Abstract.
Septic arthritis is a medical emergency, and if not treated appropriately, it can be associated with high morbidity and mortality. Melioidosis, a serious infectious disease caused by the Gram-negative bacillus Burkholderia pseudomallei, is highly endemic in South and Southeast Asia and northern Australia. We reviewed the medical charts of adult patients admitted with bacterial septic arthritis at Sunpasitthiprasong Hospital, Ubon Ratchathani, northeast Thailand from January 2012 to December 2014. Bacterial septic arthritis was defined as one or more hot swollen joints with isolation of a pathogenic organism from an affected joint or from blood. A total of 154 patients with septic arthritis were retrospectively evaluated. The most common causes were B. pseudomallei (48%, N = 74), Streptococcus spp. (29%, N = 44), and Staphylococcus aureus (10%, N = 16). Prevalence of diabetes, bacteremia, and pneumonia was higher in B. pseudomallei septic arthritis than in septic arthritis caused by the other bacteria (all P < 0.01). Seventy three percent (54/74) of patients infected with B. pseudomallei and 69% (55/80) of patients with the other bacteria received effective antimicrobials on the first day of admission (P = 0.60), but in-hospital mortality of the former group was considerably higher (34% versus 14%, P = 0.004). In conclusion, B. pseudomallei septic arthritis is common and associated with high mortality in northeast Thailand. Emergence of Streptococcus arthritis is observed. Difficulty in diagnosing melioidosis and identifying B. pseudomallei in areas where health care workers are not familiar with the disease is discussed. In melioidosis-endemic regions, parenteral ceftazidime could be considered as empirical antimicrobial therapy for patients with septic arthritis and underlying diseases.
INTRODUCTION
Septic arthritis is a common medical emergency and is associated with substantial morbidity and mortality.1 The case fatality rate (CFR) for septic arthritis is about 5–15%, and irreversible loss of joint function develops in 25–50% of those who survive.2 The infection is usually caused by hematogenous spread during a transient or persistent bacteremia, or by direct inoculation occurring with trauma or iatrogenically. The most frequent causative organisms identified internationally are Staphylococcus aureus and Streptococcus spp., whereas Gram-negative bacteria such as Escherichia coli and Pseudomonas spp. are more common in the older population and in those with recurrent urinary tract infection. Neisseria gonorrhoeae is now a rare cause of septic arthritis in Europe and North America,1 but is still prevalent in some parts of the world.3,4 Therefore, the initial antibiotic choice in patients suspected with septic arthritis is based on clinical risk factors and local epidemiology.1
Melioidosis is an infection cause by Burkholderia pseudomallei, a Gram-negative bacillus founded in soil and water. The disease is also increasingly reported as a cause of septic arthritis in those who are living in or traveling to those endemic countries.3,5,6 Burkholderia pseudomallei is intrinsically resistant to a wide range of antimicrobials, including gentamicin and most third-generation cephalosporins except ceftazidime. Effective parenteral antibiotics include ceftazidime and carbepenem.7 Even with effective antimicrobial treatment, the CFR of melioidosis is high, ranging from 14% in northern Australia8 to 40% in northeast Thailand.9 Melioidosis is predicted to be endemic in 82 tropical countries, with a high incidence rate in South Asia, Southeast Asia, and northern Australia.10 Diabetes mellitus is the most important risk factor, and is found in about 50% of melioidosis patients.9 Other known risk factors include exposure to soil or water, male gender, older age, excess alcohol consumption, chronic liver disease, chronic lung disease, chronic renal disease, and thalassemia. Nonetheless, about 20% of adult melioidosis patients have no recognized risk factors.9,11
Here, we review cases presenting with septic arthritis to a tertiary referral hospital in northeast Thailand. We also compare clinical aspects, management, and outcomes between those caused by B. pseudomallei and the other bacterial pathogens.
MATERIALS AND METHODS
Study patients.
A retrospective study was conducted to review all medical records of adult patients (age > 15 years old) admitted with septic arthritis at Sunpasitthiprasong Hospital, Ubon Ratchathani, northeast Thailand, from January 1, 2012 to December 31, 2014. Cases were searched via the hospital admission database for patients with a final diagnosis of pyogenic arthritis (International Classification of Disease, tenth Revision [ICD-10] diagnostic code of M00 or M01) and via the microbiological laboratory database searching for patients with synovial fluid culture positive for any organism. Culture-confirmed bacterial septic arthritis was defined as one or more joints with typical features of septic arthritis, and isolation of a pathogenic organism from an affected joint or from blood. Typical features of septic arthritis were defined as at least one of the compatible clinical symptoms and signs: redness, swelling, heat, pain, limited range of motion, and imaging documentation.
