Abstract
Purpose of review
Persuasive statistics support the clinical observation that because of cardiovascular comorbidities patients with inflammatory joint disease die significantly earlier despite anti-inflammatory therapy.
Recent findings
The reason for this earlier death is multifactorial and involves a combination of a complex genetic background, environmental influences, classical cardiovascular risk factors and the impact of anti-inflammatory therapy. We will describe the importance of several new mechanisms, especially the diverse intercellular communication routes including extracellular vesicles and microRNAs that support the development of cardiovascular comorbidities.
Summary
The aim of this review is to give an updated overview about the known risk factors in the development of cardiovascular comorbidities with the latest insights about their mechanism of action. Furthermore, the impact of newly identified risk factors and significance will be discussed.
Keywords: cardiovascular comorbidities, extracellular vesicles, microRNA, psoriatic arthritis, rheumatoid arthritis, systemic lupus erythematosus
INTRODUCTION
Before the appearance of the first disease-modifying drugs (DMARDs), the diagnosis of rheumatoid arthritis inevitably lead to a painful, progressive inflammatory arthropathy with joint erosion, deformation and loss-of-function. Nowadays with aggressive treatment (treat-to-target strategy), low disease activity or even remission have become realistic goals, improving the quality of life of these patients. Despite significant advances in treatment, patients die significantly earlier than the general population because of cardiovascular comorbidities, mostly in connection with accelerated atherosclerosis [1▪]. This clinical finding was underpinned by several epidemiological studies, which were followed by a new era of investigations aiming to understand the underlying pathophysiological mechanisms of this phenomenon. Lindhardsen et al.[2] examined the risk of acute myocardial infarction (AMI) in rheumatoid arthritis and stated that the risk of AMI was as high as the risk of AMI in patients with diabetes mellitus. Further results were recently published by Ruscitti et al., presenting the results of a one-year prospective single centre study of patients suffering from rheumatoid arthritis. They quantified the increased risk for cardiovascular events (CVEs) and showed that the percentage of patients suffering a CVE and/or displaying subclinical atherosclerosis doubled within 12 months [3]. Another epidemiological study from Spain measured comorbidities in rheumatoid arthritis patients with a mean disease duration of 10 years and demonstrated a 51% prevalence of a Framingham Risk Score over 20%, resulting in a frequency of 5 and 1% of AMI and stroke, respectively [4▪].
With a mean follow-up of 5.8 years in an international cohort, diverse risk factors and CVD outcomes were collected from 5638 patients with rheumatoid arthritis and found that a total of 70% of CVD events were attributable to classical cardiovascular risk factors, Disease Activity Score and seropositivity combined. This demonstrates how important it is to closely monitor disease activity and cardiovascular risk factors in rheumatoid arthritis patients, but it also shows the need for defining the ‘missing’ 30% responsible for cardiovascular comorbidities [5].
The present review aims to summarize current knowledge about the contributing factors for cardiovascular comorbidities, from the genetic background and known cardiovascular risk factors through to the newest research results, highlighting particularly those related to extracellular vesicles, microRNAs (miRNAs) and their synergy, resulting in the higher morbidity and mortality rates in these patients.
Box 1.
no caption available
GENETIC BACKGROUND OF CARDIOVASCULAR COMPLICATIONS IN AUTOIMMUNE JOINT DISEASE
The investigation of the genetic background of complex diseases is challenging. The search for genetic components for comorbidities of these autoimmune disorders (which are also of multifactorial origin) is even more challenging. The genetic basis of autoimmune joint disease is confirmed by classical genetic studies, for example twin and family studies [6]. A twin study conducted in 2000, which included over 13 000 twin pairs from Finland and the United Kingdom, estimated a contribution of genetic factors of around 60% [7].
A large number of candidate gene studies investigated the most different genes and their single nucleotide polymorphisms (SNP) in the development of cardiovascular comorbidities in patients with rheumatoid arthritis [8–10]. Starting points of candidate gene association studies are the physiology and pathophysiology of genes encoding for proteins with known function and their involvement in the development of a given phenotype. Thus, genetic loci are selected upon their potential biological function. There is a recent excellent summary of the results of these studies [1▪] showing that in addition to the well known HLA-DRB1∗ 01/04 shared epitope, several other genetic variants located inside and outside of the HLA region on the sixth chromosome may be of influence on the risk of cardiovascular disease (CVD) in rheumatoid arthritis. In addition, most variants outside the HLA region with a positive correlation to the elevated cardiovascular risk among rheumatoid arthritis patients on the basis of this review, are connected to genes encoding for proteins of cells and molecules of the immune system, including the tumor necrosis factor (TNF) superfamily genes, cytokines and related genes, chemokines, or adipokines [1▪]. Furthermore, correlations have also been found with variants of genes involved in nitric oxide synthesis [11] and vitamin D levels [12]. Finally, there are some additional exciting potential associations with other, seemingly unrelated genes, such as MTHFR, important in the homocysteine plasma level homeostasis [13].
Ten genes (CRP, HNF1A, LEPR, GCKR, NLRP3, IL1F10, PPP1R3B, ASCL1, HNF4A, and SALL1) known to have an impact on the serum level of CRP in nonrheumatic Caucasians were genotyped in rheumatoid arthritis patients. It was assessed whether they were of influence on the development of CVEs and subclinical atherosclerosis in this special clinical subgroup. Interestingly, no association could be shown between these genes and CVEs in rheumatoid arthritis [14].
CLASSICAL RISK FACTORS OF CARDIOVASCULAR DISEASES ARE MORE COMMON AMONG PATIENTS WITH AUTOIMMUNE DISORDERS
Smoking
Smoking is a well known risk factor of both autoimmune diseases and accelerated atherosclerosis. In autoimmune diseases, smoking was shown to modulate the immune system in various ways, namely the induction of the inflammatory response, alteration of cytokine balance, induction of apoptosis, and DNA damage resulting in the formation of anti-DNA antibodies [15]. There are a variety of studies that have addressed this area [16–18]. The harmful effect of smoking has been described in early atherogenesis especially on endothelial cells [19]. Effects are mediated through low NO bioavailability, followed by increased adhesion molecule expression and subsequent endothelial dysfunction. A procoagulant and inflammatory milieu is generated by the increased adherence of platelets and macrophages. Macrophages migrate under the endothelial cells, take up oxidized lipoproteins and transform into foam cells.
Several studies reported interactions between smoking and different factors that are predictive of the cardiovascular outcome [rheumatoid factor; anti-citrullinated protein antibodies (ACPA) positivity, rheumatoid nodules, anti-TNF treatment, rheumatoid cachexia] [20]. This complicates the estimation of the extent of contribution of smoking to cardiovascular risk in patients with rheumatoid arthritis. Importantly, not only smoking, but also second-hand (passive) smoking has an impact on disease activity in women with rheumatoid arthritis [21▪].
