Abstract
In March 2013, diagnosis of the first reported case of human infection with a novel avian-origin influenza A(H7N9) virus occurred in eastern China. Most human cases have resulted in severe respiratory illness and, in some instances, death. Currently there are no licensed vaccines against H7N9 virus, which continues to cause sporadic human infections. Recombinant virus-like particles (VLPs) have been previously shown to be safe and effective vaccines for influenza. In this study, we evaluated the immunogenicity and protective efficacy of a H7N9 VLP vaccine in the ferret challenge model. Purified recombinant H7N9 VLPs morphologically resembled influenza virions and elicited high-titer serum hemagglutination inhibition (HI) and neutralizing antibodies specific for A/Anhui/1/2013 (H7N9) virus. H7N9 VLP-immunized ferrets subsequently challenged with homologous virus displayed reductions in fever, weight loss, and virus shedding compared to these parameters in unimmunized control ferrets. H7N9 VLP was also effective in protecting against lung and tracheal infection. The addition of either ISCOMATRIX or Matrix-M1 adjuvant improved immunogenicity and protection of the VLP vaccine against H7N9 virus. These results provide support for the development of a safe and effective human VLP vaccine with potent adjuvants against avian influenza H7N9 virus with pandemic potential.
Keywords: Influenza, Virus, Vaccine, Avian influenza, Ferret
1. Introduction
An outbreak of human infection caused by a novel avian-origin influenza A H7N9 virus emerged in eastern China in the spring of 2013 [1]. In late March, the notification of three human cases of H7N9 in Shanghai and Anhui, all of whom died after acute respiratory distress syndrome (ARDS) and multi-organ failure, raised public health concerns [1]. As of March 2015, the World Health Organization (WHO) had reported over 500 infections and over 200 deaths, mostly in China (http://www.who.int/en/). Most human infections have been the result of exposure to infected poultry or with environments where animals are housed [1,2]. Multiple reassortment events resulted in a novel H7N9 virus with six internal genes derived from an avian influenza A(H9N2) virus [1,3–5]. Despite the considerable morbidity and mortality caused by H7N9 virus, no sustained human-to-human transmission of this subtype has been documented. However, because humans are immunologically naïve to this subtype, the emergence of a transmissible H7N9 virus presents a significant public health concern.
While H7N9 cases have remained sporadic, routine pandemic preparedness measures, including vaccine development, have continued to date. However, there are currently no approved human vaccines for H7N9 viruses. Traditional egg-based vaccine manufacturing methods require time-consuming propagation and purification, taking at least 6 months to produce large quantities of influenza vaccine. The development of safe vaccines by reassortment and reverse genetics methods also requires additional evaluation for adequate safety [6]. Experimental vaccines have been previously developed for H7 subtype viruses; however H7N9 candidate vaccines have not been sufficiently evaluated in preclinical and clinical settings [7–10]. Recombinant virus-like particles (VLPs) are considered a promising egg-independent vaccine approach that has been previously shown to be a safe and effective vaccine against influenza [11–17]. An H7N9 VLP vaccine candidate was rapidly developed in early 2013 and shown to induce protective immunity against H7N9 virus in mice [10]. Although VLP vaccines alone are sufficiently immunogenic, the addition of adjuvants can improve the performance of vaccines and reduce the required dosage. One strategy to improve vaccine efficacy is by the addition of saponin-based adjuvants such as ISCOMATRIX and Matrix-M1 [18]. Saponin-based adjuvants have the ability to enhance both humoral and cellular immune responses, including CD8+ T-cell immunity [18,19].
Ferrets are considered to be the most suitable animal model for influenza vaccine efficacy studies [13,20–22]. Using this and other animal models, experimental influenza VLP vaccines have shown protective efficacy against potentially pandemic H5N1 and H9N2 viruses, as well as the 2009 pandemic H1N1 virus [7,13,15,23,24,16]. In this study, influenza virus genes cloned into a baculovirus expression vector to produce H7N9 VLP in Spodoptera frugiperda (Sf9) insect cells, were evaluated for its immunogenicity and vaccine efficacy in the ferret challenge model. We demonstrate that the experimental H7N9 VLP vaccine induced pre-challenge serum neutralizing antibody titers of ≥80 which were associated with protection of ferrets. Influenza vaccination with H7N9 VLP vaccine also prevented significant virus replication in the lung and trachea of ferrets upon subsequent challenge. The saponin-based ISCOMATRIX and Matrix-M1 adjuvants, shown previously to be safe and well tolerated in humans [19], were found to enhance the immunogenicity and protection efficacy of H7N9 VLPs.
2. Materials and methods
2.1. Viruses and cloning of HA, NA, and M1 genes
Influenza hemagglutinin (HA) H7 and neuraminidase (NA) N9 sequences of A/Anhui/1/2013 (Anhui/1; H7N9) virus were obtained from the GISAID Epiflu database (www.gisaid.org) with accession numbers EPI439507 and EPI439509, respectively. Both genes were codon-optimized for high-level expression in Sf9 insect cells (ATCC, Manassas, VA) and synthesized biochemically (Genscript, Piscataway, NJ). The matrix (M1) sequence, GenBank accession no. ABI36004, was from A/Indonesia/5/2005 (H5N1) virus and was codon-optimized for high-level expression and synthesized (Geneart, Regensburg, Germany). In order to generate VLPs, full-length H7, N9 and M1 genes were cloned into baculovirus (rBV) pFastBac1 transfer vectors between BamHI and HindIII sites downstream from a polyhedrin promoter, as described elsewhere [10,15]. Recombinant rBV expressing H7, N9, and M1 genes were generated by using a Bac-to-Bac baculovirus expression system (Life Technologies, Carlsbad, CA). The titers of rBV preparations were determined by plaque assay in Sf9 cells and expressed as plaque forming units (pfu)/ml. For expression of VLPs, Sf9 cells were maintained as suspension cultures in HyQ-SFX insect serum free medium (HyClone, Logan, UT) at 27 ± 2 °C. Recombinant baculovirus stocks were prepared by infecting cells at a low multiplicity of infection (MOI) of ≤0.01 pfu per cell and harvested at 68–72 h post-infection.
