Abstract
Background
Quantitative magnetic resonance (QMR) has been increasingly used to measure human body composition, but its use and validation in children is limited.
Objective
We compared body composition measurement by QMR and air displacement plethysmography (ADP) in preschool children from Singapore’s multi-ethnic Asian population [n= 152; mean ± SD age: 5.0 ± 0.1 years].
Methods
Agreements between QMR- and ADP-based fat mass and fat mass index (FMI) were assessed using intraclass correlation coefficient (ICC), reduced major axis regression (RMAR), and Bland-Altman plot analyses. Analyses were stratified for the child’s sex.
Results
Substantial agreement was observed between QMR- and ADP-based fat mass (ICC: 0.85) and FMI (ICC: 0.82). RMAR analysis suggested that QMR measurements were generally lower than ADP measurements. Bland-Altman analysis similarly revealed that QMR-based fat mass were [mean difference (95% limits of agreement)] -0.5 (-2.1 to +1.1) kg lower than ADP-based fat mass and QMR-based FMI were -0.4 (-1.8 to +0.9) kg/m2 lower than ADP-based FMI. Stratification by offspring sex revealed better agreement of QMR and ADP measurements in girls than in boys.
Conclusions
QMR-based fat mass and FMI showed substantial agreement with, but was generally lower than, ADP-based measures in young Asian children.
Keywords: Body composition, quantitative magnetic resonance, QMR, air displacement plethysmography, BodPod, children
Introduction
Childhood obesity has become a global epidemic. This bodes badly for future health, given its associations with higher risk of chronic diseases such as type 2 diabetes and stroke in adulthood (1,2). Anthropometric measures such as body mass index (BMI) are often used to reflect adiposity, owing to their feasibility and low cost. However, a higher BMI may not only arise from greater body fat, but also from higher lean mass and/or bone mass, making it an imperfect measure of adiposity (3,4). The differential magnitude and velocity of fat and fat free mass accretion during different growth periods further complicate interpretation of changes in childhood BMI over time (5,6). Identifying better methods for measuring body composition in children is therefore an important research goal.
Cadaver analysis is the most accurate way to measure body composition (7), but other methods are necessary in live human subjects. In humans, the four-compartment (4-C) model, which assesses body mass and volume (using air displacement plethysmography, ADP), total water (using deuterium dilution), and bone mineral density (using dual-energy x-ray absorptiometry, DXA), is the criterion method for in vivo body composition measurement (8,9). Nonetheless, the 4-C model is costly, requires several instruments, and imposes a substantial subject burden, thereby impeding its use in large epidemiological studies in children.
Most research studies assessing body composition in children therefore base their measurements on individual techniques such as bioelectrical impedance analysis (BIA), DXA or ADP. However, these methods also have limitations. For instance, BIA and ADP are two-compartment models that assume a constant density of fat mass and fat free mass (FFM). Yet this assumption for FFM is often violated in disease states and in obese subjects with abnormal FFM hydration (7,10). DXA exposes subjects to (low-dose) X-ray radiation, thus raising parental concerns about potential adverse health effects and limiting its use for longitudinal tracking of body composition in children (11). Moreover, both ADP and DXA require subjects to remain motionless to obtain high-quality measurements—a major challenge in young children.
A new technology called quantitative magnetic resonance (QMR) (12) is accepted as an improved measure of in vivo body composition in animals (in terms of precision, speed, and ease of use) and has been increasingly adopted in human studies. Compared to ADP and DXA, QMR is faster (<3 minutes measurement time), less invasive (no ionizing radiation) and does not require subjects to remain motionless, making it a potential method to track children’s body composition over time. Thus far, only one study has assessed the validity of QMR measurement in Western infants and children (11), which reported good agreement between fat mass measured by QMR and the 4-C model in 57 older children (≥6 years) (11). It remains unclear whether QMR is suitable for measuring body composition in younger children, however. Moreover, Asians are known to have a different amount and distribution of body fat compared to Caucasians of similar BMI (13–15). To our knowledge, no study has assessed the validity of QMR measurements in Asian subjects at any age. To fill these gaps, we compared QMR and ADP measurements of body composition in young children from a multi-ethnic Asian cohort in Singapore.
Methods
Study children were participants in the Growing Up in Singapore Towards Healthy Outcomes (GUSTO) mother-offspring cohort, described in detail elsewhere (16). Briefly, from June 2009 until September 2010, pregnant mothers (<14 weeks gestational weeks) residing in Singapore and of Chinese, Malay, or Indian descent were recruited from two major public maternity wards at the KK Women’s and Children’s Hospital (KKH) and National University Hospital (NUH). Ethical approval was granted by Institutional Review Board of the respective hospitals and all mothers provided written informed consent at recruitment.
