randomized-control trials, interruptions based on structured timeperiods or specific CD4 cell counts are associated with higher risk of morbidity and mortality due to opportunistic infections and non-HIV-related diseases.1–4
While untreated, patients with HIV and hepatitis B virus (HBV) co-infection are known to have a higher risk of cirrhosis and liver-related mortality compared with those with HIV mono-infection.5,6 Nevertheless, this excess risk subsides with the use of potent anti-HBV agents in combination with ART.7,8 Treatment interruptions could then be considered particularly harmful for co-infected individuals. Data from the Strategic Management of Antiretroviral Therapy (SMART) study have indeed demonstrated heightened risk of mortality unrelated to opportunistic infections in HIV–HBV, including HIV–hepatitis C virus (HCV), co-infected patients who underwent treatment interruptions.9 Furthermore, interrupting ART in these patients often evoked rebounds in HBV DNA replication and flares in transaminase levels, suggesting higher risk of severe liver-related consequences.10
It is currently unknown whether the rates of serious morbidity and mortality are increased with treatment interruptions in HIV–HBV co-infected patients from sub-Saharan Africa (SSA). Because the epidemiology of HBV infection is vastly different between SSA and Europe or North America, with the former having more individuals horizontally infected during youth and controlling HBV infection well before HIV acquisition,11 there could be inherent differences in how HBV manifests during treatment interruptions. For instance, most co-infected patients in SSA are hepatitis B “e” antigen (HBeAg) negative, who generally harbor low levels of HBV DNA replication and transaminase levels.12 Treatment interruptions could have similar effects between HIV mono-infected and HIV–HBV co-infected patients exhibiting more inactive phases of infection.
We used unique data from a large randomized-control trial in Côte d’Ivoire, in which several immunological and clinical endpoints were evaluated during treatment interruptions, to determine whether rates of these events differed between HIV–HBV co-infected and HIV mono-infected participants. We intended to further explore the role of HBV DNA replication in their occurrence.
MATERIALS AND METHODS
Study population.
Patients participated in a prospective, randomized, open-label, multicenter trial in Abidjan, Côte d’Ivoire (NCT00158405).1,2 Briefly, the aim of this trial was to evaluate the benefits and risks of treatment interruptions. Study inclusion criteria were as follows: age ≥ 18 years, HIV-1 or mixed HIV-1/2 infection, ART-naive, and CD4+ cell count between 150 and 350/mm3 or CD4% between 12.5% and 20.0%. Patients with any one of the following were not included in the trial: residence outside of Abidjan, unwillingness to participate, pregnancy, severe renal or hepatic disease, severe psychiatric disorder, any ongoing severe clinical features of undiagnosed origin, severe hematological disorder (hemoglobin < 7.5 g/dL, platelet count < 30,000/mm3, or neutrophil count < 750/mm3), or Karnofsky score < 50.
All participants gave written informed consent, and the study protocol was approved by the Ministry of Health of Côte d’Ivoire and the French National Agency for Research on AIDS and Viral Hepatitis (ANRS, Paris, France).
Treatment interruption arms.
A total of 840 patients started ART at inclusion, receiving zidovudine and lamivudine with either efavirenz or lopinavir/ritonavir. After a 6- to 18-month phase of continuous-ART(C-ART), those who fulfilled randomization criteria (CD4 > 350/mm3, plasma HIV-1 RNA < 300 copies/mL) were randomized, respectively, 1:3:2 to one of three arms: C-ART, CD4-guided ART interruptions (CD4GT) (reintroduction when CD4 < 250/mm3, interruption when CD4 > 350/mm3), or fixed-schedule ART interruptions (2-months-off and 4-months-on or “2/4-ART”). Those who did not reach randomization criteria underwent C-ART. Study randomization and follow-up procedures have been detailed elsewhere.1,2
Assessing HBV parameters and liver enzymes.
All patients were tested for hepatitis B surface antigen (HBsAg) at study inclusion (Mini Vidas® assay; Biomerieux, Marcy l’Etoile, France), which was then confirmed using the Architect i2000 assay (Abbott Laboratories, Rungis, France).13
From samples stored at −80°C, HBeAg and anti-HBe antibodies were detected at study inclusion using the Elecsys assay (Roche Diagnostics, Meylan, France). HBV DNA viral loads (VL) were quantified at study inclusion and every 12 months during follow-up using an in-house polymerase chain reaction (PCR)–based assay (detection threshold: 12 copies/mL).14
Plasma alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were collected at study inclusion, although only AST levels were available every 6 months during follow-up. Elevated transaminase levels were defined as an AST or ALT > 40 IU/L.
Assessing HIV parameters.
CD4+ cell counts (True Count technique on FACScan, Becton Dickinson, Aalst-Enembobegem, Belgium) were obtained at study inclusion, every 3 months before randomization, and every 2 months after randomization. Plasma HIV-1 RNA was quantified using real-time PCR15 and was measured at study inclusion and every 6 months thereafter.
Statistical analysis.
Follow-up began at randomization and continued until study termination (October 31, 2005 for those enrolled in the CD4-guided arm, which was found to have higher severe morbidity during an interim analysis and stopped prematurely by the Data and Safety Monitoring Board, and March 24, 2007 for all other arms), permanent treatment discontinuation, treatment switch, or death; whichever occurred first. All analyses were conducted using STATA statistical package (v13.1, College Station, TX), and significance was determined using a P value < 0.05.
