Abstract
Purpose
Gene therapy (GT) has offered immense hope to individuals who are visually impaired due to RPE65 mutations. While GT has shown great success in clinical trials enrolling these individuals, evidence for stability and durability of this treatment over time is still unknown. Here we explore the value of functional magnetic resonance imaging (fMRI) as an objective measure to independently assess the longevity of retinal GT.
Design
Individuals with RPE65 mutations, who underwent GT in their worse-seeing eye in a Phase I clinical trial, received a second subretinal injection in their contralateral eye in a follow-on clinical trial. fMRI was longitudinally performed to assess brain responses of RPE65 patients after stimulation of their most recently treated eye before and 1–3 years after GT.
Subjects
Seven RPE65 subjects who were part of the follow-on clinical trial were separately consented to participate in a longitudinal neuroimaging fMRI study.
Methods
All subjects underwent fMRI utilizing a 3-Tesla MRI system and a 32-channel head coil. Subjects’ cortical activations were assessed using a block design paradigm of contrast reversing checkerboard stimuli delivered using an MRI compatible video system.
Main Outcome Measures
The primary parameters being measured in this study are the qualitative and quantitative fMRI cortical activations produced by our subject population in response to the visual task checkerboard stimulus.
Results
fMRI results showed minimal or no cortical responses before GT. Significant increase in cortical activation lasting at least three years after GT was observed for all subjects. Repeated measures analysis showed significant associations between cortical activations and clinical measures such as full field light sensitivity threshold (FST) for white, red, and blue colors, visual field (VF), and pupillary light reflex (PLR).
Conclusions
RPE65-subjects showed intact visual pathways, which became responsive and strengthened after treatment. fMRI results independently revealed the efficacy and durability of a one-time subretinal injection. The fMRI results paralleled those recently reported during the long-term clinical evaluations of the same subjects.1 Results from this study demonstrate that fMRI may play an important role in providing complementary information to patients’ ophthalmic clinical evaluation and has utility as an outcome measure for future retinal intervention studies.
Leber’s congenital amaurosis (LCA) is a rare blinding disease, usually inherited in an autosomal recessive fashion. It is symptomatic at birth or in the first few months of life and affects around 1 in 81,000 people.2 LCA has been associated with at least 18 different genes.3, 4 The gene encoding retinal pigment epithelium-specific protein 65kDa (RPE65) is involved in one of the more common forms of LCA called LCA2. RPE65 mutations can also cause retinitis pigmentosa and other early-onset autosomal recessive retinal degenerations.5, 6 Individuals with RPE65 mutations are good candidates for gene transfer therapy as the degeneration of retinal cells is slow, providing an extended potential time window for intervention. Recent studies in both animal models7–11 of LCA and humans12–17 have demonstrated success in restoring retinal and visual function using measures such as visual acuity, visual fields, light sensitivity, pupillary light reflex, and/or mobility. There are several clinical trials that have carried out gene therapy for individuals with RPE65-mediated disease (www.clinicaltrials.gov).18 The program at the Children’s Hospital of Philadelphia/University of Pennsylvania is the first to carry out administration of AAV2-hRPE65v2 to the contralateral eye.
Until now, it was not known whether severe impairment of the visual pathway due to congenital or early-onset inherited retinal degeneration would limit the responsiveness of vision processing neurons in the occipital cortex. Recently we showed that in humans with LCA due to RPE65 mutations, the visual cortex can be made responsive to visual input through unilateral ocular gene therapy, even after prolonged visual deprivation of up to 35 years.19 In our previous studies, we employed dim light stimuli since it is known that young RPE65 individuals have some ability to see and navigate under brightly lit conditions.20–22 Also, to account for variability in the disease stage among study participants and to correlate fMRI results with each subject’s psychophysical measures, functional analyses were carried out separately for each individual participant.
