Abstract
Insomnia is the most frequently encountered sleep complaint worldwide. While many prescription drugs are used to treat insomnia, extracts of valerian (Valeriana officinalis L., Valerianaceae) are also used for the treatment of insomnia and restlessness. To determine novel mechanisms of action, radioligand binding studies were performed with valerian extracts (100% methanol, 50% methanol, dichloromethane [DCM], and petroleum ether [PE]) at the melatonin, glutamate, and GABAA receptors, and 8 serotonin receptor subtypes. Both DCM and PE extracts had strong binding affinity to the 5-HT5a receptor, but only weak binding affinity to the 5-HT2b and the serotonin transporter. Subsequent binding studies focused on the 5-HT5a receptor due to the distribution of this receptor in the suprachiasmatic nucleus of the brain, which is implicated in the sleep–wake cycle. The PE extract inhibited [3H]lysergic acid diethylamide (LSD) binding to the human 5-HT5a receptor (86% at 50 μg/ml) and the DCM extract inhibited LSD binding by 51%. Generation of an IC50 curve for the PE extract produced a biphasic curve, thus GTP shift experiments were also performed. In the absence of GTP, the competition curve was biphasic (two affinity sites) with an IC50 of 15.7 ng/ml for the high-affinity state and 27.7 μg/ml for the low-affinity state. The addition of GTP (100 AM) resulted in a right-hand shift of the binding curve with an IC50 of 11.4 μg/ml. Valerenic acid, the active constituent of both extracts, had an IC50 of 17.2 AM. These results indicate that valerian and valerenic acid are new partial agonists of the 5-HT5a receptor.
Keywords: Circadian rhythm, Insomnia, Serotonin, 5-HT5A, Valerian, Valerenic acid
1. Introduction
Insomnia is considered the most frequent sleep complaint and affects nearly all populations throughout the world, particularly the elderly [28]. Menopausal women often suffer from sleep disturbances with frequent awakenings, likely due to disturbed expression of daily biological rhythms [4,30]. Some of the more commonly used hypnotic drugs to treat insomnia are benzodiazepines, which are associated with adverse events such as tolerance, dependence, and morning sleepiness [28]. Many menopausal women prefer not to use benzodiazepines but use valerian preparations instead, and report that these products are well tolerated, effective for the treatment of insomnia, while not inducing impairment of vigilance or cognitive and psycho-motor performance [17,29,30].
Extracts from the roots of valerian (Valeriana officinalis L., Valerianaceae) have long been used in alternative medicine for the treatment of insomnia and are the most well recognized herbal sedatives worldwide. Approximately 15 controlled clinical trials have assessed the efficacy of various valerian extracts, and the German Commission E has approved its use for the treatment of restlessness and sleeping disorders [4]. Numerous clinical trials support the use of valerian and have demonstrated an improvement in sleep latency and quality in both healthy volunteers and patients with sleep disorder [4,29,33]. Currently, there is no scientific agreement on the mechanism of action of valerian's sedating activity or the compounds responsible. Many potential mechanisms for the pharmacological activity of valerian have been proposed, including agonistic activities on the GABA, adenosine, barbiturate, and benzodiazepine receptors [34,40,46]. However, the concentrations used in these investigations were extremely high in the older reports and more recent reports put some of these data into question [12,40].
Although serotonin is well known to modulate a variety of physiological and behavioral processes including sleep– wake cycles, and circadian rhythms, the effect of valerian on serotonin receptor binding has not been completely elucidated [9,24]. Serotonin (5-HT, 5-hydroxytryptamine) impacts numerous sensory, motor, and cortical functions by activating multiple 5-HT receptor subtypes [9,24,35]. Abnormalities of these receptor systems have been implicated in many psychiatric disorders including anxiety, depression, as well as disorders of cognition, stress, and sleep [9,28,35]. Serotonin was initially thought to be a true neuromodulator of sleep because the destruction of 5-HT neurons of the raphe system or the inhibition of 5-HT synthesis with p-chloro-phenylalanine induced severe insomnia that could be reversed by restoring 5-HT synthesis [2]. More recent experiments suggest that the release of 5-HT during the sleep–wakefulness cycle initiates a cascade of genomic events in some hypnogenic neurons located in the preoptic area and the neighboring suprachiasmatic nucleus including vasoactive intestinal polypeptide and GABAergic mechanism [1,3,9,15,28,35].
Our previous work has shown that dichloromethane and petroleum ether extracts of valerian bind weakly (51% at 50 μg/ml) to the 5-HT2b receptor but have significant binding (80%) at the 5-HT5a receptor [12]. When compared with other serotonin receptors, little is known about the 5-HT5a receptor, but it is believed to be involved in circadian (sleep–wake) rhythms, anxiety, and explorative behavior [15,18,20,42]. Distribution of the 5-HT5a receptor is widespread throughout the rat brain, but the receptor concentrations are particularly intense in the suprachiasmatic nucleus of the hypothalamus, the site of the biological clock that drives circadian rhythms. Although the involvement of the 5-HT5a receptors is a more recent hypothesis, support for the 5-HT5a-mediated function in the SCN has been shown by strong distinct immunoreactivity in three neural components of the circadian timing system—the intergeniculate leaflet, median raphe, and dorsal raphe nucleus, in addition to the SCN [42].