Patients were grouped as HIV mono-infected or HIV–HBV co-infected, the latter of which was further stratified on HBV-VL > 104 copies/mL (“high baseline HBV DNA replication”) and HBV-VL ≤ 104 copies/mL (“low baseline HBV DNA replication”) at ART-initiation. The choice of threshold was determined from a previous study in SSA, demonstrating higher mortality rates at these levels.16 Characteristics between groups were compared at randomization using Kruskal–Wallis test for continuous variables and Pearson χ2 or Fisher’s exact test for categorical variables.
We considered endpoints from a previous intent-to-treat analysis in the Trivacan study1,2: 1) CD4 cell count < 350/mm3 at month 24, 2) incidence of overall mortality, 3) incidence of serious HIV-related morbidity, combining any event leading to death or classified as a World Health Organization (WHO) clinical stage 3 or 4 event; and 4) incidence of serious non-HIV-related morbidity, defined as any morbidity event that led to death and/or hospital admission that were not documented as WHO stage 2–4 events. Proportions of patients reaching the CD4 endpoint were compared between infection groups using Pearson’s χ2 test. A logistic regression model was used to calculate odds ratios (OR) and their 95% confidence intervals (CI) comparing interruption arms to C-ART within infection groups. Incidence rates (IR) of serious morbidity and mortality were expressed in events per 100 person/years. Cox proportional hazards models were used to calculate hazards ratios (HR) and their 95% CI comparing interruption arms to C-ART within infection groups. To identify differences in measures of effect across HBV infection strata, an interaction term combining infection group and randomization arm was included for all models.
In the post hoc analysis, we used time until CD4+ cell count < 200/mm3 as an end-point. HR and 95% CI were estimated for several determinants using Cox proportional hazards model, while accounting for tied failures using the Efron method. A multivariable model was constructed by including all variables with a P value < 0.1 from a univariable analysis and selecting covariates in a backwards-stepwise fashion. Parameters affecting only the co-infected study population (i.e., HBeAg-status, HBV DNA levels at inclusion) were assessed separately in the multivariable model.
RESULTS
Description of the study population.
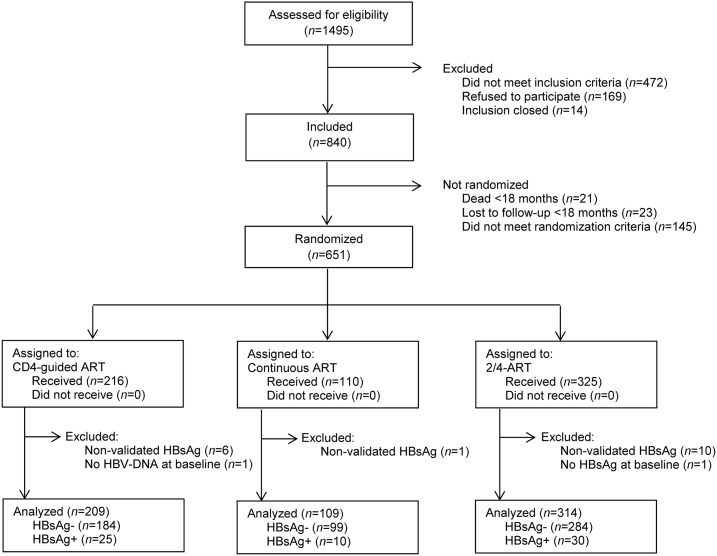
Figure 1 depicts participant flow at each stage of the study. Of the 651 individuals randomized between August 2003 and March 2005, 19 (2.9%) were not included in analysis because of the following reasons: did not have an available HBsAg-status at inclusion (N = 1), did not have confirmed a HBsAg-status (N = 17), or did not have available HBV DNA at inclusion (N = 1). In total, 632 patients were included who had been randomized to receive 2/4-ART (N = 314), CD4-guided ART (N = 209), or C-ART (N = 109). No significant differences were observed in the HIV–HBV co-infection status across study arms (overall HBsAg-positive prevalence = 10.3%, P for overall comparison = 0.6).
Figure 1.
Participant flow of the study. Description of participant flow from enrollment to inclusion and analysis.
Table 1 provides a description of the study population at randomization, while comparing infection groups. HIV–HBV co-infected patients were significantly more likely to be male, smoke, and have elevated transaminase levels versus HIV mono-infected patients. Co-infected patients with high baseline HBV DNA VL, compared with all others, had significantly lower nadir CD4+ cell count (median = 220 versus 262/mm3, respectively, P = 0.004), HIV RNA VL at ART-initiation (median = 5.44 versus 4.94 log10 copies/mL, respectively, P < 0.001), higher ALT (median = 29 versus 20 IU/L, respectively, P = 0.007), and higher AST levels (median = 27 versus 24 IU/L, respectively, P = 0.02).
Table 1.