In our initial report, treated and untreated eyes within the same RPE65 subjects were compared to assess the efficacy of gene therapy. Although there is a high degree of symmetry in disease progression between the two eyes, lack of baseline fMRI data for the initial injection made it difficult to reach a definitive conclusion on the magnitude and timing of the reported functional improvements. In the FO Phase I clinical trial, the same participants who originally received a subretinal injection to their worse-seeing eye were candidates to receive administration of the AAV2-hRPE65v2 vector to their previously untreated contralateral eye. Neuroimaging results from 3 adult RPE65 subjects of the FO study were subsequently reported comparing the baseline cortical response of the contralateral eye with short-term effects of retinal gene therapy on the human visual cortex.23 This report demonstrated that the visual cortex is extremely responsive to the stimulation of the photoreceptors via retinal gene therapy. As compared to baseline, RPE65 subjects showed significant cortical activations at one and three months after gene therapy. The FO study also demonstrated that prior exposure to AAV2 vector did not result in any adverse effects to the second administration of AAV2-hRPE65v2 due to potential immunologic complications.23 The current study takes a step beyond examining the short-term effects of retinal gene therapy and evaluates the human brain responses in a large population of RPE65 participants over a three-year time span. We hypothesize that fMRI results to be similar to those recently reported RPE65 FO clinical trial1 and it would independently demonstrate the long-lasting effects of a one-time retinal gene therapy.
Methods
Study Participants
Subjects were enrolled and evaluated as described in ClinicalTrials.gov #NCT01208389 (http://www.med.upenn.edu/carot/) at baseline and 1–3 years after surgical administration of AAV-hRPE65v2 to the fellow eye in the FO study. While RPE65 subjects in the initial Phase I clinical trial had received different doses and volumes of AAV2-hRPE65v2 in their first eye,17, 24 all subjects received the high dose of 1.5E11 vector genomes (vg) in 300 μl for the FO (Table 1). (The approximate location of the subretinal injection for the contralateral eye of all RPE65 subjects is presented in a recent report outlining a three-year longitudinal clinical outcome of the FO clinical trial).1 Overall, all RPE65 subjects except for one (CH10) received their subretinal injection as close as possible to the superior macula location.1
Table 1.
Characteristics of Study Participants.
| Subject ID | Age (Yrs) at Re-administration and Baseline fMRI | Re-administered Eye | RPE65 mutation(s) |
|---|---|---|---|
| NP02 | 30 | Left | E102K/E102K |
| CH08 | 12 | Left | F530fs/F530fs |
| CH09 | 11 | Right | R124X/K297del1aggA |
| CH10 | 14 | Left | IVS1+5g>a/F530del1ttc |
| CH11 | 26 | Left | V473D/V473D |
| CH12 | 46 | Left | K303X/W431C |
| NP15 | 14 | Left | D167W/H313R |
Seven of the original 10 subjects who participated in the Phase I neuroimaging study19 were evaluated in the FO second eye study (Table 1). From the 10 original participants, CH13 was not eligible for intervention due to glaucoma in the contralateral/uninjected eye. CH06 elected not to continue with the neuroimaging study. Longitudinal fMRI results from NP01 are also not included in the current report due to the fact that NP01 had a history of smoking and chronic smoking is known to abate cortical blood flow and has a dramatic effect on the fMRI cortical activations.25 NP03 and NP04 did not participate in any neuroimaging studies. After providing a complete description of the study, written informed consent (and when necessary, parental consent and child assent) was obtained from all subjects for the longitudinal neuroimaging study. The Review Board at the Children’s Hospital of Philadelphia (CHOP) approved all study procedures. All subjects were clinically assessed as part of their qualification to enter the clinical trial for retinal gene therapy.16, 17, 24 This study was HIPPA compliant.
Magnetic Resonance Imaging
MRI scans were conducted at CHOP on a research dedicated 3T Siemens Verio system using a 32-channel head coil. All scans were carried out by a single operator and monitored to be free of artifacts at the time of acquisition.
fMRI Sequence
Functional data were acquired using blood oxygenation level-dependent (BOLD) imaging, acquiring 3 mm isotropic resolution (matrix, 64 × 64; TR/TE, 3,000/30 ms) with a total acquisition time of 4:39 min. To permit T1 saturation, three additional volumes were acquired at the beginning of the fMRI experiment but were not used in image analysis. A transistor-transistor logic (TTL) pulse was used to automatically start the stimuli in sync with the start of fMRI acquisition. An MRI compatible response device (a button that the subject pushed when recognizing the stimulus) was used to record subject responses. Subjects were instructed to press the button once when the checkerboard first appeared.