2.2. Preparation of H7N9 VLPs
For production of H7N9 VLPs, Sf9 cells were adjusted to 2 × 106 cells/ml and co-infected at a MOI of 0.5 pfu per cell for 68–72 h with rBVs expressing H7, N9, and M1 genes. The H7N9 VLPs were harvested from Sf9 growth medium supernatant, concentrated and purified. Briefly, infected Sf9 cells were incubated with continuous agitation at 27 ± 2 °C and harvested by centrifugation at 4000 × g for 15 min. Cell culture supernatant containing VLP and baculovirus particles was initially concentrated and dialyzed against buffer using hollow fiber tangential flow filtration. Then, separation of VLP from baculovirus and other host contaminants was accomplished by using anion exchange followed by gel filtration chromatography. Purified H7N9 VLPs in PBS were filter sterilized using a 0.2 μm filter and stored at 2–8 °C until vaccinations.
2.3. Characterization of VLPs
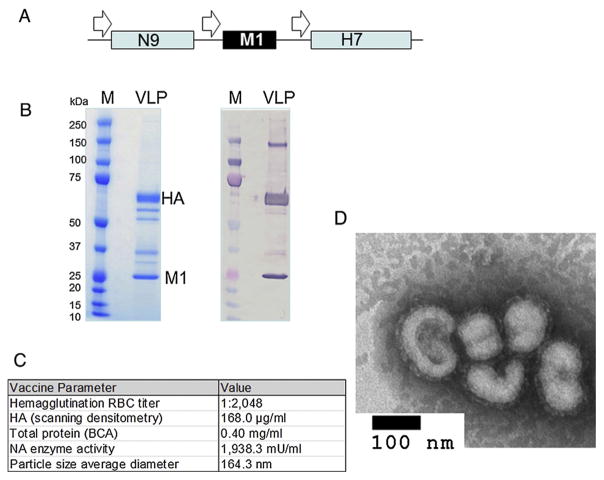
VLPs were examined by SDS-PAGE using 4–12% polyacrylamide gels (Life Technologies, Carlsbad, CA) followed by staining with Gel-Code Blue stain (Pierce, Rockford, IL). The content of H7 within VLPs was quantitated by densitometry analysis of stained gels. VLPs were also evaluated for total protein concentration (BCA bicinchoninic acid protein assay, Pierce Biochemicals, Rockford, IL) and particle size by dynamic light scattering with a ZETASizer Nano (Malvern Instruments, PA) using standard manufacturer recommended methods. Western blot was done using rabbit anti H7N7 for HA (Sino Biologicals, Beijing, China) and mouse anti-influenza A M1 for M1 (AbD Serotec, Kidlington, UK).
For transmission electron microscopy, H7N9 VLPs were adsorbed onto freshly discharged 400 mesh carbon parlodion-coated copper grids (Poly-Sciences, Warrington, PA). The grids were rinsed with buffer containing 20 mM Tris, pH 7.4, and 120 mM KCl and negatively stained with 1% phosphotungstic acid, then dried by aspiration. VLPs were visualized on a Hitachi H-7600 transmission electron microscope (Hitachi High Technologies America, Schaumburg, IL) operating at 80 kV and digitally captured with a CCD camera at 1 k × 1 k resolution (Advanced Microscopy Techniques Corp., Danvers, MA).
The hemagglutination titer was determined by serially diluting VLPs at two-fold increments in 50 μl in a 96-well plate. To each VLP dilution, 50 μl of 0.75% horse red blood cell (RBC) working solution was added, mixtures of VLPs and RBCs were gently agitated and the plate was incubated at 25 °C for 30–60 min before examination recommended by the WHO [17]. Negative hemagglutination results appeared as dots in the center of the wells. The titer was calculated as the highest dilution factor that produced a positive reading. For hemagglutination assays, dilutions of VLPs were mixed with horse RBCs.
The neuraminidase enzymatic activity of VLPs was determined by using a fluorescence-based NA assay (NA-Fluor, Life Technologies) with methyl umbelliferone N-acetyl neuraminic acid (MUNANA; Sigma, St Louis, MO) as a substrate according to manufacturer’s instructions.
2.4. Vaccinations and challenge
All animal experiments were performed under the guidance of the Centers for Disease Control and Prevention’s Institutional Animal Care and Use Committee and were conducted in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facility. Adult male Fitch ferrets, 4–5 months of age (Triple F Farms, Sayre, PA), serologically negative by hemagglutination inhibition (HI) assay for currently circulating influenza viruses, were used in this study. Groups of six ferrets received two intramuscular (i.m.) inoculations (4–5 weeks apart) of 15 μg of total HA in the VLPs with and without adjuvant in a 0.5 ml volume. PBS treated ferrets served as unimmunized controls. The adjuvants, Matrix-M1 (Novavax, AB, Uppsala, Sweden) and ISCOMATRIXTM (CSL Biotherapies Inc., King of Prussia, Pennsylvania, USA; ISCOMATRIX is a registered trademark of ISCOTEC Ab, a CSL company) were mixed with antigen for 20–30 min prior to injection, as described by the manufacture. Adjuvants were added in a 1:1 v/v ratio giving a final concentration of 60 ISCO units (IU)/0.5 ml for ISCOMATRIX and 50 μg/0.5 ml for Matrix-M1 adjuvant. The vaccine dose of 15 μg and i.m. route of administration is consistent with current human vaccination practices [25]. Prior to primary vaccination, vaccine boost, and viral challenge, all ferrets were bled for collection of serum to assess responses to vaccination and then challenged intranasally (5 weeks following boost) with 106 pfu of H7N9 virus in a total volume of 1 ml (500 μl per nostril). Following challenge, ferrets were monitored daily for changes in body weight and temperature, as well as clinical signs of illness. Nasal wash samples were collected at 2, 4, 6, and 8 days post-challenge (p.c.) and titrated in a standard plaque assay to determine viral titers in the upper respiratory tract. The statistical significance of differences in weight loss, temperature changes, and virus titers between vaccinated and PBS-control animals were determined by ANOVA.
Six ferrets per group were additionally challenged with homologous virus and euthanized days 3 or 5 p.c. (3 per time point) for collection of nasal wash, nasal turbinates, trachea, and lung as previously described [26]. All samples were immediately frozen at −80 °C until use. Tissues were homogenized in 1 ml cold PBS and titrated by standard plaque assay in MDCK cells as previously described [27]. The limit of virus detection was 10 pfu.