Description of the study population at randomization per infection group
| HIV–HBV co-infected | HIV mono-infected | P* | |||
|---|---|---|---|---|---|
| HBV DNA ≤ 104 copies/mL | HBV DNA > 104 copies/mL | Total | |||
| (N = 32) | (N = 33) | (N = 65) | (N = 567) | ||
| Gender, M/F (% males) | 14/18 (43.8) | 10/23 (30.3) | 24/41 (36.9) | 122/445 (21.5) | 0.005 |
| Age, years† | 34 (30–37) | 36 (33–39) | 35 (32–38) | 34 (29–40) | 0.2 |
| Current smoker‡ | 3 (9.4) | 3 (9.4) | 6 (9.4) | 21 (3.7) | 0.04 |
| BMI, kg/m2† | 21 (20–24) | 21 (19–22) | 21 (19–23) | 22 (20–24) | 0.4 |
| WHO clinical stage‡ | 0.3 | ||||
| Stage I | 7 (21.9) | 5 (15.1) | 12 (18.5) | 137 (24.2) | |
| Stage II | 17 (53.1) | 11 (33.3) | 28 (43.1) | 227 (40.0) | |
| Stage III | 8 (25.0) | 14 (42.4) | 22 (33.9) | 160 (28.2) | |
| Stage IV | 0 (0) | 3 (9.1) | 3 (4.6) | 43 (7.6) | |
| HIV RNA copies/mL at ART-initiation† | 4.94 (4.22–5.28) | 5.44 (4.98–5.78) | 5.15 (4.64–5.57) | 4.95 (4.34–5.45) | 0.06 |
| HIV RNA > 300 copies/mL‡ | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ntp |
| Current CD4+ count, mm−3† | 481 (412–560) | 424 (396–551) | 457 (405–555) | 456 (375–561) | 0.6 |
| Nadir CD4+ count, mm−3† | 268 (211–335) | 220 (171–288) | 235 (185–299) | 262 (202–334) | 0.10 |
| Time on ART, months† | 7.2 (7.1–10.1) | 7.2 (7.1–10.1) | 7.2 (7.1–10.1) | 7.2 (7.1–10.2) | 0.5 |
| ART protocol arm‡ | 0.6 | ||||
| Continuous | 6 (18.8) | 4 (12.1) | 10 (15.4) | 99 (17.5) | |
| CD4-guided | 11 (34.4) | 14 (42.4) | 25 (38.5) | 184 (32.5) | |
| 2/4-ART | 15 (46.9) | 15 (45.5) | 30 (46.2) | 284 (50.1) | |
| HBeAg‡ | 1 (3.1) | 19 (57.6) | 20 (30.8) | – | ntp |
| ALT, IU/L† [N = 480] | 21 (16–27) | 29 (20–51) | 25 (17–38) | 20 (15–29) | 0.04 |
| AST, IU/L† | 26 (22–31) | 27 (21–38) | 26 (21–32) | 23 (20–30) | 0.02 |
| Elevated transaminasesठ| 5 (15.6) | 7 (21.2) | 12 (18.5) | 57 (10.1) | 0.04 |
ALT = alanine aminotransferase; ART = antiretroviral therapy; AST = aspartate aminotransferase; BMI = body mass index; HBeAg = hepatitis B “e” antigen; HBV = hepatitis B virus; HIV = human immunodeficiency virus; WHO = World Health Organization.
Significance between HIV–HBV co-infected and HIV mono-infected groups determined using Kruskal–Wallis test for continuous variables and Pearson χ2 test or Fisher’s exact test for categorical variables. ntp, no test performed.
Median (interquartile range).
Number (%).
Defined as ALT or AST > 40 IU/L.
Of the 65 co-infected patients, 20 (30.8%) had HBeAg-positive serology, 31 (47.7%) detectable HBV DNA (median VL = 7.30 log10 copies/mL, interquartile range = 5.57–8.01), and 12 (18.5%) elevated transaminase levels. Importantly, no significant differences in any of these parameters were observed between study arms (Supplemental Table 1).
Description of patient follow-up.
Patients were followed for a median 2.0 (range = 0.2–3.1) years (2.0 years for HIV-HBV co-infected and 2.0 years for HIV mono-infected patients), totaling 1,261 person/years (126 person/years in co-infected and 1,135 person/years in mono-infected patients). Rates of loss-to-follow-up were slightly higher among co-infected versus mono-infected patients (1.6/100 person/years versus 1.0/100 person/years, respectively), yet there was no significant difference (P = 0.4).
In co-infected patients, 20 patients (30.8%) had rebounds > 1.0 log10 copies/mL of HBV DNA during follow-up (IR = 9.9/100 person/years), with no difference between interruption arms (log-rank test: P = 0.9). Overall, six patients (1.0%) had ALT/AST levels > 120 IU/L at baseline. Among the 626 remaining patients, 46 had a flare in ALT/AST levels > 120 IU/L during follow-up (IR = 2.2/100 person/years), which occurred predominately in co-infected patients (low baseline HBV DNA replication IR = 3.8/100 person/years, high baseline HBV DNA replication IR = 11.0/100 person/years) (log-rank test: P < 0.001). Most of these events were observed in patients undergoing CD4GT (HR = 2.95, 95% CI = 1.01–8.61) and 2/4-ART (HR = 1.74, 95% CI = 0.59–5.13) compared with C-ART.
CD4 cell count outcome and co-infection status.
In intent-to-treat analysis of the primary outcome (Table 2), 14 (23.7%) HIV–HBV co-infected and 98 (18.0%) HIV mono-infected patients had CD4 cell counts < 350/mm3 at 24 months from randomization (P = 0.3). This endpoint was more likely observed in co-infected patients with high baseline HBV DNA replication (N = 8, 27.6%), yet was not significantly different when compared with HIV mono-infected patients (P = 0.2, Table 2).
Table 2.
