Abstract.
With prompt administration of appropriate antimicrobial therapy and access to modern intensive care support, fatal pediatric melioidosis is very unusual. We describe cases of two children in whom the possibility of melioidosis was recognized relatively early, but who died of the disease, despite receiving optimal supportive care. We discuss the resulting implications for bacterial virulence factors in disease pathogenesis.
INTRODUCTION
Burkholderia pseudomallei, the Gram-negative bacterium responsible for the disease melioidosis, is endemic in Northern Australia and Southeast Asia. The vast majority of infections are asymptomatic; however, in the susceptible host, infection can result in overwhelming sepsis and death.1,2
Melioidosis is predominantly a disease of adults. It is less common in children and has a lower case-fatality rate, although this varies by geographical location.3 In Southeast Asia, up to 35% of children with melioidosis die,4 but in Australia, where there is better access to health care and superior intensive care support, fatal pediatric melioidosis is exceedingly rare.2
In January 2017, two children presented with melioidosis to Cairns Hospital, a regional referral center in Far North Queensland (FNQ), Australia. Both developed rapidly overwhelming sepsis and died despite optimal intensive care support.
CASE ONE
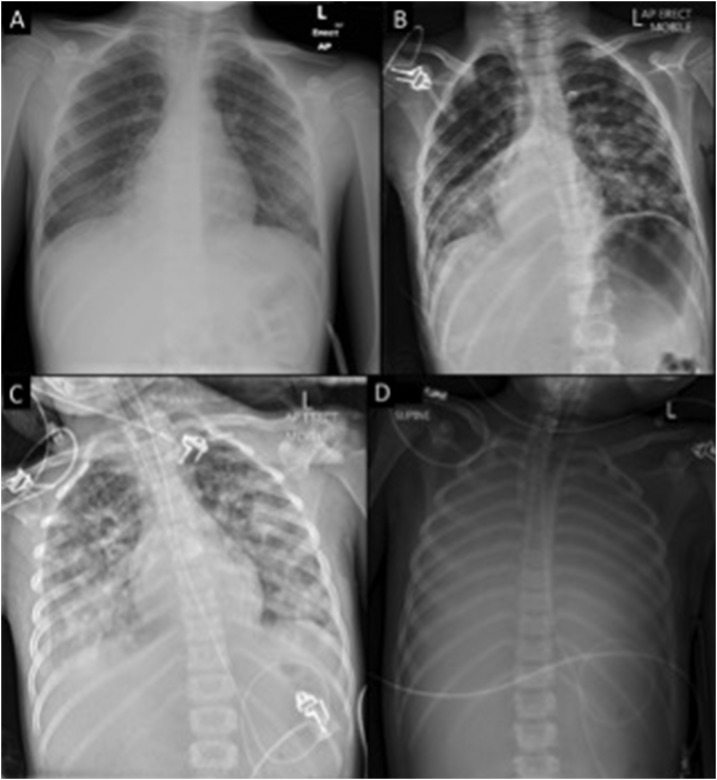
A 6-year-old male, Indigenous Australian from a remote FNQ community presented to his local health clinic with a 3-day history of fever, headache, cough, vomiting, and diarrhea. He was febrile (40.1°C), his heart rate was 122 beats per minute, his blood pressure was 105/69 mmHg, his respiratory rate was 26 breaths per minute, and his oxygen saturation was 99% on room air. A full blood count was normal, but a chest X-ray showed patchy right middle lobe consolidation (Figure 1). Blood, urine, and throat swab samples were collected for culture, empirical intravenous ceftriaxone and azithromycin were commenced, and he was referred for further management. On arrival at Cairns Hospital, 22 hours after his initial presentation, he was afebrile (36.4°C), his heart rate was 126 beats per minute, his blood pressure was 86/51 mmHg, his respiratory rate was 30 breaths per minute, and his oxygen saturation was 95% on room air. He was admitted to the pediatric ward, but despite adequate fluid resuscitation, in the ensuing 6 hours septic shock developed; meropenem, vancomycin and lincomycin were commenced, and he was admitted to the intensive care unit (ICU) for intubation, ventilation, and vasopressor support. Investigations demonstrated pancytopenia (hemoglobin 112 g/L; white cell count 0.8 × 109/L; platelet count 126 × 109/L) and a coagulopathy (international normalized ratio [INR] 1.9). Computerized tomography (CT) revealed a normal brain, dense consolidation in the right middle and bilateral lower lobes of his chest (Figure 1), but an unremarkable abdomen and pelvis. Over the subsequent 12 hours, he required high doses of norepinephrine, epinephrine and vasopressin, developed anuric renal failure and worsening coagulopathy (INR 4.9, fibrinogen 0.8 g/L, platelets 23 × 109/L). He was profoundly hypoxemic (PaO2 32 mm of Hg on FiO2 1.0) despite appropriate ventilation, muscle relaxation, recruitment maneuvers, high positive end-expiratory pressure (PEEP), and