Abstract.
Recent World Health Organization (WHO) guidelines recommend antiretroviral therapy (ART) for all HIV-infected people; previously CD4+ T lymphocyte quantification (CD4 count) or clinical staging determined eligibility for children ≥ 5 years old in low- and middle-income countries. We examined positive predictive value (PPV) of a rapid diagnostic test (RDT) algorithm and ART eligibility for hospitalized children with newly diagnosed HIV infection. We enrolled 363 hospitalized Malawian children age 2 months to 16 years with two serial positive HIV RDT from 2013 to 2015. Children aged ≤ 18 months whose nucleic acid testing was negative or unavailable were later excluded from the analysis (N = 16). If RNA PCR was undetectable, human immunodeficiency virus (HIV) enzyme immunoassay (EIA) and western blot (WB) were performed. Those with negative or discordant EIA and WB were considered HIV negative and excluded from further analysis (N = 6). ART eligibility was assessed using age, CD4 count, and clinical HIV stage. Among 150 patients with HIV RNA PCR results, 15 had undetectable HIV RNA. Of those, EIA and WB were positive in nine patients and negative or discordant in six patients. PPV of serial RDT was 90% versus RNA PCR alone and 96% versus combined RNA PCR, EIA, and WB. Of all patients aged ≥ 5 years, 8.9% were ineligible for ART under previous WHO guidelines. Improved HIV testing algorithms are needed for accurate diagnosis of HIV infection in children as prevalence of pediatric HIV declines. Universal treatment will significantly increase the numbers of older children who qualify for ART.
INTRODUCTION
Of the more than 36 million people living with human immunodeficiency virus (HIV) worldwide, an estimated 1.8 million are children under age 15 years.1,2 Scale-up of prevention of mother-to-child transmission (PMTCT) efforts have prevented an estimated 1.3 million new HIV infections among children between 2010 and 2015.3 This was accomplished, in part, through expanded HIV rapid diagnostic testing (RDT), and global adoption of Malawi’s Option B+ strategy (universal lifelong antiretroviral therapy [ART] eligibility for all HIV-infected pregnant and breastfeeding women) by the World Health Organization (WHO).3–5 However, inconsistent delivery of HIV testing and lack of resources to perform confirmatory testing using HIV enzyme immunoassay (EIA), western blot (WB), or nucleic acid testing pose a continued challenge for the accurate identification and linkage to care for mothers and children living with HIV.4,6,7 As a result of these challenges, many children remain undiagnosed or improperly diagnosed and less than one half of children living with HIV are receiving ART.1,4
HIV diagnostic testing algorithms are based on recommendations of national experts and international agencies such as the WHO, guided by cost effectiveness, feasibility, and resources in low- and middle-income countries. In Malawi, national guidelines recommend HIV DNA PCR for definitive diagnosis of HIV infection in treatment-naïve children < 12 months.8 For treatment-naïve children ≥ 12 months old, a serial RDT algorithm similar to the algorithm recommended by the WHO for high prevalence areas is recommended: if the first HIV RDT is positive, a second RDT from a different manufacturer is used to confirm results. If the results are discordant, RDTs from the two different manufacturers are then run in parallel. If results are discordant again, testing is repeated at the next healthcare visit or the patient is directed to a referral laboratory, if available, for nucleic acid testing.8,9 Retesting before ART initiation, and at 24 months of age for children taking ART in the first 2 years of life, is recommended, and is performed using the same RDT algorithm.8,9 WHO recommendations for diagnosis of HIV in children are similar, but recommend confirmation of positive serial RDT (or other serologic test) with nucleic acid testing in children ≤ 18 months.9 The WHO recommends RDT platforms with ≥ 99% sensitivity, ≥ 98% specificity, and aims for a positive predictive value (PPV) of ≥ 99% for RDT testing algorithms.9
Guidelines for treatment of children with HIV in low- and middle-income countries have been revised over time in response to greater availability of RDTs and ART, resulting in expanded eligibility for older children. In 2010, the WHO recommended universal ART only for children 2 years old and younger.10 In 2013, the WHO expanded eligibility to recommend universal ART for children under age 5 years, but children older than 5 years qualified for treatment only if they had advanced clinical HIV disease (stage 3 or 4) or absolute CD4+ T lymphocyte count (CD4 count) ≤ 500 cells/mm3.11 Although studies suggest that CD4 count is preferred over clinical staging for determination of immunosuppression, CD4 testing availability is limited in some low-income countries, and for certain clinical conditions (such as tuberculosis) prompt initiation of ART is recommended regardless of CD4 count, so clinical staging remained part of WHO recommendations.11–13 In 2015 and 2016, the WHO released updated guidelines recommending universal ART for all HIV-infected persons regardless of age, clinical disease, or CD4 count, eliminating the need for immunologic or clinical staging before ART initiation.14,15
The hospital setting represents a valuable opportunity for HIV testing and prompt initiation of ART in children.16–19 Prevalence of HIV in sub-Saharan Africa is higher in hospitalized children than the general pediatric population, and HIV-infected hospitalized children have higher mortality than HIV-uninfected hospitalized children.17–19 High rates of pneumonia, sepsis, malnutrition, gastrointestinal illness, meningitis, and skin infections have been described in untreated HIV-infected hospitalized children.17–19 Many of these studies were done before the implementation of PMTCT and pediatric ART guidelines, so it is not known if the clinical presentation of untreated HIV-infected children has changed and how many children would qualify for treatment using 2013 WHO guidelines versus universal ART.18,19 The aim of this study was to use serial HIV RDT to diagnose HIV infection in hospitalized Malawian children and using immunologic and clinical staging, determine how many additional children will qualify for treatment as universal ART guidelines are adopted. A post hoc secondary aim was to analyze the PPV of the 2-RDT algorithm for HIV diagnosis as compared with nucleic acid testing.
