Abstract.
A case of disseminated cysticercosis of brain, muscle subcutaneous tissue is reported and the use of musculoskeletal ultrasound in the diagnosis of the condition emphasized.
CASE PRESENTATION
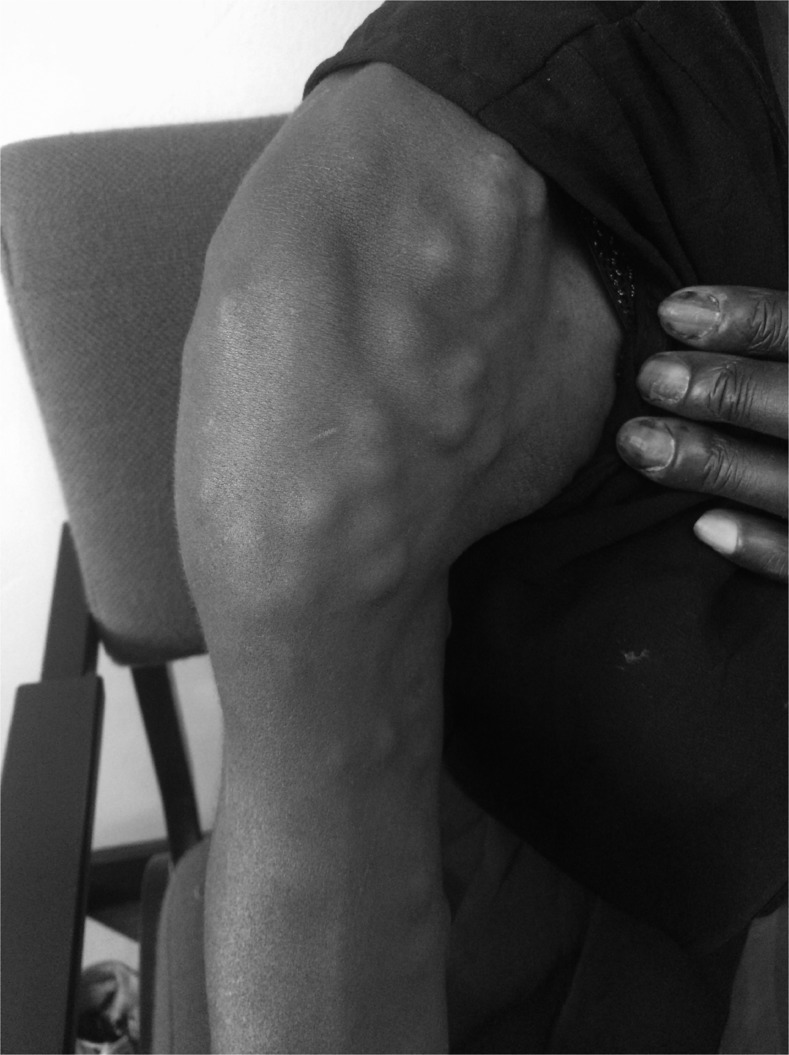
A 41-year-old woman was seen at the Lighthouse Anti-Retroviral Therapy (ART) clinic in Lilongwe, Malawi. She was HIV-positive and had been on ART (TDF/3TC/EFV) for the past 6 years and had been well adherent, which a suppressed viral load and a current CD4 count of 471 cells/mm3 confirmed. Over the past 4 years, she had developed multiple subcutaneous nodules (Figure 1), which made people look at her “in a strange way.” A previous biopsy performed at an external hospital had been not diagnostic. The nodules were clearly visible and palpated on all parts of the body. They were firm, nonmobile and subcutaneous in location.
Figure 1.
Shoulder of the patient with multiple nodules. Similar nodules were seen disseminated on the whole body and in the face.
