Clinical medicine and medical research have long occupied disease-specific specialties. A visitor to any hospital or university in a developed country will likely find a cardiology department or an institute of cancer research but frequently these are widely separated or even in different buildings. This is reflected in clinical research where cancer is a frequent exclusion criterion from cardiovascular trials and likewise patients with established heart disease are often routinely excluded from cancer studies. As a result our understanding of how cancer, cardiovascular disease and their treatments interact remains relatively limited. This is despite the fact that improved treatments for both conditions have resulted in substantially improved survival rates and as a result, an ever larger population of patients will now experience both conditions in their lifetime.
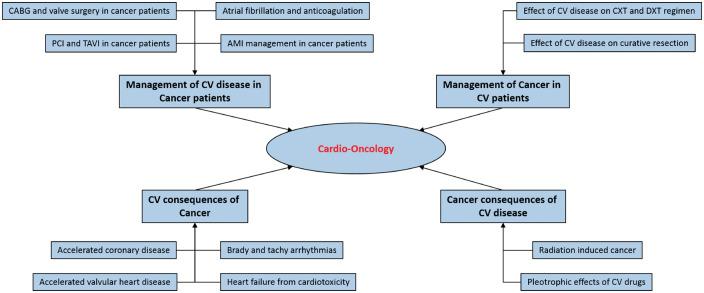
Cardio-oncology as a clinical and research speciality began with an initial limited focus on the cardio-toxic effects of chemotherapeutic agents such as the anthracyclines but must now evolve to encompass the full complexity of clinical interactions between cancer and cardiovascular diseases. It can be divided into four key areas (Figure 1):
Figure 1.
Schematic of the key clinical and research elements of cardio-oncology. AMI, acute myocardial infarction; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation; CXT, chemotherapy; DXT, radiotherapy; CV, cardiovascular.
Management of cardiovascular disease in cancer patients
Atrial fibrillation
Research by Melloni et al in this issue (ref) contributes to increasing evidence that cancer and cardiovascular disease frequently coexist and may interact in ways which affect patient outcomes. The authors investigate the impact of a prior cancer diagnosis on anticoagulation and bleeding in patients recruited to the ORBIT-AF registry. The first key finding is that almost a quarter of patients (23.8%) had a history of cancer at enrolment confirming that, far from a trivial subgroup, co-morbid patients constitute a substantial minority of this population. It is perhaps reassuring that there was little evidence of therapeutic discrimination in the cancer population, although this may reflect an inevitable selection bias towards guideline-based practice in a registry population. The authors go on to show a significantly higher risk of major bleeding in the cancer patient population (hazard ratio = 1.21, 95% confidence interval 1.04–1.40; P = 0.0155). Many questions remain. No data were available to the authors on cancer status, site, stage, or treatment. This cancer population will be very heterogeneous and the context of the additional major bleeds (e.g. gastrointestinal cancer, metastatic disease, etc.) remains speculative. This is observational and not randomized data. The cancer patients were older, more multi-morbid and had both higher thromboembolic and higher bleeding risk score values. However, whilst caution is required prior to translation into clinical practice, these data do suggest the cancer-AF population may have important differences in their risk of anticoagulation, a finding which merits further investigation.
Stable ischaemic and valvular heart disease
A prior cancer diagnosis also has the potential to influence treatment choices for other cardiovascular diseases. This is particularly likely where operative or procedural interventions are under consideration. There is however a paucity of data from contemporary patients with advanced stable valvular or coronary artery disease who have co-existent cancer, although outcomes from cardiac surgery are reportedly worse following thoracic radiotherapy than for the general surgical population.1 It is not known if inappropriate prognostic risk stratification could be leading to some eligible cancer patients being denied surgical or procedural options to treat their cardiovascular disease.
Acute coronary syndromes
Outcomes of primary percutaneous coronary intervention (PPCI) for acute myocardial infarction (AMI) in a cancer subpopulation are reported to be significantly worse than in non-cancer patients.2 Adoption of critical elements of current guideline-based approaches to the management of AMI (such as the use of PPCI with drug eluting stents and the duration of dual antiplatelet therapy) may be influenced by concerns about the potentially adverse impact of an inter-current cancer diagnosis. This may be exaggerated by an inappropriately negative perception of prognosis amongst specialists not involved in the contemporary day-to-day management of cancer patients. There is evidence that patients with cancer who present with AMI are treated less intensively.2 It remains unclear if this is appropriate.