Rates of endpoints stratified by co-infection status
| Outcome | Infection group | P* | Infection group (considering viral replication) | P† | ||
|---|---|---|---|---|---|---|
| HIV–HBV | HIV | HIV–HBV, VL > 104 copies/mL | HIV | |||
| CD4+ cell count < 350/mm3 at 24 months‡, n (%) | (N = 59) | (N = 544) | (N = 29) | (N = 544) | ||
| Total | 14 (23.7) | 98 (18.0) | 0.3 | 8 (27.6) | 98 (18.0) | 0.2 |
| Continuous | 1 (11.1) | 5 (5.2) | 0.4 | 0 (0) | 5 (5.2) | 0.9 |
| 2/4-ART | 3 (10.7) | 40 (14.5) | 0.8 | 2 (14.3) | 40 (14.5) | 0.9 |
| CD4-guided | 10 (45.5) | 53 (31.0) | 0.17 | 6 (50.0) | 53 (31.0) | 0.17 |
| Death, n events (IR/100 person/years) | (N = 65) | (N = 567) | (N = 33) | (N = 567) | ||
| Total | 3 (1.6) | 5 (0.3) | 0.02 | 3 (4.8) | 5 (0.3) | 0.001 |
| Continuous | 1 (5.1) | 0 (0) | ntp | 1 (14.4) | 0 (0) | ntp |
| 2/4-ART | 1 (1.7) | 2 (0.4) | 0.19 | 1 (3.4) | 2 (0.4) | 0.06 |
| CD4-guided | 1 (2.0) | 3 (0.8) | 0.4 | 1 (3.8) | 3 (0.8) | 0.19 |
| Serious HIV-related morbidity§, n events (IR/100 person/years) | (N = 65) | (N = 567) | (N = 33) | (N = 567) | ||
| Total | 15 (13.2) | 101 (10.0) | 0.3 | 13 (24.0) | 101 (10.0) | 0.002 |
| Continuous | 2 (11.0) | 12 (6.5) | 0.7 | 2 (35.5) | 12 (6.5) | 0.02 |
| 2/4-ART | 5 (9.3) | 48 (9.4) | 0.9 | 4 (14.7) | 48 (9.4) | 0.4 |
| CD4-guided | 8 (19.1) | 41 (13.0) | 0.3 | 7 (33.1) | 41 (13.0) | 0.02 |
| Serious non-HIV-related morbidity‖, n events (IR/100 person/years) | (N = 65) | (N = 567) | (N = 33) | (N = 567) | ||
| Total | 13 (11.5) | 137 (13.9) | 0.5 | 7 (10.7) | 137 (13.9) | 0.7 |
| Continuous | 2 (12.4) | 24 (13.7) | 0.9 | 1 (17.8) | 24 (13.7) | 0.8 |
| 2/4-ART | 4 (9.0) | 40 (12.5) | 0.5 | 3 (12.4) | 40 (12.5) | 0.6 |
| CD4-guided | 7 (13.4) | 73 (14.9) | 0.8 | 3 (11.1) | 73 (14.9) | 0.9 |
ART = antiretroviral therapy; HBV = hepatitis B virus; HIV = human immunodeficiency virus; IR = incidence rates; VL = viral loads.
Comparisons were made between HIV–HBV vs. HIV mono-infected patients.
Comparisons were made between HIV–HBV co-infected patients with high HBV DNA VL vs. HIV mono-infected patients. P values obtained from Pearson’s χ2 test or Fisher’s exact test for categorical variables and from Wald χ2 test after fitting a Cox proportional hazards model. ntp, no test performed.
Of the 632 patients randomized, 29 patients (continuous, N = 3; 2/4-ART, N = 10; CD4-guided, N = 16) were excluded from the CD4 cell count < 350 cells/mm3 analysis because they died (continuous, N = 1; 2/4-ART, N = 3; CD4-guided, N = 4) or were lost to follow-up (continuous, N = 1; 2/4-ART, N = 5; CD4-guided, N = 8) before month 24 or because their CD4 cell count data were missing at month 24 (continuous, N = 1; 2/4-ART, N = 2; CD4-guided, N = 4). The remaining 603 patients (continuous, N = 106; 2/4-ART, N = 304; CD4-guided, N = 193) were included in the intent-to-treat analysis.
Any event leading to death or any morbidity event classified as a World Health Organization (WHO) stage 3 or 4 event.
Any morbidity event that led to death and/or hospital admission and that were not documented as WHO stage 2–4 events.
CD4 cell count < 350/mm3 at 24-months occurred more frequently in patients randomized to either treatment interruption arm (2/4-ART and CD4-guided) compared with those on C-ART for both HIV mono-infected (OR = 4.83, 95% CI = 1.91–12.23, P = 0.001) and co-infected individuals (OR = 2.81, 95% CI = 0.32–24.69, P = 0.4). No interaction was observed between treatment arms and co-infection groups (P = 0.3), even when stratifying on levels of baseline HBV DNA replication (P = 0.3).
In post hoc multivariable analysis (Supplemental Table 2), rates of progressing toward a CD4 cell count < 200/mm3 were significantly associated with being randomized to an interruption arm (P < 0.001), having a lower body mass index (P = 0.04), lower CD4 cell count (P < 0.001), and higher AST levels (P < 0.001) at study inclusion. Among only HIV–HBV co-infected patients, there were no significant differences in achieving this endpoint based on HBeAg-positive serology (adjusted-HR = 2.39, 95% CI = 0.85–6.74, P = 0.10) or higher HBV DNA levels (adjusted-HR = 1.17 per log10 copies/mL, 95% CI = 0.91–1.50, P = 0.2) when included in the multivariable model.
Mortality and co-infection status.