inhaled prostacyclin. Hemodynamic instability precluded prone positioning. The decision was made to aeromedically retrieve him on veno-arterial extracorporeal membrane oxygenation (VA-ECMO) to the nearest pediatric ECMO center, 1,000 miles away. VA-ECMO was commenced successfully by the retrieval team 20 hours after initial ICU admission (Figure 1). Burkholderia pseudomallei sensitive to meropenem and sulfamethoxazole/trimethoprim was identified in his initial blood cultures; meropenem was continued. Soon after arrival to the pediatric ECMO center, he developed a dilated right pupil. CT revealed a right middle cerebral artery infarct with associated edema but preserved cerebral blood flow. His severe coagulopathy precluded decompressive craniotomy and so his elevated intracranial pressure was managed medically with neuroprotective measures, including hypertonic saline. Despite maximal support he continued to deteriorate and developed bilateral dilated pupils. CT angiogram showed no intracerebral blood flow; circulatory death occurred on withdrawing VA-ECMO 4 days after his initial presentation.
Figure 1.
Case 1 chest X-ray series (A) initial (B) 12 hours post initial presentation (C) immediately before extracorporeal membrane oxygenation (ECMO) initiation (50 hours after initial presentation) (D) during ECMO therapy.
CASE TWO
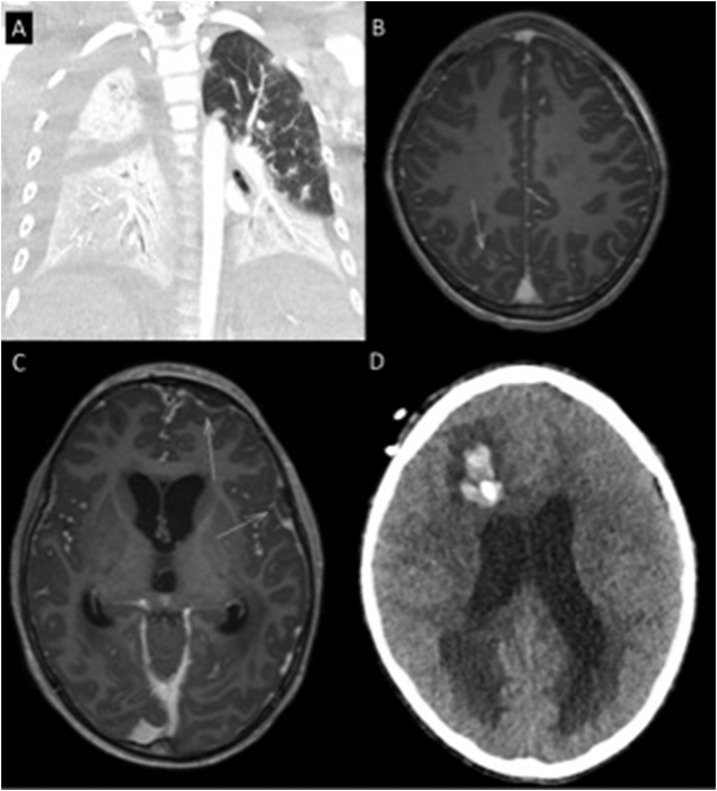
A 10-year-old boy from Papua New Guinea (PNG), presented to the health clinic on a small island near the Australian border. He had a 4-day history of fever (40°C) and increasing drowsiness (Glasgow Coma Score [GCS] 14). On presentation his heart rate was 167 beats per minute but his blood pressure was 123/93 mmHg, and he had no oxygen requirement. He was malnourished, with a weight below the third percentile. He had sustained a forehead hematoma after a 1.5 meter fall 5 days earlier. His wound had received traditional medical care including “scratching” with sticks. He received ceftriaxone at the health clinic in PNG before being transferred to the nearest Australian hospital in the Torres Strait Islands. On arrival in Australia—4 hours after presentation to the clinic in PNG—he was afebrile but his GCS had deteriorated to 8 (motor response 4, verbal response 2, and eye response 2); his blood pressure was 95/60 mmHg, his heart rate was 130 beats per minute, and his oxygen saturation was 85% on room air. Within an hour, he was intubated and empirical meropenem and vancomycin had been commenced. He was evacuated aeromedically for ICU care. During transfer, he developed shock and profound hypoxia. On arrival at our hospital, he was pancytopenic (hemoglobin 95 g/L, leukocyte count 0.5 × 109/L, and platelet count 12 × 109/L). CT imaging showed bilateral lung consolidation (Figure 2) but a normal brain and abdomen. Empirical lincomycin, azithromycin, and oseltamivir were added to his meropenem and vancomycin. His respiratory failure evolved, requiring paralysis, high PEEP, and continuous inhaled prostacyclin. He required high-dose norepinephrine, epinephrine, and vasopressin. His initial blood cultures from the Torres Strait hospital grew B. pseudomallei, sensitive to meropenem and sulfamethoxazole/trimethoprim. His marked thrombocytopenia, requiring regular platelet transfusions, precluded addition of sulfamethoxazole/trimethoprim and so meropenem monotherapy was continued.