Insulin resistance
Despite the updated recommendations from European League Against Rheumatism (EULAR) for the management of cardiovascular risk factors in patients with inflammatory arthritis, type 2 diabetes (T2D) is still underdiagnosed und undertreated. Ruscitti et al.[22] claim the poor clinical response for this as the main risk factor. A recent cross-sectional study demonstrated that the prevalence of both T2D and impaired fasting glucose (IFG) was higher in patients with rheumatoid arthritis compared with age-matched and sex-matched controls [23]. Furthermore, they were associated with both rheumatoid arthritis-specific features and traditional cardiovascular risk factors [23].
Dyslipidaemia
Adverse changes in the lipid profile are one of the main risk factors of cardiovascular morbidity and mortality. However, it is not evident to which level targeting the different lipoprotein subpopulations reduces the risk of CVEs. In a recently published prospective study based on data from over 50 000 patients with hypertension, dyslipidaemia or diabetes mellitus, an association was found between high-density lipoprotein (HDL)-cholesterol, total/HDL-cholesterol and triglyceride/HDL-cholesterol ratios, and a higher risk for CVD, in contrast to other common lipid profile biomarkers [24]. Recent observations show that small dense low-density lipoprotein (LDL) particles (sdLDL) are elevated not only in diverse metabolic disorders, but also in rheumatoid arthritis and psoriatic arthritis (PsA) [25]. This lipoprotein subgroup is especially important as it seems to be particularly atherogenic, and seems to be a good predictor of significant coronary artery stenosis. This is because of its high susceptibility to oxidation, high endothelial permeability, and decreased LDL receptor affinity [26].
Sudden cardiac death is twice as common among rheumatoid arthritis patients as in the general population. Regarding this, the importance of close lipid management is highlighted by the recent results of Turk et al.[27], showing a close relationship between prolonged QRS time and elevated total cholesterol.
Arterial hypertension
A study of cross-sectional design with multistage sampling, involving 2455 Chinese hypertensive patients, clearly demonstrated the importance of patient compliance. The percentage of noncompliant patients and the rate of suboptimal blood pressure control were both above 45%. In addition, multimorbidity was also more frequent in these patients, accentuating the importance of more clinical attention to this subgroup of patients [28▪▪].
Among classical cardiovascular risk factors, hypertension has the highest incidence and prevalence both in rheumatoid arthritis (74 cases per 1000 patient-years; 18.6% of patients) and psoriatic arthritis (79.8 cases per 1000 patient-years; 19.9% of patients) [29]. Not only blood pressure itself but arterial inflammation is more prevalent in patients with rheumatoid arthritis and is independently associated with both traditional cardiovascular risk factors and rheumatoid arthritis-disease characteristics [30▪▪].
Another important aspect of close cardiovascular risk management is demonstrated in a recent analysis of retrobulbar blood flow and choroidal thickness of rheumatoid arthritis patients, which showed a significantly higher peak systolic velocity of the ophthalmic and central retinal artery in rheumatoid arthritis patients compared with healthy controls [31].
Physical activity
Carlsson et al. stimulated peripheral blood mononuclear cells from children with high versus average physical activity. The authors found that high physical activity was associated with lower immune reactivity toward autoantigens GAD65, HSP60, and IA-2 and also with lower spontaneous pro-inflammatory immune activity [32▪].
However, physical activity in juvenile idiopathic arthritis (JIA) patients can be a double-edged sword as at times of acute flare, exercise may be very painful for these patients. Also, physical activity may exacerbate underlying inflammatory processes. Through exercise, the secretion of various hormones, miRNAs, and peptides are influenced and it seems that muscle cell-derived IL-6 has a central role in the fine balancing of anti-inflammatory and pro-inflammatory cytokines [33].
Hyperhomocysteinaemia
Taking into consideration that the risk for cardiovascular comorbidities is still underestimated in patients with rheumatoid arthritis, it is thought-provoking that in the Journal of Rheumatology in 1998, attention had already been drawn to the importance of folic acid supplementation to prevent folate deficiency and hyperhomocysteinaemia and, if necessary, to prevent Methotrexate (MTX) toxicity [34]. Essouma and Noubiap [35] emphasized the importance of the bidirectional link between immunoinflammatory activation and hyperhomocysteinaemia. Hyperhomocysteinaemia may lead to nuclear kappa B enhancement and vice versa, chronic immune activation causes hyperhomocysteinaemia through vitamin B (including folic acid) depletion. The authors also underline the importance of folic acid supplementation in preventing cardiovascular complications in rheumatoid arthritis [35]. In cutaneous lupus erythematosus, the level of homocysteine is correlated with disease severity [36]. The C677T polymorphism in the MTHFR gene, important in the re-methylation of homocysteine, varies depending on geography and ethnicity [37].
Vitamin D level
The CIMESTRA trial has recently shown the importance of optimal vitamin D serum levels in patients with rheumatoid arthritis. The study found that low baseline vitamin D metabolite levels associate with long-term CVEs in patients suffering from rheumatoid arthritis [38▪]. Neuropathic pain is often a therapeutic challenge in chronic inflammatory diseases. In a recent cross-sectional study, patients suffering from rheumatoid arthritis filled out the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) questionnaire and their serum vitamin D levels were measured. An association was shown between low-serum vitamin D levels and increased neuropathic pain, which underlines again the importance of optimal vitamin D serum levels [39].
Obstructive sleeping apnoea
A current population-based study showed that obstructive sleep apnoea has a higher incidence in patients with rheumatoid arthritis as compared with age-matched and sex-matched controls. Considering the importance of obstructive sleep apnoea in predicting the future CVD risk, it may be important to screen patients for obstructive sleep apnoea [40▪].
Diet
The higher risk of cardiovascular comorbidities among patients with inflammatory joint disease consuming higher amounts of sodium is not the only consequence: sodium has an impact on the Th17 pathway activation and it can, thus, promote autoimmunity. A recent study shows an increased sodium excretion in patients with early rheumatoid arthritis [41]. Not only in-vitro evidence shows an anti-inflammatory effect of trans-resveratrol, the major cardioprotective component of red wine, but in preclinical models of osteoarthritis and rheumatoid arthritis. A joint protective effect through decreased production of pro-inflammatory and pro-degenerative soluble factors of trans-resveratrol was shown [42]. Fish consumption seems to be protective not only in decreasing the CVD risk through, for example, lowering triglycerides and increasing HDL serum levels [43], but has also been shown to impact on rheumatoid arthritis, associated with lower disease activity [44].