As shown in Table 2, three and five deaths were observed among HIV–HBV co-infected and HIV mono-infected patients, respectively, resulting in a significantly higher IR in those with co-infection (P = 0.02). All deaths among co-infected patients occurred in those with high baseline HBV DNA replication (IR = 4.8 versus 0.3/100 person/years for HIV mono-infected patients, P = 0.001, Table 2). The causes of death during follow-up were tuberculosis (N = 3), advanced liver cirrhosis (N = 1), pneumococcocal meningitis (N = 1), fever with meningeal syndrome (N = 1), and unspecified causes (N = 2) (Table 3). Any formal statistical analysis on causes of death was precluded by the small numbers of observations.
Table 3.
Causes of mortality and serious morbidity between infection groups
| Infection group | P* | Infection group (considering viral replication) | P† | |||
|---|---|---|---|---|---|---|
| HIV–HBV | HIV | HIV–HBV, VL > 104 copies/mL | HIV | |||
| n (IR/100 person/years) | (N = 65) | (N = 567) | (N = 33) | (N = 567) | ||
| Overall mortality | ||||||
| Tuberculosis | 2 (1.6) | 1 (0.1) | 0.02 | 2 (3.2) | 1 (0.1) | 0.004 |
| Liver related | 1 (0.8) | 0 (0) | – | 1 (1.6) | 0 (0) | – |
| Bacterial diseases | 0 (0) | 2 (0.2) | – | 0 (0) | 2 (0.2) | – |
| Unknown | 0 (0) | 2 (0.2) | – | 0 (0) | 2 (0.2) | – |
| Serious HIV-related morbidity | ||||||
| Death | 1 (0.9) | 3 (0.3) | 0.3 | 1 (1.8) | 3 (0.3) | 0.12 |
| Tuberculosis | 3 (2.6) | 17 (1.7) | 0.5 | 2 (3.7) | 17 (1.7) | 0.3 |
| Bacterial diseases | 8 (7.0) | 33 (3.3) | 0.05 | 7 (12.9) | 33 (3.3) | 0.001 |
| Oropharyngeal candidiasis | 3 (2.6) | 46 (4.5) | 0.3 | 3 (5.5) | 46 (4.5) | 0.8 |
| Other‡ | 0 (0) | 2 (0.2) | – | 0 (0) | 2 (0.2) | – |
| Serious non-HIV-related morbidity | ||||||
| Malarial infection | 2 (1.8) | 12 (1.2) | 0.6 | 2 (3.5) | 12 (1.2) | 0.17 |
| Bacterial diseases | 1 (0.9) | 7 (0.7) | 0.9 | 0 (0) | 7 (0.7) | – |
| Non-specific | 6 (5.3) | 90 (9.2) | 0.19 | 2 (3.5) | 90 (9.2) | 0.17 |
| Other | 4 (3.5) | 28 (2.8) | 0.7 | 3 (5.3) | 28 (2.8) | 0.3 |
HBV = hepatitis B virus; HIV = human immunodeficiency virus; IR = incidence rates; VL = viral loads.
Comparisons were made between HIV–HBV vs. HIV mono-infected patients.
Comparisons were made between HIV–HBV co-infected patients with high HBV DNA VL vs. HIV mono-infected patients. P values obtained from Wald χ2 test after fitting a Cox proportional hazards model.
Prolonged vaginal candidiasis (N = 1), isosporiasis (N = 1).
Serious HIV-related morbidity outcomes and co-infection status.
In total (Table 2), at least one serious HIV-related event occurred in 15 HIV–HBV co-infected (three of whom died) and 101 HIV mono-infected patients (five of whom died), resulting in a slightly higher IR during co-infection versus mono-infection (P = 0.3). This endpoint was significantly more frequent in co-infected patients with high baseline HBV DNA replication compared with those with HIV mono-infection (P = 0.002, Table 2). This increased risk was largely due to bacterial infections (Table 3), the most common of which were invasive bacterial diseases (N = 19) including pyelonephritis (N = 7), enteritis (N = 5), pneumonia (N = 4), and other invasive infections (N = 3).
Serious HIV-related morbidity was more frequently observed in HIV mono-infected patients randomized to the CD4-guided (HR = 1.97, 95% CI = 1.03–3.76, P = 0.04) but not 2/4-ART arm (HR = 1.43, 95% CI = 0.76–2.68, P = 0.3). Similar magnitudes of effect were observed in HIV–HBV co-infected patients (CD4-guided HR = 1.67, 95% CI = 0.34–8.13, P = 0.5; 2/4-ART HR = 0.81, 95% CI = 0.15–4.28, P = 0.8). Accordingly, no interaction was observed between treatment arms and co-infection groups (P = 0.4), even when stratifying on levels of baseline HBV DNA replication (P = 0.2).
Serious non-HIV-related morbidity outcomes and co-infection status.
Overall, at least one serious non-HIV-related event occurred in 13 HIV–HBV co-infected and 137 HIV mono-infected patients (none of whom died for both groups), with no significant difference in IR between co-infection versus mono-infection (P = 0.3, Table 2). There was also no significant difference in the incidence of non-HIV morbidity between patients with high baseline HBV DNA replication compared with those with HIV mono-infection (P = 0.7, Table 2). The major cause of non-HIV-related morbidity were nonspecific (Table 3), with the most frequent being fever of unknown origin (N = 61), gastroenteritis (N = 14), and gynecological (N = 11).
There was no significant increase in serious non-HIV-related morbidity when randomized to either treatment interruption arm (2/4-ART and CD4-guided) compared with C-ART for both HIV mono-infected (HR = 1.02, 95% CI = 0.66–1.58, P = 0.9) and HIV–HBV co-infected patients (HR = 0.90, 95% CI = 0.19–4.28, P = 0.9), resulting in a nonsignificant interaction between treatment arms and co-infection groups with or without consideration of baseline HBV DNA replication (P > 0.9).