Participants
At age 5 years, the children were invited back for a clinic visit, when we measured their body composition and anthropometry. A total of 668 participants attended the clinic visit in KKH, where both the ADP and QMR machines were hosted, providing the pool of participants for this study. Of these children, 256 completed the QMR, and 251 completed the ADP measurement; 152 had both QMR and ADP measurements and were thus included in the current analysis. The flow of participants is shown in Figure S1. Although clinic visits commenced in December 2014, QMR measurements were not started until 3 June 2015 for logistical reasons, resulting in 125 participants who were not approached for QMR scans. The completion rate was therefore 47% [256/ (668-125)*100%] for QMR and 38% (251/ 668*100%) for ADP. The main reason for parental refusal of measurement for both ADP and QMR was children’s fear of the closed chamber. Other major reasons included unwillingness of children to change into the tight-fitting clothing required for ADP and parents’ concerns about magnetic field exposure for QMR. Based on Pearson's χ2 tests for categorical variables and independent sample t-tests for continuous variables, children with neither ADP nor QMR measurement were more likely to be boys and slightly older, as compared with children with either measurement (Table S1). Other characteristics, including age- and sex-specific BMI z-score (derived using a Singapore standard (17)) and proportions of obese and overweight (defined as BMI z-score > 95th and 85th percentile, respectively), did not differ between children who had neither body composition measurement vs. those with at least one of the measurements.
Body composition
For QMR measurement, the EchoMRI-Adolescent Humans Body Composition Analyzer (EchoMRI Corporation, Singapore) was used. Quality control measures were performed daily according to the manufacturer’s recommendations. The machine was calibrated each day with 8 bottles of canola oil of known mass (36 kg in total), and a routine system test was conducted daily. Participants were measured in light clothing and in supine position. Before the measurement, children were asked to empty their bladders. Although slight motion during QMR scanning process is tolerated, the children were instructed to minimize their movement. The QMR measurement is brief and can be completed in less than 3 minutes.
For ADP, the BodPod body composition tracking system version 5.2.0 (Cosmed, Rome, Italy) was used. The system was calibrated each day before use; a cylinder with known volume (50L) was used to calibrate the chamber, and two 10 kg weights were used to calibrate the scale. The participants changed into tight-fitting clothing prior to measurement, including a tight-fitting cap to minimize air trapping within the hair. Body mass was measured using the BodPod’s precise electronic scale (6-20 seconds, depending on participant’s movement), while body volume was measured in the BodPod chamber. Two volume measurements of approximately 50 seconds each were obtained; when the two measures differed, a third measurement was obtained. The total ADP measurement time is about 5 minutes. Body density, and subsequently % body fat and absolute fat mass, was then calculated from the body mass and volume. Further technical details of QMR and ADP measurements are included in Material S1.
Anthropometry
The children’s weight and height were measured using a calibrated digital scale (SECA weighing scale model 813, SECA Corp., Hamburg, Germany) and stadiometer (SECA model 213), respectively. The fat mass index (FMI) was derived using the formula fat mass/square of height (kg/m2). Abdominal circumference was measured using an inelastic measuring tape (Butterfly brand, China) and recorded to the nearest 1 mm. These measurements were taken in duplicate using a standardized protocol and then averaged (18). Subscapular skinfold (SS) and triceps skinfold (TS) thicknesses were measured using Holtain skinfold calipers (Holtain Ltd, Crymych, UK) in triplicate, with the two closest values then averaged. The skinfold measurements were taken on the right side of the body and recorded to the nearest 0.2 mm. The sum of skinfold thicknesses (SST) was subsequently derived by adding TS and SS.
Statistical analysis
Characteristics of study children are summarized using mean ± SD for continuous variables and n (%) for categorical variables. Paired t-test was used to test differences in ADP and QMR (see Figure S2 for justification). The intraclass correlation coefficient (ICC), reduced major axis regression (RMAR), and Bland-Altman plot were used to assess the agreement between QMR and ADP measurements of fat mass and FMI. For ICC, we used a two-way mixed effects model assessing absolute agreement of the two measurement methods. RMAR is used to describe association between two continuous variables when both are prone to measurement error, i.e., when neither can be considered a ‘gold standard’ (19,20). The RMAR slope and the y-intercept allow assessment of bias. When a fixed bias is present, the RMAR slope will be parallel to the line of identity (y= x) but with an intercept other than 0. In contrast, when the slope is any value other than 1, a proportional bias (measurement difference depends on the magnitude of measurement) is present (19). Lastly, for the Bland-Altman plot, the difference of fat mass or FMI (ADP-QMR) was plotted against the average fat mass or FMI based on the two methods; the mean difference and 95% limits of agreement (LOA; mean difference ± 1.96 SD) were then calculated. To assess the LOA acceptability, we compared our results with the estimates calculated from Andres et al. study (11), which assessed agreements of QMR and ADP with the 4-C model in older children. Because previous studies have shown that agreement of body composition measurements can differ in boys and girls (21,22), we also stratified our analyses of agreement by sex.