MATERIALS AND METHODS
Children, 2 months to 16 years old with positive serial RDT admitted to the pediatric wards at Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi, from February 2013 through June 2015 were enrolled. Patients were excluded from the analysis if they were ≤ 18 months and confirmatory nucleic acid testing (HIV DNA or RNA PCR) was negative or unavailable, if they died or were discharged before testing, or if they had a prior diagnosis of HIV (Figure 1). For children with two positive HIV RDTs but undetectable HIV RNA PCR, confirmatory EIA (Bio-Rad GS HIV-1/2 Plus O) and WB (Bio-Rad GS HIV-1) testing was performed at the University of North Carolina Project Laboratory, Lilongwe, Malawi; those children with negative or discordant EIA and WB testing and undetectable HIV RNA PCR were considered HIV uninfected and excluded from further analysis (Figure 1). This study was approved by the Institutional Review Boards of the University of Malawi College of Medicine, Partners Healthcare and the Albert Einstein College of Medicine. Consent and assent (for those children aged ≥ 7 years) was obtained from patients and guardians.
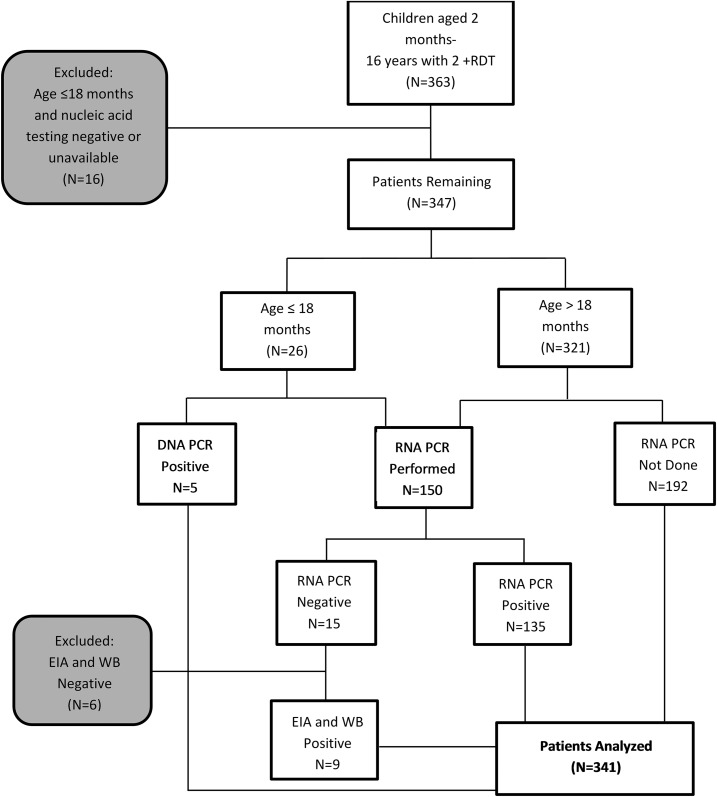
Figure 1.
Inclusion of study subjects. Three hundred and sixty three children who tested positive for HIV by rapid diagnostic testing (RDT) were enrolled. Sixteen were excluded because they were 18 months of age or younger with negative or unavailable confirmatory DNA or RNA PCR test. Of the remaining 347 patients, 150 had sufficient plasma for HIV viral load (HIV VL). Of these 150 children, 15 had undetectable HIV RNA PCR. Confirmatory HIV testing by enzyme immunoassay (EIA) and western blot (WB) was performed on archived plasma from these patients. Of the 15, nine had positive EIA and WB results. Of the remaining six samples, three had negative EIA and WB, two had positive EIA and negative WB and one had negative EIA and indeterminate WB with one band positive.
HIV counselors approached all admitted patients with unknown HIV status (no documented HIV testing results performed after age 24 months) for HIV testing by RDT (Determine HIV-1/2 [Abbott], followed by Uni-Gold HIV [Trinity Biotech] if positive initial test), and subsequently provided counseling and test results as recommended in local provider-initiated testing and counseling guidelines.8 Children with two serial positive HIV RDTs were offered enrollment in the study. The study nurse performed clinical staging based on Malawi HIV guidelines8 and completed a data collection form recording age, height, weight, and discharge diagnosis. Blood was drawn for absolute and percent CD4+ T-cell lymphocyte counts (FACScount platform, QECH laboratories), dried blood spots for confirmatory HIV DNA PCR in children ≤ 12 months as per local guidelines, and if sufficient blood was available, plasma was archived for HIV-1 Real Time RNA PCR (HIV RNA PCR, Abbott m2000, UNC Project Laboratory). Staging and test results were provided to guardians and patients with documentation in the hospital record and individual health passport. Study data were collected and managed using REDCap™ electronic data capture tools. QECH HIV counselors completed training and demonstrated competency in HIV RDT testing before study initiation.