DISCUSSION
The Trivacan study was the first large, prospective study from SSA to clearly demonstrate the detrimental effects of treatment interruptions on HIV-related morbidity, promptly leading to strong recommendations for C-ART in international guidelines.17 In this substudy, we extend these findings by showing increased rates of HIV-related morbidity in co-infected patients specifically when their HBV VL is high, although this was not the case for non-HIV-related morbidity. The relative associations for both endpoints were consistent in CD4+ cell guided and structured treatment interruption arms. These results bolster our understanding of the potential consequences of treatment interruptions in co-infected patients and to some degree, of HBV treatment outcomes in SSA.
When examining the causes more closely, bacterial diseases unrelated to tuberculosis were clearly responsible for the increased risk of HIV-related morbidity. Bacterial infections represent a considerable healthcare burden to HIV-infected individuals from SSA.18 Nevertheless, the reasons for accelerated risk toward these infections among co-infected patients with high HBV VL are unclear. During viral hepatitis, bacterial infections linked to cirrhosis generally involve gram-negative microorganisms in the urinary or digestive tracts.19 As these infections were not common in our study, any role of advanced liver disease would be unlikely. Most infections were, however, invasive and pulmonary- or renal-related, which would be more in line with acquired immunodeficiency syndrome (AIDS)-associated illnesses. Indeed, this concurs with previous research underpinning the increased progression to AIDS-defining events in untreated co-infected patients with particularly more active forms of HBV infection.20
With only one liver-related death observed during follow-up, the causes of severe morbidity and mortality in those with high HBV VL would again appear more related to HIV than HBV. A large body of epidemiological evidence from SSA has supported the higher risk of all-cause mortality in HIV–HBV co-infected versus HIV mono-infected patients after initiating ART,6,21,22 especially, at higher levels of HBV DNA16 or when undergoing non-tenofovir containing ART.23 Unfortunately, the causes of mortality are lacking in many of these studies, making it difficult to infer further on the pathological components leading to death. Control of HBV replication is evidently a key component in chronic HBV infection because elevated HBV DNA levels have been consistently associated with poorer liver-related outcomes.24 HBV DNA replication was for the most part controlled during study follow-up, and hepatic flares were not particularly common, adding evidence that low rates of liver-related morbidity and mortality would be expected.
These results are in contrast to the SMART study,9 in which non-HIV-related mortality, and not mortality due to opportunistic infections, was higher in patients with HIV and viral hepatitis co-infection. It should be noted, nonetheless, that the SMART study only included WHO grade 4 opportunistic diseases in their definition, whereas the Trivacan study identified those belonging to either grades 3 or 4. Recognizing opportunistic infections at both grades are important in the setting of SSA because together they pose heightened risk of overall mortality after ART-initiation.25 Furthermore, the analysis from the SMART study combined those with HBV- and/or HCV-infection9 and the most common causes of death in co-infected patients (i.e., unknown, substance abuse, and non-AIDS-related cancer) are more representative of HIV–HCV co-infected individuals.26 Our analysis only focused on differences between HIV–HBV and HIV-infected patients in a study population with a negligible prevalence of HCV-infection.27
Interestingly, co-infection did not appear to influence the association between treatment interruptions and CD4+ cell count < 350/mm3 at 24-months, nor did it increase the risk of obtaining a CD4+ count < 200/mm3 during follow-up. HIV–HBV co-infection has been shown to decelerate immunorestoration after ART-initiation.28 Lower CD4+ cell counts are also strongly linked with active HBV DNA replication29 and lower nadir CD4+ counts with persistent HBV DNA viremia during tenofovir-containing ART.30 Taken together, this evidence would normally point toward greater difficulty among HIV–HBV co-infected patients in maintaining higher CD4+ cell counts. Nevertheless, the effects listed previously were observed at CD4+ thresholds < 200/mm3, whereas most patients included in our study had nadir CD4+ cell counts higher than this level. Any effect of HIV–HBV co-infection on immunological endpoints would be then assumed minimal.
Although treatment interruptions are strongly discouraged, they do have particular relevance to contemporary patients in SSA. More specifically, variable adherence and short-term interruptions are prevalent31 and 6–21% of HIV-infected adults attending ART-facilities are lost to follow-up.32 In addition, when compared with HIV mono-infected patients, those with HIV–HBV co-infection have higher rates of discontinuing regular attendance at the clinic,22 which could make them more vulnerable to temporary treatment discontinuation. Coupled with the heightened risk of HIV-related morbidity associated with co-infection at high baseline HBV DNA levels, these factors need strong consideration during patient management.
Certain limitations of our study need to be addressed. First, patients with severe liver diseases, or rather, those presenting with clear signs of hepatic insufficiency, were excluded. Our study population was then more likely to represent co-infected patients asymptomatic for HBV infection. Having data on liver fibrosis would have helped provide further understanding of HBV-disease severity in this study population, thereby allowing exploration of any link between fibrosis/cirrhosis and bacterial infections; but was not possible. Second, we used an in-house PCR technique to quantify HBV DNA, and thus, VL from other commercial PCR-assays might not be fully comparable. Third, HBV DNA was not quantified immediately after treatment interruption, only at yearly visits, and thus the incidence of > 1.0 log10 copies/mL rebounds in HBV viral replication could have been underestimated. Fourth, we did not have serological or virological data on HCV infection. Although HCV prevalence would be expected to be low in this setting,27 we cannot say for certain whether it had any effect on our results. Fifth, follow-up lasted only for a median 2 years, which might fail to represent the effects of co-infection on long-term survival. Studies with longer follow-up are needed to clarify this issue. Finally, despite being one of the larger studies with comprehensive data on HBV infection, the small number of co-infected patients could have reduced statistical power for certain analyses, especially those involving infection group × randomization arm interactions.