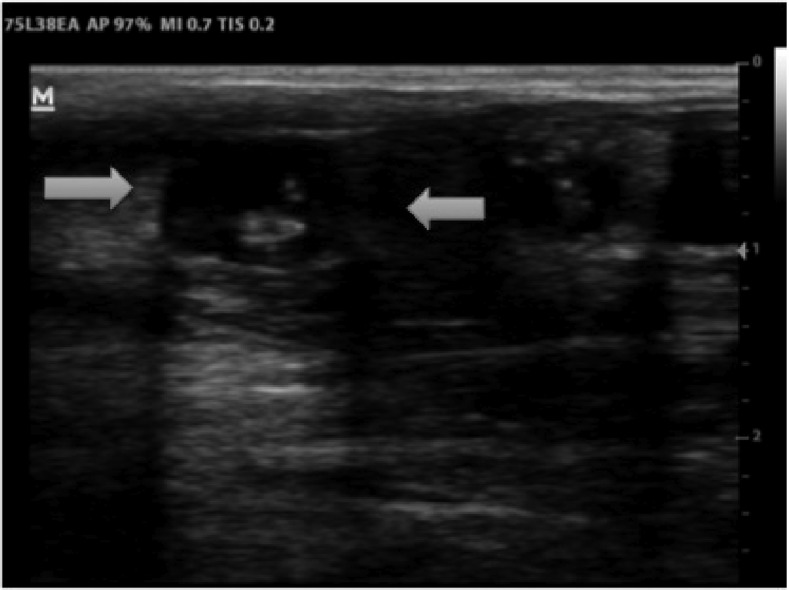
A musculoskeletal (MSK) ultrasound of the nodules was performed using a 7-MHz linear probe (DP-30; Mindray, Shenzen, China). This revealed predominately cystic lesions, most of which contained a central echogenic region (Figure 2, Supplemental Video 1). An abdominal scan using a 5-MHz probe identified multiple cysts in the liver, pancreas, and spleen.
Figure 2.
Ultrasound of the nodules at time of diagnosis (7 MHz linear probe): Echofree cysts (8 × 10 mm) are visible with an echogenic central region (= “scolex”).
Based on the ultrasound appearance of the cysts, a presumptive diagnosis of cysticercosis was made, and she was questioned about any neurological symptoms. She reported that she was recently told that she “was epileptic now”. In total, she had had four seizures, all in the past year; she was not currently on antiepileptic drugs therapy.
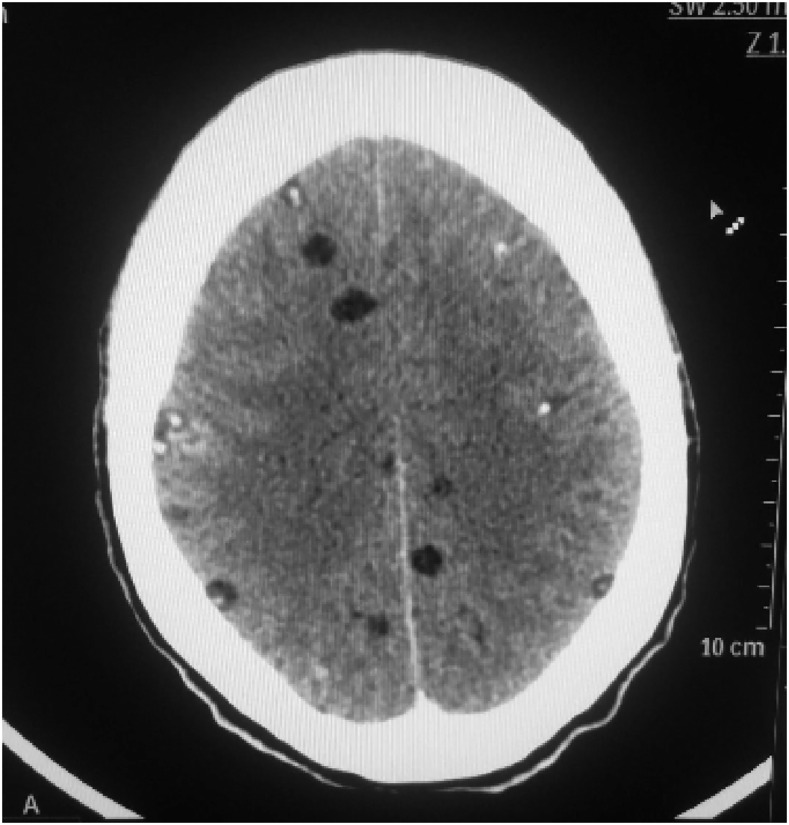
The patient was started on valproate 200 mg twice a day; after 1 week, she was admitted to the ward for CT scan of the brain and antihelminthic treatment. The CT scan revealed extensive brain involvement (Figure 3) with more than 50 cysts. Discussion with the patient regarding the risks and benefits of antiparasitic treatment was undertaken. The patient had suffered significant stigma due to the appearance of the cutaneous cysts and desired treatment beyond antiepileptics. In addition, her ART regimen meant that antiepileptic options were extremely limited because of potential drug interactions.