2.5. Serological assays
All sera were initially diluted 1:10 in receptor-destroying enzyme from Vibrio cholerae (Denka Seiken, Tokyo, Japan). Both HI and neutralization assays were performed. The HI assay was performed using 0.5% turkey or 1% horse erythrocytes with 4 hemagglutination units (HAU) of homologous viruses using standard methods [15]. Titers of virus neutralizing (VN) antibody were determined essentially as described [28] and expressed as the reciprocal of the highest dilution of serum that neutralized 100 pfu of virus in MDCK cell cultures. The neutralization titers were presented as the geometric mean titers (GMT) from vaccinated or control ferrets.
3. Results
3.1. Characterization of H7N9 VLP vaccine
Influenza H7N9 VLPs were constructed in a rBV expression system with the sequences of the virus genes for HA, NA and M1 (Fig. 1A). The H7 and N9 proteins were derived from sequences of A/Anhui/1/2013 (Anhui/1; H7N9) virus and the conserved M1 protein, derived from an H5N1 virus, was previously shown to be sufficient for successful preparation of chimeric VLPs [10]. The presence of H7 and M1 proteins in VLPs was confirmed by SDS-PAGE and Western blot with antibodies specific for HA and M1 (Fig. 1B). Using a universal NA antibody, it was previously shown that the N9 within VLPs co-migrated with HA on the gel [10], and the HA:NA mass ratio is 5.6:1 as determined previously by capillary gel electrophoresis [10]. The expressed H7 HA of the VLPs represented uncleaved HA0 polypeptide of 62–64 kilodaltons (kDa). The M1 protein, which has been previously shown to be a major structural component of influenza VLP complexes [7,15], is 252 aa in length with the expected molecular mass of approximately 25 kDa. Functionally, the VLPs exhibited high titer hemagglutination activity (HAU = 2048 per 0.40 mg/ml total VLP protein), as titrated with horse RBCs, confirming that the incorporated HA protein retained its stability and binding capacity (Fig. 1C). The H7N9 VLPs also displayed NA enzymatic activity in a standard fluorescence-based influenza neuraminidase assay (Fig. 1C). The size and morphology of VLPs were further examined by negative-staining transmission electron microscopy (TEM) (Fig. 1D). The H7N9 VLPs were identified as largely spherical, typical influenza-like enveloped particles, which average approximately 164 nm in diameter with characteristic spikes of HA protruding from the VLP envelope.
Fig. 1.
Recombinant baculovirus (rBV) construct and expression of A/Anhui/1/2013 (H7N9) VLPs in Sf9 cells. (A) Influenza HA, NA and M1 were codon-optimized for high-level expression in Sf9 cells and synthesized biochemically. The HA, NA, and M1 genes were combined within rBV in a tandem fashion so that each gene was expressed from its own expression cassette that included polyhedrin promoter and polyadenylation signal. (B) Expression of H7N9 VLPs by SDS-PAGE (left panel) and Western blot. The molecular weight (MW) of uncleaved HA0 was approximately 62–64 kilodalton (kDa). Western blot was done using rabbit anti-H7N7 and mouse anti-influenza A M1 (right panel). (C) Properties and functions of the H7N9 VLPs, and (D) negative staining electron microscopy of H7N9 VLPs. Bar, 100 nm.
3.2. Immunogenicity and protective efficacy of H7N9 VLPs in ferrets
In the first experiment, ferrets received two intramuscular (i.m.) vaccinations of H7N9 VLPs in the presence or absence of ISCOMATRIX adjuvant; control animals received PBS in place of vaccine. There were no adverse effects at the injection site in any of the ferrets following immunization. The HI and neutralization antibody responses to H7N9 virus were measured among individual serum samples collected just prior to vaccine boost and virus challenge. As expected, no antibody responses to H7N9 were detected in the serum of control animals. A single 15 μg VLP vaccine dose elicited a detectable neutralizing antibody response in all vaccinated ferrets (geometric mean titer [GMT] = 16) (Table 1). The addition of ISCOMATRIX to the VLP vaccine increased neutralizing titers (≥40) (GMT = 63) to Anhui/1 (H7N9) virus. A vaccine boost led to substantial increases in neutralizing antibody titers that ranged from 80 to 160 (GMT = 113) in the unadjuvanted group and 160 to 320 (GMT = 254) among ferrets that received VLP with ISCOMATRIX. HI antibody titers measured in sera of adjuvanted and non-adjuvanted ferrets followed a similar pattern to the neutralizing antibody response, albeit at lower titers (Table 1). All vaccinated ferrets displayed pre-challenge HI antibody titers of ≥80 to Anhui/1 virus; the pre-challenge HI titers were highest among the VLP/ISCOMATRIX-vaccinated ferrets (GMT = 142) compared to ferrets in the unadjuvanted group (GMT = 80). Overall, these data indicate that the VLP vaccine elicited high-titer serum neutralizing antibodies specific for H7N9 virus and the addition of ISCOMATRIX augmented the antibody response to vaccination.
Table 1.
Geometric mean of neutralizing (neut) and hemagglutination inhibition (HI) serum antibody titers following H7N9 VLP inoculation in ferrets.a
| Vaccine group | Neut titerb | HI titerb | ||
|---|---|---|---|---|
|
|
|
|||
| Pre-boost | Pre-challenge | Pre-boost | Pre-challenge | |
| Experiment 1 | ||||
| VLP | 16 (10–20) | 113 (80–160) | 9 (5–10) | 80 (80) |
| VLP + ISCOMATRIX | 63 (40–80) | 254 (160–320) | 35 (20–40) | 142 (80–160) |
| PBS | <10 | <10 | <10 | <10 |
| Experiment 2 | ||||
| VLP | 20 (10–20) | 101 (80–160) | 7 (5–10) | 63 (40–80) |
| VLP + M1 | 50 (40–80) | 285 (160–320) | 25 (20–40) | 202 (160–320) |
| PBS | <10 | <10 | <10 | <10 |
Ferrets were vaccinated i.m. two times with the 15 μg of H7N9 VLP.
Samples were tested for HI and neutralization antibody activity against A/Anhui/1/2013 virus. Geometric mean titers (with range in parentheses) are shown as the reciprocal of the highest dilution of serum that completely inhibits HI or that neutralized 100 pfu of infectious virus (neut).