Finally, to assess the relative precision of QMR and ADP, FMI based on both measurements was plotted against common adiposity measures (BMI, SST, and abdominal circumference). The spreads of data of the scatter plots were visually inspected. Linear regressions were fitted for the adiposity measures and FMI based on both instruments, with the resulting predicted values (and their 95% CI) overlaid onto the scatter plots. To assess model fit, we also compared the root mean square error (RMSE), i.e., the standard deviation of unexplained variance in a regression model; a lower RMSE value indicates a better model fit. All statistical analyses were performed using the statistical software package STATA version 13.1 (StataCorp., Texas, USA).
Results
Table 1 shows the characteristics of the children who participated in this study. Study children (45% boys) had a mean ± SD age of 5.0 ± 0.1 years and body weight of 18.4 ± 3.2 kg. About half of the children were Chinese, the remainder being of Malay or Indian ethnicity. The mean fat mass (FMI) based on the QMR method [3.9 kg (3.3 kg/m2)] were lower than those based on the ADP method [4.4 kg (3.7 kg/m2)].
Table 1.
Characteristics of participants (total n= 152).
| Characteristics | n (%) or mean ± SD |
|---|---|
| Sex | |
| Male | 68 (45%) |
| Ethnicity | |
| Chinese | 73 (48%) |
| Malay | 51 (34%) |
| Indian | 28 (18%) |
| Age at measurement, y | 5.0 ± 0.1 |
| Body weight, kg | 18.4 ± 3.2 |