Figure 3.
Brain CT scan with multiple, intraprenchymal cysticrecosis cysts.
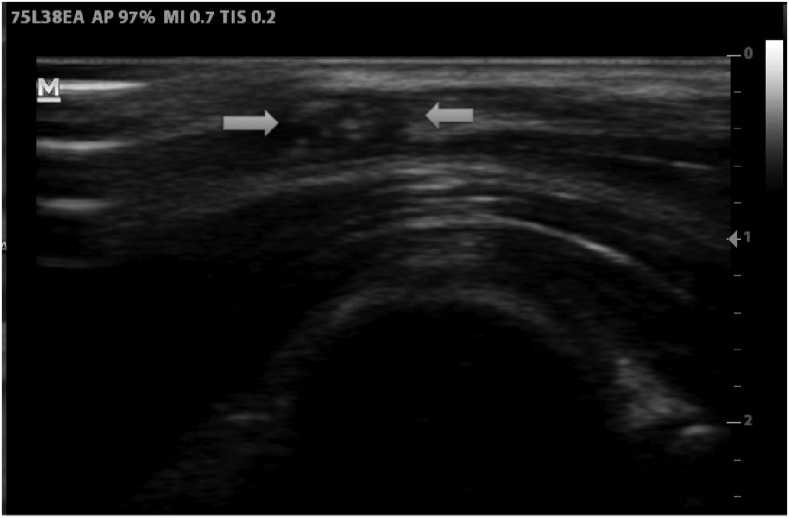
Albendazole 400 mg twice a day and praziquantel 1.2 g once a day (1 week) were prescribed in addition to prednisolone 40 mg/day. On the third day of treatment, the patient developed severe body ache, headache, vomiting, and an additional 20 mg of steroid was given. On the sixth day, she reported no more vomiting and said that she felt “just fine.” To the delight of the patient, the nodules were already significantly reduced in size and hardly visible. On the following day, she was discharged home. Albendazole was continued for further 2 weeks and the steroids were tapered. As prophylaxis against further fits valproate was continued for 6 months. On a follow-up after 3 weeks of treatment, the sonographic appearance had changed substantially with the nodules being smaller and less well defined (Figure 4, Supplemental Video 2).
Figure 4.
Ultrasound of the nodules after 3 weeks of treatment (7 MHz linear probe): Cysts are smaller, more echogenic and less defined as sign of cyst degeneration.
DISCUSSION
Cysticercosis is an infection with the larval form of the pork tapeworm Taenia solium. It is endemic in Southeast Asia, Mexico, Central and South America, and Africa. In Sub-Saharan Africa, 0.76–2.46 million people were estimated to have epilepsy due to neurocysticercosis.1 Extensive research has been done in South America on the presentation and findings in patients with neurocysticercosis; unfortunately, data on neurocysticercosis (NCC) presentation and epidemiology in Africa is limited.1
Humans can be either the definitive host, or act as an intermediate host. After ingestion of inadequately cooked infected pork meat, cysticerci develop into the adult tapeworms of T. solium in the human bowel which rarely causes any noticeable symptoms. The eggs of the tapeworm are excreted with the feces; the intermediate host (usually the pig) then ingests them. Ingested eggs hatch in the pig’s small intestine, penetrate the mucosa and reach various tissues, resulting in porcine cysticercosis and completing the life cycle. Humans can accidentally serve as intermediate hosts, when eggs are ingested with contaminated food or water. Seroprevelance data demonstrating hotspot gradients surrounding tapeworm carriers suggest that most individuals are probably directly infected from another human carrier of the T. solium tapeworm.2
Neurocysticercosis is the most common parasitic infestation of the human central nervous system3 and a common cause of epilepsy in endemic areas. The diagnosis is relatively difficult to make solely on a clinical basis because the manifestations are not specific, and visualization of the organism usually is not feasible. Plain radiographs rarely show cysticerci except in chronic cases when they are calcified.3 Advanced testing such as a serum western blot, or antigen detection are available for disease detection and following patients after treatment, respectively. Unfortunately, most of these tests are unavailable in Sub-Saharan Africa.1 Neuroimaging is often essential for confirmation of the diagnosis.4
NCC is defined as the presence of at least one NCC-like lesion (a round-shaped hypodensity on brain computed tomography (CT) with a hyperdense dot representing the head of the cysticercus [scolex]), usually not bigger than 1 cm in diameter and without perifocal edema.1 CT scanners are only available in few centers in Sub-Saharan Africa and usually at relatively high cost for the patient. As a result, many cases of cysticercosis remain undiagnosed for long time, as our case illustrates.