We next determined the level of protection against homologous virus challenge induced by H7N9 VLP vaccine. Vaccine protection was measured by reduction in fever, weight loss, and viral shedding in ferrets following a stringent challenge dose (106 pfu) of Anhui/1 virus. Unimmunized control ferrets challenged with Anhui/1 virus displayed fever, weight loss and transient inactivity. On day 2 post-challenge (p.c.), all control ferrets exhibited an early spike in body temperature, ranging from 0.8 to 2.9 °C over baseline (mean maximum = 2.3 °C) (Table 2); elevated temperatures (>1.0 °C over baseline) were maintained for 4 days p.c. (not shown). VLP- and VLP/ISCOMATRIX-vaccinated animals also displayed an early (day 2 p.c.) spike in body temperature (mean maximum = 2.0 °C and 1.3 °C, respectively), however body temperatures returned to baseline by day 4 p.c. and both vaccinated groups displayed significantly lower (p ≤0.05) temperatures compared to control ferrets. Significant differences in weight loss were also observed between vaccinated and control ferrets (Table 2 and Fig. 2). Control animals exhibited 12.7% mean maximum weight loss compared with 7.4% and 3.5% weight loss for VLP- and VLP/ISCOMATRIX-vaccinated ferrets, respectively.
Table 2.
Clinical symptoms observed in VLP-immune ferrets challenged with H7N9 virus.
| Vaccine groupa | Clinical symptoms of H7N9 challenged ferrets | ||
|---|---|---|---|
|
| |||
| Mean temp increase over baseline (3C)b | Mean % weight lossc | Mean peak viral titerd | |
| VLP | 2.0 | 7.4 | 5.0 ± 0.18 |
| VLP + ISCOMATRIX | 1.3 | 3.5 | 4.2 ± 0.04 |
| PBS | 2.3 | 12.7 | 6.5 ± 0.11 |
Ferrets were vaccinated i.m. two times with the 15 μg of H7N9 VLP.
Temperature increases over ferret baseline of 38.5 ± 0.2 °C, all maximum temperatures from day 2 p.c.
Mean maximum weight loss percentage values shown (day 7 or 8 post-challenge) for 6 ferrets per group.
Virus detected in nasal wash samples, mean peak titer shown as log10 pfu/ml including standard deviation. All peak titers from day 2 p.c.
Fig. 2.
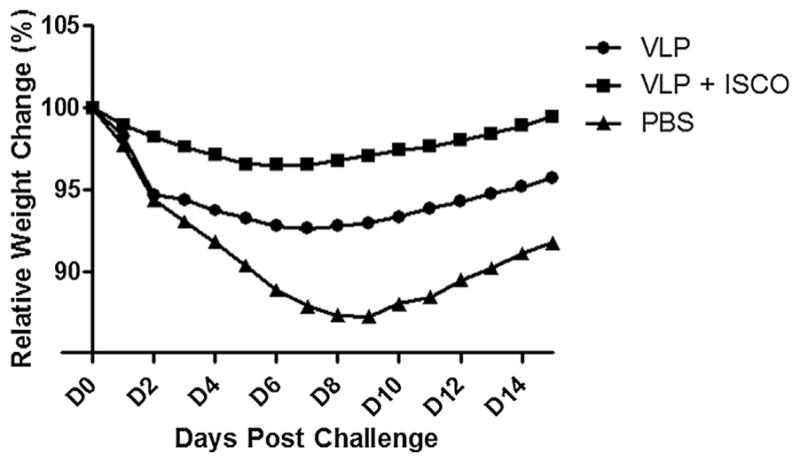
Morbidity of VLP vaccinated ferrets following Anhui/1 virus challenge. Ferrets (6 per group) received two intramuscular inoculations (4 weeks apart) in the presence or absence of ISCOMATRIX adjuvant and control animals received PBS in place of vaccine. Following H7N9 virus challenge ferrets were weighed daily to measure morbidity. Average weights in each vaccine group were measured for the duration of the study, and percent original body weight was calculated based on the average starting weight for each group on day 0. Statistical significance from PBS group was determined by analysis of area under curve (AUC) with ANOVA and Bonferroni post-test; p < 0.05 for VLP versus PBS, p < 0.001 for VLP + ISCO versus PBS group.
The extent of virus replication in the upper respiratory tract was determined by titrating nasal wash samples collected from immune and control ferrets on alternate days post-challenge (p.c.) In comparison to control animals, H7N9 VLP-vaccinated ferrets displayed a significant reduction in viral shedding on days 2, 4 and 6 p.c. (Table 2 and Fig. 3). The addition of ISCOMATRIX adjuvant to H7N9 VLP vaccine provided enhanced protection against virus shedding compared with VLP only. Viral titers measured in VLP/ISCOMATRIX-vaccinated ferrets were significantly reduced on all days measured (2, 4 and 6 p.c.) and these ferrets exhibited a faster rate of virus clearance compared with PBS controls (Fig. 3). Taken together, these data demonstrate that the VLPs, especially when adjuvanted, offered reductions against substantial fever, weight loss, and viral shedding following H7N9 virus challenge.
Fig. 3.
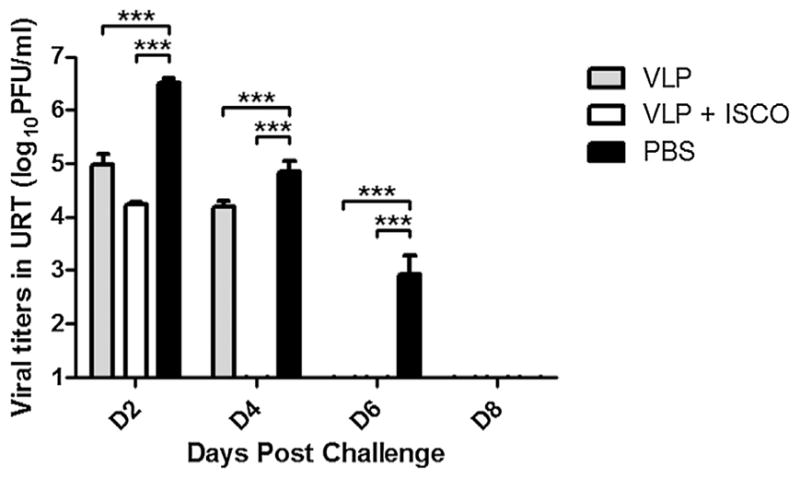
H7N9 VLP vaccine efficacy following Anhui/1/virus challenge. Ferrets (6 per group) received two intramuscular inoculations (4 weeks apart) in the presence or absence of ISCOMATRIX adjuvant and control animals received PBS in place of vaccine. The nasal cavities of challenged ferrets were washed with 1 ml PBS every other day beginning on day 2 p.c. and virus titers were determined. Statistical significances were determined by two-way ANOVA. ***Indicates statistical significance (p < 0.001) in viral titers between PBS and VLP-vaccinated groups for each day post-challenge.