Considering that our previous work indicated that valerian extracts bind predominantly to the 5-HT5a receptor, the purpose of this work was to determine if the valerian extract acts as an agonist of this receptor. To this end, bioassay-guided fractionation of the DCM and PE extracts to isolate and identify the chemical constituents responsible for this activity was also performed.
2. Materials and methods
2.1. Materials and reagents
All chemicals and reagents were purchased from Fisher (Hanover Park, IL) or Sigma (St. Louis, MO) unless otherwise indicated. All cell culture media were obtained from Life Technologies (Carlsbad, CA). FBS was acquired from Atlanta Biologicals (Norcross, GA). [3H]Lysergic acid diethylamide (LSD), [3H]hydroxytryptamine (5-HT), and [3H]-8-hydroxy-2-(di-N-propylamino)tetralin (8-OH-DPAT) were obtained from NEN Life Science Products (Boston, MA). 5-HT5a membranes were purchased from Perkin-Elmer (Shelton, CT), while valerian reference compounds (valerenic acid, hydroxyvalerenic acid, and acetoxyvalerenic acid) were obtained from ChromaDex (Santa Ana, CA). The roots and rhizomes of valerian were obtained and identified from the field station of the University of Illinois at Chicago. Voucher specimens have been deposited at the University of Illinois at Chicago, College of Pharmacy.
2.2. Extraction and isolation
Valerian extracts for screening purposes were prepared from dried powdered underground parts of V. officinalis L. by successively extracting 100 g dried powdered material each time three times at 500 ml of the following solvents and an ultraturrax at ambient temperature: (1) dichloro-methane (DCM), (2) petroleum ether (PE), (3) methanol (MeOH), and in the end with methanol 50% (MeOH 50%). Moreover, an extract was prepared by extracting 20 g dried powdered underground parts of V. officinalis only with methanol using the same procedure. Bioassay-guided fractionation: 1300 g of V. officinalis underground parts was exhaustively extracted in two parts each time three times at 1500 ml petroleum ether and an ultraturrax at ambient temperature. The extraction yield was 1.3% and 1.4%, respectively. The dried petroleum ether extract was separated by silica gel vacuum column chromatography with a petroleum ether and chloroform gradient (2.5% to 5.0% steps). The active fraction (13.3 g) was further fractionated by a second silica gel vacuum column with hexane and ethyl acetate as gradient (2.5% to 5.0% steps). Valerenic acid (4.2 mg) was isolated from subfraction 5 by semipreparative HPLC with a water/acetonitrile gradient (75–85% acetonitrile in 28 min). Besides valerenic acid, the separation resulted in two compound mixtures (0.9 mg and 1.4 mg) and one isomeric mixture (1.4 mg). The content of valerenic acid was determined by HPLC using the method with an external standard curve for valerenic acid [7]. RP-Column a 1:1 combination of the dichloroform (DCM) and PE extract, a 1:2 mixture of the methanol (MeOH) and MeOH 50% and a 100% MeOH.
2.3. Serotonin receptor binding assays
Initial radioligand binding studies were performed by MDS Pharma Services (Bothell, WA) as previously described for serotonin receptor subtypes 1A, 1B, 2B, 2C, 3, 5A, 6, 7, serotonin transporter [5,6,8,23,31,32,37–41,43–45,47]. Significant binding affinity was only observed at the 5-HT5a receptor (>50%) for the PE and DCM extracts at a concentration of 50 μg/ml [12]. Similar results were reported by Schumacher et al. [40]. Therefore, this investigation focused only on the 5-HT5a receptor assay and GTP shift experiments.
For the 5-HT5a receptor binding experiments, additional assays were performed with minor modifications using human 5-HT5a receptor expressed in Chinese hamster ovary (CHO-K1) cell membranes. The human 5-HT5a-transfected CHO-KI cells were grown in Ham's F12 media supplemented with 10% FBS, MEM sodium pyruvate (1 mM), gentamicin (50 mg/ml), and penicillin/streptomycin (50 U/ml). Cells were scraped from culture dishes at full confluence. These cells were homogenized and centrifuged twice at 12,000 ×g for 20 min. The pellets were dissolved in TEM buffer (75 mM Tris, 1 mM EDTA, 12.5 mM of MgCl2, pH 7.4) and stored at −80°C. Protein concentrations were determined by the Lowry method using bovine serum albumin as standard. All preparations were kept on ice. [3H]LSD (1.64 nM) incubated at 37 °C for 60 min in incubation buffer (50 mM TRIS (base), 10 mM MgCl2·6H2O, 0.5 mM EDTA). After a 1-h incubation at 37°C, the mixtures for both receptors were filtered over 934-AH Whatman filters that had been presoaked in 0.5% polyethylenimine (PEI) and washed two times in ice-cold 50 mM Tris buffer (pH 7.4) using a 96-well Tomtec-Harvester (Orange, CT). Each filter was dried, suspended in Wallac microbeta plate scintillation fluid (Perkin-Elmer Life Sciences, Boston, MA), and counted with a Wallac 1450 Microbeta liquid scintillation counter (Perkin-Elmer Life Sciences, Boston, MA). 5-Hydroxytryptamine (serotonin, 5-HT) (250 nM) was used to define nonspecific binding, which accounted for <10% of the total binding. The percent inhibition of [3H]-LSD binding to the 5-HT5a receptor was determined as [1 – (dpmsample – dpmblank)/(dpmDMSO – dpmblank)] × 100. The inhibition of [3H]-LSD binding (%) of the sample was calculated in comparison with the inhibition of 1 μM 5-HT (100%). For the most potent compounds, IC50 values were determined by evaluation of the percent inhibition of [3H]-LSD binding in a number of serial dilutions. The data represent the average of triplicate determinations.