In conclusion, HIV–HBV co-infected patients with elevated HBV replication at ART-initiation have a higher rate of serious HIV-related morbidity during treatment. This heightened risk is generally similar across interruption arms. Notwithstanding the importance of liver-related disease attributed to HBV infection, our results underscore a particular danger of bacterial infections facing treated individuals with active HBV co-infection from SSA, stressing the need for detecting HBsAg and initiating ART early-on in this group of patients.
Supplementary Material
Acknowledgments:
We thank all patients who participated in the Trivacan trial. We also gratefully acknowledge the valuable contributions of the SMIT, CeDReS, CEPREF, USAC, CIRBA, CNTS, the Programme PACCI team, as well as INSERM exU593 and U897 teams (Abanou Matthieu, Adou Isabelle, Aman Adou, Bakayoko Ibouraîma, Bombo Léontine, Cissé Edwidge, Coulibaly Ali, Djédjé Lucien, Djobi-Djo Edouard, Goly Jocelyn, Kassi Marie-Cécile, Koffi Justine, Koffi-N’Dri Aholi, Konan Sylvie, Konaté Mamadou, Kouadio Bertin, Kouamé Martin, Kouadio Martin, Kouadio Victoire, Kouakou Adrienne, Kouakou Yao, Kouamé Antoine, Kouamé Ferdinand, Kouamé Gérald, Kouamé Justine, Labibi Georgette, Lehou Jean, Moh Jules, Moussa-Doumbia Mariam, Martin Marie-Pierre, N’Dri Marie Julie, Nalourgou Tuo, N’Chot Célestin, N’Goran Brou, Nogbout Marie-Pascale, Orne-Gliemann Joanna, Ouattara Bakary, Ouattara Minata, Oupoh Joséphine, Sidibé Abdelh, Siloué Bertine, Soro Adidiata, Tchehy Amah-Cécile, Yao Juliette, Yoro Guei, and Zaho Marcel); and Bristol-Myers Squibb for providing Zerit and Videx during the study and Merck Sharp & Dohme for the donation of Stocrin. We also gratefully acknowledge Bristol-Myers Squibb for providing Zerit and Videx and Merck Sharp & Dohme for the donation of Stocrin.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1.Danel C, et al. ; Trivacan ANRS 1269 Trial Group , 2006. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet 367: 1981–1989. [DOI] [PubMed] [Google Scholar]
- 2.Danel C, et al. ; Trivacan ANRS 1269 Trial Group , 2009. Two-months-off, four-months-on antiretroviral regimen increases the risk of resistance, compared with continuous therapy: a randomized trial involving West African adults. J Infect Dis 199: 66–76. [DOI] [PubMed] [Google Scholar]
- 3.Ananworanich J, et al. ; Staccato Study Group ; Swiss HIV Cohort Study , 2006. CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: results of the Staccato randomised trial. Lancet 368: 459–465. [DOI] [PubMed] [Google Scholar]
- 4.El-Sadr WM, et al. ; Strategies for Management of Antiretroviral Therapy (SMART) Study Group , 2006. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med 355: 2283–2296. [DOI] [PubMed] [Google Scholar]
- 5.Falade-Nwulia O, Seaberg EC, Rinaldo CR, Badri S, Witt M, Thio CL, 2012. Comparative risk of liver-related mortality from chronic hepatitis B versus chronic hepatitis C virus infection. Clin Infect Dis 55: 507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kouamé GM, et al. 2017. Higher mortality despite early ART in HIV and hepatitis B virus coinfected patients with high HBV replication. Clin Infect Dis, 10.1093/cid/cix747. [DOI] [PubMed] [Google Scholar]
- 7.Piroth L, et al. ; GERMIVIC Study Group , 2010. Management and treatment of chronic hepatitis B virus infection in HIV positive and negative patients: the EPIB 2008 study. J Hepatol 53: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 8.Piroth L, et al. ; GERMIVIC Study Group , 2015. Therapeutic management and evolution of chronic hepatitis B: does HIV still have an impact? The EPIB 2012 study. Liver Int 35: 1950–1958. [DOI] [PubMed] [Google Scholar]
- 9.Tedaldi E, et al. ; SMART Study Group and International Network for Strategic Initiatives in Global HIV Trials (INSIGHT) , 2008. Opportunistic disease and mortality in patients coinfected with hepatitis B or C virus in the strategic management of antiretroviral therapy (SMART) study. Clin Infect Dis 47: 1468–1475. [DOI] [PubMed] [Google Scholar]
- 10.Dore GJ, et al. ; SMART INSIGHT Study Group , 2010. Frequent hepatitis B virus rebound among HIV-hepatitis B virus-coinfected patients following antiretroviral therapy interruption. AIDS 24: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthews PC, Geretti AM, Goulder PJR, Klenerman P, 2014. Epidemiology and impact of HIV coinfection with hepatitis B and hepatitis C viruses in Sub-Saharan Africa. J Clin Virol 61: 20–33. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann CJ, Thio CL, 2007. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis 7: 402–409. [DOI] [PubMed] [Google Scholar]
- 13.Boyd A, et al. ; ANRS 12240 VarBVA Study , 2015. Identifying patients infected with hepatitis B virus in Sub-Saharan Africa: potential for misclassification. Diagn Microbiol Infect Dis 83: 248–251. [DOI] [PubMed] [Google Scholar]