3.3. The effect of H7N9 VLP vaccination on the virus replication in the ferret respiratory tract
In the second experiment, VLP vaccine efficacy was assessed using a second saponin-based adjuvant, Matrix-M1. The extent of VLP vaccine protection against H7N9 virus was measured as viral titers in lung and upper (nasal turbinate and trachea) respiratory tract tissues at the peak of virus replication. Ferrets received two i.m. vaccinations of H7N9 VLPs in the presence or absence of Matrix-M1 adjuvant and control animals received PBS in place of vaccine. Similar to the first vaccine experiment, the neutralizing antibody responses to H7N9 virus were measured in individual serum samples collected prior to vaccine boost and challenge (Table 1, experiment 2). A single immunization elicited a detectable neutralizing antibody response in all VLP-M1 vaccinated and five of six VLP-only vaccinated ferrets. Following booster vaccination, neutralizing antibody responses to H7N9 virus were substantially elevated in all vaccinated ferrets. The pre-challenge neutralizing antibody titers against Anhui/1 virus ranged from 80 to 160 (GMT = 101) in the VLP vaccinated ferrets and 160 to 320 (GMT = 285) VLP-M1 vaccinated animals.
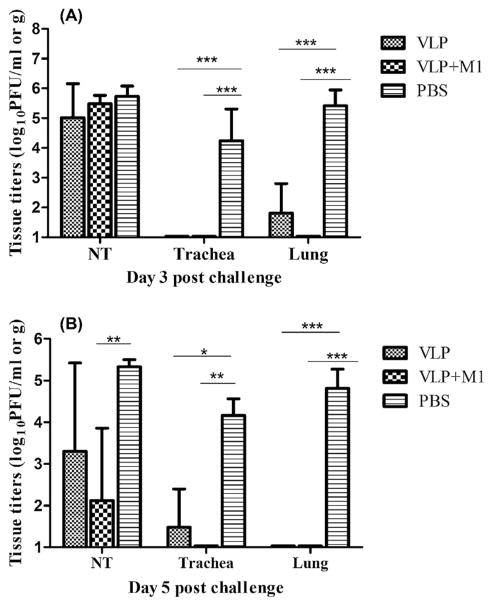
Vaccinated and control ferrets were similarly challenged with 106 pfu of Anhui/1 virus five weeks after vaccine boost. On days 3 and 5 p.c., unimmunized control animals showed high titers (≥4.2 mean log10 pfu/ml) of infectious virus in each of the three respiratory tract tissues (Fig. 4). Conversely, mean tissue titers among VLP vaccinated ferrets were lower and significant reductions were achieved in trachea (p < 0.05) and lung tissues (p < 0.001) days 3 and 5 p.c. Notably, no infectious virus was recovered from lung tissues of any ferrets administered VLP vaccine plus M1 adjuvant. Among ferrets that received VLP vaccine only, no virus was detected in lungs on day 5 p.c. and the mean lung virus titers at day 3 p.c. were 2200-fold less than unimmunized controls. Virus titers in nasal turbinates (NT) were significantly reduced on day 5 p.c. among ferrets inoculated VLP vaccine plus M1 adjuvant (Fig. 4B). VLP-M1 vaccination also resulted in a significant reduction in virus shedding measured on days 3 and 5 p.c. (Fig. 5). Taken together, these data suggest that VLP immunization also prevented significant virus replication in the ferret lung and trachea and the addition of M1 adjuvant enhanced the protective efficacy of the VLP vaccine.
Fig. 4.
H7N9 VLP vaccine protection against lung replication. Ferrets (6 per group) received two intramuscular inoculations (5 weeks apart) in the presence or absence of Matrix-M1 adjuvant and control animals received PBS in place of vaccine. Animals were challenged i.n. with 106 pfu of influenza A/Anhui/1/2013 (H7N9) virus. Viral titers of influenza virus in tissues on day 3 (A), and day 5 post-challenge (B) were determined by a standard plaque assay. Infectious virus was detectable in lung tissues of two of three vaccinated ferrets on day 3. The data shown are mean values plus standard deviations (error bars) for 3 ferrets per group for each time period. The differences in viral titers between groups were analyzed by two-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 5.
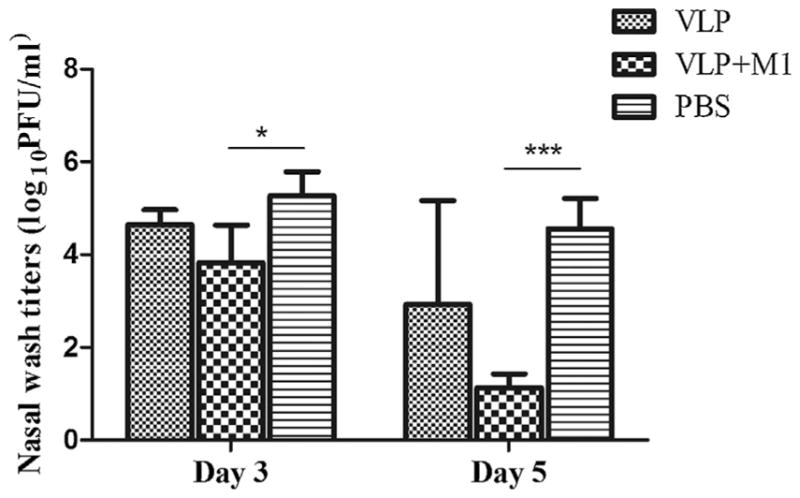
H7N9 VLP vaccine protection against virus shedding. Ferrets (6 per group) received two intramuscular inoculations (5 weeks apart) in the presence or absence of Matrix-M1 adjuvant and control animals received PBS in place of vaccine. Animals were challenged i.n. with 106 pfu of Anhui/1 virus. Nasal washes were collected prior to euthanasia (3 ferrets/time point) and titrated in a standard plaque assay. The differences in viral titers between groups were analyzed by two-way ANOVA. *p < 0.05, ***p < 0.001.