Figure 2.
Case 2 (A) computerized tomography (CT) chest bilateral infiltrates (B) magnetic resonance imaging (MRI) brain (postcontrast T1 axial) intracerebral abscess highlighted with arrow (C) MRI brain (postcontrast T1 axial) subdural empyemas highlighted with arrows (D) CT brain hydrocephalus and intracerebral hemorrhage associated with external ventricular drain.
He improved, permitting weaning of his vasopressor and ventilator support, although his profound thrombocytopenia persisted. However, on day 5, he developed worsening respiratory failure, necessitating reintroduction of inhaled prostacyclin and prone positioning. He was transferred to the nearest specialized pediatric ICU, 200 miles away. He developed multiple complications over the next 7 days despite ongoing antimicrobial treatment with high-dose meropenem and trimethoprim-sulfamethoxazole. Magnetic resonance imaging (MRI) of the brain showed a subdural empyema, a parieto-occipital intracerebral abscess, and a possible pituitary abscess with associated communicating hydrocephalus and early cerebellar tonsillar herniation (Figure 2). External ventricular drains were inserted. He developed multiple soft tissue abscesses including a soleal abscess requiring drainage, bilateral infrascapular infection, left hip septic arthritis, and osteomyelitis of the skull and the right tibia. His neurological status deteriorated further and repeat MRI showed persisting hydrocephalus, worsening tonsillar herniation, and a left frontal infarct. Given his poor prognosis, after discussion with his family, the decision was made to withdraw treatment. He died 2 weeks after his initial presentation.
DISCUSSION
Earlier disease recognition and improvements in ICU care have seen a dramatic fall in melioidosis-related mortality in Australia in recent decades.4,5 A fatal case of pediatric melioidosis is now very unusual in the country. In a series from the Royal Darwin Hospital, the Australian hospital with the highest caseload of melioidosis, all five children admitted to ICU during the 24-year study period survived.6
Children with melioidosis present differently to adults. Most adults are bacteremic and pneumonia is the most frequent clinical manifestation. However, in the largest Australian pediatric series, pneumonia and bacteremia occurred rarely, instead skin and soft tissue infections were the most common clinical findings.6
The presentation of two children in this series, and their deaths in the same month despite optimal ICU management, is therefore striking. Both had severe multilobar pneumonia resulting in respiratory failure and bacteremia which precipitated shock. The first child had no predisposing factors or clear inoculating events. Appropriate antimicrobial therapy, vasopressor, and ventilatory support were commenced in a tertiary referral center within hours of his sepsis being recognized, but he continued to deteriorate. This is the first case—to our knowledge—of ECMO being used in the management of melioidosis and demonstrates the excellent level of support that patients are able to receive in Australia, although sadly in this case, it was to no avail. The second child was malnourished and presented relatively late in his disease course. Although meropenem was commenced within hours of his acute deterioration, his infection continued to evolve.