INFLAMMATION
Neutrophil to lymphocyte ratio is not only a reliable marker for inflammation in neoplastic and cardiovascular disorders, but a recent study shows its reliability also for disease activity in rheumatoid arthritis [45]. Low disease activity, defined by a disease activity score (DAS, 28) lower than 3.2 results in a reduced risk in cardiovascular complication in patients suffering from rheumatoid arthritis [46▪]. The magnitude and period of time of elevated CRP serum levels correlates with an increased risk of cardiovascular complications in rheumatoid arthritis [47]. Myocardial infarction is one of the main complications of accelerated atherosclerosis in rheumatoid arthritis patients and CRP serum level is also associated with AMI [48]. Thus, Meissner et al. declare that it is seemingly irrelevant which DMARD is administered but the goal must be the quickest effective disease control.
Symmetric dimethylarginine (SDMA) and asymmetric dimethylarginine (ADMA) emerge as novel biomarkers of CVDs. Their levels are also abnormal in patients with rheumatoid arthritis. Dimitroulas et al.[49] analyzed their presence in rheumatoid arthritis patients and the results revealed that these molecules may promote endothelial injury in rheumatoid arthritis patients as a result of systemic inflammation during active disease periods.
DOES TREATMENT INFLUENCE CARDIOVASCULAR RISK?
Treatment of inflammatory joint disease should aim to reduce the cardiovascular risk accompanied with the disease, which is best achieved with a treat-to-target approach [50]. This is underpinned by a time-dependent Cox regression analysis of the Nijmegen early rheumatoid arthritis inception cohort, which showed that low disease activity was significantly associated with reduced risk of first CVE [46▪]. The effect of glucocorticosteroids on the cardiovascular risk is still questionable. On the one hand, glucocorticosteroid-induced hypertension, insulin resistance, and metabolic effects (especially insulin resistance and obesity) increase the cardiovascular risk, but on the other hand, attenuating inflammation is beneficial [51,52]. Nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk of CVD in the general population; diclofenac has a similar cardiovascular risk to rofecoxib [53]. The cardiovascular safety profile of COX2 selective and nonselective NSAIDs in rheumatoid arthritis and osteoarthritis was recently published; celecoxib was found to be noninferior to naproxen or ibuprofen in this study [54]. NSAIDs, especially COX2 inhibitors, increase the cardiovascular risk in rheumatoid arthritis [51].
Probably because of a blood pressure lowering and anti-inflammatory effect, methotrexate therapy seems to decrease the cardiovascular risk in rheumatoid arthritis [55].
Although TNF inhibitors frequently increase the total cholesterol, triglyceride, HDL and LDL cholesterol levels, accumulating evidence suggest the beneficial effect of these biologicals on cardiovascular risk [51]. Interestingly, whenever compared with TNF inhibitors, tocilizumab is associated with an even higher increase in blood cholesterol and triglyceride levels [56]. A recently published multidatabase cohort study suggests that the cardiovascular risk of rheumatoid arthritis patients treated with tofacitinib versus TNF inhibitors is similar [57]. These observations suggest that glucocorticosteroids and NSAIDs should be tapered as soon as possible. Appropriate combinations of synthetic and biological DMARDs, in addition to optimizing lipid levels and hypertension, may provide the best cardiovascular outcome in rheumatoid arthritis.
NEW PARTICIPANTS IN THE COMPLEX BACKGROUND OF CARDIOVASCULAR COMORBIDITIES IN AUTOIMMUNE DISEASES: EXTRACELLULAR VESICLES
In addition to cytokines and chemokines, extracellular vesicles are new mediators of intercellular communication [58]. Extracellular vesicles are highly diverse, heterogeneous, membrane-surrounded, subcellular structures that can be found in all body fluids. Currently, there is no molecular marker or marker panel that precisely discriminates extracellular vesicle subpopulations; based on their size, extracellular vesicles can be classified as small extracellular vesicles/exosomes (30–150 nm), intermediate sized extracellular vesicles/microvesicles/microparticles (100–1000 nm) and large extracellular vesicles such as apoptotic bodies (1–5 μm). Extracellular vesicles may target both neighbouring or remote cells, and by transferring DNA, RNA or proteins, extracellular vesicles may regulate multiple functions of target cells [58,59].
Extracellular vesicles are secreted by all human cells; blood-derived extracellular vesicles mainly originate from platelets, red blood cells, monocytes, lymphocytes, granulocytes, and endothelial cells. In addition to their broad physiological functions, extracellular vesicles play a central role in the pathogenesis of several diseases including cardiovascular and immunological conditions [60,61]. Although the lack of standardized isolation and characterization methods still hampers the widespread use of extracellular vesicles as diagnostic and prognostic biomarkers, a characteristic extracellular vesicle profile has been described in autoimmune diseases [60,62]. Increased levels of both phosphatidylserine-positive and phosphatidylserine-negative microvesicles, without association with the disease activity, was recently described in SLE [63].
Numerous observations support the multifaceted role of extracellular vesicles in CVD as well [64▪]. Extracellular vesicles have been claimed to promote plaque stability [65]. Small extracellular vesicles containing insulin-like growth factor 1 receptor (IGF-1R) and miR-29a have been found to have a cardioprotective effect in rats [66]. On the other hand, mainly platelet-derived human microvesicles are thrombogenic [67]. Elevated levels of endothelial cell-derived and platelet-derived extracellular vesicles were observed in acute coronary syndrome [68]. Interestingly, according to recently published observations, although smoking promoted both extracellular vesicle release by leukocytes, platelets and endothelial cells, and vascular inflammation, these effects were prevented by red wine [69]. Extracellular vesicles may also significantly modulate the effect of cytokines [70].
A highly unexpected observation was recently found by Sodar et al.[71], namely that LDL mimics extracellular vesicles derived from blood plasma and may be copurified, which underlines their potential role whenever examining factors contributing to the development of CVD in rheumatic conditions/disorders.
Although there is little direct evidence, these data strongly support the potential role of extracellular vesicles in vascular comorbidity of autoimmune diseases. Extracellular vesicles may provide a link between inflammation and thromboembolic risk (Fig. 1).
FIGURE 1.