4. Discussion
Similar to avian influenza H5N1 subtype viruses, avian H7 viruses have caused outbreaks and human infection in recent years and continue to pose a public health threat. The continuing occurrence of human H7N9 infections and the suboptimal immunogenicity of H7 vaccines underscore the need for continued development of improved influenza vaccines [29–31]. Although it has been shown that a H7N3 live attenuated influenza vaccine can be protective against H7N9 virus due to the presence of conserved antigenic sites on the H7 HA [5], an H7N9-specific vaccine is needed for optimal protection. The WHO reported that a comparison of A(H7N9) viruses isolated from humans, poultry and environmental samples using HI assays shows that limited antigenic diversity exists among this group of viruses (http://www.who.int/en/). Thus, based on genetic and antigenic analysis of H7N9 viruses, the WHO has recommended that A/Anhui/1/2013-like virus be used for the development of influenza H7N9 vaccines for pandemic preparedness purposes. Using the ferret model, regarded as the most relevant model for influenza vaccine efficacy assessments, we showed that an H7N9 VLP vaccine induced high levels of virus-neutralizing antibodies to Anhui/1 virus, which were augmented by the addition of saponin-based adjuvants, ISCOMATRIX or Matrix-M1. H7N9 VLP vaccination was effective at significantly reducing viral shedding and lung viral loads associated with H7N9 virus infection.
Several experimental H7 influenza vaccines have been developed and evaluated. They include a live attenuated H7N7 candidate vaccine virus containing a modified HA from the highly pathogenic A/Netherlands/219/2003 (H7N7) virus that protected mice, ferrets, and monkeys from virus challenge [32]. Moreover, a live attenuated H7N3 influenza virus vaccine was well tolerated and immunogenic in a Phase I clinical trial in healthy adults [33]. Conversely, a clinical trial of an inactivated H7N7 virus vaccine in healthy young adults exhibited low immunogenicity despite vaccinations with two doses of up to 90 μg of HA [34]. Recent results from a phase 1 study in healthy adults demonstrated that an adjuvant (MF59) was required to achieve significant HI antibody responses to H7N9 virus [35]. Evaluations for in vitro correlates of immunogenicity of inactivated H5, H7 and H9 vaccines in humans revealed that low HA titer was associated with low immunogenicity, whereas the presence of particles or split virus pieces was associated with higher immunogenicity [36]. This suggested that correct conformation and localization of H7 within the intact virus particle vaccine is important for optimal immunogenicity. Another type of vaccine is the cell-based H7N1 split influenza virion, which was shown to confer protection in the mouse and ferret challenge models [37]. More recently, protection against H7N9 viruses has been reported using viral vector vaccines [38,39].
In the present study, recombinant VLPs were generated from structural proteins of the Anhui/1 H7N9 virus. The size and morphology of the H7N9 VLPs resembled influenza virions and confirmation of HA0 on the outer surface of VLPs along with hemagglutination and neuraminidase functions, suggested proper H7N9 VLP self-assembly. Although negative-staining TEM was not performed on the VLP/adjuvant formulations, previously unpublished TEM images on other VLPs in saponin-based adjuvants have shown no obvious interactions or changes in VLP morphology compared to VLP without adjuvant (Smith, personal communication). We tested the ability of adjuvanted and non-adjuvanted H7N9 VLPs to provide protection in a stringent (106 pfu) challenge model. We used 15 μg of total HA in the VLPs, which represents the standard dose per virus contained in licensed inactivated seasonal influenza vaccines. In the first vaccine experiment, all ferrets that received VLP plus ISCOMATRIX adjuvant were well protected against weight loss and prolonged viral shedding. Ferrets vaccinated with adjuvant generally had higher levels of HI and neutralizing antibodies than did non-adjuvant vaccinated ferrets. Even without adjuvant, the VLP vaccine elicited pre-challenge HI titers of ≥40, which is generally accepted to represent a 50% protective titer for seasonal influenza viruses in adult populations [40]. An enhancing effect of ISCOMATRIX adjuvant on the immunogenicity of VLP vaccination has been demonstrated in mice [10] and in humans [19]. It is generally believed that ISCOMATRIX adjuvant possesses two main functions: efficient antigen delivery (i.e. promotes trafficking of vaccine particles directly into draining lymph nodes) and potent immune activation by inducing both Th1 and Th2 type responses [41]. As ferret specific reagents become more widely available, the utility of this challenge model to investigate T-cell functions with respect to adjuvant use will increase.
The clinical course of H7N9 virus infection in humans can result in the progression of severe pneumonia and ARDS [1]. Further progression of lung injury may result in inadequate gas exchange, lower respiration, and ultimately death. Thus, unlike seasonal influenza virus infection, H7N9 virus can efficiently replicate in lung tissue resulting in an inflammatory response and potentially decreased lung function [42]. Therefore, it was important to examine whether H7N9 VLP vaccine provided significant protection in the lung. Strikingly, only trace amounts of infectious virus were found in lung tissue of two of three vaccinated ferrets on day 3 p.c. and no virus was measured (above the limit of detection) on day 5 p.c. H7N9 VLP vaccination also prevented significant virus replication in the trachea tissues. Similar to the immunogenicity-enhancing effect of ISCOMATRIX, the Matrix-M1 adjuvant induced an enhanced neutralizing antibody response and improved VLP vaccine protection against H7N9. Although the precise mechanism of Matrix-M1 action has not been well studied, most likely this adjuvant functions similar to other saponin-based adjuvants by improving antigen delivery and stimulating immune responses.
Influenza VLPs are relatively easy to develop and manufacture, offering certain advantages over current influenza vaccine technologies [15,43]. VLP vaccine technology is particularly advantageous since it provides cost-effective manufacturing processes that alleviate safety restrictions associated with live virus vaccines. Moreover, the non-egg based manufacturing process could be used to help meet the demand for influenza preparedness and surge capacity against emerging avian influenza viruses, such as H7N9. Influenza VLPs can also accommodate multiple subtypes of HA in their envelopes. In a recent study, novel multi-subtype VLPs, which co-localized H5, H7, and H9 subtypes of HA within the envelope protected ferrets from experimental infection challenge with three avian influenza viruses [7,8].