Children meeting eligibility criteria for treatment were referred for ART. WHO guidelines for pediatric ART eligibility changed shortly after initiation of the study; enrolled children were offered treatment according to the updated 2013 guidelines on their adoption.
Statistical methods.
Mann–Whitney U test was used when comparing continuous variables between two groups. Continuous variables between ≥ 3 groups were analyzed using Kruskal–Wallis test and Dunn’s test for post hoc pairwise comparisons. Pearson’s χ2 test was used to compare categorical variables. P value of ≤ 0.05 was used to determine statistical significance. Multiplicity adjusted P values were used for the Dunn’s test for post hoc comparisons. Spearman correlation was used to evaluate associations. Two PPV of the serial RDT were computed: one against RNA PCR alone and the other against combined RNA PCR, EIA, and WB. ART eligibility was assessed using age, CD4 count, and clinical HIV stage. Patients confirmed HIV negative by combined RNA PCR, EIA, and WB were excluded from this analysis. As a sensitivity analysis, ART eligibility was assessed for the subset of patients with confirmed HIV positivity (detectable HIV DNA or RNA). All analyses were performed with GraphPad Prism version 6.07.
RESULTS
We consented and enrolled 363 children with two serial positive RDT, of whom 16 were excluded from data analysis because they were ≤ 18 months and confirmatory nucleic acid testing (HIV DNA or RNA PCR) was negative or unavailable. After excluding the 16 patients ≤ 18 months without confirmatory nucleic acid testing, 347 patients were analyzed (Figure 1).
HIV quantification and correlation with RDT results.
HIV RNA PCR was measured for a subset of children. Of the 150 children with available HIV RNA PCR results, 15 had undetectable HIV RNA (< 400 copies), prompting additional testing by EIA and WB. Of those 15 children with undetectable HIV RNA, nine had positive EIA and WB results, and repeat RDTs performed on available archived plasma were positive (Table 1). Of the remaining six patients with undetectable HIV RNA PCR, three had negative EIA and WB, two had positive EIA and negative WB, and one had negative EIA and indeterminate WB (one band positive) (Table 2). Repeat HIV RDTs performed on archived plasma were negative for all six patients. The PPV of two serial RDTs was 90% versus RNA PCR alone, and 96% versus combined RNA PCR, EIA, and WB.
Table 1.
Characteristics of nine children with two positive HIV RDTs, positive HIV enzyme immunoassay and western blot results, but undetectable HIV RNA PCR
| Age (months) | Gender | Discharge diagnosis | WHO clinical stage | Reason for stage | CD4 absolute (cells/mm3) | CD4% |
|---|---|---|---|---|---|---|
| 21 | Female | Pneumonia | 3 | MW, SBP | 154 | 34.4 |
| 27 | Male | Other malaria | 3 | MW | Missing | Missing |
| 48 | Male | Cerebral malaria | 1 | AS | 683 | ND |
| 60 | Male | Other malaria | 1 | AS | 948 | ND |
| 63 | Female | Missing | 1 | AS | 412 | 17.3 |
| 144 | Female | Marasmus | 4 | SM | 133 | ND |
| 156 | Male | Other malaria | 1 | AS | 374 | 20.5 |
| 168 | Female | Recurrent anemia | 4 | SM | 622 | ND |
| 168 | Female | Meningitis | 4 | SBI | 748 | 42.1 |
RDT = rapid diagnostic test; WHO = World Health Organization; CD4 = CD4+ T-lymphocytes; SBP = severe bacterial pneumonia; MW = moderate wasting; ND = not done; AS = asymptomatic; SM = severe malnutrition; SBI = Severe bacterial infection.
Table 2.
Characteristics of six children with two positive HIV RDTs, negative or indeterminate HIV enzyme immunoassay and western blot testing, and undetectable HIV RNA PCR
| Age (Months) | Gender | Discharge diagnosis | WHO clinical stage | Reason for stage | CD4 absolute (cells/mm3) | CD4% | EIA | WB |
|---|---|---|---|---|---|---|---|---|
| 24 | Male | Pneumonia | 4 | SM | 305 | 11.5 | Positive | Negative |
| 36 | Male | Pneumonia | 3 | RP | 866 | 31.4 | Negative | Negative |
| 36 | Female | Sepsis | 4 | SBI | 1,168 | 52.7 | Negative | Negative |
| 36 | Male | Missing | 1 | AS | 3,547 | 40.2 | Negative | Indeterminate |
| 56 | Male | Typhoid | 4 | SBI | 669 | 14.4 | Negative | Negative |
| 57 | Male | Pneumonia | 3 | RP | 1,234 | 42.6 | Positive | Negative |
RDT = rapid diagnostic test; WHO = World Health Organization; CD4 = CD4+ T-lymphocytes; EIA = enzyme immunoassay; WB = western blot; SM = severe malnutrition; RP = recurrent pneumonia; SBI = severe bacterial infection; AS = asymptomatic.
After excluding the six patients with undetectable HIV RNA PCR and negative confirmatory testing from further analysis, data from the remaining 341 HIV-positive patients were analyzed. A sensitivity analysis was also performed on the subset of 140 patients with detectable HIV DNA or RNA testing with similar results (Supplemental Figure 1).