| Height, cm | 108.8 ± 4.7 |
| Abdominal circumference, cm | 52.3 ± 5.2 |
| BMI z-score | 0.02 ± 0.92 |
| Fat mass, kg | |
| QMR | 3.9 ± 1.7*** |
| ADP | 4.4 ± 1.7 |
| Fat mass index, kg/m2 | |
| QMR | 3.3 ± 1.3*** |
| ADP | 3.7 ± 1.3 |
P< 0.001; P-values were derived from paired t-test comparing fat mass and fat mass index based on QMR and ADP (similarly, all P< 0.001 for Wilcoxon signed-rank tests, a non-parametric equivalent of paired t-test). ADP, air displacement plethysmography; QMR, quantitative magnetic resonance.
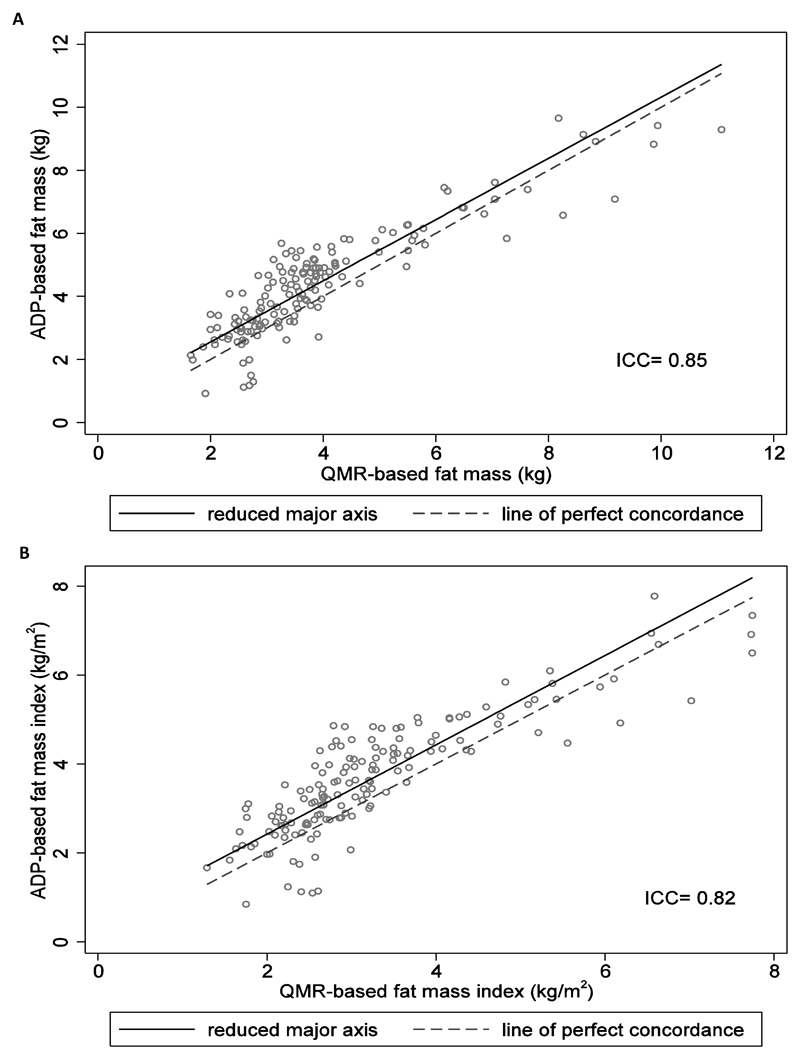
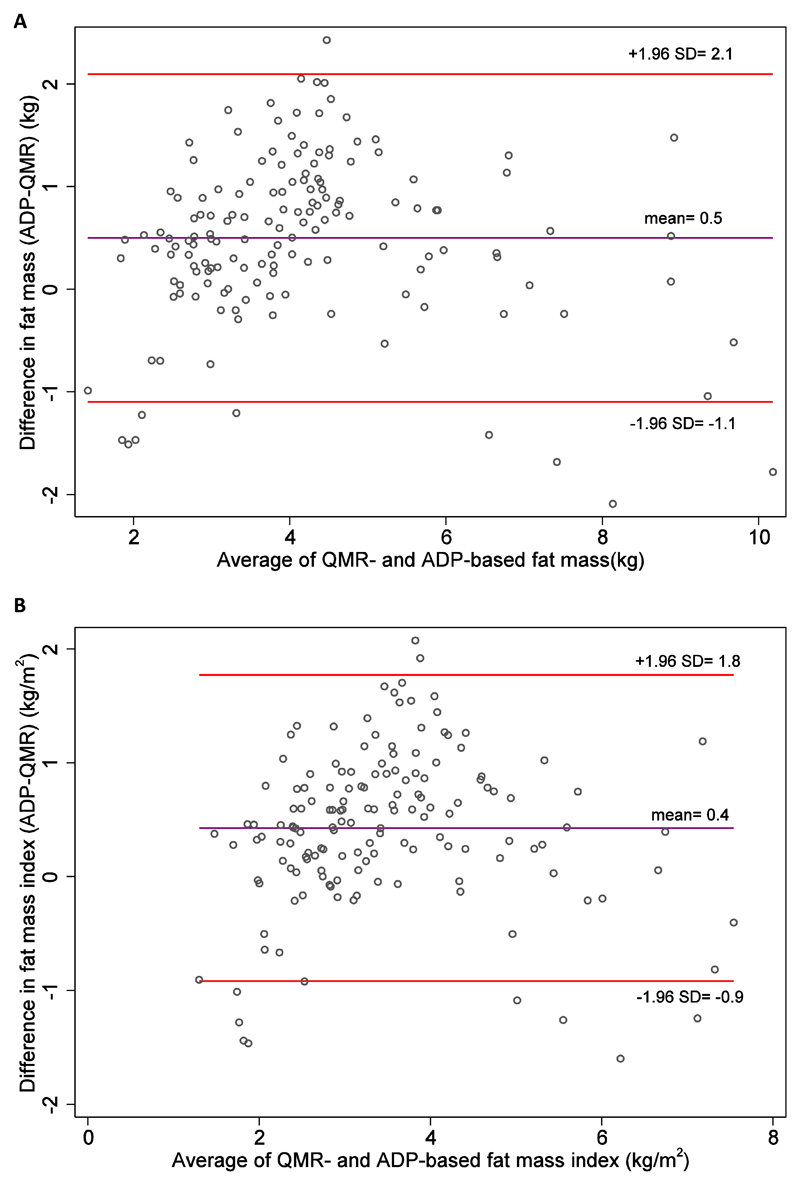
The two-way scatter plots of fat mass and FMI based on ADP and QMR are shown in Figure 1. The absolute agreement between QMR and ADP measurements was high (ICC= 0.85 for fat mass and 0.82 for FMI). However, RMAR analysis suggested a systematic bias, in that ADP measurements were systematically higher than QMR measurements (fat mass: slope= 1.0, intercept= 0.6; FMI: slope= 1.0, intercept= 0.4). Bland-Altman analysis revealed mean differences (95% LOAs) of +0.5 (-1.1 to +2.1) kg for fat mass and +0.4 (-0.9 to +1.8) kg/m2 for FMI (Figure 2; estimates based on ADP minus QMR measurement). The 95% LOAs for fat mass were narrower than those in Andres et al. study with the 4-C model in older children (-1.6 to +2.9 kg for QMR-4C and -3.2 to +1.0 kg for ADP-4C), suggesting that our 95% LOAs were reasonable.
Figure 1.