2.4. GTP shift experiments
Inhibition of binding of [3H]LSD (68.2 Ci/mmol, Perkin-Elmer) by the petroleum ether extract was measured in the presence and in the absence of 100 μM GTP. 5-HT (500 μM) was used to define nonspecific binding [8,40]. The assay was carried out under the same conditions as described for the assay above.
2.5. Data analysis
The data obtained in our laboratory represent the average ± SD of at least triplicate determinations. Curve fitting was performed using Akaike's Information Criteria (AICs), GraphPad Prism version 4.01 for Windows, GraphPad Software, San Diego CA, www.graphpad.com. Kd, IC50, and Ki values were determined using the same program.
3. Results
Extracts of valerian (100 MeOH, 50% MeOH, DCM and PE) were initially screened to determine if these extracts contained any potential ligand(s) of the melatonin, serotonin, benzodiazepine, glutamate, and GABAA receptors, or serotonin transporter [12]. No significant binding was found for any of the extracts at the 5-HT1A, 1B, 2C, 3, 6, 7, melatonin, benzodiazepine, or glutamine receptors. Only the PE and the DCM extracts (50 μg/ml) were active in the 5-HT2b, 5-HT5a receptor assays, and the serotonin transporter assay. A combination of the PE/DCM extracts bound to the serotonin 5-HT2b (51% binding), serotonin transporter (53% binding), and in the 5-HT5a receptor binding assay (80% binding) at a concentration of 50 μg/ml (Fig. 1). When tested separately, the PE extract exhibited 86% binding at the 5-HT5a receptor as compared with a 51% binding of the DCM extract at a concentration of 50 μg/ml. None of the other extracts tested were active (data not shown). The 100% methanol and 50% methanol extracts were not active in any of the receptors or transporter tested.
Fig. 1.
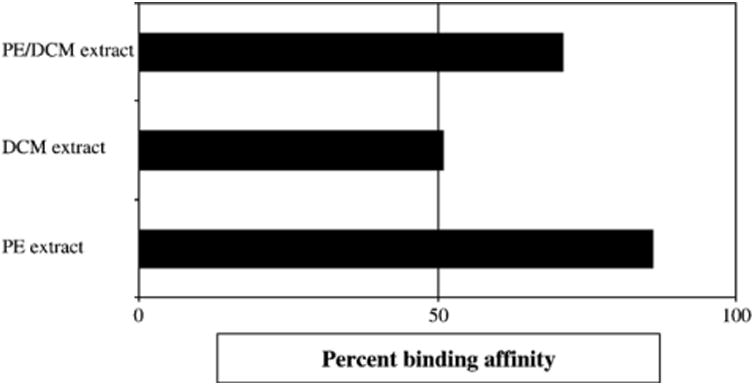
Percent binding affinity of the dichloromethane/petroleum ether extract combination, the dichloromethane extract, and petroleum ether extract to the 5-HT5a receptor. Extracts were tested at concentrations of 50 μg/ml in duplicate.
Due to the dense distribution of the 5-HT5a receptor to areas in the brain responsible for circadian timing and the sleep–wake cycle, the binding of the PE extract to the 5-HT5a receptor was investigated in further detail. To validate this method, serotonin (5-HT) was tested as an inhibitor [3H]LSD binding to the 5-HT5a receptor. A binding assay was established and validated by creating a saturation curve with [3H]LSD. Experimentally generated Ki values for [3H]LSD were consistent with the literature (Ki = 1.8 nM). The PE extract displaced the [3H]LSD from the 5-HT5a receptor with an IC50 curve that depicted a biphasic shape and best fit a two-site competition equation using the Akaike's Information Criteria (GraphPad Prism 4.01 for Windows). The PE extract displaced radioligands from the 5-HT5a receptor with an IC50 of 15.7 ng/ml (Ki: 9.48 ng/ml) for the high-affinity state and 27.7 μg/ml (Ki: 16.7 μg/ml) for the low-affinity state (Fig. 2). Since the 5-HT5a receptor belongs to the G-protein-coupled transmembrane proteins, GTP converts two classes of binding sites to a single lower affinity class of sites and leads to a right shift in case of an agonistic activity at the receptor. The IC50 curves for the PE extract in the absence and presence of GTP are depicted in Fig. 3. GTP can cause an uncoupling of the receptor from the G-protein leading to a shift of the receptor from the high- to the low-affinity state for agonists [40]. The addition of GTP resulted in a rightward shift of the PE extract binding curve to a low-affinity state or monophasic with an IC50 of 11.4 μg/ml (Ki = 6.88 μg/ml). These data suggested that chemical components of the PE valerian extract function as an agonist of the human 5-HT5a receptor.