Patient characteristics.
Of the 341 HIV-positive children analyzed, 324 children had absolute CD4+ T lymphocyte results, 245 had CD4+ T lymphocyte percent results, and 337 had HIV clinical stage data. Patient characteristics are summarized for the whole sample and by discharge diagnosis (Table 3). Median age of the cohort was 60 months (range 2 months to 16 years). The most common discharge diagnoses were presumed or confirmed sepsis (N = 68, 19.9%), pneumonia (N = 67, 19.6%), malaria (N = 66, 19.4%), and malnutrition (N = 52, 15.2%). 79% of children had severe HIV clinical disease (stage 3 or 4). Median absolute CD4 count was 512 cells/mm3 (range 1–3888 cells/mm3) and median CD4% was 18.0% (range 0.4–69%). HIV RNA PCR ranged from undetectable to > 10,000,000 copies/mL, with geometric mean of 182,271 copies/mL.
Table 3.
Characteristics of hospitalized children with new HIV diagnosis
| Discharge diagnosis (n, %) | Median age* (months) | Median absolute CD4* (cells/mm3) | Median CD4%* | WHO HIV clinical stage 3 or 4 (percent) | Geometric mean HIV RNA PCR* (copies/mL) |
|---|---|---|---|---|---|
| All (N = 341, 100%) | 60 [29.5–120.0] | 512 [247.8–934.5] | 18.0 [10.4–26.6] | 79.8 | 182,271 [59,080–581,640] |
| Sepsis (N = 68, 19.9%) | 72 [29.3–143.8] | 511 [258.0–899.0] | 18.0 [10.3–25.5] | 98.5 | 157,420 [76,930–535,080] |
| Pneumonia (N = 67, 19.6%) | 60 [28.0–120.0] | 619 [296.0–1,032.0] | 18.2 [10.9–27.1] | 100 | 122,180 [30,855–473,855] |
| Malaria (N = 66, 19.4%) | 72 [36.0–105.0] | 486 [311.0–874.0] | 20.3 [14.9–30.2] | 20.3 | 311,190 [75,733–1,545,000] |
| Malnutrition (N = 52, 15.2%) | 32 [24.0–84.0] | 766 [189.0–1,100.0] | 17.4 [8.9–25.7] | 100 | 375,360 [87,575–962,785] |
| Meningitis (N = 25, 7.3%) | 72 [48.0–126.0] | 557 [341.5–774.0] | 26.9 [15.4–30.7] | 100 | 157,175 [46,965–589,185] |
| Tuberculosis (N = 17, 5.0%) | 120 [96.0–150.0] | 272 [51.0–603.5] | 11.4 [4.7–24.0] | 100 | 76,575 [54,930–101,198] |
| Gastroenteritis (N = 8, 2.3%) | 36 [17.5–129.0] | 617 [256.0–741.3] | 15.0 [6.2–26.1] | 62.5 | 366,780 [115,535–501,378] |
| Other/Missing (N = 38, 11.1%) | 48 [28.5–120.0] | 320 [104.0–595.5] | 13.6 [7.7–23.2] | 55.8 | 93,460 [42,440–432,025] |
CD4 = CD4+ T-lymphocytes; WHO = World Health Organization.
[interquartile range].
Differences in patient characteristics between discharge diagnoses.
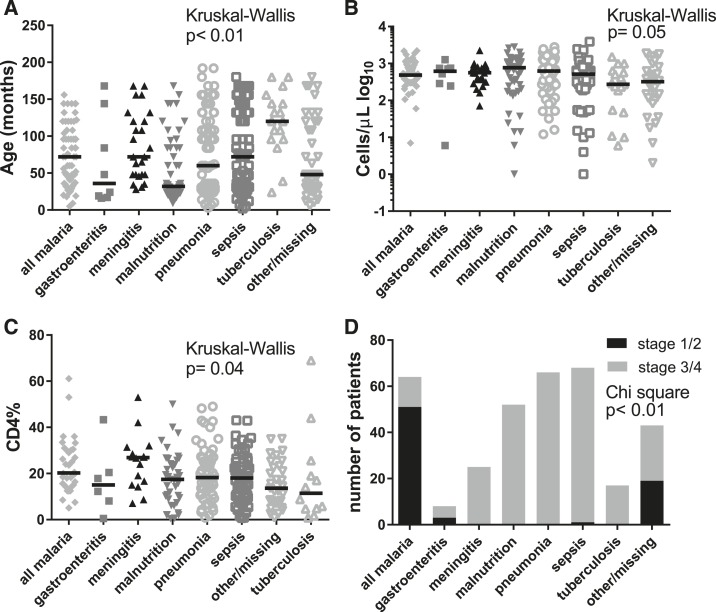
There were significant differences in age across discharge diagnoses (P < 0.01, KW), driven by older age in children with presumed or confirmed tuberculosis and younger age in children with malnutrition (Figure 2A). Absolute CD4 count differed by discharge diagnosis (P = 0.05, KW), although CD4% did not (Figure 2B and C). Overall, age correlated negatively with CD4 count (Spearman r = −0.45, 95% confidence interval [CI]: −0.54–0.36, P < 0.01), but did not correlate with CD4%. CD4 counts positively correlated with CD4% when looking at all children, and when children < 5 and ≥ 5 years old were analyzed separately (Supplemental Figure 2A–C) (Spearman r = 0.61, 95% CI: 0.52–0.68, P < 0.01). There was no significant difference in HIV RNA PCR across diagnoses (Supplemental Figure 2D).