The emergence of a novel reassortant avian influenza H7N9 virus that continues to transmit zoonotically to humans represents a significant threat to human health [1]. The data presented here suggest that Anhui/1 VLPs represent a useful vaccine for H7N9 influenza virus. Moreover, ISCOMATRIX and Matrix-M1 adjuvants improved immunogenicity and protection of the VLP vaccine. Such a vaccine capable of reducing virus shedding and virus load in lung tissue may attenuate disease caused by H7N9 virus and may also reduce potential transmission of virus to susceptible hosts.
Acknowledgments
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
References
- 1.Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, et al. Human infection with a novel avian-origin influenza A(H7N9) virus. N Engl J Med. 2013;368:1888–97. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Emergence of avian influenza A(H7N9) virus causing severe human illness – China, February–April 2013. MMWR Morb Mortal Wkly Rep. 2013 May;62(18):366–71. [PMC free article] [PubMed] [Google Scholar]
- 3.Lee RT, Gunalan V, Van TD, Le LT, Eisenhaber F, Maurer-Stroh S. A new piece in the puzzle of the novel avian-origin influenza A(H7N9) virus. Biol Direct. 2013;8:26. doi: 10.1186/1745-6150-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam TT, Wang J, Shen Y, Zhou B, Duan L, Cheung CL, et al. The genesis and source of the H7N9 influenza viruses causing human infections in China. Nature. 2013;502:241–4. doi: 10.1038/nature12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joseph T, McAuliffe J, Lu B, Vogel L, Swayne D, Jin H, et al. A live attenuated cold-adapted influenza A H7N3 virus vaccine provides protection against homologous and heterologous H7 viruses in mice and ferrets. Virology. 2008;378:123–32. doi: 10.1016/j.virol.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Update of WHO biosafety risk assessment and guidelines for the production and quality control of human influenza vaccines against avian influenza A(H7N9) virus. 2013. [Google Scholar]
- 7.Pushko P, Pearce MB, Ahmad A, Tretyakova I, Smith G, Belser JA, et al. Influenza virus-like particle can accommodate multiple subtypes of hemagglutinin and protect from multiple influenza types and subtypes. Vaccine. 2011;29:5911–8. doi: 10.1016/j.vaccine.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 8.Tretyakova I, Pearce MB, Florese R, Tumpey TM, Pushko P. Intranasal vaccination with H5, H7 and H9 hemagglutinins co-localized in a virus-like particle protects ferrets from multiple avian influenza viruses. Virology. 2013;442:67–73. doi: 10.1016/j.virol.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 9.Rudenko L, Isakova-Sivak I, Donina S. H7N3 live attenuated influenza vaccine has a potential to protect against new H7N9 avian influenza virus. Vaccine. 2013;31:4702–5. doi: 10.1016/j.vaccine.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 10.Smith GE, Flyer DC, Raghunandan R, Liu Y, Wei Z, Wu Y, et al. Development of influenza H7N9 virus like particle (VLP) vaccine: homologous A/Anhui/1/2013 (H7N9) protection and heterologous A/chicken/Jalisco/CPA1/2012 (H7N3) cross-protection in vaccinated mice challenged with H7N9 virus. Vaccine. 2013;31:4305–13. doi: 10.1016/j.vaccine.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 11.Galarza JM, Latham T, Cupo A. Virus-like particle (VLP) vaccine conferred complete protection against a lethal influenza virus challenge. Viral Immunol. 2005;18:244–51. doi: 10.1089/vim.2005.18.244. [DOI] [PubMed] [Google Scholar]
- 12.Kang SM, Pushko P, Bright RA, Smith G, Compans RW. Influenza virus-like particles as pandemic vaccines. Curr Top Microbiol Immunol. 2009;333:269–89. doi: 10.1007/978-3-540-92165-3_14. [DOI] [PubMed] [Google Scholar]
- 13.Perrone LA, Ahmad A, Veguilla V, Lu X, Smith G, Katz JM, et al. Intranasal vaccination with 1918 influenza virus-like particles protects mice and ferrets from lethal 1918 and H5N1 influenza virus challenge. J Virol. 2009;83:5726–34. doi: 10.1128/JVI.00207-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bright RA, Carter DM, Daniluk S, Toapanta FR, Ahmad A, Gavrilov V, et al. Influenza virus-like particles elicit broader immune responses than whole virion inactivated influenza virus or recombinant hemagglutinin. Vaccine. 2007;25:3871–8. doi: 10.1016/j.vaccine.2007.01.106. [DOI] [PubMed] [Google Scholar]
- 15.Pushko P, Tumpey TM, Bu F, Knell J, Robinson R, Smith G. Influenza virus-like particles comprised of the HA, NA, and M1 proteins of H9N2 influenza virus induce protective immune responses in BALB/c mice. Vaccine. 2005;23:5751–9. doi: 10.1016/j.vaccine.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 16.Quan FS, Vunnava A, Compans RW, Kang SM. Virus-like particle vaccine protects against 2009 H1N1 pandemic influenza virus in mice. PLoS ONE. 2010;5(2):e9161. doi: 10.1371/journal.pone.0009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross TM, Mahmood K, Crevar CJ, Schneider-Ohrum K, Heaton PM, Bright RA. A trivalent virus-like particle vaccine elicits protective immune responses against seasonal influenza strains in mice and ferrets. PLoS ONE. 2009;4(6):e6032. doi: 10.1371/journal.pone.0006032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maraskovsky E, Schnurr M, Wilson NS, Robson NC, Boyle J, Drane D. Development of prophylactic and therapeutic vaccines using the ISCOMATRIX adjuvant. Immunol Cell Biol. 2009;87:371–6. doi: 10.1038/icb.2009.21. [DOI] [PubMed] [Google Scholar]
- 19.Fries LF, Smith GE, Glenn GM. A recombinant virus like particle influenza A(H7N9) vaccine. N Engl J Med. 2013;369:2564–6. doi: 10.1056/NEJMc1313186. [DOI] [PubMed] [Google Scholar]
- 20.Huang SS, Banner D, Fang Y, Ng DC, Kanagasabai T, Kelvin DJ, et al. Comparative analyses of pandemic H1N1 and seasonal H1N1, H3N2, and influenza B infections depict distinct clinical pictures in ferrets. PLoS ONE. 2011;6(11):e27512. doi: 10.1371/journal.pone.0027512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng X, Eisenbraun M, Xu Q, Zhou H, Kulkarni D, Subbarao K, et al. H5N1 vaccine-specific B cell responses in ferrets primed with live attenuated seasonal influenza vaccines. PLoS ONE. 2009;4(2):e4436. doi: 10.1371/journal.pone.0004436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belser JA, Szretter KJ, Katz JM, Tumpey TM. Use of animal models to understand the pandemic potential of highly pathogenic avian influenza viruses. Adv Virus Res. 2009;73:55–97. doi: 10.1016/S0065-3527(09)73002-7. [DOI] [PubMed] [Google Scholar]