fMRI Paradigm
In the past, while using simple contrast reversing checkerboard stimuli, we have been successful in showing the efficacy of gene therapy in this subject population.19, 23 Similar to our earlier study,19, 23 the current report employed dim stimuli that went unperceived by most of the subjects at baseline before retinal intervention. The purpose of this dim stimuli presentation was to assess the ability of subjects in perceiving dim light after gene therapy. The fMRI paradigm consisted of 15-second blocks of flickering (8-Hz) black and white checkerboards, which consisted of three contrasts of high, medium, and low, interleaved with 15 seconds of blank (black) screens.19, 23 Subjects were asked to fixate on a yellow cross in the center of the checkerboard patterns, or, if they could not see the cross, to look straight ahead to their central vision. Subjects were additionally asked to press the response button immediately after presentation of the visual stimuli and to hold the response button if they experienced phosphenes.26 Resonance Technology VisuaStim27 (www.mrivideo.com, Northridge, CA) goggles featuring a digital display and a 30° horizontal field of view was used to present the fMRI stimuli. The visual paradigm was programmed in E-Prime (www.psychology-software-tools.com, Pittsburgh, PA).28
fMRI Processing
All fMRI analyses were performed using the general linear model (GLM) and the contrast of active blocks (checkerboard stimuli) minus the rest blocks (blank, black screen) as implemented in BrainVoyagerQX.29 To account for variability in the disease stage amongst study subjects, fMRI data were analyzed individually (see Supplementary Information available at www.aaojournal.org) for each subject (and not grouped as is done in most fMRI studies). A single subject approach is appropriate due to the fact that RPE65 subjects differed by age/disease progression and thus, the area of the retina in which there was evidence of sufficient (albeit unhealthy) retinal cells. Therefore, it is reasonable to expect that each individual subject will have a unique response to gene therapy and accordingly a unique fMRI cortical activation pattern. However, correlational analyses with clinical measures were carried out on a group level.
Real Time fMRI
The research MR system at CHOP is equipped with a Siemens fMRI software that allows real time monitoring of the subjects’ performance during fMRI experiments as well as their translational and rotational head movements.23, 26 Using the real time feature, fMRI acquisition with ≥0.6 mm translational or ≥0.6 degrees for rotational movement was terminated, the subject was informed to stay still, and then the experiment would be restarted.
3D T1 Weighted (MPRAGE) Imaging
A 3D isotropic structural high resolution T1 sequence was acquired with inversion preparation pulse (IR-Prep: TR = 2080 ms, TE = 2.54 ms, BW =180 Hz/Px, matrix size = 320×320, FOV = 256×256 mm2, 192 axial slices, slice thickness = 0.8 mm, inversion time = 1200 ms with Flip Angle =8°, NEX = 1, Echo Spacing= 7.8, iPAT = 2 and scan time = 7:04 minutes). This sequence was obtained for visual activation localization and generation of inflated hemispheres.29
Vision Testing and Ocular Examination
RPE65 subjects underwent a comprehensive clinical evaluation as part of an approved clinical trial protocol. In addition to other measures, subjects’ clinical evaluations included multiple clinical tests of visual function including evaluation of visual acuity (VA), visual field (VF), pupillometry light reflex (PLR) evaluating for difference in responses between the two eyes, and full sensitivity testing (FST) for white, red and blue light sources.1, 17, 24 Spark Therapeutics, Inc. carried out measures of VA, VF, FSTs and other testing under sponsorship. For this current study, amplitudes of the pupillary light reflex were measured in each eye individually at baseline to the second eye injection and then in follow-up testing. The PLR results were measured independently from the FO clinical trial retinal and visual function data carried out by the Spark Therapeutics. Results from the above mentioned clinical tests were obtained for FO baseline and 1–3 years post gene therapy evaluation to assess possible correlations between these clinical measures and fMRI results.