Fig. 2.
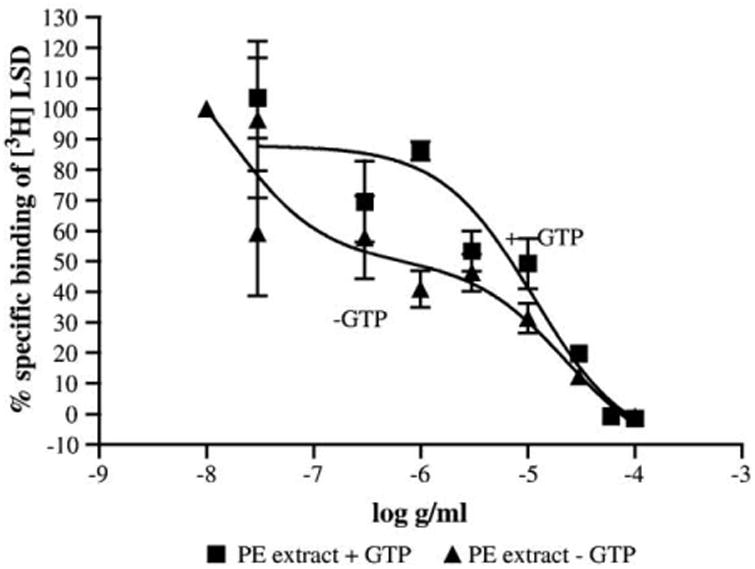
Binding of the PE extract of valerian to human 5-HT5a-transfected CHO membranes in the presence of [3H]LSD and in the presence or absence of GTP (100 μM). GTP results in a right-hand shift of the IC50 curve, indicating that the extract acts as an agonist at the 5-HT5a receptor.
Fig. 3.
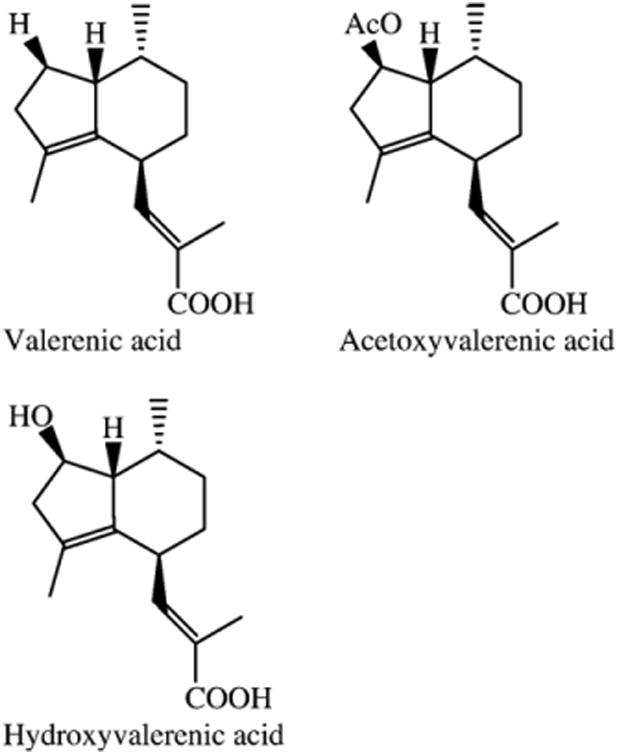
Major sesquiterpenes present in the PE and DCM valerian extracts. (1) Valerenic acid, (2) acetoxyvalerenic acid, and (3) hydroxyvalerenic acid.
In order to identify the active chemical compounds from valerian that bound to the 5-HT5a receptor, both the PE and DCM extracts were fractionated and pure compounds were isolated. In contrast to the methanol extracts tested, both the DCM and PE extracts contained valerenic acid (Fig. 3), with the PE extract characterized by its high valerenic acid content. In addition to valerenic acid, the PE extract also contained trace amounts of acetoxy-valerenic acid (Fig. 3) and a mixture of valepotriates. Hydroxyvalerenic acid (Fig. 3) was not found in the PE but did occur in the DCM extract. To isolate the active 5-HT5a receptor binding ligand from the PE extract, bioassay-guided fractionation was conducted leading to several active fractions, all containing valerenic acid. Receptor binding comparisons of the three major sesquiterpenes, valerenic acid, hydroxyl valerenic acid, and acetoxy-valerenic acid, confirmed that valerenic acid was the active constituent (see Fig. 4 and Table 1). The semipreparative HPLC fractionation of the active fraction yielded valerenic acid as the major active compound, as well as valepotriate isomeric mixture, which also exhibited some activity (62%) at a concentration of 50 μg/ml in the 5-HT5a receptor binding assay. Valerenic acid exhibited significant affinity for the 5-HT5a receptor (80% at 50 μg/ml), with an IC50 value for valerenic acid of 17.2 μM (Ki: 10.7 μM).
Fig. 4.