Figure 2.
Age distribution (A), CD4+ T cell absolute and CD4% (B, C) and HIV clinical stage (D) based on discharge diagnosis. Horizontal bars denote median values. (A) Age distribution based on discharge diagnosis. Age differed between groups (P < 0.01, Kruskal–Wallis test), driven by the older age of children with tuberculosis and younger age of children with malnutrition. Children with meningitis were older than children with malnutrition (multiplicity adjusted P < 0.04, Dunn’s Test), whereas children with tuberculosis were older than children with malnutrition (multiplicity adjusted P < 0.01, Dunn’s Test). (B) CD4+ T lymphocyte absolute count based on discharge diagnosis. CD4 count differed between groups (P = 0.05, Kruskal–Wallis test; although no significant difference was detected by post hoc Dunn’s test of multiple comparisons). Children with tuberculosis had lower median CD4 count (272 cells/µL) than children with malnutrition (766 cells/µL) or malaria (486 cells/µL, P = 0.04 and P = 0.02, respectively using Mann–Whitney U test). Note: scale of y axis is log10. (C) CD4+ T lymphocyte percent based on discharge diagnosis. CD4% did not differ between groups (P = 0.04, Kruskal–Wallis; although no significant difference was detected by post hoc Dunn’s test of multiple comparisons). Because of changes in Queen Elizabeth Central Hospital (QECH) testing procedure, not all children had CD4% performed. Children with tuberculosis had the lowest CD4% (11.4%). (D) HIV clinical stage and discharge diagnoses. HIV clinical stage differed based on discharge diagnosis (Chi-square, P < 0.01). Nearly all children with malnutrition, pneumonia, meningitis, tuberculosis, and sepsis had advanced clinical HIV disease severity (stage 3 or 4), whereas 79.7% of children with malaria had mild or asymptomatic clinical HIV disease (stage 1 or 2).
HIV clinical stage differed by discharge diagnosis (Chi-square, P < 0.01) (Figure 2D). All children with pneumonia, malnutrition, meningitis, and tuberculosis met criteria for HIV clinical stage 3 or 4, as these diagnoses are associated with severe immunodeficiency per clinical guidelines. Conversely, 80% of children with malaria had clinical stage 1 or 2 HIV disease. HIV clinical stage did not correlate with absolute CD4 count (Spearman r = −0.04, 95% CI: −0.15–0.07, P = 0.50) or CD4% (Spearman r = −0.05, 95% CI: −0.18–0.08, P = 0.43).
ART eligibility.
Among children ≥ 5 years who did not qualify for ART based on age alone under 2013 guidelines (N = 180), 11.1% (N = 20) were eligible for ART based on CD4 count but would have not qualified based on clinical stage alone. 8.9% of children ≥ 5 years (N = 16) did not qualify for ART based on CD4 or clinical stage, but would qualify for treatment with the adoption of universal treatment eligibility.
DISCUSSION
We observed a higher-than-expected proportion of patients with positive HIV RDT and undetectable HIV RNA PCR in our study based on WHO guidelines for testing algorithms.9 Of 150 subjects with sufficient plasma samples for HIV RNA PCR, 9 (6.0%) were HIV seropositive by RDT, EIA, and WB, but had undetectable HIV RNA PCR (Table 2). It is possible that these children could control HIV viremia without treatment and based on CD4 count could be slow progressors, long term nonprogressors, or elite-controllers.20–22 Alternatively, these children could have positive HIV serology due to persistent maternal antibodies. Studies in Malawi and the United States have shown that the median age of seroreversion has increased over time, and a significant number of HIV-uninfected exposed children 18 months and older have positive HIV antibody testing due to persistent maternal antibody.23,24 In children with late seroreversion, serial RDT is likely to be persistently positive if repeated before ART initiation as guidelines recommend, and this problem can be expected to grow if successful PMTCT efforts result in greater numbers of HIV-exposed uninfected children.
Among the 150 subjects with HIV RNA PCR data, there were also six (4.0%) with undetectable HIV RNA PCR and negative EIA and/or WB. This could reflect systems or operational errors such as inaccurate charting or interpretation of faint positive bands on RDT test kits. Alternatively, these could represent false positive RDT results, an increasingly recognized problem in low- and middle-income countries.25–27
Although cost efficient and easily performed in the field, the sole use of RDTs for HIV diagnosis has significant limitations. Personnel must be properly trained to read and record the results correctly. Faintly positive test bands that are difficult to interpret have been described as a cause of incorrectly reported positive results, particularly when using whole blood.27–29 False positive RDTs due to heightened B-lymphocyte activation are well described in those with intercurrent illness, particularly parasitic infections such as malaria, and this effect is more pronounced in children.29–31
HIV RDT false positive tests are of particular concern in children. RDTs are highly sensitive and specific; the reported sensitivity of both Determine and Unigold HIV RDTs is 100% (95% CI: 95.5–100.0), and specificity is 99.4% (95% CI: 96.7–100.0) and 100% (95% CI: 97.9–100.0), respectively, when using whole blood.32 However, as HIV prevalence falls with successful PMTCT efforts and adoption of Option B+, the PPV of HIV RDTs will be significantly reduced, resulting in a greater number of false positive tests.30,33 Compared with the WHO standard of 99% sensitivity and 98% specificity, the PPV of an HIV RDT is 72.2% if population HIV prevalence is 5%, and the PPV drops to 33.4% if population HIV prevalence is 1%.9 In our study, the PPV of the 2-RDT algorithm was 90% versus RNA PCR alone and 96% versus combined RNA PCR, EIA, and WB, which does not meet the goal of 99% set by the WHO. With a 90–96% PPV, for every 1,000 children with a positive HIV RDT screen, 40–100 results would be falsely positive.