Results
fMRI Results
fMRI results presented here are the sum of high- and medium-contrast stimuli in response to stimulation of the newly treated eye using a flickering checkerboard paradigm.19, 23 We did not include the results from the low contrast stimuli because almost no RPE65 subject was able to see the lowest contrast (dimmest stimulus) and fMRI analysis of low contrast stimulus demonstrated minor or no cortical activations. We also do not discuss the results from the eyes injected prior to the FO study, as there was no baseline fMRI assessment performed before initial administration of gene therapy. Detailed fMRI results for individual patients are presented in the Supplementary Information which is available online at www.aaojournal.org. Overall, functional MRI after gene therapy administration to the contralateral eye showed significant cortical activation in and around the visual cortex for all RPE65 subjects for full-field contrast-reversing (8Hz) checkerboard stimuli at high- and medium-contrasts. Presentation of the same stimuli at baseline (before gene therapy) showed minimal cortical activations (except in NP15) for high and medium-contrast stimuli. As shown in Figure 1, compared to baseline, all RPE65 subjects showed significantly elevated levels of cortical activations at one year and continued this trend for their fMRI evaluations at years two and three. As expected, patterns of activations varied in each individual depending on their age, the progression of disease and location of their subretinal injection. Generally, subjects showed compensatory activations primarily in and around the extrastriatal cortex outside the primary visual cortex at FO baseline (see the first column of Figure 1) (except NP15).
Figure 1. Longitudinal fMRI results for individual RPE65 participants superimposed on their inflated cortex.
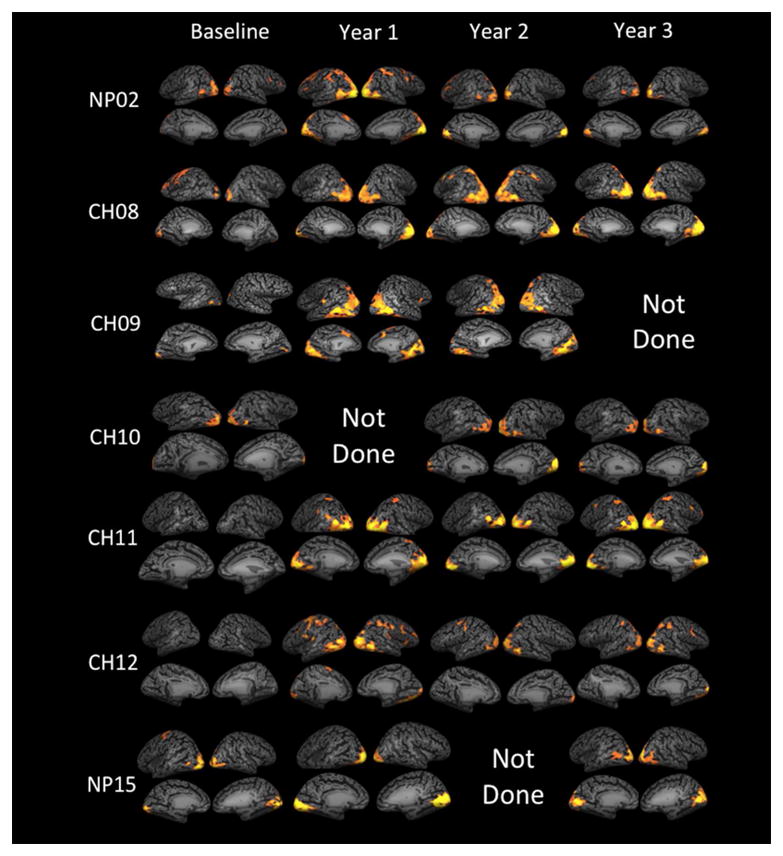
fMRI results for 7 RPE65 subjects at baseline, one year, two years, and three years post retinal gene augmentation therapy are presented in Figure 1. Cortical activations are assessed in response to the stimulation of the newly treated eye for both the high and medium contrast stimuli.19 Results are presented for the left eye for all but one participant (CH09) who received re-administration to his right eye. fMRI results for all subjects were corrected for multiple comparisons using false discover rate (fdr < 5%) and a corrected P < 0.004, with a continuous connected area threshold (cca ≥ 100 mm2) that further controls for multiple comparison type 1 error.19, 23 In order to show cortical activations at baseline, fMRI results required considerably relaxed statistical threshold of an uncorrected P<0.05 and cca ≥ 50 except for NP15 and NP02. Using the above statistics, statistically significant areas of cortical activations are shown as yellow/orange clusters overlaid onto the medial and lateral representations of inflated cortex for each individual subjects. Overall comparison of the baseline and three year follow up fMRI results show noticeable increase in widespread bilateral activations in all areas of visual cortex extending from medial to lateral and posterior to anterior aspects of the occipital cortex one year after gene therapy for majority of the subjects. As shown in Figure 1, activation pattern vary for individual subject depending on his/he age and disease progression or other reasons. Changes in cortical activations over time for baseline and one year after retinal gene therapy using responses to only the high contrast stimuli (here we used responses to the high and medium contrasts) were reported in a recent report by Bennett et al. (2016).