Scatter plots of (A) fat mass and (B) fat mass index based on ADP plotted against fat mass and fat mass index based on QMR. The dotted line represents line of identity (y= x) while the solid line represents reduced major axis regression line. ADP, air displacement plethysmography; QMR, quantitative magnetic resonance; ICC, intraclass correlation coefficient.
Figure 2.
Bland-Altman plots comparing (A) fat mass and (B) fat mass index based on ADP and QMR. The middle line represents mean difference in fat mass and fat mass index between ADP and QMR measurements (ADP minus QMR). The top and bottom lines represent ±1.96 SD from the mean (the bounded area represents 95% limit of agreement). ADP, air displacement plethysmography; QMR, quantitative magnetic resonance.
When plotted against common adiposity measures, the spread of FMI was narrower for QMR than for ADP measurements. The resulting 95% prediction intervals were also narrower, and the RMSE lower (indicating better fit), for QMR than for ADP models (Figure S3).
The agreement between QMR and ADP measurements was better in girls (ICC: 0.89 and 0.87 for fat mass and FMI, respectively) than in boys (ICC: 0.81 and 0.76) (Table S2). The confidence intervals for ICC overlapped between boys and girls, but the point estimate for boys lay outside of the confidence intervals of girls (Table S2). In addition, the mean differences between the QMR- vs. ADP-based fat mass and FMI were lower, and the corresponding 95% LOA narrower, in girls than in boys.
Discussion
In this study of Asian preschool children, we observed that QMR-based fat mass and FMI showed substantial agreement with those based on ADP. Moreover, QMR-based fat mass and FMI were consistently lower than those based on ADP. To our knowledge, ours is the first study that compares QMR and ADP measurements in Asians at any age. Asians are known to have a different body shape and fat distribution from Caucasians, and these differences are likely to influence body composition (23).
We are aware of only one previous study that assessed the validity of QMR measurements in children (11). In that U.S. study, fat mass based on QMR showed the least deviation (+10%) from the 4-C model in 57 children aged 6 years or above, followed by the deuterium dilution technique (+16.1%), ADP (-18.1%), and DXA (+26.5%). Moreover, that study also reported the highest precision for QMR measurements (coefficient of variation= 1.4% for fat mass, as compared with 6.4% for ADP measurements). However, since QMR overestimated and ADP underestimated fat mass determined by the 4-C model, the direction of agreement in that study is different from our finding that QMR-based fat mass was consistently lower than that based on ADP. The aforementioned U.S. study included children up to 16 years, and it is likely that the wide age range (6-16 years old) affected the direction of agreement. Indeed, in contrast with that U.S. study, a slight overestimation of fat mass (about 1%) by ADP compared with the 4-C model was observed in another U.S. study of children aged 2-6 years (24). Interestingly, validation studies in adults have reported that QMR tends to underestimate fat mass compared with the 4-C model (25,26). The discrepancy in results might also be due to non-standardized protocols in different studies and differences in body shapes between Asians and Caucasians. Collectively, the combined results suggest that agreement between body composition measurement techniques may differ at different ages and in different populations, emphasizing the need for validation studies in children, and especially in Asian children. However, owing to the low deviation of QMR from the 4-C model in Caucasian children and good agreement between QMR and ADP measurements in our (Asian) study, the combined results suggest that QMR is a valid and precise measure for tracking childhood body composition.
Previous studies have reported better agreement between QMR- and 4-C model-based fat mass in adult (aged 23-55) women than in men (25,26). To our knowledge, our study is the first to note similar sex differences in children. Two-compartment models like the ADP assume constant fat mass and fat free mass density, but known sex differences in fat free mass density (27,28) may have resulted in differential measurement error and consequently differential agreement.
The QMR technique has several advantages over commonly used body composition measurement methods. QMR measurement is non-invasive, does not use ionizing radiation, and can be completed within 3 minutes. Moreover, a small amount of body movement has little effect on the measurement, a significant advantage over ADP and DXA techniques (which require complete immobility for high-quality measurements), particularly at young ages. Because the QMR technology is based on relaxation properties of hydrogen nuclei in different tissues, it does not assume constant fat and fat-free mass density and is less influenced by variation in body volume and density. QMR measurement can also quantify lean body mass (without solid components mainly located in bones) unlike ADP, although this measure requires further validation (29). In our study, the completion rate for QMR (47%) is better than ADP (38%), which may due partly to less subject demand (no need to change into tight-fitting clothing which was required for ADP, better tolerance of subjects’ movements compared to ADP) and shorter measurement time associated with QMR. Collectively, these advantages of QMR make it a suitable instrument to track body composition in children.