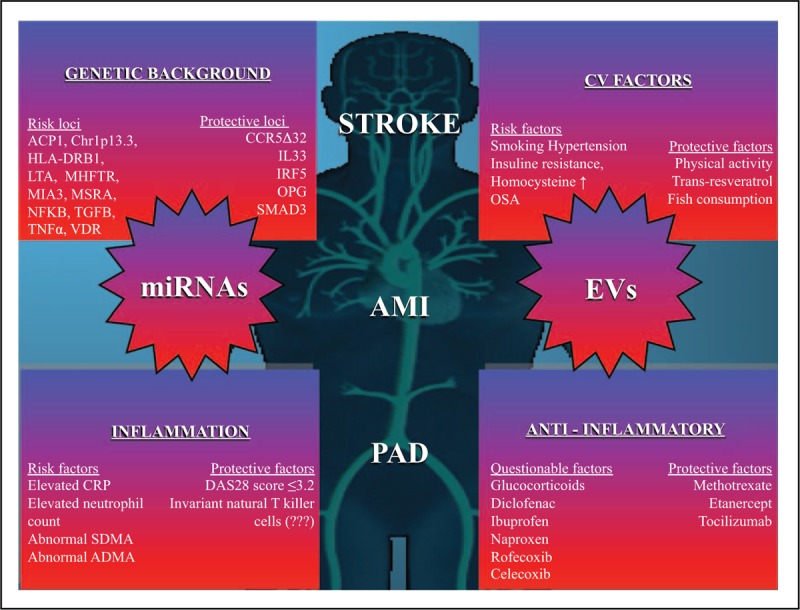
The figure demonstrates the known factors to our best current knowledge being of influence on the development of cardiovascular comorbidities such as stroke, acute myocardial infarction or peripherial arterial disease. PAD, peripherial arterial disease.
ANOTHER NEW PARTICIPANT: MICRORNAS
Estimates show that as high as 10–30% of protein coding genes are regulated by micro-RNAs [72], which are small regulator RNA molecules composed of 21–24 nucleotides. Salmena et al.[73] introduced the term ‘competing endogenous RNAs,’ describing the complex communication system between the different subtypes of RNA molecules. During CD4 T-cell activation, posttranscriptional urydylation by the enzymes TUT4 and TUT7 are responsible for the fine tuning of miRNA levels [74]. MiRNAs are relatively stable molecules and their measurement is reliably reproducible [75]. It also now known that the most diverse illnesses are all characterized by specific changes in the miRNA profile, which makes them a very promising tool for diagnostic purposes [76,77].
Serum miR-210 and miR-155 levels could be shown to be reliable biomarkers for the diagnosis of rheumatoid arthritis [78]. There have been studies looking for miRNA biomarkers for subclinical atherosclerosis in rheumatoid arthritis, but until now only little or no association was found, whenever assessing miR-15a-5p, miR-24-3p, miR-26a-5p, miR-125a-5p, miR-146a-5p, miR-155-5p, and miR-223-3p [79].
An interesting link between different risk factors was found in a study measuring vitamin D levels in SLE patients and correlating them with certain miRNA levels in patients’ T cells. An association between vitamin D concentrations and measured miRNA levels (miRNA-377, miRNA-342, miRNA-10a, miRNA-374b, miRNA-125a, and miRNA-410) was observed – not only comparing SLE patients with healthy controls, but also between patients differing in their vitamin D serum levels and also in cultured T cells from SLE patients, wherever the correlation was dose-dependent and time-dependent [80▪▪].
COOPERATION OF DIFFERENT MESSENGERS? EXTRACELLULAR VESICLES TRANSPORT IMPORTANT MICRORNAS
In atherosclerosis, Nguyen et al.[81▪▪] have demonstrated that extracellular vesicles originating from atherogenic macrophages transfer certain miRNAs (in particular miR-146a). The important role of miRNA-126-3p and miRNA-126-5p, transferred by extracellular vesicles originating from endothelial cells after AMI, was also demonstrated by Akbar et al.[82] and showed that these messengers promote the recruitment of transcriptionally activated splenic monocytes to the heart.
Systematic characterization of microvesicles and exosomes derived from T lymphocytes of healthy and SLE patients revealed that, depending on stimuli, extracellular vesicles carry a specific RNA profile and a deregulation of miR-155∗, miR-34b, and miR-34a could be shown. This again underlines the importance of intercellular communication via extracellular vesicles and miRNAs in autoimmune diseases [83▪▪].
CONCLUSION
Cardiovascular comorbidities of autoimmune diseases are the result of different contributing factors and their synergistic effects
The present review contains numerous studies investigating multiple independent risk factors of cardiovascular comorbidities in autoimmune diseases, focusing on conditions involving the joints. We wish to underline the complex interactions and the importance of their total effect on the phenotype discussed in this review ([84–98]; Table 1). Our suggestion is to focus on new mechanisms emerging, especially the common intercellular communication system of extracellular vesicles and miRNAs as no study yet considers these factors together in the development of cardiovascular comorbidities in patients with inflammatory joint disease.
Table 1.
List of risk factors influencing the development of cardiovascular comorbidities in inflammatory joint diseases
| Factors | Effect | References |
| Genetic background | ||
| ACP1 – *C haplotype | Risk factor | Teruel et al. [84] |
| CCR5Δ32 | Protective factor | Rodrígúez-Rodríguez et al. [85] |
| Chr1p13.3 – rs599839 – G allele | Risk factor | López-Mejías et al. [86] |
| HLA-DRB1*01*04 | Risk factor | Mattey et al. [87] |
| IL33 – rs3939286 – T allele | Protective factor | López-Mejías et al. [88] |
| IRF5 – GTG haplotype | Protective factor | Garcia-Bermúdez et al. [89] |
| LTA – 252GG | Risk factor | Panoulas et al. [90] |
| MHFTR – rs1801131 – C allele | Risk factor | Abd El-Aziz et al. [91] |
| MIA3 – rs17465637 – A allele | Risk factor | Garcia-Bermúdez et al. [92] |
| MSRA – rs10903323 – G allele | Risk factor | Garcia-Bermúdez et al. [93] |
| NFKB – rs28362491 – -94ATTG ins/del | Risk factor | López-Mejías et al. [94] |
| OPG – CGA haplotype | Protective factor | Genre et al. [95] |
| SMAD3 – rs17228212 – C allele | Protective factor | Garcia-Bermúdez et al. [96] |
| TGFB – rs1800470TC | Risk factor | Chen et al. [97] |
| TNFα – rs1800629 – A allele | Risk factor | Rodríguez-Rodríguez et al. [8] |
| VDR – GATG haplotype | Risk factor | López-Mejias et al. [12] |
| ZC3HC1 – rs11556924 – TT genotype | Risk factor | Lopez-Mejias et al. [98] |
| Classical cardovascular risk factors | ||
| Smoking | Risk factor | Murphy et al. [20] |
| Insulin resistance | Risk factor | Ruscitti et al. [22] |
| Dyslipidaemia | Risk factor | Gerber et al. [25] |
| Arterial hypertension | Risk factor | Radner et al. [29], Geraldino-Pardilla et al. [30▪▪] |
| Physical activity | Protecting factor | Carlsson et al. [32▪], Antunes et al. [33] |
| Hyperhomocysteinaemia | Risk factor | Morgan et al. [34], Essouma and Noubiap [35] |
| Low baseline vitamin D level | Risk factor | Herly et al. [38▪] |
| Obstructive sleeping apnoe | Risk factor | Wilton et al. [40▪] |
| Sodium intake | Risk factor | Marouen et al. [41] |
| Trans-resveratrol | Protective factor | Nguyen et al. [42] |
| Fish consumption | Protective factor | Alhassan et al. [43], Tedeschi et al. [44] |
| Therapy | ||
| Corticostreoids | Complex effect | Roubille et al. [51], van Sijl et al. [52] |
| NSAIDs | ||
| Celecoxib | Complex effect | Nissen et al. [54] |
| Naproxen | Complex effect | Nissen et al. [5] |
| Ibuprofen | Complex effect | Nissen et al. [54] |
| Methotrexate | Protective factor | Mangoni et al. [55] |
| TNF inhibitors | Protective factor | Roubille et al. [51] |
| Tocilizumab | Complex effect | Gabay et al. [56], Kim et al. [57] |
| New modalities | ||
| Extracellular vesicles | Complex effect | No publication yet |
| miRNAs | Complex effect | No publication yet |
Acknowledgements
The authors would like to thank gratefully to David Darling for his valuable constructive comments.