Data collection.
Demographic data, presenting symptoms and signs, duration of symptoms before admission, underlying disease, history of previous joint surgery or injection, and imaging results were collected. The involved joint and microbiological results for synovial fluid and blood, treatment, and outcome at hospital discharge and at 1-year follow up were recorded.
Parenteral antimicrobials prescribed for patients with septic arthritis were considered effective if the isolated organism were susceptible to that antimicrobial on the susceptible testing. Only ceftazidime, carbapenem drug, and amoxicillin/clavulanic acid were considered effective against B. pseudomallei. Arthrotomy was defined as an open joint procedure. Arthroscopic washout was not available in the hospital during the study period.
Cumulative incidences of bacteremia.
We estimated cumulative incidences of bacteremia to compare the most common causes of septic arthritis and bacteremia in the same setting. The overall cumulative incidence was estimated by the total number of new bacteremia cases divided by the total population of Ubon Ratchathani at the beginning of the study period. The total number of new bacteremia cases from January 2012 to December 2014 was estimated from the microbiological laboratory database. Ubon Ratchathani province had an estimated population in 2012 of 1.86 million. Because of the difficulty in establishing their clinical significance, organisms frequently associated with contamination including coagulase-negative staphylococci, viridans group streptococci, Corynebacterium spp., Bacillus spp., Diptheroid spp., Micrococcus spp., and Propionibacterium spp. were excluded from the analysis for cumulative incidences of bacteremia. Only the first episode of bacteremia for each patient was included in the analysis.
Statistical analysis.
Categorical variables were compared using χ2 test or Fisher’s exact test. Continuous variables were compared using Mann–Whitney U test. Interquartile ranges (IQRs) are presented as 25th and 75th percentiles. Logistic regression models were used to calculate odds ratios. All analyses were performed using STATA version 14.0 (StataCorp LP, College station, TX). Because the cumulative incidences of Streptococcus septic arthritis was much higher than S. aureus arthritis in our setting during the study period, we conducted additional analysis by comparing clinical manifestations between septic arthritis caused by Streptococcus spp. and S. aureus.
Ethics statement.
Approval for the study was obtained from the Ethics Committee of Sunpasitthiprasong Hospital (059/2014).
RESULTS
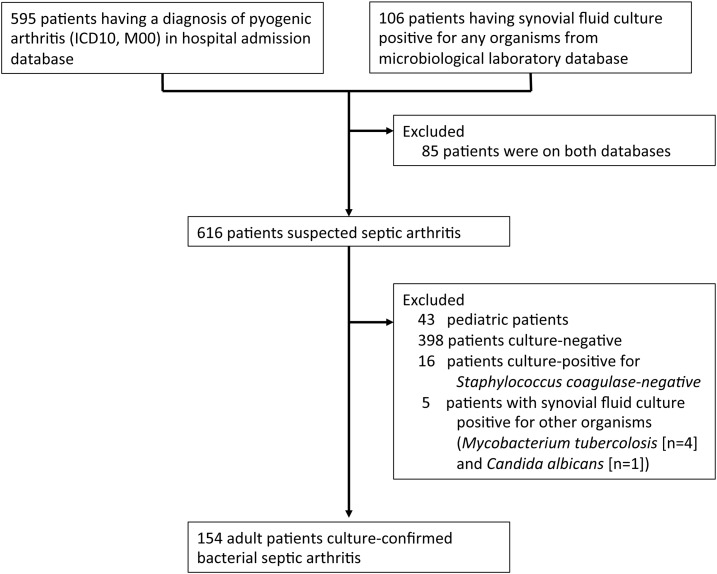
A total of 616 medical case records were identified by the search of both the hospital admission database for patients with a final diagnosis of pyogenic arthritis and the microbiological hospital database for patients with synovial fluid culture-positive for any bacteria (Figure 1). A total of 43 cases were pediatric patients, 398 cases were culture-negative for any organism, 16 cases were culture-positive for Staphylococcus coagulase negative (considered as bacterial contaminants in the context of native joints), four cases had synovial fluid culture-positive for Mycobacterium tuberculosis, and one case was positive for Candida albicans. Therefore, 154 adult patients with culture-confirmed bacterial septic arthritis were included in the final analysis.