- 14.Boyd A, et al. ; ANRS 12240 VarBVA Study 2015. Low risk of lamivudine-resistant HBV and hepatic flares in treated HIV-HBV-coinfected patients from Côte d’Ivoire. Antivir Ther 20: 643–654. [DOI] [PubMed] [Google Scholar]
- 15.Rouet F, et al. 2005. Transfer and evaluation of an automated, low-cost real-time reverse transcription-PCR test for diagnosis and monitoring of human immunodeficiency virus type 1 infection in a West African resource-limited setting. J Clin Microbiol 43: 2709–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velen K, Charalambous S, Innes C, Churchyard GJ, Hoffmann CJ, 2016. Chronic hepatitis B increases mortality and complexity among HIV-coinfected patients in South Africa: a cohort study. HIV Med 17: 702–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammer SM, et al. ; International AIDS Society-USA 2008. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA 300: 555–570. [DOI] [PubMed] [Google Scholar]
- 18.Anglaret X, et al. ; ANRS 12222 Morbidity/Mortality Study Group , 2012. AIDS and non-AIDS morbidity and mortality across the spectrum of CD4 cell counts in HIV-infected adults before starting antiretroviral therapy in Cote d’Ivoire. Clin Infect Dis 54: 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nahon P, et al. ; ANRS CO12 CirVir and Microcir Groups 2017. Bacterial infection in compensated viral cirrhosis impairs 5-year survival (ANRS CO12 CirVir prospective cohort). Gut 66: 330–341. [DOI] [PubMed] [Google Scholar]
- 20.Chun HM, et al. Infectious Disease Clinical Research Program HIV Working Group , 2012. Hepatitis B virus coinfection negatively impacts HIV outcomes in HIV seroconverters. J Infect Dis 205: 185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthews GV, et al. ; PHIDISA II Study Team , 2011. Impact of lamivudine on HIV and hepatitis B virus-related outcomes in HIV/hepatitis B virus individuals in a randomized clinical trial of antiretroviral therapy in southern Africa. AIDS 25: 1727–1735. [DOI] [PubMed] [Google Scholar]
- 22.Sarfo FS, Kasim A, Phillips R, Geretti AM, Chadwick DR, 2014. Long-term responses to first-line antiretroviral therapy in HIV and hepatitis B co-infection in Ghana. J Infect 69: 481–489. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins C, Christian B, Ye J, Nagu T, Aris E, Chalamilla G, Spiegelman D, Mugusi F, Mehta S, Fawzi W, 2013. Prevalence of hepatitis B co-infection and response to antiretroviral therapy among HIV-infected patients in Tanzania. AIDS 27: 919–927. [DOI] [PubMed] [Google Scholar]
- 24.Chen C-J, Yang H-I, Iloeje UH; REVEAL-HBV Study Group , 2009. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology 49 (Suppl 5): S72–S84. [DOI] [PubMed] [Google Scholar]
- 25.Moh R, et al. 2007. Incidence and determinants of mortality and morbidity following early antiretroviral therapy initiation in HIV-infected adults in West Africa. AIDS 21: 2483–2491. [DOI] [PubMed] [Google Scholar]
- 26.Smith C, et al. ; Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study Group , 2010. Factors associated with specific causes of death amongst HIV-positive individuals in the D:A:D Study. AIDS 24: 1537–1548. [DOI] [PubMed] [Google Scholar]
- 27.Riou J, Aït Ahmed M, Blake A, Vozlinsky S, Brichler S, Eholié S, Boëlle P-Y, Fontanet A; HCV Epidemiology in Africa Group , 2016. Hepatitis C virus seroprevalence in adults in Africa: a systematic review and meta-analysis. J Viral Hepat 23: 244–255. [DOI] [PubMed] [Google Scholar]
- 28.Wandeler G, et al. ; Swiss HIV Cohort Study , 2013. Hepatitis B virus infection is associated with impaired immunological recovery during antiretroviral therapy in the Swiss HIV cohort study. J Infect Dis 208: 1454–1458. [DOI] [PubMed] [Google Scholar]
- 29.Matthews GV, et al. 2013. Patterns and causes of suboptimal response to tenofovir-based therapy in individuals coinfected with HIV and hepatitis B virus. Clin Infect Dis 56: e87–e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyd A, Gozlan J, Maylin S, Delaugerre C, Peytavin G, Girard P-M, Zoulim F, Lacombe K, 2014. Persistent viremia in human immunodeficiency virus/hepatitis B coinfected patients undergoing long-term tenofovir: virological and clinical implications. Hepatology 60: 497–507. [DOI] [PubMed] [Google Scholar]
- 31.Boyer S, Clerc I, Bonono C-R, Marcellin F, Bilé P-C, Ventelou B, 2011. Non-adherence to antiretroviral treatment and unplanned treatment interruption among people living with HIV/AIDS in Cameroon: individual and healthcare supply-related factors. Soc Sci Med 72: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 32.Auld AF, et al. 2014. Antiretroviral therapy enrollment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults--seven African countries, 2004–2013. MMWR Morb Mortal Wkly Rep 63: 1097–1103. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.