This pattern of activation dramatically changed after retinal gene therapy, showing a significant increase in the amount of activation within the primary visual areas for all subjects except CH12, who is the oldest study participant with advanced retinal degeneration (47 years old) (see columns 2–4 of Figure 1). Annual fMRI evaluation for a few subjects could not be obtained for some of their time points due to subject’s unavailability or a change in their status preventing fMRI examination (e.g. having dental braces). Three of the participants (NP02, CH08 and CH11) intermittently experienced phosphenes.26 To control for effects of this phenomenon during the course of fMRI, subjects were directed to press a button if they were experiencing phosphenes. The experiment was repeated if subjects reported seeing phosphenes during the stimulus presentation. However, some of the longitudinal results, particularly from these three subjects, might be attributed to unreported phosphene events. All fMRI analyses were performed using the same statistical threshold of fdr (5%) corrected p<0.004 and an extent threshold (continuous connected area [cca]) of ≥100 continuous voxels. The following is a detailed explanation of the results for each RPE65 subject before and after gene therapy.
Changes of the visual cortex activation over time
The mean and standard error for the cortical activations for baseline and 1–3 years after the delivery of subretinal injection are presented in the Supplementary Table S1 (available at www.aaojournal.org). The graphical representation of the changes in the mean value of cortical activations over time for the whole visual cortex and activation of its medial surface are presented in Figure 2 panels A and B respectively. Changes in cortical activations over time for baseline and one year after retinal gene therapy using responses to only the high contrast stimuli (here we used responses to the high and medium contrasts) were reported in a recent report by Bennett et al. (2016).
Figure 2. Longitudinal changes in mean and standard error of the left, right and total visual cortical activations.
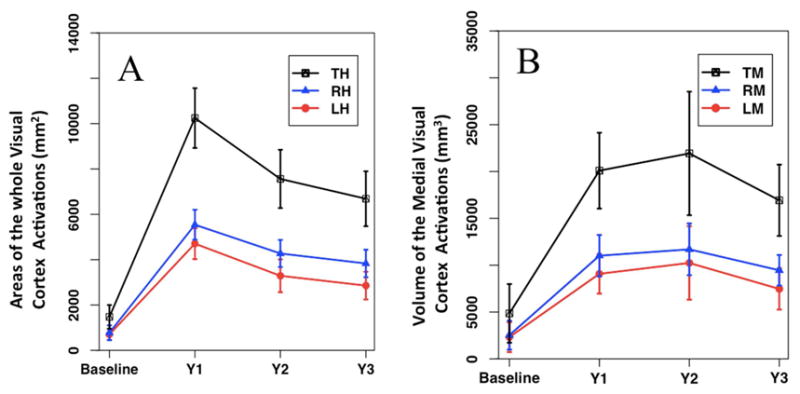
Longitudinal cortical activation results in response to a contrast-reversing checkerboard stimulus, for RPE65 patients at baseline and 1–3 years after receiving gene therapy in their contralateral eyes. Panel A shows the mean ± SE (standard error) of the area of cortical activations for the entire area of the left (LH), right (RH) hemispheres and total visual cortex (TH) over time. Panel B shows the mean ± SE of the volume of cortical activations within the medial surface of the visual cortex for the left (LM), right (RM) and total medial activations (TM). As compared to the baseline, cortical activations at years 1–3 for both the medial and total visual cortex activations were all statistically significant (p< 0.05).
The association between total visual cortex activations and clinical measures
Longitudinal regression analysis using the linear mixed effect models (using random intercept) was performed with image parameters as predictors and clinical measures as outcome variables to assess the association between the activations across the entire surface area of the visual cortex and each of the clinical measure over time. The results of the mixed effect models that evaluate the association between the entire areas of the right hemisphere (RH), left hemisphere (LH), and total hemisphere (TH) activations over time and all available clinical measures are presented in Table 2. As shown in Table 2, all clinical measures were significantly correlated with the longitudinal fMRI results except for the visual field and visual acuity.
Table 2. Regression analysis results between overall cortical activations and clinical measures.