Figure 1.
Study flow diagram.
A total of 106 (69%) patients had a positive synovial fluid culture, and 113 (73%) patients had a positive blood culture (Table 1). The most common causative pathogens were B. pseudomallei (48%, N = 74), Streptococcus spp. (29%, N = 44) and S. aureus (10%, N = 16). Other pathogenic organisms observed included Klebsiella spp. (5), E. coli (4), Enterococcus spp. (3), Salmonella spp. (3), Pseudomonas aeruginosa (2), Enterobacter spp. (2) and Acinetobacter spp. (1). Of 16 patients infected with S. aureus, three (19%) were caused by methicillin-resistant S. aureus. Two were patients with end-stage renal disease on hemodialysis, and the other one was a paraplegic patient with necrotizing fasciitis. Of four E. coli and five Klebsiella spp. cases, two (50%) and one (20%) were infected with extended-spectrum beta-lactamases–producing isolates, respectively.
Table 1.
Causative organisms
| Organisms | Number of patients (%, N = 154) | Synovial fluid culture positive | Blood culture positive |
|---|---|---|---|
| Burkholderia pseudomallei | 74 (48) | 55 | 64 |
| Streptococcus spp. | 44 (29) | 21 | 32 |
| Staphylococcus aureus | 16 (10) | 12 | 13 |
| Klebsiella spp. | 5 (3) | 4 | 2 |
| Escherichia coli | 4 (3) | 4 | 0 |
| Enterococcus spp. | 3 (2) | 2 | 1 |
| Salmonella spp. | 3 (2) | 3 | 0 |
| Pseudomonas aeruginosa | 2 (1) | 2 | 0 |
| Enterobacter spp. | 2 (1) | 2 | 0 |
| Acinetobacter spp. | 1 (1) | 0 | 1 |
Table 2 shows the demographic of the study population. A total of 92 (60%) patients were male, and the median age at presentation was 55 years old (IQR 45–66 years). Gender and age were not different between B. pseudomallei septic arthritis and those caused by the other bacterial pathogens. Three-quarters of B. pseudomallei septic arthritis presented during rainy season (defined as the 6-month period from June to November) whereas half of those caused by other bacterial pathogens presented during rainy season (73% versus 58%, P = 0.06). Diabetes mellitus was more common in those caused by B. pseudomallei than in those caused by the other bacterial pathogens (88% versus 19%, P < 0.001). Percentages of patients without any underlying risk factors were higher in those caused by the other bacterial pathogens than in those caused by B. pseudomallei (56% versus 4%, P < 0.001). A previous episode of melioidosis was observed only in those who presented with B. pseudomallei septic arthritis (9% versus 0%, P = 0.005), whereas two patients who had joint prosthesis were infected with Streptococcus spp. (N = 1) and E. coli (N = 1).
Table 2.
Case demographics
| Variables | Burkholderia pseudomallei septic arthritis (N = 74) | Other septic arthritis (N = 80) | P value* |
|---|---|---|---|
| Male gender | 42 (57%) | 50 (63%) | 0.51 |
| Age (year, median, IQR) | 53 (44–61) | 58 (48–67) | 0.22 |
| Presenting during rainy season† | 54 (73%) | 46 (58%) | 0.06 |
| Risk factors for development of septic arthritis | |||
| Diabetes mellitus | 65 (88%) | 15 (19%) | < 0.001 |
| Chronic kidney disease | 13 (18%) | 9 (11%) | 0.36 |
| Cutaneous ulcers | 8 (11%) | 13 (16%) | 0.33 |
| Gouty arthritis | 4 (5%) | 9 (11%) | 0.36 |
| Rheumatoid arthritis | 2 (3%) | 1 (1%) | 0.61 |
| Alcoholism | 0 | 3 (4%) | 0.25 |
| Joint prosthesis | 0 | 2 (3%) | > 0.99 |
| Intravenous drug abuse | 0 | 0 | NA |
| History of melioidosis | 7 (9%) | 0 | 0.005 |
| None | 3 (4%) | 45 (56%) | < 0.001 |
IQR = interquartile range.