Strengths and limitations
Our study is strengthened by its relatively large sample of Asian children. However, because our study is embedded in a mother-offspring cohort requiring deep phenotyping (including nutritional, neurodevelopment, and allergy assessment) during follow-up visits, repeated measurements of body composition at the same age were not possible, so as to avoid excessive burden on the child and family. Absolute precision (based on repeated measures) of ADP and QMR could therefore not be ascertained. In our attempt to assess relative precision of QMR compared with ADP, we found that the spread of QMR-based fat mass and FMI was narrower when plotted against common adiposity measures, and that RMSE values for all QMR models were lower, strongly suggesting better precision of the QMR vs. the ADP measurement. The absolute precision of QMR measurements has been consistently shown to be superior to other conventional techniques (11,23,25,26). Furthermore, approximately half of the eligible participants had neither QMR nor ADP measurements, owing to parental refusal or logistical reasons. However, children without these measurements did not differ in weight, height, BMI, and proportions of obese/overweight vs. those with either measurement, suggesting that our analysis population is fairly representative of the range of body composition at this age and a low likelihood of selection bias. The key limitation of the QMR system is its substantially higher capital cost than a BodPod machine. Children’s fear of the closed chamber is a major limitation for both QMR and BodPod (albeit better completion rate observed for QMR), indicating that proper training of staff to explain the procedure and child-friendly decoration of the machines are needed to improve take-up rate.
Conclusions
Fat mass and FMI based on QMR showed substantial agreement with those based on ADP. This observation, coupled with the high precision of QMR measurement and its suitability and ability to track childhood body composition longitudinally, suggests that QMR may be ideal for measuring body composition in children. The current childhood obesity epidemic calls for improved methods for measuring body composition in children. QMR’s relatively high cost may be justifiable for long-term, fast, and precise tracking of childhood body composition.
Supplementary Material
What is already known about this subject?
Accurate assessment of body composition in children is essential to study the associations of early life factors with childhood adiposity and the adverse consequences of excessive adiposity.
Recently, quantitative magnetic resonance (QMR) has been increasingly used to measure human body composition.
However, the use and validation of QMR in measuring body composition in children is limited.
What this study adds
In a cohort of multiethnic Asian preschool children, QMR-based fat mass and fat mass index showed substantial agreement with, but was generally lower than, air displacement plethysmography-based measures.
Considering QMR’s improved precision, non-invasiveness and brief measurement time, it appears to be a suitable method for measuring body composition in children.
Acknowledgement
The authors thank the GUSTO study group, which includes Allan Sheppard, Amutha Chinnadurai, Anne Eng Neo Goh, Anne Rifkin-Graboi, Anqi Qiu, Arijit Biswas, Bee Wah Lee, Birit F.P. Broekman, Boon Long Quah, Borys Shuter, Carolina Un Lam, Chai Kiat Chng, Cheryl Ngo, Choon Looi Bong, Claudia Chi, Cornelia Yin Ing Chee, Yam Thiam Daniel Goh, Doris Fok, E Shyong Tai, Elaine Tham, Elaine Quah Phaik Ling, Evelyn Xiu Ling Loo, Falk Mueller-Riemenschneider, George Seow Heong Yeo, Helen Chen, Heng Hao Tan, Hugo P S van Bever, Iliana Magiati, Inez Bik Yun Wong, Ivy Yee-Man Lau, Jeevesh Kapur, Jenny L. Richmond, Jerry Kok Yen Chan, Joanna D. Holbrook, Joanne Yoong, Joao N. Ferreira, Jonathan Tze Liang Choo, Jonathan Y. Bernard, Joshua J. Gooley, Kenneth Kwek, Krishnamoorthy Niduvaje, Kuan Jin Lee, Leher Singh, Lieng Hsi Ling, Lin Lin Su, Lourdes Mary Daniel, Mark Hanson, Mary Foong-Fong Chong, Mary Rauff, Mei Chien Chua, Melvin Khee-Shing Leow, Michael Meaney, Neerja Karnani, Ngee Lek, Oon Hoe Teoh, P. C. Wong, Paulin Tay Straughan, Peter D. Gluckman, Pratibha Agarwal, Queenie Ling Jun Li, Rob M. van Dam, Salome A. Rebello, Seang-Mei Saw, See Ling Loy, S. Sendhil Velan, Seng Bin Ang, Shang Chee Chong, Sharon Ng, Shiao-Yng Chan, Shirong Cai, Shu-E Soh, Sok Bee Lim, Stella Tsotsi, Chin-Ying Stephen Hsu, Sue Anne Toh, Swee Chye Quek, Walter Stunkel, Wayne Cutfield, Wee Meng Han, Wei Wei Pang, Yin Bun Cheung, Yiong Huak Chan, and Zhongwei Huang.