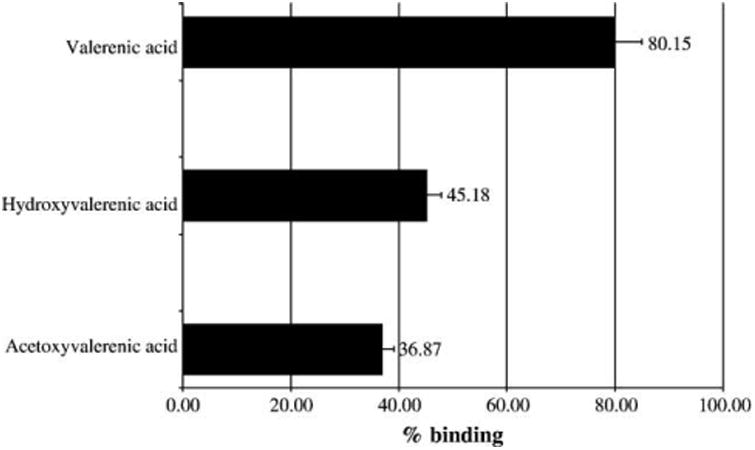
Binding affinity of the sesquiterpenes valerenic acid, hydroxyvalerenic acid, and acetoxyvalerenic acid to the 5-HT5a receptor tested at a concentration of 50 μg/ml, n = 3.
Table 1.
Correlation between the valerenic acid concentration and the 5-HT5a receptor binding activity of the PE- and DCM extracts of different V. officinalis plant samples
| Extract | Milligram valerenic acid/100 mg extract | Activity in 5-HT5a assay, percent specific binding |
|---|---|---|
| Petroleum ether (PE) extracts were tested at a concentration of 20 μg/ml | ||
| PE 1 | 7.33 | 64.93 |
| PE 2 | 8.21 | 76.02 |
| PE 3 | 6.23 | 40.4 |
| Dichloromethane (DCM) extracts were tested at a concentration of 50 μg/ml | ||
| DCM 1 | 2.88 | 70.88 |
| DCM 2 | 3.39 | 71.38 |
| DCM 3 | 1.94 | 46.83 |
The valerenic acid content was determined by external calibration (r = 0.96), every data point was at least obtained by triplicate determination.
To correlate the valerenic acid content of various valerian extracts with the 5-HT5a receptor binding activity, we tested the PE and DCM extracts of three different batches of plant materials. The results clearly demonstrate that there is a direct correlation between the valerenic acid content of these extracts and the 5-HT5a receptor binding activity (Table 1). Extracts prepared from valerian roots having a high concentration of valerenic acid were associated with a higher significant affinity for the 5-HT5a receptor.
4. Discussion
Valerian has been used as a sedative for the treatment of insomnia and restlessness since the second century [26, 27,29,33]. Currently, valerian is approved in Europe as a sedative by the German Commission E. Approximately 15 controlled clinical trials have been published to date and suggest that treatment with valerian extracts improves sleep structure and sleep perception of insomnia in healthy patients and in patients suffering from sleep disorders, without producing the traditional sedative side effects [10,13–16,21,22,25,45]. Valerian extracts have also been used for the relief of sleep disorders associated with aging and menopause [19,43]. Interestingly, Vorbach et al. [45] reported that approximately 2–4 weeks of therapy with valerian is needed to achieve significant improvements in sleep disturbances. Clinically, valerian does not cause dependency, and there does not appear to be any additive effects with alcohol [4]. Thus, the mechanism by which valerian exerts its sedative activity may be unlike other sleep aids.
Many potential mechanisms for the pharmacological activities of valerian have been proposed, including agonistic activities on the GABA, adenosine, barbiturate, and benzodiazepine receptors [4,29,48]. However, newer studies have questioned these data [12,40]. Schumacher et al. [40] found no significant binding of valerian compounds to either benzodiazepine or GABAA receptors in concentrations up to 100 μM. These data are supported by our previous work [12]. Furthermore, the concentrations of valerian extracts used in at least one study were extremely high [48]. This study reported that valerian extracts bound to the GABA receptor in vitro; however, the concentration of valerian extract used was 3 mg/ml (29.6%) [48]. Thus, considering the concentration used and the low binding affinity, the results are questionable.
In contrast to other plausible mechanisms, the effects of valerian on the serotonin receptor have not been fully investigated. Serotonin is well known to modulate sleep– wake cycles and circadian rhythms in the human brain via a G-protein-coupled receptor family [24,28]. Reports on the binding affinity of valerian to serotonin receptors are few. In one previous investigation, several compounds isolated from a valerian extract were reported to have no significant affinity for the 5-HT1a receptor [40]. This is also in agreement with our work, as none of our valerian extracts bound to the 5-HT1a receptor in concentrations up to 50 μg/ml [12]. Only the DCM and PE extracts of valerian had high binding affinity for the 5-HT5a receptor. Binding of valerian extracts to the 5-HT5a receptor has not been previously reported, and thus may represent a new mechanism of action. Valerenic acid, one of the major constituents of valerian, is the primary active compound; however, a synergistic mechanism involving other compounds present in the PE extract cannot currently be ruled out. Valerenic acid was present in both the DCM and PE extracts of valerian; however, the PE extracts contained a higher concentration of valerenic acid than the DCM extracts, thus explaining the higher binding affinity to the 5-HT5a receptor. Since the binding affinity of the extracts was dependent on the concentration of valerenic acid, and neither methanol nor 50% methanol extracts contained valerenic acid, they were not active in this assay.