Management of cancer in patients with cardiovascular disease
The approach to cancer treatment depends on the cancer site and stage as well as the histological and molecular phenotype. Crucially however, patient co-morbidities are central to decision-making about treatment strategy. Potentially curative cancer treatment with surgery, chemotherapy, radiotherapy, and targeted agents (or combinations of these modalities) is frequently more physiologically demanding than palliative treatment which is intended to relieve symptoms and prolong life where possible. As with cardiothoracic surgery above, perceptions of co-morbid risk are therefore a key determinant of treatment decisions. Central to this is a history of cardiovascular disease. This may, for example, explain some of the regional variations reported in colorectal hepatic metastatectomy3 and lung cancer resection rates.4 There is little evidence to support percutaneous coronary revascularization prior to cancer surgery. However, anecdotally patients are frequently referred for consideration of this.5 It also remains unclear if delays in curative cancer treatment to allow for detailed functional assessment of underlying ischaemic heart disease are outweighed by the benefits of enhanced risk stratification. A better understanding of the impact of ischaemic heart disease on the risk of curative cancer strategies, including surgery, has considerable potential to enhance patient-centred (stratified) care.
Cardiovascular sequelae in cancer patients
Cardiomyopathies and heart failure
Many common chemotherapeutic agents used for the treatment of cancer have substantial short- and longer-term risks of cardiotoxicity.6 Perhaps the best characterized example is anthracycline induced cardiomyopathy.7 The effects are cumulative and dose dependent with evidence of diastolic dysfunction occurring at lower doses than systolic dysfunction. Whilst there can be a considerable delay before the onset of overt cardiomyopathy, recent data suggest initial subclinical onset within the first year after treatment.8 A high proportion (82%) respond, at least partially, to treatment although this response may not be maintained in the long term.9 Cyclophosphamide and the antimetabolites are also associated with cardiomyopathies. When used together, adverse effects may be synergistic.7 The relative contribution of other treatable cardiovascular risk factors remains poorly quantified and may be relevant as these agents are increasingly used in populations with pre-existent cardiovascular disease.
Accelerated ischaemic and valvular heart disease
An increased incidence of ischaemic heart disease has been reported in survivors of childhood cancer.10,11 Thoracic radiotherapy in particular is associated with accelerated atherosclerotic coronary disease,12 whilst chemotherapeutic agents have been associated with early acute myocardial infarction (although their contribution to late onset accelerated coronary disease is less clear). It is clear this increased risk with radiotherapy is sustained over long periods after the index treatment and interacts adversely with other established cardiovascular risk factors. Thoracic radiotherapy is also associated with valvular heart disease.13 Both aortic and mitral valves may be affected, but with significant latency and accelerated progression. Once again established cardiovascular risk factors appear to exacerbate this process.
Other cardiovascular conditions
Bradyarrhythmias have been reported in a number of anti-cancer treatments including thalidomide. There are also reports of tachyarrhythmias, associated with prolongation of the QT interval. Interaction with pre-existent medications is a frequent contributory element. It has recently been reported that young adult survivors of cancer experience excess hospitalizations for a whole range of cardiovascular conditions including hypertension, pulmonary heart disease, and conduction disorders.11
Cancer sequelae in cardiovascular patients
Just as cancer treatments are associated with long-term cardiovascular sequelae, there is emerging evidence that established cardiovascular treatments impact on cancer outcomes. For example, radiation exposure during cardiovascular interventions increases cancer risk.14,15 The risk appears greater in younger patients (especially children) and women.15 Conversely, epidemiological studies in patients prescribed aspirin for cardiovascular indications describe a beneficial effect on cancer outcomes and a reduction in metastatic spread of solid tumours.16 Other established cardiovascular drugs whose anticancer properties are under investigation include beta blockers, angiotensin converting enzyme (ACE) inhibitors, and statins.
Summary
Understanding of key interactions between cardiovascular disease, cancer and their respective treatments remains a key unmet clinical need and is essential to continued improvements in the outcomes of patients living with both conditions. Insights from observational studies such as the work of Melloni et al and. Big Data projects such as the VICORI initiative (virtual cardio-oncology research institute—a UK programme to link national cardiovascular and oncology audit data) promise to greatly enhance our understanding. Ultimately progress towards closer integration of clinical care between specialties holds potential for future hypothesis driven cardio-oncology research studies to directly inform clinical practice
Acknowledgements
D. A. and M. D. P. lead the VICORI initiative co-funded by the British Heart Foundation and Cancer Research UK. Support is additionally acknowledged from the Department of Cardiovascular Medicine at the University of Leicester and the NIHR Leicester Cardiovascular Biomedical Research Unit
Conflicts of interest: D.A. has received research grant funding from Astra Zeneca inc. for research into the anti-metastatic effects of antiplatelet drugs.