P value derived using χ2 test or Fisher’s exact test.
From June to November.
Overall, three quarters (76%; 118/154) presented with monoarthritis, and a quarter (23%; 35/154) presented with polyarthritis (Table 3). The most frequently involved joint was knee (64%; 98/154), and the percentage of involvement was lower in those infected with B. pseudomallei compared with other bacterial pathogens (53% versus 74%, P = 0.01). Hip was the fourth common joint involved (12%; 19/154), and the percentage of involvement was more common in those infected with B. pseudomallei compared with other bacterial pathogens (19% versus 6%, P = 0.03). Ten patients had osteomyelitis confirmed by standard radiography (N = 8) or magnetic resonance imaging (N = 2). Bacteremia and pneumonia were more common in those infected with B. pseudomallei than those infected by the other bacterial pathogens (P = 0.001 and < 0.001, respectively). Hepatosplenic abscess was observed in 31% of melioidosis patients who had abdominal imaging performed (16/51), whereas this was not observed in those caused by other bacterial pathogens (0/8, P = 0.09). Central nervous system (CNS) infection was observed in three melioidosis patients and three nonmelioidosis patients (Streptococcus spp. [N = 2] and S. aureus [N = 1]). CNS infection included brain abscess (N = 2), meningitis (N = 2), epidural abscess (N = 1), meningitis (N = 1), and meningoencephalitis (N = 1). A total of three melioidosis patients and two patients caused by other bacterial pathogens developed symptoms and signs of arthritis after hospital admission (4% versus 3%, P = 0.67).
Table 3.
Sites of infection
| Variables | Burkholderia pseudomallei septic arthritis (%, N = 74) | Other septic arthritis (%, N = 80) | P value* |
|---|---|---|---|
| No. of joints involved | |||
| 1 (monoarthritis) | 61 (82) | 58 (73) | 0.18 |
| ≥ 2 (Polyarthritis) | 13 (18) | 22 (28) | – |
| Location of joints | |||
| Knee | 39 (53) | 59 (74) | 0.01 |
| Shoulder | 14 (19) | 9 (11) | 0.23 |
| Hip | 14 (19) | 5 (6) | 0.03 |
| Ankle | 11 (15) | 14 (18) | 0.67 |
| Wrist | 5 (7) | 12 (15) | 0.13 |
| Elbow | 4 (5) | 2 (3) | 0.43 |
| Sternoclavicular | 1 (1) | 4 (5) | 0.37 |
| Osteomyelitis | 3 (4) | 7 (9) | 0.33 |
| Other organ involvements | |||
| Blood† | 64/74 (86) | 49/79 (62) | 0.001 |
| Lung | 19 (26) | 1 (1) | < 0.001 |
| Soft tissue | 13 (18) | 16 (20) | 0.84 |
| Skin | 3 (4) | 8 (10) | 0.21 |
| Urinary tract | 8 (11) | 2 (3) | 0.05 |
| Bone | 3 (4) | 7 (8) | 0.33 |
| Central nervous system | 3 (4) | 3 (4) | > 0.99 |
| Lymph node | 2 (3) | 0 | 0.23 |
| Liver or splenic abscess‡ | 16/51 (31) | 0/8 (0) | 0.09 |
P value derived using χ2 test or Fisher’s exact test.
Blood culture was not performed in one case.
In patients who had abdominal imaging by ultrasonography, CT scan, or MRI.
The joint was aspirated in 112 patients (72%). The synovial fluid was recorded as pus without a white blood cell count report in 22 patients (12 melioidosis and 10 nonmelioidosis patients, P = 0.67), and the white blood cell count was not recorded in another 13 patients. Of 77 patients with a white blood cell count available, the median white blood cell count was not different between melioidosis and nonmelioidosis patients (29,965 [IQR 9,910–105,000] versus 35,600 [IQR 12,400–173,000]/mm3, P = 0.47). The median proportion of neutrophils was also not different between the two groups (91% [IQR 81–94%] versus 90% [IQR 80–95%], P = 0.97).