Due to of the association of G-proteins with the 5-HT5a receptor, GTP binding studies were used to determine if valerian extracts exhibited agonist or antagonist activity. While agonists stimulate the binding of GTP to the G-protein, neutral antagonists have no effect, and antagonists with inverse agonistic activity reduce GTP binding. Thus, addition of GTP induces an uncoupling of the receptor from the G-protein leading to a shift of the receptor from the high- to the low-affinity state for agonists [40]. In our experiments, the binding curve for the PE extract was biphasic in the absence of GTP, and addition of GTP resulted in a rightward shift of the PE extract binding curve to a low-affinity state. These data support the hypothesis that valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor, and suggest an entirely novel mechanism to explain the sedative effects of valerian. The 5-HT5a receptor is expressed in many brain regions, including several important neural components of the circadian timekeeping system, namely the suprachiasmatic nucleus (SCN, in the hypothalamus), the intergeniculate leaflet, the dorsal raphe nucleus, and the median raphe nucleus [11,15,36]. The SCN is thought to tag the 24-h endogenous circadian clock that regulates sleep and wakefulness [11]. Thus, it has been proposed that the 5-HT5a receptor may play a role in the serotonergic regulation of circadian timekeeping [15,18,36,42]. Confirmation of this mechanism of action should be performed in vivo using 5-HT5a receptor knockout mice [20].
Acknowledgments
This study was partly funded by NIH Grant P50 AT00155 jointly provided to the UIC/NIH Center for Botanical Dietary Supplements Research by the Office of Dietary Supplements, National Institute for General Medical Sciences, Office for Research on Women's Health, National Center for Complementary, and Alternative Medicine. The contents are solely the responsibility of the authors and do not necessarily represent the views of the funding agencies.
References
- 1.Andre P, Arrighi P. Modulation of Purkinje cell response to glutamate during the sleep–waking cycle. Neuroscience. 2001;105:731–746. doi: 10.1016/s0306-4522(01)00208-1. [DOI] [PubMed] [Google Scholar]
- 2.Bellino FL. Biology of Menopause. Springer-Verlag; New York: 2000. p. 325. [Google Scholar]
- 3.Bentivoglio M, Grassi-Zucconi G. The pioneering experimental studies on sleep deprivation. Sleep. 1997;20:570–576. doi: 10.1093/sleep/20.7.570. [DOI] [PubMed] [Google Scholar]
- 4.Blumenthal M, editor. Therapeutic Guide to Herbal Medicines. American Botanical Council; Austin, TX: 1998. The Complete German Commission E Monographs. [Google Scholar]
- 5.Boess FG, Steele JA, Liu D, Reid J, Glencorse TA, Martin IL. Analysis of the ligand binding site of the 5-HT3 receptor using site-directed mutagenesis: importance of glutamate 106. Neuropharmacology. 1997;36:637–647. doi: 10.1016/s0028-3908(97)00044-0. [DOI] [PubMed] [Google Scholar]
- 6.Bonhaus DW, DeSouza A, Salazar FHR, Matsuoka BD, Zuppan P, Chan HW, Eglen RM. The pharmacology and distribution of human 5-hydroxytryptamine 2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Brit J Pharmacol. 1995;115:622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bos R, Woerdenbag HJ, Hendriks H, Zwaving JH, De Smet PAM, Tittel G, Wikstroem HV, Scheffer JJC. Analytical aspects of phytotherapeutic valerian preparations. Phytochem Anal. 1996;7:143–151. [Google Scholar]
- 8.Burdette J, Liu JH, Chen SN, Fabricant DS, Piersen CE, Barker EL, Pezzuto JM. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem. 2003;51:5561–5670. doi: 10.1021/jf034264r. [DOI] [PubMed] [Google Scholar]
- 9.Chaouloff F, Berton O, Mormede P. Serotonin and stress. Neuropsychopharmacology. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 10.Cropley M, Cave Z, Ellis J, Middleton RW. Effect of kava and valerian on human physiological and psychological responses to mental stress assessed under laboratory conditions. Phytother Res. 2002;16:23–27. doi: 10.1002/ptr.1002. [DOI] [PubMed] [Google Scholar]
- 11.Dawson K. Temporal organization of the brain: neurocognitive mechanisms and clinical implications. Brain Cogn. 2004;54:75–94. doi: 10.1016/s0278-2626(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 12.Dietz B, Pauli G, Mahady GB. Valerian extracts bind to the serotonin receptor. Proceedings of the Annual American Society of Pharmacognosy Meeting; Chapel Hill, NC. July 2003. [Google Scholar]