Sonographic features of cysticercosis in the muscle have been described previously3 but are not widely known. The characteristic findings also seen in our case are small, well-defined elliptical lesions of approximately 10 mm in diameter with an eccentric, echogenic, pedunculated structure inside, that represents the scolex.4,5 Over time, fluid may leak out of the cyst, or the cysts may calcify leading to a less pathognomonic appearance.
As ultrasound is more widely available than CT scanners in most areas where neurocysticercosis is endemic, this imaging modality may assist with diagnosis in low-resource settings. One could imagine that MSK ultrasound could be used as an initial screening tool in patients with newly diagnosed epilepsy in endemic areas. Finding muscular cysts might help physician target patients who are most in need of referral for advanced neuroimaging. There is limited evidence regarding which muscles are most commonly involved in human subjects although one might hypothesize that large muscle groups such as the quadriceps, hamstring, and biceps would be the most appropriate to screen as part of a POCUS protocol.
In Peru, a recent study of pigs demonstrated that muscular ultrasound was both sensitive and specific for the presence of high cyst burden.6 It remains to be seen if this is also true for human neurocysticercosis. More research needs to be done regarding the applicability and safety of ultrasound as a screening tool in regions with high rates of epilepsy and limited access to more advanced neuroimaging. Certainly, it poses a diagnostic option for patients with palpable nodules as seen in our case. Cysticercosis should therefore be considered as a potential application for point-of-care ultrasound for infectious diseases, which in recent years became more frequently described7 and more widely used in regions where other image modalities are limited.
Supplementary Material
Note: Supplemental videos appear at www.ajtmh.org.
REFERENCES
- 1.Winkler AS, 2012. Neurocysticercosis in sub-Saharan Africa: a review of prevalence, clinical characteristics, diagnosis, and management. Pathog Glob Health 106: 261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lescano AG, Garcia HH, Gilman RH, Gavidia CM, Tsang VC, Rodriguez S, Moulton LH, Villaran MV, Montano SM, Gonzalez AE; Cysticercosis Working Group in Peru , 2009. Taenia solium cysticercosis hotspots surrounding tapeworm carriers: clustering on human seroprevalence but not on seizures. PLoS Negl Trop Dis 3: e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asrani A, Morani A, 2004. Primary sonographic diagnosis of disseminated muscular cysticercosis. J Ultrasound Med 23: 1245–1248. [DOI] [PubMed] [Google Scholar]
- 4.Garcia HH, Nash TE, Del Brutto OH, 2014. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 13: 1207–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vijayaraghavan SB, 2004. Sonographic appearances in cysticercosis. J Ultrasound Med 23: 423–427. [DOI] [PubMed] [Google Scholar]
- 6.Flecker RH, et al. 2017. Assessing ultrasonography as a diagnostic tool for porcine cysticercosis. PLoS Negl Trop Dis 11: e0005282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bélard S, Tamarozzi F, Bustinduy AL, Wallrauch C, Grobusch MP, Kuhn W, Brunetti E, Joekes E, Heller T, 2016. Point-of-care ultrasound assessment of tropical infectious diseases—a review of applications and perspectives. Am J Trop Med Hyg 94: 8–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.