Although both children were neutropenic, neither received granulocyte colony-stimulating factor (G-CSF), which one retrospective series suggested improved outcomes in melioidosis.7 However, a subsequent randomized control trial8 demonstrated no mortality benefit with G-CSF administration and so, although the drug was considered in these cases, it was not administered to either child.
It has long been recognized that the clinical presentation of melioidosis can vary enormously.9 The strong association with the wet season and classical risk factors of diabetes mellitus, hazardous alcohol use and chronic lung and kidney disease emphasize the importance of environmental and host factors.10 However, it is likely that the pathogenic potential of different strains of the organism also plays a role. Burkholderia pseudomallei has a highly variable genome. Indeed, the bacterium has one of the most complex genomes sequenced to date.11 Multiple potential virulence factors have been described for B. pseudomallei, but their association with specific clinical manifestations has not yet been defined.12 In FNQ, the rates of bacteremia in adults with melioidosis are among the highest ever reported, although whether this relates to differences in the pathogenic potential of local B. pseudomallei strains, or differences in local case finding, remains to be elucidated.10
Melioidosis is rare in children in Australia, and this contributed to the delay in the initiation of appropriate antibiotic therapy, decreasing the children’s likelihood of survival.13 However, their subsequent rapid deterioration and deaths emphasize the lethal potential of the organism, even where optimal medical support is available.
REFERENCES
- 1.Gibney KB, Cheng AC, Currie BJ, 2008. Cutaneous melioidosis in the tropical top end of Australia: a prospective study and review of the literature. Clin Infect Dis 47: 603–609. [DOI] [PubMed] [Google Scholar]
- 2.Currie BJ, Ward L, Cheng A, 2010. The epidemiology and clinical spectrum of melioidosis: 540 cases from the 20 year Darwin prospective study. PLoS Negl Trop Dis 4: e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Limmathurotsakul D, Peacock SJ, 2011. Melioidosis: a clinical overview. Br Med Bull 99: 125–139. [DOI] [PubMed] [Google Scholar]
- 4.Udayan U, Chandrakar S, Dias A, Dias M, 2015. A new threat to children: melioidosis. Pediatr Infect Dis 6: 135–138. [Google Scholar]
- 5.Stephens DP, Thomas JH, Ward LM, Currie BJ, 2016. Melioidosis causing critical illness: a review of 24 years of experience from the royal darwin hospital ICU. Crit Care Med 44: 1500–1505. [DOI] [PubMed] [Google Scholar]
- 6.McLeod C, Morris PS, Bauert PA, Kilburn CJ, Ward LM, Baird RW, Currie BJ, 2015. Clinical presentation and medical management of melioidosis in children: a 24-year prospective study in the northern territory of Australia and review of the literature. Clin Infect Dis 60: 21–26. [DOI] [PubMed] [Google Scholar]
- 7.Cheng AC, Stephens DP, Anstey NM, Currie BJ, 2004. Adjunctive granulocyte colony-stimulating factor for treatment of septic shock due to melioidosis. Clin Infect Dis 38: 32–37. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AC, et al. 2007. A randomized controlled trial of granulocyte colony-stimulating factor for the treatment of severe sepsis due to melioidosis in Thailand. Clin Infect Dis 45: 308–314. [DOI] [PubMed] [Google Scholar]
- 9.Cheng AC, Currie BJ, 2005. Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 18: 383–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart JD, Smith S, Binotto E, McBride WJ, Currie BJ, Hanson J, 2017. The epidemiology and clinical features of melioidosis in far north Queensland: implications for patient management. PLoS Negl Trop Dis 11: e0005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden MT, et al. 2004. Genomic plasticity of the causative agent of melioidosis, Burkholderia pseudomallei. Proc Natl Acad Sci USA 101: 14240–14245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiersinga WJ, Currie BJ, Peacock SJ, 2012. Melioidosis. N Engl J Med 367: 1035–1044. [DOI] [PubMed] [Google Scholar]
- 13.Rhodes A, et al. 2017. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 45: 486–552. [DOI] [PubMed] [Google Scholar]