Children meeting the WHO criteria for HIV infection could be treated with ART indefinitely based on erroneous HIV RDT results. Although WHO guidelines recommend repeat serial RDT for confirmation of HIV diagnosis before ART initiation, only 20% of the country-specific testing strategies currently adhere to these guidelines,9 and repeat testing may be persistently positive in HIV-exposed uninfected children. Although supplemental confirmatory testing is costly, these costs may be smaller than the financial cost and potential toxicity of lifelong ART as HIV prevalence falls. Incorrect HIV diagnosis places a significant burden on the healthcare system and individuals who are incorrectly diagnosed.34
In this study, 11.1% of children qualified for ART by CD4 count criteria who would have been ineligible using clinical staging alone, and another 8.9% were ineligible for ART by CD4 count and clinical stage under 2013 guidelines. A combined 20% of potentially ART-ineligible children in our study would qualify for ART under 2015 guidelines, indicating that a significant proportion of hospitalized children would benefit from prompt HIV diagnosis if diagnostic algorithms are improved and as a universal treatment strategy is adopted in Malawi. This number is consistent with a prior study estimating an additional 17% of children were in need of ART after eligibility expansion in 2010.35
As described in other studies, HIV clinical staging did not correlate with immunologic parameters in our population.12,13 Malawi HIV staging guidelines classify children with refractory malnutrition or severe bacterial infections as stage 3 or 4; however, in sub-Saharan Africa, these conditions are common in children, and may not represent advanced HIV infection.8 Acute illness, especially infection, is associated with lower CD4 count, and could alter immunologic staging in the inpatient setting.36,37 If universal ART is adopted, the limitations of both immunologic and clinical staging to determine ART eligibility in the pediatric inpatient setting would be avoided.
Sepsis, pneumonia, malaria, and malnutrition were the most common discharge diagnoses among newly diagnosed HIV-infected children in this study. These diagnoses frequently affect children under 5 years old and are common causes of childhood morbidity and mortality in sub-Saharan Africa, highlighting the benefit of universal HIV screening in the hospital setting for all admitted patients, regardless of the reason for hospitalization.38 The spectrum of clinical disease seen in our study is similar to studies done before the implementation of PMTCT efforts.18
Children diagnosed with tuberculosis, meningitis, and malaria were older than children with other illnesses. Older age of children presenting with tuberculosis may reflect the duration of HIV infection,39 whereas older children presenting with malaria is consistent with prior observations that HIV-infected children with severe malaria including cerebral malaria are older than HIV-uninfected children with severe malaria.40,41
Limitations.
HIV testing of all admitted children was incomplete, because of the limited schedule of trained HIV counselors to perform pretest and posttest counseling and testing in the hospital, or inability to obtain consent from an appropriate guardian, as noted in similar settings.42 Those children who died or were discharged before blood drawn for testing were not included. Thus, we were unable to accurately measure HIV prevalence or mortality in children admitted to the hospital with newly diagnosed HIV. As recommended by the WHO, a more accurate assessment of HIV prevalence in pediatric populations is needed.43
We were unable to perform HIV RNA PCR in all children because of the inability to obtain sufficient volumes of blood. We were thus unable to calculate the sensitivity or specificity of the 2-RDT algorithm used in this study, and it is possible that a small number of children who were included in the data analysis and had CD4 testing but not HIV RNA PCR had falsely positive results. Results were statistically similar when analysis was performed for only those subjects with detectable HIV DNA or RNA PCR results, although there were no differences in CD4 count or percent between diagnosis groups, possibly because of smaller sample size (Supplemental Figure 1).
HIV screening of mothers of HIV RDT-reactive children and delayed confirmatory convalescent HIV RDTs after complete resolution of acute illness (when possible) are routinely performed at QECH to identify potential false positive tests before ART initiation. However, the results of this testing were not available for this study. Further evaluation of the effect of this practice on RDT PPV is needed.
In summary, HIV RDT algorithms may be suboptimal for HIV diagnosis in hospitalized children in the setting of acute illness, falling HIV prevalence, and delayed seroreversion. ART eligibility in pediatric inpatients can be influenced by acute illness, with poor correlation between clinical and immunologic staging. We estimate that 8.9% of the previously ineligible Malawian children aged ≥ 5 years would qualify for the treatment if the updated WHO guidelines are implemented. Further study of pediatric HIV testing algorithms is needed in low- and middle-income countries in the era of universal ART eligibility.