- 13.Donath F, Quispe S, Diefenbach K, Maurer A, Fietze I, Roots I. Critical evaluation of the effect of valerian extract on sleep structure and sleep quality (see comment) Pharmacopsychiatry. 2000;33:47–53. doi: 10.1055/s-2000-7972. [DOI] [PubMed] [Google Scholar]
- 14.Dorn M. Wirksamkeit und Vertraglichkeit von Baldrian versus Oxazepam bei nichtorganischen und nichtpsychiatrischen Insomnien: Eine randomisierte, doppelblinde, klinische Vergleichsstudie. Forsch Komplement Med Klass Nat Heilkd. 2000;7:79–84. doi: 10.1159/000021314. [DOI] [PubMed] [Google Scholar]
- 15.Duncan MJ, Jennes L, Jefferson JB, Brownfield MS. Localization of serotonin(5A) receptors in discrete regions of the circadian timing system in the Syrian hamster. Brain Res. 2000;869:178–185. doi: 10.1016/s0006-8993(00)02383-0. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez S, Paladini AC, Marder M. Sedative and sleep-enhancing properties of linarin, a flavonoid-isolated from Valeriana officinalis. Pharmacol Biochem Behav. 2004;77:399–404. doi: 10.1016/j.pbb.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Gerhardt U, Linnenbrink N, Geroghiadou Ch, Hobi V. Vigilanz-mindernde Effekte zweier pflanzlicher Schlafmittel (Effects of two plant-based remedies on vigilance) Schweiz Rundsch Med. 1996;85:473–481. [PubMed] [Google Scholar]
- 18.Geurts FJ, De Sutter E, Timmermans JP. Localization of 5-HT2a, 5-HT3 receptor, 5-HT5a receptor, 5-HT5a receptor-like immunoreactivity in the rat cerebellum. J Chem Neuroanat. 2004;24:65–74. doi: 10.1016/s0891-0618(02)00020-0. [DOI] [PubMed] [Google Scholar]
- 19.Glass JR, Sproule BA, Herrmann N, Streiner D, Busto UE. Acute pharmacological effects of temazepam, diphenhydramine, and valerian in healthy elderly subjects. J Clin Psychopharmacol. 2003;23:260–268. doi: 10.1097/01.jcp.0000084033.22282.b6. [DOI] [PubMed] [Google Scholar]
- 20.Grailhe R, Waeber C, Dulawa SC, Hornung JP, Gu H. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT5a receptor. Neuron. 1999;22:581–591. doi: 10.1016/s0896-6273(00)80712-6. [DOI] [PubMed] [Google Scholar]
- 21.Hallam KT, Olver JS, McGrath C, Norman TR. Comparative cognitive and psychomotor effects of single doses of Valeriana officinalis and triazolam in healthy volunteers. Hum Psychopharmacol. 2003;18:619–625. doi: 10.1002/hup.542. [DOI] [PubMed] [Google Scholar]
- 22.Herrera-Arellano A, Luna-Villegas G, Cuevas-Uriostegui ML, Alvarez L, Vargas-Pineda G, Zamilpa-Alvarez A, Tortoriello J. Polysomnographic evaluation of the hypnotic effect of Valeriana edulis standardized extract in patients suffering from insomnia. Planta Med. 2001;67:695–699. doi: 10.1055/s-2001-18344. [DOI] [PubMed] [Google Scholar]
- 23.Hoyer D. Characterization of the 5-HT1B recognition site in rat brain: binding studies with (—) [125] iodocyanopindolol. Eur J Pharmacol. 1985;118:1–12. doi: 10.1016/0014-2999(85)90657-0. [DOI] [PubMed] [Google Scholar]
- 24.Jouvet M, Story SA. Sleep and serotonin: an unfinished story. Neuropsychopharmacology. 1999;21:24S–27S. doi: 10.1016/S0893-133X(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 25.Kuhlmann J, Berger W, Podzuweit H, Schmidt U. The influence of valerian treatment on “reaction time, alertness and concentration” in volunteers. Pharmacopsychiatry. 1999;32:235–241. doi: 10.1055/s-2007-991100. [DOI] [PubMed] [Google Scholar]
- 26.Leathwood PD, Chauffard F. Aqueous extract of valerian reduces latency to fall asleep in man. Planta Med. 1985;51:144–148. doi: 10.1055/s-2007-969430. [DOI] [PubMed] [Google Scholar]
- 27.Leathwood PD, Chauffard F, Heck E, Munoz-Box R. Aqueous extract of valerian root (Valeriana officinalis L.) improves sleep quality in man. Pharmacol Biochem Behav. 1981;17:66–71. doi: 10.1016/0091-3057(82)90264-7. [DOI] [PubMed] [Google Scholar]
- 28.Lustberg L, Reynolds CF., III Depression and insomnia: questions of cause and effect. Sleep Med Rev. 2000;4:253–262. doi: 10.1053/smrv.1999.0075. [DOI] [PubMed] [Google Scholar]
- 29.Mahady GB, Fong HHS, Farnsworth NR, editors. Valerian, Botanical Dietary Supplements: Quality, Safety and Efficacy. Swets and Zeitlinger B.V. Lisse; The Netherlands: 2001. p. 271. [Google Scholar]
- 30.Mahady GB, Parrot J, Lee C, Yun GS, Dan A. Botanical dietary supplement use in peri- and postmenopausal women. Menopause. 2003;10:65–72. doi: 10.1097/00042192-200310010-00011. [DOI] [PubMed] [Google Scholar]