- 23.Bright RA, Carter DM, Crevar CJ, Toapanta FR, Steckbeck JD, Cole KS, et al. Cross-clade protective immune responses to influenza viruses with H5N1 HA and NA elicited by an influenza virus-like particle. PLoS ONE. 2008;3(1):e1501. doi: 10.1371/journal.pone.0001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pushko P, Kort T, Nathan M, Pearce MB, Smith G, Tumpey TM. Recombinant H1N1 virus-like particle vaccine elicits protective immunity in ferrets against the 2009 pandemic H1N1 influenza virus. Vaccine. 2010;28:4771–6. doi: 10.1016/j.vaccine.2010.04.093. [DOI] [PubMed] [Google Scholar]
- 25.Cox NJ, Subbarao K. Influenza. Lancet. 1999 Oct;354(9186):1277–82. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 26.Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, et al. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol. 2005;79:11788–800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng H, Goldsmith C, Thawatsupha P, Chittaganpitch M, Waicharoen S, Zaki S, et al. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type i interferon response in polarized human bronchial epithelial cells. J Virol. 2007;81:12439–49. doi: 10.1128/JVI.01134-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mozdzanowska K, Furchner M, Washko G, Mozdzanowski J, Gerhard W. A pulmonary influenza virus infection in SCID mice can be cured by treatment with hemagglutinin-specific antibodies that display very low virus-neutralizing activity in vitro. J Virol. 1997;71:4347–55. doi: 10.1128/jvi.71.6.4347-4355.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.WHO. Antigenic and genetic characteristics of A(H5N1), A(H7N3), A(H9N2) and variant influenza viruses and candidate vaccine viruses developed for potential use in human vaccines. 2013. [Google Scholar]
- 30.Belser JA, Blixt O, Chen LM, Pappas C, Maines TR, Van Hoeven N, et al. Contemporary North American influenza H7 viruses possess human receptor specificity: implications for virus transmissibility. Proc Natl Acad Sci U S A. 2008;105:7558–63. doi: 10.1073/pnas.0801259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pappas C, Matsuoka Y, Swayne DE, Donis RO. Development and evaluation of an Influenza virus subtype H7N2 vaccine candidate for pandemic preparedness. Clin Vaccine Immunol. 2007;14:1425–32. doi: 10.1128/CVI.00174-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min JY, Vogel L, Matsuoka Y, Lu B, Swayne D, Jin H, et al. A live attenuated H7N7 candidate vaccine virus induces neutralizing antibody that confers protection from challenge in mice, ferrets, and monkeys. J Virol. 2010;84:11950–60. doi: 10.1128/JVI.01305-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talaat KR, Karron RA, Callahan KA, Luke CJ, DiLorenzo SC, Chen GL, et al. A live attenuated H7N3 influenza virus vaccine is well tolerated and immunogenic in a Phase I trial in healthy adults. Vaccine. 2009;27:3744–53. doi: 10.1016/j.vaccine.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Couch RB, Patel SM, Wade-Bowers CL, Nino D. A randomized clinical trial of an inactivated avian influenza A (H7N7) vaccine. PLoS ONE. 2012;7(12):e49704. doi: 10.1371/journal.pone.0049704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bart SA, Hohenboken M, Della Cioppa G, Narasimhan V, Dormitzer PR, Kanesa-Thasan N. A cell culture-derived MF59-adjuvanted pandemic A/H7N9 vaccine is immunogenic in adults. Sci Transl Med. 2014;6:234ra55. doi: 10.1126/scitranslmed.3008761. [DOI] [PubMed] [Google Scholar]
- 36.Couch RB, Decker WK, Utama B, Atmar RL, Nino D, Feng JQ, et al. Evaluations for in vitro correlates of immunogenicity of inactivated influenza a h5, h7 and h9 vaccines in humans. PLoS ONE. 2012;7(12):e50830. doi: 10.1371/journal.pone.0050830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox RJ, Major D, Hauge S, Madhun AS, Brokstad KA, Kuhne M, et al. A cell-based H7N1 split influenza virion vaccine confers protection in mouse and ferret challenge models. Influenza Other Respir Viruses. 2009;3:107–17. doi: 10.1111/j.1750-2659.2009.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goff PH, Krammer F, Hai R, Seibert CW, Margine I, Garcia-Sastre A, et al. Induction of cross-reactive antibodies to novel H7N9 influenza virus by recombinant Newcastle disease virus expressing a North American lineage H7 subtype hemagglutinin. J Virol. 2013;87:8235–40. doi: 10.1128/JVI.01085-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreijtz JH, Wiersma LC, De Gruyter HL, Vogelzang-van Trierum SE, van Amerongen G, Stittelaar KJ, et al. A single immunization with modified vaccinia virus ankara-based influenza virus H7 vaccine affords protection in the influenza A (H7N9) pneumonia ferret model. J Infect Dis. 2015;211:791–800. doi: 10.1093/infdis/jiu528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katz JM, Hancock K, Xu X. Serologic assays for influenza surveillance, diagnosis and vaccine evaluation. Expert Rev Anti Infect Ther. 2011;9:669–83. doi: 10.1586/eri.11.51. [DOI] [PubMed] [Google Scholar]
- 41.Wilson NS, Yang B, Morelli AB, Koernig S, Yang A, Loeser S, et al. ISCOMATRIX vaccines mediate CD8+ T-cell cross-priming by a MyD88-dependent signaling pathway. Immunol Cell Biol. 2012;90:540–52. doi: 10.1038/icb.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belser JA, Tumpey TM. Tropism of H7N9 influenza viruses in the human respiratory tract. Lancet Respir Med. 2013;1:501–2. doi: 10.1016/S2213-2600(13)70161-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe T, Watanabe S, Neumann G, Kida H, Kawaoka Y. Immunogenicity and protective efficacy of replication-incompetent influenza virus-like particles. J Virol. 2002;76:767–73. doi: 10.1128/JVI.76.2.767-773.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]