Supplementary Material
Acknowledgments:
We thank the patients, their families, and the health providers at Queen Elizabeth Central Hospital, the Blantyre Malaria Project, and the Malawi-Liverpool Wellcome Trust Clinical Research Programme. We thank Rob Krysiak and Gerald Tegha of the UNC Malawi Project Laboratory in Lilongwe for facilitating HIV viral load and confirmatory testing. We thank Neil Kennedy, Queen Dube, Josephine Langton, and Macpherson Mallewa of the University of Malawi College of Medicine Department of Paediatrics and Child Health for valuable insights and discussions about our work.
Note: Supplemental figures appear at www.ajtmh.org.
Disclaimer: Kim serves on the Scientific Advisory Board of the Sanford Guide to Antimicrobial Therapy Guide.
REFERENCES
- 1.UNAIDS, 2016. Fact Sheet. Geneva, Switzerland: United Nations. [Google Scholar]
- 2.UNAIDS, 2016. Children and HIV Fact Sheet. Geneva, Switzerland: United Nations. [Google Scholar]
- 3.UNAIDS, 2016. The Prevention Gap Report. Geneva, Switzerland: United Nations. [Google Scholar]
- 4.WHO/UNAIDS, 2014. 2014 Global Update on the Health Sector Response to HIV. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 5.UNAIDS, 2014. The Gap Report. Geneva, Switzerland: United Nations. [Google Scholar]
- 6.UNAIDS, 2013. Global Report: UNAIDS Report on The Global AIDS Epidemic 2013. Geneva, Switzerland: United Nations. [Google Scholar]
- 7.Hsiao NY, Stinson K, Myer L, 2013. Linkage of HIV-infected infants from diagnosis to antiretroviral therapy services across the Western Cape, South Africa. PLoS One 8: e55308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malawi Ministry of Health, 2014. Clinical Management of HIV in Children and Adults. Lilongwe, Malawi: Malawi Ministry of Health. [Google Scholar]
- 9.WHO, 2015. Consolidated Guidelines on HIV Testing Services. Geneva, Switzerland: WHO. [Google Scholar]
- 10.WHO, 2010. Antiretroviral Therapy for HIV Infection in Infants and Children: Towards Universal Access: Recommendations for a Public Health Approach: 2010 Revision. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 11.WHO, 2013. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 12.Kagaayi J, Makumbi F, Nakigozi G, Wawer MJ, Gray RH, Serwadda D, Reynolds SJ, 2007. WHO HIV clinical staging or CD4 cell counts for antiretroviral therapy eligibility assessment? An evaluation in rural Rakai district, Uganda. AIDS 21: 1208–1210. [DOI] [PubMed] [Google Scholar]
- 13.O’Hare B, Milner DA, Jr, Newberry L, Pelani I, Malisita K, 2014. Discordance between clinical and immunological ART eligibility criteria for children in Malawi. BMC Res Notes 7: 666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO, 2015. Guideline on When to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 15.WHO, 2016. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 16.Kankasa C, et al. 2009. Routine offering of HIV testing to hospitalized pediatric patients at university teaching hospital, Lusaka, Zambia: acceptability and feasibility. J Acquir Immune Defic Syndr 51: 202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamla D, et al. 2013. Evidence from the field: missed opportunities for identifying and linking HIV-infected children for early initiation of ART. AIDS 27 (Suppl 2): S139–S146. [DOI] [PubMed] [Google Scholar]
- 18.Rogerson SR, Gladstone M, Callaghan M, Erhart L, Rogerson SJ, Borgstein E, Broadhead RL, 2004. HIV infection among paediatric in-patients in Blantyre, Malawi. Trans R Soc Trop Med Hyg 98: 544–552. [DOI] [PubMed] [Google Scholar]
- 19.Zwi K, Pettifor J, Soderlund N, Meyers T, 2000. HIV infection and in-hospital mortality at an academic hospital in South Africa. Arch Dis Child 83: 227–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grabar S, Selinger-Leneman H, Abgrall S, Pialoux G, Weiss L, Costagliola D, 2009. Prevalence and comparative characteristics of long-term nonprogressors and HIV controller patients in the French Hospital Database on HIV. AIDS 23: 1163–1169. [DOI] [PubMed] [Google Scholar]
- 21.Pantaleo G, et al. 1995. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med 332: 209–216. [DOI] [PubMed] [Google Scholar]
- 22.Paul ME, Mao C, Charurat M, Serchuck L, Foca M, Hayani K, Handelsman EL, Diaz C, McIntosh K, Shearer WT, 2005. Predictors of immunologic long-term nonprogression in HIV-infected children: implications for initiating therapy. J Allergy Clin Immunol 115: 848–855. [DOI] [PubMed] [Google Scholar]
- 23.Gulia J, Kumwenda N, Li Q, Taha TE, 2007. HIV seroreversion time in HIV-1-uninfected children born to HIV-1-infected mothers in Malawi. J Acquir Immune Defic Syndr 46: 332–337. [DOI] [PubMed] [Google Scholar]