The linear regression analysis results for the association between the entire visual cortex activation areas of the LH, RH, and TH and each of the RPE65 clinical measures.
| Clinical Measures | Area of Cortical Activation in mm2 (LH) | Area of Cortical Activation in mm2 (RH) | Area of Cortical Activation in mm2 (LH + RH) | |||
|---|---|---|---|---|---|---|
| Regression coefficient (SE)§ | P | Regression coefficient (SE)§ | P | Regression coefficient (SE)§ | P | |
| Visual field (VF) (Degrees) | 0.75 (1.08) | 0.50 | 0.78 (1.14) | 0.50 | 1.45 (2.14) | 0.51 |
| Full Field Stimulus Threshold (FST) White light (dB) | 74.24 (21.13) | 0.003 | 72.61 (24.58) | 0.009 | 146.85 (44.91) | 0.005 |
| Full Field Stimulus Threshold (FST) Red light (dB) | 101.97 (40.15) | 0.02 | 96.02 (45.48) | 0.0499 | 197.24 (84.03) | 0.03 |
| Full Field Stimulus Threshold (FST) Blue light (dB) | 75.63 (20.86) | 0.002 | 73.65 (24.38) | 0.008 | 149.28 (44.44) | 0.004 |
| Pupillary Light Reflex (PLR) (mm × 100) | 39.99 (14.52) | 0.02 | 53.71 (14.90) | 0.004 | 93.93 (29.10) | 0.007 |
| Visual acuity (VA) (LogMAR × 10) | −3.14 (73.69) | 0.97 | 28.72 (80.01) | 0.72 | 25.58 (151.68) | 0.87 |
From linear mixed effect model with each of measure of area of cortical activation as dependent variables and each of clinical measures as independent variables.
The FST measures for the white light was associated with the LH (rc= 74.24, p<0.003), RH (rc=72.61, p<0.009) and TH (rc= 146.85, p<0.005) and it also correlated for the red light ((LH (rc=101.97, p<0.02), RH (rc=96.02, p<0.05), and TH (rc=197.24, p<0.03)) and blue light ((LH (rc=75.63, p<0.002), RH (rc=73.65, p< 0.008) and TH (rc=149.28, p<0.004)). Total occipital cortical activation areas were strongly associated with temporal changes in the pupillary light reflex (PLR) of the RPE65 subjects ((LH (rc=39.99, p<0.02), RH (rc = 53.71, p<0.004), and TH (rc= 93.93, p<0.007)). The graphical representation of the association between the area of activations for TH, and FST measures of the white, red, and blue lights for all their longitudinal measurements are presented in the top section of Figure 3. The graphical results for the association between the TH area of activations and PLR, VF and VA measures are presented in the top section of Figure 4. All other graphs depicting the association between the LH and RH areas of activations and FST, PLR, VF and VA measures are presented in the Supplementary Figures S3–S6 (available at www.aaojournal.org).
Figure 3. Association between FST clinical measures and visual cortical activations.
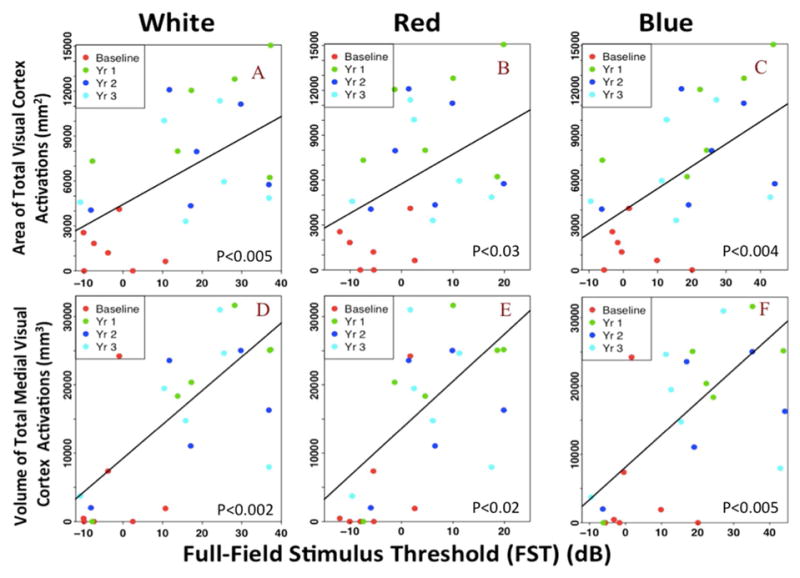
Scatterplot with linear regression lines from the mixed effect model for the association of cortical activations and FST measures using white, red and blue light sources. The top portion presents the association between the FST measures of (A) white, (B) red, and (C) blue light stimuli and the area of the total visual cortex activations. The bottom section shows the association between the FST measures of (D) white, (E) red, and (F) blue lights and the volume of the total medial visual cortex activations. Both values of the cortical activations are significantly associated with the increase in light sensitivity in RPE65 patients over time. However, the activation of the medial cortex are more strongly associated with the FST light sensitivity measures (see Discussion).