Financial support:
This research is supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore- NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research (A*STAR), Singapore, and Nestec. Study sponsors were not involved in the design of the study, statistical analysis and results interpretation. KMG is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and by the European Union's Seventh Framework Programme (FP7/2007-2013), projects EarlyNutrition and ODIN (Food-Based Solutions for Optimal Vitamin D Nutrition and Health through the Life Cycle) under grant agreements nos 289346 and 613977.
Abbreviations used
- 4-C
four-compartment
- ADP
air displacement plethysmography
- BIA
bioelectrical impedance analysis
- BMI
body mass index
- DXA
dual-energy x-ray absorptiometry
- FFM
fat free mass
- FMI
fat mass index
- GUSTO
Growing Up in Singapore Towards healthy Outcomes
- ICC
intraclass correlation coefficient
- LOA
limit of agreement
- KKH
KK Women's and Children's Hospital
- NUH
National University Hospital
- QMR
quantitative magnetic resonance
- RMAR
reduced major axis regression
- RMSE
root mean squared error
- SS
subscapular skinfold
- SST
sum of skinfold
- TS
triceps skinfold
Footnotes
Authors’ contribution:
L-WC analyzed and interpreted the data and wrote the first draft of the paper. LW-C and M-TT acquired data for QMR and ADP measurements, respectively. L-WC, M-TT, and IMA contributed to data collection, cleaning, and analysis. LP-CS, KHT, VSR, PDG, Y-SC, FY, KMG, and YSL designed and led the GUSTO study. MVF and CHJ led the magnetic resonance imaging domain and body composition domain, respectively, in the GUSTO study. MSK, FY, and YSL advised on interpretation of results. All authors critically revised the manuscript. FY and YSL had primary responsibility for the final content. FY and YSL are joint corresponding authors. All authors have read and approved the final manuscript.
Conflicts of interest:
KMG, Y-SC, and YSL have received reimbursement for speaking at conferences sponsored by companies selling nutritional products. KMG and Y-SC are part of an academic consortium that has received research funding from Abbott Nutrition, Nestec and Danone. The other authors have no financial or personal conflict of interest to declare.
References
- 1.Lobstein T, Jackson-Leach R, Moodie ML, et al. Child and adolescent obesity: part of a bigger picture. Lancet. 2015;385(9986):2510–2520. doi: 10.1016/S0140-6736(14)61746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The Lancet. Managing the tide of childhood obesity. Lancet (London, England) 2015;385(9986):2434. doi: 10.1016/S0140-6736(15)61122-9. [DOI] [PubMed] [Google Scholar]
- 3.Wells JCK. Toward body composition reference data for infants, children, and adolescents. Adv Nutr. 2014;5(3):320S–9S. doi: 10.3945/an.113.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Javed A, Jumean M, Murad MH, et al. Diagnostic performance of body mass index to identify obesity as defined by body adiposity in children and adolescents: a systematic review and meta-analysis. Pediatr Obes. 2015;10(3):234–244. doi: 10.1111/ijpo.242. [DOI] [PubMed] [Google Scholar]
- 5.Malone SK, Zemel BS. Measurement and Interpretation of Body Mass Index During Childhood and Adolescence. J Sch Nurs. 2015;31(4):261–271. doi: 10.1177/1059840514548801. [DOI] [PubMed] [Google Scholar]
- 6.Freedman DS, Wang J, Maynard LM, et al. Relation of BMI to fat and fat-free mass among children and adolescents. Int J Obes. 2005;29(1):1–8. doi: 10.1038/sj.ijo.0802735. [DOI] [PubMed] [Google Scholar]
- 7.Wells JCK, Fewtrell MS. Measuring body composition. Arch Dis Child. 2006;91(7):612–617. doi: 10.1136/adc.2005.085522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuller NJ, Jebb SA, Laskey MA, Coward WA, Elia M. Four-component model for the assessment of body composition in humans: comparison with alternative methods, and evaluation of the density and hydration of fat-free mass. Clin Sci (Lond) 1992;82(6):687–693. doi: 10.1042/cs0820687. [DOI] [PubMed] [Google Scholar]
- 9.Chomtho S, Wells JC, Williams JE, Davies PS, Lucas A, Fewtrell MS. Infant growth and later body composition: evidence from the 4-component model. Am J Clin Nutr. 2008;87(6):1776–1784. doi: 10.1093/ajcn/87.6.1776. [DOI] [PubMed] [Google Scholar]
- 10.Deurenberg P. Limitations of the bioelectrical impedance method for the assessment of body fat in severe obesity. Am J Clin Nutr. 1996;64(3 Suppl):449S–452S. doi: 10.1093/ajcn/64.3.449S. [DOI] [PubMed] [Google Scholar]