Overall, 109 patients (71%) received effective antimicrobials on the day of hospital admission, and the median time from admission to effective antimicrobials were not different between the two groups (P = 0.60, Table 4). Percentages of patients who received arthrotomy washout was not significantly different between the two groups (P = 0.13). In the univariable model, the CFR of patients infected with B. pseudomallei was higher than those caused by other bacterial pathogens (34% versus 14%, P = 0.004), and CFR of patients with blood culture positive was higher than those without blood culture positive (28% [32/113] versus 10% [4/41], P = 0.02). In the multivariable logistic regression model, there was strong and borderline evidence showing that B. pseudomallei septic arthritis (adjusted odds ratio [aOR] 2.6; 95% CI 1.2–6.0, P = 0.02) and blood culture positivity (aOR 2.7; 95% CI 0.9–8.6, P = 0.09) were associated with in-hospital mortality, respectively.
Table 4.
Treatment and outcomes
| Variables | Burkholderia pseudomallei septic arthritis (N = 74) | Other septic arthritis (N = 80) | P value* |
|---|---|---|---|
| Time from admission to effective antimicrobials (days, median, IQR, range) | 0 (0–1, 0–16) | 0 (0–1, 0–12) | 0.67 |
| Received effective antimicrobial on the admission day | 54 (73%) | 55 (69%) | 0.60 |
| Arthrotomy | 53 (72%) | 47 (59%) | 0.13 |
| In-hospital mortality | 25 (34%) | 11 (14%) | 0.004 |
| Duration of hospitalization in those who survived (days, median, IQR) | 22 (14–32) | 15 (9–31) | 0.14 |
| 1-year outcome | |||
| Recurrent septic arthritis | 1/29 (3%) | 0/26 (0%) | > 0.99 |
| Deformity | 2/29 (7%) | 2/26 (7%) | > 0.99 |
| Limited range of motion without deformity | 3/29 (10%) | 7/26 (27%) | 0.16 |
IQR = interquartile range.
P value derived using χ2 test or Fisher’s exact test, or using Mann–Whitney test for the continuous variables.
Data for 1-year outcome was available in 55 of 114 (48%) patients who survived. Recurrent septic arthritis, deformity, and limit range of motion without deformity were observed in 1, 4, and 10 patients, respectively (Table 4), and those 1-year outcomes were not significantly different between melioidosis and nonmelioidosis patients. Among nonmelioidosis patients, the proportion of patients with deformity or limited range of motion without deformity was also not significantly different among cases caused by Streptococcus spp. (38%, 6/16) and cases caused by S. aureus (38%, 3/8).
We also evaluated the incidences of bacteremia in the hospitals during the study period and found that the most common causes of bacteremia were E. coli (N = 1,221, 19%), B. pseudomallei (N = 836, 13%), S. aureus (N = 719, 11%), Streptococcus spp. (N = 654, 10%), and Pseudomonas spp. (N = 592, 9%). The overall cumulative incidences were 65.6, 44.9, 38.7, 35.2, and 31.8 per 100,000 population during the 3-year study period, respectively. We also compared clinical manifestations between septic arthritis caused by Streptococcus spp. and S. aureus, and those were not significantly different between the two groups.
DISCUSSION
Our study shows that B. pseudomallei is the most common cause of septic arthritis in northeast Thailand, and patients with septic arthritis caused by B. pseudomallei have a higher CFR compared with those caused by other bacterial pathogens. In Europe and the United States, the most common cause of nongonococcal septic arthritis is S. aurues, and Streptococcus spp. are the next most common one.12 However, in our study, Streptococcus spp. are more common than S. aureus among non-B. pseudomallei septic arthritis. We did not observe the increase of cumulative incidence of Streptococcus bacteremia in general. The high proportion of Streptococcus septic arthritis in our study could relate to the reports of the emergence of group B Streptococcus13 and Streptococcus suis14 as causes of septic arthritis in Thailand. Further studies are needed to evaluate the emergence of Streptococcus arthritis in tropical developing countries. In melioidosis-endemic regions, parenteral ceftazidime could be considered as empirical antimicrobial therapy for patients with septic arthritis and underlying diseases. Choice of empirical antibiotic may need adjustment on the basis of local antimicrobial sensitivity profile of common pathogens for septic arthritis,15,16 and de-escalation to the most appropriate single-agent therapy should be performed as soon as the causative pathogen can be identified.