Financial support and sponsorship
This work was supported by the National Research, Development and Innovation Fund of Hungary; with the following grants NVKP_16-1-2016-0017, OTKA NN 111023, OTKA-119459, PD-OTKA-121187, OTKA-111958 and OTKA-120237, Medinprot Synergy V grant, NKM-69/2016/CNR-HAS Joint Project 2016–2018 ‘NutriCargo: Characterization of plant secreted nanovesicles’, B66D16000360005; VEKOP-2.3.3-15-2016-00016 and VEKOP-2.3.2-16-2016-00002.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Footnotes
György Nagy and Nóra Németh contributed equally to this work.
REFERENCES
- 1▪.López-Mejías R, Castaneda S, Gonzalez-Juanatey C, et al. Cardiovascular risk assessment in patients with rheumatoid arthritis: the relevance of clinical, genetic and serological markers. Autoimmun Rev 2016; 15:1013–1030. [DOI] [PubMed] [Google Scholar]; A very detailed review, especially of the most important genetic markers in the cardiovascular risk assessment in patients with rheumatoid arthritis.
- 2.Lindhardsen J, Ahlehoff O, Gislason GH, et al. The risk of myocardial infarction in rheumatoid arthritis and diabetes mellitus: a Danish nationwide cohort study. Ann Rheum Dis 2011; 70:929–934. [DOI] [PubMed] [Google Scholar]
- 3.Ruscitti P, Cipriani P, Masedu F, et al. Increased cardiovascular events and subclinical atherosclerosis in rheumatoid arthritis patients: 1 year prospective single centre study. PLoS One 2017; 12:e0170108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4▪.Balsa A, Lojo-Oliveira L, Alperi-Lopez M, et al. Prevalence of comorbidities in rheumatoid arthritis and evaluation of their monitoring in clinical practice: The Spanish Cohort of the COMORA Study. Reumatol Clin 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]; In addition to monitoring the prevalence of comorbidities, the study also shows the evaulation and thus, the high impact of their monitoring in clinical practice.
- 5.Crowson CS, Rollefstad S, Ikdahl E, et al. A Trans-Atlantic Cardiovascular Consortium for Rheumatoid Arthritis (ATACC-RA). Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann Rheum Dis 2017; 77:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Worthington J, Ollier WE, Leach MK, et al. The Arthritis and Rheumatism Council's National Repository of Family Material: pedigrees from the first 100 rheumatoid arthritis families containing affected sibling pairs. Br J Rheumatol 1994; 33:970–976. [DOI] [PubMed] [Google Scholar]
- 7.MacGregor AJ, Snieder H, Rigby AS, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 2000; 43:30–37. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Rodriguez L, Gonzalez-Juanatey C, Palomino-Morales R, et al. TNFA -308 (rs1800629) polymorphism is associated with a higher risk of cardiovascular disease in patients with rheumatoid arthritis. Atherosclerosis 2011; 216:125–130. [DOI] [PubMed] [Google Scholar]
- 9.Lopez-Mejias R, García-Bermúdez M, González-Juanatey C, et al. NFKB1-94ATTG ins/del polymorphism (rs28362491) is associated with cardiovascular disease in patients with rheumatoid arthritis. Atherosclerosis 2012; 224:426–429. [DOI] [PubMed] [Google Scholar]
- 10.Farragher TM, Goodson NJ, Naseem H, et al. Association of the HLA-DRB1 gene with premature death, particularly from cardiovascular disease, in patients with rheumatoid arthritis and inflammatory polyarthritis. Arthritis Rheum 2008; 58:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bukach OP, Fediv OO, Syndorchuk LP, et al. Changes of lipid metabolism in patients with rheumatoid arthritis and concomitant arterial hypertension, abdominal obesity and diabetes mellitus type 2 depending on the gene polymorphism T-786C of endothelial nitric oxide synthase. Int J endocrine 2017; 13:79–84. [Google Scholar]
- 12.López-Mejías R, Genre F, Remuzgo-Martinez S, et al. Vitamin D receptor GATG haplotype association with atherosclerotic disease in patients with rheumatoid arthritis. Atherosclerosis 2016; 245:139–142. [DOI] [PubMed] [Google Scholar]
- 13.Remuzgo-Martínez S, Genre F, López-Mejías R, et al. Decreased expression of methylene tetrahydrofolate reductase (MTHFR) gene in patients with rheumatoid arthritis. Clin Exp Rheumatol 2016; 34:106–110. [PubMed] [Google Scholar]
- 14.Lopez-Mejias R, Genre F, Remuzgo-Martinez S, et al. Influence of elevated-CRP level-related polymorphisms in nonrheumatic Caucasians on the risk of subclinical atherosclerosis and cardiovascular disease in rheumatoid arthritis. Sci Rep 2016; 6:31979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harel-Meir M, Sherer Y, Shoenfeld Y, et al. Tobacco smoking and autoimmune rheumatic diseases. Nat Clin Pract Rheumatol 2007; 3:707–715. [DOI] [PubMed] [Google Scholar]
- 16.Lewis JB, Hirschi KM, Arroyo JA, et al. Plausible roles for RAGE in conditions exacerbated by direct and indirect (secondhand) smoke exposure. Int J Mol Sci 2017; 18: pii: E652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed RM, Dransfield MT, Eberlein M, et al. Gender differences in first and secondhand smoke exposure, spirometric lung function and cardiometabolic health in the old order Amish: A novel population without female smoking. PLoS One 2017; 12:e0174354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Hariri M, Zibara K, Farhat W, et al. Cigarette Smoking-Induced Cardiac Hypertrophy, Vascular Inflammation and Injury Are Attenuated by Antioxidant Supplementation in an Animal Model. Frontiers in pharmacology 2016; 7:397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messner B, Bernhard D. Smoking and cardiovascular disease: mechanisms of endothelial dysfunction and early atherogenesis. Arterioscler Thromb Vasc Biol 2014; 34:509–515. [DOI] [PubMed] [Google Scholar]
- 20.Murphy D, Mattey D, Hutchinson D, et al. Anti-citrullinated protein antibody positive rheumatoid arthritis is primarily determined by rheumatoid factor titre and the shared epitope rather than smoking per se. PLoS One 2017; 12:e0180655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Hammam N, Gheita TA. Impact of secondhand smoking on disease activity in women with rheumatoid arthritis. Clin Rheumatol 2017; 36:2412–2420. [DOI] [PubMed] [Google Scholar]; The study draws attention to the worsening effect of secondhand smoking especially in women with rheumatoid arthritis.