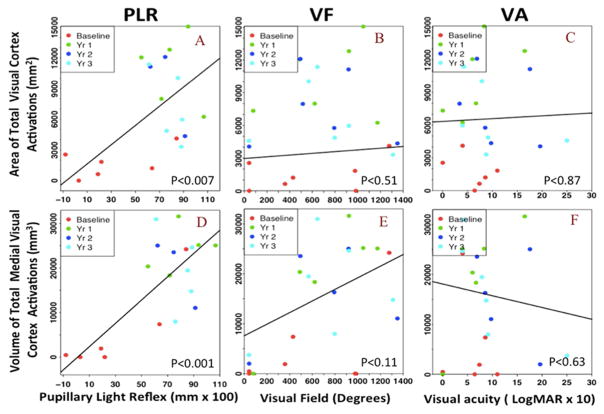
Figure 4. Association between PLR, VF and VA clinical measures and visual cortical activations.
Scatter plot with linear regression lines from the mixed effect model for the association of cortical activations with the PLR, VF and VA measures. The top portion shows the association of the PLR (A), VF (B), and VA (C) measures with the area of the total visual cortex activations. The bottom portion shows the association between the PLR (D), VF (E), and VA (F) measures with the volume of the total medial visual cortex activations. Both measures of the cortical activations are significantly associated with PLR of the RPE65 patients over time. However, the medial activations are more strongly associated with the VF over time. No association was observed with the VA for either the area of the total visual cortex and the volume of the medial activations.
The association between the activations of the medial visual cortex and clinical measures
The association between the clinical measures and the volume of activations located mainly within the medial surface of the visual cortex (in and around the primary visual cortex) is presented in Table 3. As shown in Table 3, stronger associations were observed with the volume of the medial activations and subjects’ clinical measures as compared to regression coefficients and significance observed with the total visual cortex surface activations and subjects’ clinical measures (compare with values in Table 2). This is particularly evident for the association of the medial cortical activation volume with the subjects’ visual field [left medial: LM (rc=5.05, p<0.21), right medial: RM (rc=6.38, p<0.07), total medial: TM (rc=11.62, p<0.11)]. The changes in the volume of the medial cortical activations were strongly associated with the changes observed in subjects’ FST for white light (LM (rc=227.65, p<0.01), RM (rc=264.84, p<0.001), TM (rc=495.08, p<0.002)), red light ((LM (rc=294.97, p<0.06), RM (rc=392.00, p<0.005), TM (rc=689.58, p<0.02)) and blue light ((LM (rc=212.80, p<0.02), RM (rc=262.79, p<0.001), TM (rc=475.63, p<0.005)). The strongest and the most significant association is observed between the volume of activations of the medial visual cortex and the RPE65 subjects’ pupillary light reflex results [(LM (rc=119.57, p<0.001), RM (r=139.71, p<0.001), TM (r=261.36, p<0.001)]. Similar to the total visual cortex surface activation, the volume of activations of the medial surface did not show any association with RPE65 subjects’ visual acuity measures over time. The graphical representation of association between the volume of activations for TM, and FST measures of the white, red, and blue lights over time are presented in the bottom section of Figure 3. The graphical results for the association between the TM volume of activations and PLR, VF and VA measures are presented in the bottom section of Figure 4. All other graphs depicting the association between the LM and RM volumes of activations and FST, PLR, VF and VA measures are presented in the Supplementary Figures S3–S6 (available at www.aaojournal.org).
Table 3. Regression analysis results between medial cortical activations and clinical measures.