The high proportion of septic arthritis being caused by B. pseudomallei could be mainly because the incidence rate of melioidosis in the study area is high. We found that B. pseudomallei is the second most common cause of bacteremia during the study period after only E. coli. This is consistent with the previous finding that B. pseudomallei was also reported as the second most common cause of community-acquired bacteremia between 2004 and 2010 in northeast Thailand.17 This is also supported by the report that around 8% of culture-confirmed melioidosis patients in northeast Thailand had septic arthritis.18 There is increasing evidence suggesting that B. pseudomallei with bimA and fhaB3 genes are associated with neurological and bacteremic melioidosis, respectively.19,20 It is still unknown whether any specific genotypes of B. pseudomallei are associated with septic arthritis, and further studies are needed. The number of patients with septic arthritis of joint prosthesis was very low (two patients), and that could be due to the low rate of prosthetic implants in the region.
The high CFR of septic arthritis caused by B. pseudomallei in our study is comparable with the CFR observed in all culture-confirmed melioidosis cases in Thailand9 and is consistent with the CFR observed in B. pseudomallei septic arthritis in other lower- and middle-income countries.21 Burkholderia pseudomallei is highly virulent in humans,22 and melioidosis patients often have a rapidly progressive illness, leading to multiple organ failures and high mortality. The observed CFR for septic arthritis caused by the other bacterial pathogens (14%) is in the range of 10–15% reported by other international studies.23 The higher CFR of septic arthritis caused by B. pseudomallei is consistent with the previous finding that the mortality of patients with B. pseudomallei bacteremia was higher than those with bacteremia caused by other bacterial pathogens.24 The high CFR in patients with septic arthritis and concomitant bacteremia is also consistent with the previous findings.25,26
Diagnosis of septic arthritis caused by B. pseudomallei can be challenging, particularly in areas where melioidosis is endemic but under- or never diagnosed.10,27 This is because clinical manifestations of melioidosis can be diverse, and B. pseudomallei can be commonly misidentified as a culture contaminant or as another species (e.g., Burkholderia cepacia, Bacillus spp., or Pseudomonas spp.), especially by laboratory staff unfamiliar with this organism.28 In resource-limited areas, a simple three disc diffusion test is recommended for screening all oxidase-positive Gram-negative rods, and B. pseudomallei should be suspected if the isolate is resistant to gentamicin and colistin/polymyxin, and susceptible to co-amoxiclav.28,29 Although we observed that diabetes mellitus and the presence of lung involvement is more commonly observed in septic arthritis caused by B. pseudomallei, those are not uncommon in septic arthritis caused by the other bacterial pathogens. On the contrary, history of melioidosis and the presence of liver or splenic abscess could be highly specific for melioidosis. The presence of prostatic abscess with septic arthritis should also suspect of melioidosis5,8; however, this is not observed in our study probably because prostatic abscess is not common in melioidosis patients in Thailand.30 Educating both clinicians and technicians about diagnosis of melioidosis is necessary.7 When clinicians suspect that B. pseudomallei is a possible cause of septic arthritis, clinicians should also notify microbiological laboratories so that laboratory technicians can perform appropriate testing for any oxidase-positive Gram-negative bacilli isolated and use appropriate biosafety practices to prevent laboratory exposure.7,28
The knee is the most commonly affected joint in septic arthritis caused by both B. pseudomallei and non-B. pseudomallei. This is consistent with the previous finding of B. pseudomallei septic arthritis in Australia.31 However, this is inconsistent with the previous finding in Khon Kaen, northeast Thailand, where B. pseudomallei septic arthritis was associated with an upper-extremity joint involvement.32 Immediate washout and adequate debridement of the septic joint are always needed. For those who are culture positive for B. pseudomallei, the minimum duration of antimicrobial treatment is 2 to 4 weeks of parenteral antimicrobials followed by a subsequent 90 days oral eradication phase.7,33 The recommended duration of the parenteral phase is timed from the date of most recent draining or resection where the culture of the draining or resected material grew B. pseudomallei, and the clock is reset if a subsequent specimen is culture positive.33
One-year outcomes for joint function were good for the majority of patients who were discharged alive from the hospital. This compares favorably to previous reports of outcome from septic arthritis.23 There was no difference between long-term outcomes for B. pseudomallei septic arthritis compared with other bacterial pathogens. Although S. aureus has been reported as associated with poorer long-term outcomes,34 we did not observe any difference between long-term outcomes for Streptococcus spp. and S. aureus septic arthritis.