- 22.Ruscitti P, Ursini F, Cipriani P, et al. Poor clinical response in rheumatoid arthritis is the main risk factor for diabetes development in the short-term: a 1-year, single-centre, longitudinal study. PLoS One 2017; 12:e0181203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruscitti P, Ursini F, Cipriani P, et al. Prevalence of type 2 diabetes and impaired fasting glucose in patients affected by rheumatoid arthritis: Results from a cross-sectional study. Medicine (Baltimore) 2017; 96:e7896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orozco-Beltran D, Gil-Guillen VF, Redon J, et al. Lipid profile, cardiovascular disease and mortality in a Mediterranean high-risk population: The ESCARVAL-RISK study. PLoS One 2017; 12:e0186196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerber PA, Nikolic D, Rizzo M, et al. Small, dense LDL: an update. Curr Opin Cardiol 2017; 32:454–459. [DOI] [PubMed] [Google Scholar]
- 26.Toft-Petersen AP, Tilsted HH, Aaroe J, et al. Small dense LDL particles–a predictor of coronary artery disease evaluated by invasive and CT-based techniques: a case-control study. Lipids Health Dis 2011; 10:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turk SA, Heslinga SC, Dekker J, et al. The relationship between cardiac conduction times, cardiovascular risk factors, and inflammation in patients with early arthritis. J Rheumatol 2017; 44:580–586. [DOI] [PubMed] [Google Scholar]
- 28▪▪.Li YT, Wang HH, Liu KQ, et al. Medication adherence and blood pressure control among hypertensive patients with coexisting long-term conditions in primary care settings: a cross-sectional analysis. Medicine (Baltimore) 2016; 95:e3572. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mecial adherence defines in first line, the long-term efficacy of disease control.
- 29.Radner H, Lesperance T, Accortt NA, Solomon DH, et al. Incidence and prevalence of cardiovascular risk factors among patients with rheumatoid arthritis, psoriasis, or psoriatic arthritis. Arthritis Care Res (Hoboken) 2017; 69:1510–1518. [DOI] [PubMed] [Google Scholar]
- 30▪▪.Geraldino-Pardilla L, Zartoshti A, Bag Ozbek A, et al. Arterial inflammation detected with 18 f-fluoro-deoxyglucose positron emission tomography in rheumatoid arthritis. Arthritis Rheumatol 2017; doi:10.1002/art.40345. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; Arterial inflammation may be an important connection between autoimmune diseases and high prevalence of cardiovascular comorbidities.
- 31.Kal A, Duman E, Sezenoz AS, et al. Evaluation of retrobulbar blood flow and choroidal thickness in patients with rheumatoid arthritis. Int Ophthalmol 2017; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 32▪.Carlsson E, Ludvigsson J, Huus K, Faresjo M, et al. High physical activity in young children suggests positive effects by altering autoantigen-induced immune activity. Scand J Med Sci Sports 2016; 26:441–450. [DOI] [PubMed] [Google Scholar]; Molecular evidences of the positive effect of high physical activity.
- 33.Rochette E, Duché P, Merlin E. Juvenile idiopathic arthritis and physical activity: possible inflammatory and immune modulation and tracks for interventions in young populations. Autoimmun Rev 2015; 14:726–734. [DOI] [PubMed] [Google Scholar]
- 34.Morgan SL, Baggott JE, Lee JY, Alarcon GS, et al. Folic acid supplementation prevents deficient blood folate levels and hyperhomocysteinemia during longterm, low dose methotrexate therapy for rheumatoid arthritis: implications for cardiovascular disease prevention. J Rheumatol 1998; 25:441–446. [PubMed] [Google Scholar]
- 35.Essouma M, Noubiap JJ. Therapeutic potential of folic acid supplementation for cardiovascular disease prevention through homocysteine lowering and blockade in rheumatoid arthritis patients. Biomark Res 2015; 3:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonciani D, Antiga E, Bonciolini V, et al. Homocysteine serum levels are increased and correlate with disease severity in patients with lupus erythematosus. Clin Exp Rheumatol 2016; 34:76–81. [PubMed] [Google Scholar]
- 37.Liew SC, Gupta ED. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. Eur J Med Genet 2015; 58:1–10. [DOI] [PubMed] [Google Scholar]
- 38▪.Herly M, Stengaard-Pedersen K, Horslev-Petersen K, et al. Association between baseline vitamin D metabolite levels and long-term cardiovascular events in patients with rheumatoid arthritis from the CIMESTRA trial: protocol for a cohort study with patient-record evaluated outcomes. BMJ Open 2017; 7:e014816. [DOI] [PMC free article] [PubMed] [Google Scholar]; The CIMESTRA trial shows the importance of optimal vitamin D level in the long-term outcome of CVEs in patients with rheumatoid arthritis.
- 39.Yesil H, Sungur U, Akdeniz S, et al. Association between serum vitamin D levels and neuropathic pain in rheumatoid arthritis patients: A cross-sectional study. Int J Rheum Dis 2017; doi:10.1111/1756-185X.13160. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 40▪.Wilton KM, Matteson EL, Crowson CS, et al. Risk of Obstructive Sleep Apnea and Its Association with Cardiovascular and Noncardiac Vascular Risk in Patients with Rheumatoid Arthritis: A Population-based Study. J Rheumatol 2017; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; Obstructive sleep apnea is an easily diagnosable disease with a high impact on cardiovascular risk management.