- 31.Millerk WE, Teitler M. Membrane-bound and solubilized brain 5-HT3 receptor: improved radioligand binding assay using bovine area postrma or rat cortex and the radioligand [3H]GR65630, [3H]BRL43694, and [3H]LY278584. Synapse. 1992;11:58–66. doi: 10.1002/syn.890110108. [DOI] [PubMed] [Google Scholar]
- 32.Monsma FJ, Jr, Ward RP, Hamblin MW, Sibley DR. Cloning and expression of a novel serotonin receptor with high affinity for tricyclic psychotropic drugs. Mol Pharmacol. 1993;43:320–327. [PubMed] [Google Scholar]
- 33.Morazzoni P, Bombardelli E. Valeriana officinalis: traditional use and recent evaluation of activity. Fitoterapia. 1995;96:99–112. [Google Scholar]
- 34.Mueller CE, Brattstrom A, Abourashed EA, Koetter U. Interactions of valerian extracts and a fixed valerian-hop extract combination with adenosine receptors. Life Sci. 2002;71:1939–1949. doi: 10.1016/s0024-3205(02)01964-1. [DOI] [PubMed] [Google Scholar]
- 35.Naughton M, Mulrooney JB, Leonard BE. A review of the role of serotonin receptors in psychiatric disorders. Hum Psychopharmacol. 2000;15:397–415. doi: 10.1002/1099-1077(200008)15:6<397::AID-HUP212>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 36.Oliver KR, Kinsey AM, Wainwright A, Sirinathsinghji DJ. Localization of 5-HT5a receptor-like immunoreactivity in the rat brain. Brain Res. 2000;867:131–142. doi: 10.1016/s0006-8993(00)02273-3. [DOI] [PubMed] [Google Scholar]
- 37.Pazos A, Palacios JM. Mesulergine a selective serotonin-2 ligand in the rt cortex, does not label the receptors in porcine and human cortex: evidence for species differences in brain serotonin-2 receptors. Eur J Pharmacol. 1985;106:622–628. doi: 10.1016/0014-2999(84)90056-6. [DOI] [PubMed] [Google Scholar]
- 38.Rees S, Foord St, Goodson S, Bull D, Kilpatrick G, Lee M. Cloning and characterisation of the human 5-HT5a-serotonin receptor. FEBS Lett. 1994;355:242–246. doi: 10.1016/0014-5793(94)01209-1. [DOI] [PubMed] [Google Scholar]
- 39.Roth BL, Choudhary MS, Uluer S, Monsma FJ, Jr, Shen Y, Meltzer HY, Sibley DR. Binding of typical and atypical antipsychotic agents to 5-hydroxytryptamine-6 and 5-hydroxytryptamine-7 receptors. J Pharmacol Exp Ther. 1994;268:1403–1410. [PubMed] [Google Scholar]
- 40.Schumacher BSS, Hoelzl J, Khudeir N, Hess S, Mueller CE. Lignans isolated from valerian: identification and characterization of a new olivil derivative with partial agonistic activity at A1 adenosine receptors. J Nat Prod. 2002;65:1479–1485. doi: 10.1021/np010464q. [DOI] [PubMed] [Google Scholar]
- 41.Shen Y, Metcalf MA, Jose PA, Hamblin MW, Sibley DR. Molecular cloning and expression of a 5-hydroxytryptamine 7 serotonin receptor subtype. J Biol Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- 42.Sprouse J, Reynolds L, Braselton J, Schmidt A. Serotonin-induced phase advances of SCN neuronal firing in vitro: a possible role for the 5-HT5a receptors. Synapse. 2004;54:111–118. doi: 10.1002/syn.20070. [DOI] [PubMed] [Google Scholar]
- 43.Sun J. Morning/evening menopausal formula relieves menopausal symptoms: a pilot study. J Alt Complemen Med. 2003;9:403–409. doi: 10.1089/107555303765551624. [DOI] [PubMed] [Google Scholar]
- 44.Thomas EA, Hu JL, Carson MJ, Sutcliffe JG. Pertussis toxin treatment prevents 5-HT5a receptor-mediated inhibition of cyclic AMP accumulation in rat C6 glioma cells. J Neurosci Res. 2000;61:75–81. doi: 10.1002/1097-4547(20000701)61:1<75::AID-JNR9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 45.Vorbach EU, Gortelmayer R, Bruning J. Therapie von Insomnien: Wirksamkeit und Vertrag-lichkeit eines Baldrian-Praparates. Psycho-pharmakotherapie. 1996;3:109–115. [Google Scholar]
- 46.Wheatley D. Stress-induced insomnia treated with kava and valerian: singly and in combination. Hum Psychopharmacol. 2001;16:353–356. doi: 10.1002/hup.299. [DOI] [PubMed] [Google Scholar]
- 47.Wolf WA. The serotonin 5-HT2C receptor is a prominent serotonin receptor in basal ganglia: evidence from functional studies on serotonin-mediated phosphoinositide hydrolysis. J Neurochem. 1997;169:1449–1458. doi: 10.1046/j.1471-4159.1997.69041449.x. [DOI] [PubMed] [Google Scholar]
- 48.Yuan C, Xiao Y, Aung HH, Xie J, Ang-Lee MK. The γ-aminobutyric andrenergic effects of valerian and valerenic acid on rat brainstem neuronal activity. Anaesth-Analg. 2004;98:353–358. doi: 10.1213/01.ANE.0000096189.70405.A5. [DOI] [PubMed] [Google Scholar]