A limitation of this study is that the isolates were not available for further investigations, and data of long-term outcomes were available only in a proportion of patients. It is also possible that septic arthritis caused by other bacterial pathogens is under-diagnosed in our study because those patients might have received antibiotics at primary hospitals or over the counter before blood or synovial fluid specimens were collected at our referral hospital. This is unlikely to decrease the yield of bacterial culture for B. pseudomallei because the organism is intrinsically resistant to commonly used first-line antibiotics. It is also possible that for a minority of patients with positive culture from the blood only, the bacteria isolated might not represent the cause of associated joint inflammation. Our study was not designed to have enough power to compare clinical manifestations between septic arthritis caused by Streptococcus spp. and S. aureus.
CONCLUSIONS
In conclusion, we observe a high proportion of B. pseudomallei as a cause of septic arthritis in northeast Thailand, with higher mortality than for other bacterial causes of septic arthritis. Emergence of Streptococcus arthritis is observed. In melioidosis-endemic regions, a parenteral ceftazidime could be considered as empirical antimicrobial therapy for patients with septic arthritis and underlying diseases.
Acknowledgments:
We are grateful to the patients and staff of Sunpasitthiprasong Hospital, and the Wellcome Trust-Oxford University-Mahidol University Tropical Medicine Research Program. We thank Gumphol Wongsuvan, Maliwan Hongsuwan and Prapass Wannapinij for their technical assistance.
REFERENCES
- 1.Mathews CJ, Weston VC, Jones A, Field M, Coakley G, 2010. Bacterial septic arthritis in adults. Lancet 375: 846–855. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg DL, 1998. Septic arthritis. Lancet 351: 197–202. [DOI] [PubMed] [Google Scholar]
- 3.Morgan DS, Fisher D, Merianos A, Currie BJ, 1996. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol Infect 117: 423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saraux A, et al. 1997. HIV infection as a risk factor for septic arthritis. Br J Rheumatol 36: 333–337. [DOI] [PubMed] [Google Scholar]
- 5.Thomas J, Jayachandran NV, Shenoy Chandrasekhara PK, Lakshmi V, Narsimulu G, 2008. Melioidosis—an unusual cause of septic arthritis. Clin Rheumatol 27 (Suppl 2): S59–S61. [DOI] [PubMed] [Google Scholar]
- 6.Caldera AS, Kumanan T, Corea E, 2013. A rare cause of septic arthritis: melioidosis. Trop Doct 43: 164–166. [DOI] [PubMed] [Google Scholar]
- 7.Lipsitz R, et al. 2012. Workshop on treatment of and postexposure prophylaxis for Burkholderia pseudomallei and B. mallei infection, 2010. Emerg Infect Dis 18: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currie BJ, Ward L, Cheng AC, 2010. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis 4: e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limmathurotsakul D, Wongratanacheewin S, Teerawattanasook N, Wongsuvan G, Chaisuksant S, Chetchotisakd P, Chaowagul W, Day NP, Peacock SJ, 2010. Increasing incidence of human melioidosis in northeast Thailand. Am J Trop Med Hyg 82: 1113–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Limmathurosakul D, et al. 2016. Predicted global distribution of Burkholderia pseudomallei and burden of melioidosis. Nat Microbiol 1: 15008. [DOI] [PubMed] [Google Scholar]
- 11.Currie BJ, Jacups SP, Cheng AC, Fisher DA, Anstey NM, Huffam SE, Krause VL, 2004. Melioidosis epidemiology and risk factors from a prospective whole-population study in northern Australia. Trop Med Int Health 9: 1167–1174. [DOI] [PubMed] [Google Scholar]
- 12.Shirtliff ME, Mader JT, 2002. Acute septic arthritis. Clin Microbiol Rev 15: 527–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Louthrenoo W, Kasitanon N, Wangkaew S, Hongsongkiat S, Sukitawut W, Wichainun R, 2014. Streptococcus agalactiae: an emerging cause of septic arthritis. J Clin Rheumatol 20: 74–78. [DOI] [PubMed] [Google Scholar]
- 14.Takeuchi D, et al. 2012. Population-based study of Streptococcus suis infection in humans in Phayao Province in northern Thailand. PLoS One 7: e31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell JM, Turnidge JD, Cefepime Study Group , 2001. Multicentre study of the in vitro activity of cefepime, a broad-spectrum cephalosporin, compared to other broad-spectrum agents. Pathology 33: 53–60. [PubMed] [Google Scholar]