References
- 1. Wu W, Masri A, Popovic ZB, Smedira NG, Lytle BW, Marwick TH, Griffin BP, Desai MY. Long-term survival of patients with radiation heart disease undergoing cardiac surgery: a cohort study. Circulation 2013;127:1476–1485. [DOI] [PubMed] [Google Scholar]
- 2. Velders MA, Boden H, Hofma SH, Osanto S, van der Hoeven BL, Heestermans AA, Cannegieter SC, Jukema JW, Umans VA, Schalij MJ, van Boven AJ. Outcome after ST elevation myocardial infarction in patients with cancer treated with primary percutaneous coronary intervention. Am J Cardiol 2013;112:1867–1872. [DOI] [PubMed] [Google Scholar]
- 3. Jones RP, Vauthey JN, Adam R, Rees M, Berry D, Jackson R, Grimes N, Fenwick SW, Poston GJ, Malik HZ. Effect of specialist decision-making on treatment strategies for colorectal liver metastases. Br J Surg 2012;99:1263–1269. [DOI] [PubMed] [Google Scholar]
- 4. Beckett P, Woolhouse I, Stanley R, Peake MD. Exploring variations in lung cancer care across the UK–the ‘story so far’ for the National Lung Cancer Audit. Clin Med (Lond) 2012;12:14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanon S, Rihal CS. Non-cardiac surgery after percutaneous coronary intervention. Am J Cardiol 2014;114:1613–1620. [DOI] [PubMed] [Google Scholar]
- 6. Reulen RC, Winter DL, Frobisher C, Lancashire ER, Stiller CA, Jenney ME, Skinner R, Stevens MC, Hawkins MM, British Childhood Cancer Survivor Study Steering G. Long-term cause-specific mortality among survivors of childhood cancer. JAMA 2010;304:172–179. [DOI] [PubMed] [Google Scholar]
- 7. Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol 2009;53:2231–2247. [DOI] [PubMed] [Google Scholar]
- 8. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM. Early Detection of Anthracycline Cardiotoxicity and Improvement With Heart Failure Therapy. Circulation 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 9. Lipshultz SE, Franco VI, Miller TL, Colan SD, Sallan SE. Cardiovascular disease in adult survivors of childhood cancer. Annu Rev Med 2015;66:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mulrooney DA, Nunnery SE, Armstrong GT, Ness KK, Srivastava D, Donovan FD, Kurt BA, Metzger ML, Krasin MJ, Joshi V, Durand JB, Robison LL, Hudson MM, Flamm SD. Coronary artery disease detected by coronary computed tomography angiography in adult survivors of childhood Hodgkin lymphoma. Cancer 2014;120:3536–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Laar M, Feltbower RG, Gale CP, Bowen DT, Oliver SE, Glaser A. Cardiovascular sequelae in long-term survivors of young peoples' cancer: a linked cohort study. Br J Cancer 2014;110:1338–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, Correa C, Cutter D, Gagliardi G, Gigante B, Jensen MB, Nisbet A, Peto R, Rahimi K, Taylor C, Hall P. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987–998. [DOI] [PubMed] [Google Scholar]
- 13. Cutter DJ, Schaapveld M, Darby SC, Hauptmann M, van Nimwegen FA, Krol AD, Janus CP, van Leeuwen FE, Aleman BM. Risk of valvular heart disease after treatment for Hodgkin lymphoma. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson JN, Hornik CP, Li JS, Benjamin DK Jr, Yoshizumi TT, Reiman RE, Frush DP, Hill KD. Cumulative radiation exposure and cancer risk estimation in children with heart disease. Circulation 2014;130:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lawler PR, Afilalo J, Eisenberg MJ, Pilote L. Comparison of cancer risk associated with low-dose ionizing radiation from cardiac imaging and therapeutic procedures after acute myocardial infarction in women versus men. Am J Cardiol 2013;112:1545–1550. [DOI] [PubMed] [Google Scholar]
- 16. Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet 2012;379:1591–1601. [DOI] [PubMed] [Google Scholar]