- 41.Marouen S, du Cailar G, Audo R, et al. Sodium excretion is higher in patients with rheumatoid arthritis than in matched controls. PLoS One 2017; 12:e0186157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nguyen C, Savouret JF, Widerak M, et al. Resveratrol, potential therapeutic interest in joint disorders: a critical narrative review. Nutrients 2017; 9: pii: E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alhassan A, Young J, Lean MEJ, Lara J, et al. Consumption of fish and vascular risk factors: a systematic review and meta-analysis of intervention studies. Atherosclerosis 2017; 266:87–94. [DOI] [PubMed] [Google Scholar]
- 44.Tedeschi SK, Bathon JM, Giles JT, et al. The relationship between fish consumption and disease activity in rheumatoid arthritis. Arthritis Care Res 2017; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mercan R, Bitik B, Tufan A, et al. The association between neutrophil/lymphocyte ratio and disease activity in rheumatoid arthritis and ankylosing spondylitis. J Clin Lab Anal 2016; 30:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46▪.Arts EE, Fransen J, Den Broeder AA, et al. Low disease activity (DAS28</=3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: a time-dependent Cox regression analysis in a large cohort study. Ann Rheum Dis 2017; 76:1693–1699. [DOI] [PubMed] [Google Scholar]; Low-disease activity is a keypoint in preventing CVEs in rheumatoid arthritis.
- 47.Gonzalez-Gay MA, Gonzalez-Juanatey C, Pineiro A, et al. High-grade C-reactive protein elevation correlates with accelerated atherogenesis in patients with rheumatoid arthritis. J Rheumatol 2005; 32:1219–1223. [PubMed] [Google Scholar]
- 48.Meissner Y, Zink A, Kekow J, et al. Impact of disease activity and treatment of comorbidities on the risk of myocardial infarction in rheumatoid arthritis. Arthritis Res Ther 2016; 18:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dimitroulas T, Hodson J, Sandoo A, et al. Endothelial injury in rheumatoid arthritis: a crosstalk between dimethylarginines and systemic inflammation. Arthritis Res Ther 2017; 19:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet 2017; 389:2338–2348. [DOI] [PubMed] [Google Scholar]
- 51.Roubille C, Richer V, Starnino T, et al. The effects of tumour necrosis factor inhibitors, methotrexate, nonsteroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis 2015; 74:480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Sijl AM, Boers M, Voskuyl AE, Nurmohamed MT. Confounding by indication probably distorts the relationship between steroid use and cardiovascular disease in rheumatoid arthritis: results from a prospective cohort study. PLoS One 2014; 9:e87965.Epub 2014/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schjerning Olsen AM, Fosbol EL, Gislason GH. The impact of NSAID treatment on cardiovascular risk - insight from Danish observational data. Basic Clin Pharmacol Toxicol 2014; 115:179–184. [DOI] [PubMed] [Google Scholar]
- 54.Nissen SE, Yeomans ND, Solomon DH, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med 2016; 375:2519–2529. [DOI] [PubMed] [Google Scholar]
- 55.Mangoni AA, Baghdadi LR, Shanahan EM, et al. Methotrexate, blood pressure and markers of arterial function in patients with rheumatoid arthritis: a repeated cross-sectional study. Ther Adv Musculoskelet Dis 2017; 9:213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gabay C, McInnes IB, Kavanaugh A, et al. Comparison of lipid and lipid-associated cardiovascular risk marker changes after treatment with tocilizumab or adalimumab in patients with rheumatoid arthritis. Ann Rheum Dis 2016; 75:1806–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim SC, Solomon DH, Rogers JR, et al. Cardiovascular safety of tocilizumab versus tumor necrosis factor inhibitors in patients with rheumatoid arthritis: a multi-database cohort study. Arthritis Rheumatol 2017; 69:1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gyorgy B, Szabo TG, Pasztoi M, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci 2011; 68:2667–2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kowal J, Arras G, Colombo M, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A 2016; 113:E968–E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marini M, Ibba-Manneschi L, Manetti M. Cardiac telocyte-derived exosomes and their possible implications in cardiovascular pathophysiology. Adv Exp Med Biol 2017; 998:237–254. [DOI] [PubMed] [Google Scholar]
- 61.Buzas EI, Gyorgy B, Nagy G, et al. Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol 2014; 10:356–364. [DOI] [PubMed] [Google Scholar]
- 62.Knijff-Dutmer EA, Koerts J, Nieuwland R, et al. Elevated levels of platelet microparticles are associated with disease activity in rheumatoid arthritis. Arthritis Rheum 2002; 46:1498–1503. [DOI] [PubMed] [Google Scholar]
- 63.Mobarrez F, Vikerfors A, Gustafsson JT, et al. Microparticles in the blood of patients with systemic lupus erythematosus (SLE): phenotypic characterization and clinical associations. Sci Rep 2016; 6:36025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64▪.Osteikoetxea X, Nemeth A, Sodar BW, et al. Extracellular vesicles in cardiovascular disease: are they Jedi or Sith? J Physiol 2016; 594:2881–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]; A wide spectrum overview about our current knowledge of extracellular vesicles.
- 65.Zernecke A, Bidzhekov K, Noels H, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal 2009; 2:ra81. [DOI] [PubMed] [Google Scholar]
- 66.Yamaguchi T, Izumi Y, Nakamura Y, et al. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol 2015; 178:239–246. [DOI] [PubMed] [Google Scholar]
- 67.Suades R, Padro T, Alonso R, et al. High levels of TSP1+/CD142+ platelet-derived microparticles characterise young patients with high cardiovascular risk and subclinical atherosclerosis. Thromb Haemost 2015; 114:1310–1321. [DOI] [PubMed] [Google Scholar]
- 68.Morel O, Pereira B, Averous G, et al. Increased levels of procoagulant tissue factor-bearing microparticles within the occluded coronary artery of patients with ST-segment elevation myocardial infarction: role of endothelial damage and leukocyte activation. Atherosclerosis 2009; 204:636–641. [DOI] [PubMed] [Google Scholar]
- 69.Schwarz V, Bachelier K, Schirmer SH, et al. Red wine prevents the acute negative vascular effects of smoking. Am J Med 2017; 130:95–100. [DOI] [PubMed] [Google Scholar]
- 70.Szabo GT, Tarr B, Paloczi K, et al. Critical role of extracellular vesicles in modulating the cellular effects of cytokines. Cell Mol Life Sci 2014; 71:4055–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sodar BW, Kittel A, Paloczi K, et al. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci Rep 2016; 6:24316.Epub 2016/04/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kusenda B, Mraz M, Mayer J, Pospisilova S. MicroRNA biogenesis, functionality and cancer relevance. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2006; 150:205–215. [DOI] [PubMed] [Google Scholar]
- 73.Salmena L, Poliseno L, Tay Y, et al. A ceRNA hypothesis: the Rosetta Stone of a hidden RNA language? Cell 2011; 146:353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gutierrez-Vazquez C, Enright AJ, Rodriguez-Galan A, et al. 3’ Uridylation controls mature microRNA turnover during CD4 T-cell activation. RNA 2017; 23:882–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chim SS, Shing TK, Hung EC, et al. Detection and characterization of placental microRNAs in maternal plasma. Clin Chem 2008; 54:482–490. [DOI] [PubMed] [Google Scholar]
- 76.Mansoori B, Mohammadi A, Shirjang S, Baradaran B. MicroRNAs in the diagnosis and treatment of cancer. Immunol Invest 2017; 46:880–897. [DOI] [PubMed] [Google Scholar]
- 77.Cui JY, Liang HW, Pan X L, et al. Characterization of a novel panel of plasma microRNAs that discriminates between Mycobacterium tuberculosis infection and healthy individuals. PLoS One 2017; 12:e0184113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abdul-Maksoud RS, Sediq AM, Kattaia A, et al. Serum miR-210 and miR-155 expression levels as novel biomarkers for rheumatoid arthritis diagnosis. Br J Biomed Sci 2017; 74:209–213. [DOI] [PubMed] [Google Scholar]
- 79.Ormseth MJ, Solus JF, Vickers KC, et al. Utility of select plasma microRNA for disease and cardiovascular risk assessment in patients with rheumatoid arthritis. J Rheumatol 2015; 42:1746–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80▪▪.Chen DJ, Li LJ, Yang XK, et al. Altered microRNAs expression in T cells of patients with SLE involved in the lack of vitamin D. Oncotarget 2017; 8:62099–62110. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study draws attention to the complex interaction between different risk factors such as micraRNAs, vitamin D level.