- 16.Nickerson EK, et al. 2009. Staphylococcus aureus bacteraemia in a tropical setting: patient outcome and impact of antibiotic resistance. PLoS One 4: e4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanoksil M, Jatapai A, Peacock SJ, Limmathurotsakul D, 2013. Epidemiology, microbiology and mortality associated with community-acquired bacteremia in northeast Thailand: a multicenter surveillance study. PLoS One 8: e54714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teparrakkul P, Tsai JJ, Chierakul W, Gerstenmaier JF, Wacharaprechasgu T, Piyaphanee W, Limmathurotsakul D, Chaowagul W, Day NP, Peacock SJ, 2008. Rheumatological manifestations in patients with melioidosis. Southeast Asian J Trop Med Public Health 39: 649–655. [PubMed] [Google Scholar]
- 19.Morris JL, Fane A, Sarovich DS, Price EP, Rush CM, Govan BL, Parker E, Mayo M, Currie BJ, Ketheesan N, 2017. Increased neurotropic threat from Burkholderia pseudomallei strains with a B. mallei-like variation in the bimA motility gene, Australia. Emerg Infect Dis 23: 740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarovich DS, Price EP, Webb JR, Ward LM, Voutsinos MY, Tuanyok A, Mayo M, Kaestli M, Currie BJ, 2014. Variable virulence factors in Burkholderia pseudomallei (melioidosis) associated with human disease. PLoS One 9: e91682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pandey V, Rao SP, Rao S, Acharya KK, Chhabra SS, 2010. Burkholderia pseudomallei musculoskeletal infections (melioidosis) in India. Indian J Orthop 44: 216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiersinga WJ, Currie BJ, Peacock SJ, 2012. Melioidosis. N Engl J Med 367: 1035–1044. [DOI] [PubMed] [Google Scholar]
- 23.Kaandorp CJ, Dinant HJ, van de Laar MA, Moens HJ, Prins AP, Dijkmans BA, 1997. Incidence and sources of native and prosthetic joint infection: a community based prospective survey. Ann Rheum Dis 56: 470–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suputtamongkol Y, et al. 1999. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis 29: 408–413. [DOI] [PubMed] [Google Scholar]
- 25.Lin WT, Tang HJ, Lai CC, Chao CM, 2017. Clinical manifestations and bacteriological features of culture-proven Gram-negative bacterial arthritis. J Microbiol Immunol Infect 50: 527–531. [DOI] [PubMed] [Google Scholar]
- 26.Ferrand J, El Samad Y, Brunschweiler B, Grados F, Dehamchia-Rehailia N, Sejourne A, Schmit JL, Gabrion A, Fardellone P, Paccou J, 2016. Morbimortality in adult patients with septic arthritis: a three-year hospital-based study. BMC Infect Dis 16: 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wooten MD, Panwalker AP, 2001. Septic arthritis caused by Burkholderia pseudomallei: case report and review of the literature. J Clin Rheumatol 7: 242–247. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmaster AR, et al. 2015. Melioidosis diagnostic workshop, 2013. Emerg Infect Dis 21: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dance DA, Limmathurotsakul D, Currie BJ, 2017. Burkholderia pseudomallei: challenges for the clinical microbiology laboratory-a response from the front line. J Clin Microbiol 55: 980–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng AC, Currie BJ, 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 18: 383–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morse LP, Smith J, Mehta J, Ward L, Cheng AC, Currie BJ, 2013. Osteomyelitis and septic arthritis from infection with Burkholderia pseudomallei: a 20-year prospective melioidosis study from northern Australia. J Orthop 10: 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kosuwon W, Taimglang T, Sirichativapee W, Jeeravipoolvarn P, 2003. Melioidotic septic arthritis and its risk factors. J Bone Joint Surg Am 85-A: 1058–1061. [DOI] [PubMed] [Google Scholar]
- 33.Pitman MC, Luck T, Marshall CS, Anstey NM, Ward L, Currie BJ, 2015. Intravenous therapy duration and outcomes in melioidosis: a new treatment paradigm. PLoS Negl Trop Dis 9: e0003586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goldenberg DL, Reed JI, 1985. Bacterial arthritis. N Engl J Med 312: 764–771. [DOI] [PubMed] [Google Scholar]