- 11.Andres A, Gomez-Acevedo H, Badger TM. Quantitative Nuclear Magnetic Resonance to Measure Fat Mass in Infants and Children. Obesity. 2011;19(10):2089–2095. doi: 10.1038/oby.2011.215. [DOI] [PubMed] [Google Scholar]
- 12.Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377(6):990–1002. doi: 10.1007/s00216-003-2224-3. [DOI] [PubMed] [Google Scholar]
- 13.Lakshmi S, Metcalf B, Joglekar C, Yajnik CS, Fall CH, Wilkin TJ. Differences in body composition and metabolic status between white UK and Asian Indian children (EarlyBird 24 and the Pune Maternal Nutrition Study) Pediatr Obes. 2012;7(5):347–354. doi: 10.1111/j.2047-6310.2012.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kadowaki T, Sekikawa A, Murata K, et al. Japanese men have larger areas of visceral adipose tissue than Caucasian men in the same levels of waist circumference in a population-based study. Int J Obes. 2006;30(7):1163–1165. doi: 10.1038/sj.ijo.0803248. [DOI] [PubMed] [Google Scholar]
- 15.Paley C, Hull H, Ji Y, et al. Body fat differences by self-reported race/ethnicity in healthy term newborns. Pediatr Obes. 2016;11(5):361–368. doi: 10.1111/ijpo.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soh S-E, Tint MT, Gluckman PD, et al. Cohort profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43(5):1401–1409. doi: 10.1093/ije/dyt125. [DOI] [PubMed] [Google Scholar]
- 17.National Healthcare Group Polyclinics. Age and Gender-Specific National BMI Cut-Offs (Singapore) 2010.
- 18.Hamilton CM, Strader LC, Pratt JG, et al. The PhenX Toolkit: get the most from your measures. Am J Epidemiol. 2011;174(3):253–260. doi: 10.1093/aje/kwr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yassi N, Campbell BCV, Moffat BA, et al. Know your tools—concordance of different methods for measuring brain volume change after ischemic stroke. Neuroradiology. 2015;57(7):685–695. doi: 10.1007/s00234-015-1522-8. [DOI] [PubMed] [Google Scholar]
- 20.Smith RJ. Use and misuse of the reduced major axis for line-fitting. Am J Phys Anthropol. 2009;140(3):476–486. doi: 10.1002/ajpa.21090. [DOI] [PubMed] [Google Scholar]
- 21.Tompuri TT, Lakka TA, Hakulinen M, et al. Assessment of body composition by dual-energy X-ray absorptiometry, bioimpedance analysis and anthropometrics in children: the Physical Activity and Nutrition in Children study. Clin Physiol Funct Imaging. 2015;35(1):21–33. doi: 10.1111/cpf.12118. [DOI] [PubMed] [Google Scholar]
- 22.Wells JCK, Haroun D, Williams JE, et al. Evaluation of DXA against the four-component model of body composition in obese children and adolescents aged 5–21 years. Int J Obes. 2010;34(4):649–655. doi: 10.1038/ijo.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galgani JE, Smith SR, Ravussin E. Assessment of EchoMRI-AH versus dual-energy X-ray absorptiometry to measure human body composition. Int J Obes (Lond) 2011;35(9):1241–1246. doi: 10.1038/ijo.2010.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fields DA, Allison DB. Air-displacement plethysmography pediatric option in 2-6 years old using the four-compartment model as a criterion method. Obesity (Silver Spring) 2012;20(8):1732–1737. doi: 10.1038/oby.2012.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Napolitano A, Miller SR, Murgatroyd PR, et al. Validation of a quantitative magnetic resonance method for measuring human body composition. Obesity (Silver Spring) 2008;16(1):191–198. doi: 10.1038/oby.2007.29. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher D, Thornton JC, He Q, et al. Quantitative magnetic resonance fat measurements in humans correlate with established methods but are biased. Obesity (Silver Spring) 2010;18(10):2047–2054. doi: 10.1038/oby.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Heshka S, Wang J, Wielopolski L, Heymsfield SB. Magnitude and variation of fat-free mass density: a cellular-level body composition modeling study. Am J Physiol Endocrinol Metab. 2003;284(2):E267–73. doi: 10.1152/ajpendo.00151.2002. [DOI] [PubMed] [Google Scholar]
- 28.Wells JCK, Williams JE, Chomtho S, et al. Pediatric reference data for lean tissue properties: density and hydration from age 5 to 20 y. Am J Clin Nutr. 2010;91(3):610–618. doi: 10.3945/ajcn.2009.28428. [DOI] [PubMed] [Google Scholar]
- 29.Bosy-Westphal A, Müller MJ. Assessment of fat and lean mass by quantitative magnetic resonance. Curr Opin Clin Nutr Metab Care. 2015;18(5):446–451. doi: 10.1097/MCO.0000000000000201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.