- 81▪▪.Nguyen MA, Karunakaran D, Geoffrion M, et al. Extracellular vesicles secreted by atherogenic macrophages transfer microRNA to inhibit cell migration. Arterioscler Thromb Vasc Biol 2017; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nguyen et al. show a molecular explanation to the interaction between extracellular vesicles and miRNAs in the development of atherosclerosis.
- 82.Akbar N, Digby JE, Cahill TJ, et al. Endothelium-derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction. JCI insight 2017; 2: [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83▪▪.Classen L, Tykocinski LO, Wiedmann F, et al. Extracellular vesicles mediate intercellular communication: transfer of functionally active microRNAs by microvesicles into phagocytes. Eur J Immunol 2017; 47:1535–1549. [DOI] [PubMed] [Google Scholar]; Extracellular vesicles and miRNAs are together key actors in intercellular communication.
- 84.Teruel M, Martin JE, Gonzalez-Juanatey C, et al. Association of acid phosphatase locus 1∗C allele with the risk of cardiovascular events in rheumatoid arthritis patients. Arthritis Res Ther 2011; 13:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodrígúez-Rodríguez L, Gonzalez-Juanatey C, Garcia-Bermudez M, et al. CCR5Delta32 variant and cardiovascular disease in patients with rheumatoid arthritis: a cohort study. Arthritis Res Ther 2011; 13:R133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopez-Mejias R, Gonzalez-Juanatey C, Garcia-Bermudez M, et al. The lp13.3 genomic region -rs599839- is associated with endothelial dysfunction in patients with rheumatoid arthritis. Arthritis Res Ther 2012; 14:R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mattey DL, Thomson W, Ollier WE, et al. Association of DRB1 shared epitope genotypes with early mortality in rheumatoid arthritis: results of eighteen years of followup from the early rheumatoid arthritis study. Arthritis Rheum 2007; 56:1408–1416. [DOI] [PubMed] [Google Scholar]
- 88.Lopez-Mejias R, Genre F, Remuzgo-Martinez S, et al. Protective role of the interleukin 33 rs3939286 gene polymorphism in the development of subclinical atherosclerosis in rheumatoid arthritis patients. PLoS One 2015; 10:e0143153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garcia-Bermudez M, Lopez-Mejias R, Genre F, et al. Interferon regulatory factor 5 genetic variants are associated with cardiovascular disease in patients with rheumatoid arthritis. Arthritis Res Ther 2014; 16:R146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Panoulas VF, Nikas SN, Smith JP, et al. Lymphotoxin 252A>G polymorphism is common and associates with myocardial infarction in patients with rheumatoid arthritis. Ann Rheum Dis 2008; 67:1550–1556. [DOI] [PubMed] [Google Scholar]
- 91.Abd El-Aziz TA, Mohamed RH. Influence of MTHFR C677T gene polymorphism in the development of cardiovascular disease in Egyptian patients with rheumatoid arthritis. Gene 2017; 610:127–132. [DOI] [PubMed] [Google Scholar]
- 92.Garcia-Bermudez M, Lopez-Mejias R, Gonzalez-Juanatey C, et al. Association study of MIA3 rs17465637 polymorphism with cardiovascular disease in rheumatoid arthritis patients. DNA Cell Biol 2012; 31:1412–1417. [DOI] [PubMed] [Google Scholar]
- 93.Garcia-Bermudez M, Lopez-Mejias R, Gonzalez-Juanatey C, et al. Association of the methionine sulfoxide reductase A rs10903323 gene polymorphism with cardiovascular disease in patients with rheumatoid arthritis. Scand J Rheumatol 2012; 41:350–353. [DOI] [PubMed] [Google Scholar]
- 94.Lopez-Mejias R, Garcia-Bermudez M, Gonzalez-Juanatey C, et al. NFKB1-94ATTG ins/del polymorphism (rs28362491) is associated with cardiovascular disease in patients with rheumatoid arthritis. Atherosclerosis 2012; 224:426–429. [DOI] [PubMed] [Google Scholar]
- 95.Genre F, Lopez-Mejias R, Garcia-Bermudez M, et al. Osteoprotegerin CGA haplotype protection against cerebrovascular complications in anti-CCP negative patients with rheumatoid arthritis. PLoS One 2014; 9:e106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Garcia-Bermudez M, Lopez-Mejias R, Genre F, et al. SMAD3 rs17228212 gene polymorphism is associated with reduced risk to cerebrovascular accidents and subclinical atherosclerosis in anti-CCP negative Spanish rheumatoid arthritis patients. PLoS One 2013; 8:e77695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y, Dawes PT, Packham JC, Mattey DL. Interaction between smoking and functional polymorphism in the TGFB1 gene is associated with ischaemic heart disease and myocardial infarction in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther 2012; 14:R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lopez-Mejias R, Genre F, Garcia-Bermudez M, et al. The ZC3HC1 rs11556924 polymorphism is associated with increased carotid intima-media thickness in patients with rheumatoid arthritis. Arthritis Res Ther 2013; 15:R152. [DOI] [PMC free article] [PubMed] [Google Scholar]