- 24.Gutierrez M, Ludwig DA, Khan SS, Chaparro AA, Rivera DM, Cotter AM, Scott GB, 2012. Has highly active antiretroviral therapy increased the time to seroreversion in HIV exposed but uninfected children? Clin Infect Dis 55: 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klarkowski D, Glass K, O’Brien D, Lokuge K, Piriou E, Shanks L, 2013. Variation in specificity of HIV rapid diagnostic tests over place and time: an analysis of discordancy data using a Bayesian approach. PLoS One 8: e81656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klarkowski DB, Wazome JM, Lokuge KM, Shanks L, Mills CF, O’Brien DP, 2009. The evaluation of a rapid in situ HIV confirmation test in a programme with a high failure rate of the WHO HIV two-test diagnostic algorithm. PLoS One 4: e4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shanks L, Siddiqui MR, Kliescikova J, Pearce N, Ariti C, Muluneh L, Pirou E, Ritmeijer K, Masiga J, Abebe A, 2015. Evaluation of HIV testing algorithms in Ethiopia: the role of the tie-breaker algorithm and weakly reacting test lines in contributing to a high rate of false positive HIV diagnoses. BMC Infect Dis 15: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gray RH, Makumbi F, Serwadda D, Lutalo T, Nalugoda F, Opendi P, Kigozi G, Reynolds SJ, Sewankambo NK, Wawer MJ, 2007. Limitations of rapid HIV-1 tests during screening for trials in Uganda: diagnostic test accuracy study. BMJ 335: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kroidl I, Clowes P, Mwalongo W, Maganga L, Maboko L, Kroidl AL, Geldmacher C, Machibya H, Hoelscher M, Saathoff E, 2012. Low specificity of determine HIV1/2 RDT using whole blood in south west Tanzania. PLoS One 7: e39529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klarkowski D, O’Brien DP, Shanks L, Singh KP, 2014. Causes of false-positive HIV rapid diagnostic test results. Expert Rev Anti Infect Ther 12: 49–62. [DOI] [PubMed] [Google Scholar]
- 31.Gasasira AF, Dorsey G, Kamya MR, Havlir D, Kiggundu M, Rosenthal PJ, Charlebois ED, 2006. False-positive results of enzyme immunoassays for human immunodeficiency virus in patients with uncomplicated malaria. J Clin Microbiol 44: 3021–3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO , 2015. HIV Assays: Laboratory Performance and Other Operational Characteristics: Rapid Diagnostic Tests (Combined Detection of HIV-1/2 Antibodies and Discriminatory Detection of HIV-1 and HIV-2 Antibodies): Report 18. Geneva, Switzerland: WHO. [Google Scholar]
- 33.Tu XM, Litvak E, Pagano M, 1992. Issues in human immunodeficiency virus (HIV) screening programs. Am J Epidemiol 136: 244–255. [DOI] [PubMed] [Google Scholar]
- 34.Shanks L, Klarkowski D, O’Brien DP, 2013. False positive HIV diagnoses in resource limited settings: operational lessons learned for HIV programmes. PLoS One 8: e59906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penazzato M, Crowley S, Mofenson L, Franceschetto G, Nannyonga MM, Mazza A, Giaquinto C, 2012. Programmatic impact of the evolution of WHO pediatric antiretroviral treatment guidelines for resource-limited countries (Tukula Fenna Project, Uganda). J Acquir Immune Defic Syndr 61: 522–525. [DOI] [PubMed] [Google Scholar]
- 36.Lisse I, Samb B, Whittle H, Jensen H, Soumare M, Simondon F, Aaby P, 1998. Acute and long-term changes in T-lymphocyte subsets in response to clinical and subclinical measles. A community study from rural Senegal. Scand J Infect Dis 30: 17–21. [DOI] [PubMed] [Google Scholar]
- 37.Lisse IM, Aaby P, Whittle H, Knudsen K, 1994. A community study of T lymphocyte subsets and malaria parasitaemia. Trans R Soc Trop Med Hyg 88: 709–710. [DOI] [PubMed] [Google Scholar]
- 38.WHO, 2013. Data Repository Causes of Child Mortality. Geneva, Switzerland: World Health Organization. Available at: http://www.who.int/gho/child_health/mortality/causes/en/. Accessed March 25, 2015.
- 39.Glynn JR, Murray J, Bester A, Nelson G, Shearer S, Sonnenberg P, 2008. Effects of duration of HIV infection and secondary tuberculosis transmission on tuberculosis incidence in the South African gold mines. AIDS 22: 1859–1867. [DOI] [PubMed] [Google Scholar]
- 40.Hochman SE, et al. 2015. Fatal pediatric cerebral malaria is associated with intravascular monocytes and platelets that are increased with HIV coinfection. MBio 6: e01390–e01415. Erratum in: MBio 2016;7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bronzan RN, et al. 2007. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis 195: 895–904. [DOI] [PubMed] [Google Scholar]
- 42.Kranzer K, Meghji J, Bandason T, Dauya E, Mungofa S, Busza J, Hatzold K, Kidia K, Mujuru H, Ferrand RA, 2014. Barriers to provider-initiated testing and counselling for children in a high HIV prevalence setting: a mixed methods study. PLoS Med 11: e1001649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.UNAIDS/WHO, 2013. Working Group on Global HIV/AIDS and STI Surveillance: Paediatric HIV Surveillance Among Infants and Children Less Than 18 Years of Age. Geneva, Switzerland: World Health Organization. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.