The linear regression analysis results for the association between the volumes of the visual cortex medial activations with each of the RPE65 clinical measures.
| Clinical Measures | Volume of Medial activation in mm3 (LM) | Volume of Medial activation in mm3 (RM) | Volume of Total Medial activation in mm3 (RM + LM) | |||
|---|---|---|---|---|---|---|
| Regression coefficient (SE)§ | P | Regression coefficient (SE)§ | P | Regression coefficient (SE)§ | P | |
| Visual field (VF) (Degrees) | 5.05 (3.86) | 0.21 | 6.38 (3.27) | 0.07 | 11.62 (6.95) | 0.11 |
| Full Field Stimulus Threshold (FST) White light (dB) | 227.65 (78.11) | 0.01 | 264.84 (62.77) | <0.001 | 495.08 (137.00) | 0.002 |
| Full Field Stimulus Threshold (FST) Red light (dB) | 294.97 (145.13) | 0.06 | 392.00 (120.41) | 0.005 | 689.58 (260.44) | 0.02 |
| Full Field Stimulus Threshold (FST) Blue light (dB) | 212.80 (84.41) | 0.02 | 262.79 (68.78) | 0.001 | 475.63 (149.19) | 0.005 |
| Pupillary Light Reflex (PLR) (mm × 100) | 119.57 (26.98) | <0.001 | 139.71 (26.96) | <0.001 | 261.36 (51.39) | <0.001 |
| Visual acuity (VA) (LogMAR × 10) | −142.21 (255.63) | 0.59 | −102.75 (228.04) | 0.66 | −235.33 (477.05) | 0.63 |
From linear mixed effect model with each measure of medial activations as dependent variables and each of clinical measures as independent variables.
Discussion
The present study was carried out in a population of RPE65 subjects who received administration of AAV2-hRPE6v2 (1.5 × 1011 vector genomes [vg]) to the contralateral eye as a FO study of an RPE65 gene therapy Phase I clinical trial. Previously, the same subject group had received unilateral AAV2 subretinal injections in their worse seeing eye17, 24 in a dose-escalation study, with doses ranging from 1.5 × 1010 to 0.5 × 1011 vg. Gene therapy administration to the contralateral eye occurred an average of two years after Phase I unilateral treatment administration. As part of the FO clinical trial, RPE65 subjects received FO evaluations for three years after administration of gene therapy to their contralateral eyes. Their long-term clinical evaluations demonstrated not only a high degree of safety and efficacy despite having a previous subretinal injection, but also a long lasting effect of up to three years for a one-time delivery of gene augmentation therapy.1 Separate from the main clinical trial, a longitudinal neuroimaging study was conducted to independently characterize the efficacy of this one-time subretinal injection in a subgroup of the RPE65 subjects who participated in the FO clinical trial (see Table 1). The neuroimaging study compared the spatio-temporal patterns of this subgroup’s brain activations using fMRI at baseline and annually for 3 years after receiving gene therapy. fMRI comparisons were used to assess and quantify the association between cortical activations and the subjects’ clinical measures over time. It is important to note that, as we have previously described,19 the fMRI paradigm used in our experiments was selected to be composed of stimuli with dim light, since it is known that young RPE65 subjects have some ability to see and navigate their surroundings under high-intensity lighting conditions.20–22 Also, to account for variability in disease progression among the different RPE65 subjects and to assess the association of fMRI results with a subject’s clinical measures, functional analyses were carried out separately for each individual subject.
In our previous report,19 treated and untreated eyes within individual RPE65 subjects were compared to assess the efficacy of gene therapy. However, lack of baseline data on cortical responses from an untreated eye (for the initial injection/study) prevented a true presentation of the magnitude and pattern of improvement induced by gene therapy. In the present report, we provide functional brain responses that clearly show the efficacy of retinal gene therapy and its durable effect for at least three years after a subretinal injection. Comparison of pre- and post-surgical fMRI provides further evidence for the effectiveness of gene therapy for RPE65 subjects and shows that a therapeutic effect is observed even after prior exposure to AAV2-hRPE65v2.
Consistent with our previous reports,19, 23 the longitudinal fMRI results from the second eye showed that when presented with the same visual stimuli,19 RPE65 subjects responded minimally at baseline as compared with their post intervention results. Thus, prior to receiving their subretinal injection, RPE65 subjects had minimal compensatory or no response to the checkerboard stimuli, whereas all subjects became dramatically responsive after treatment and their initial post-surgical responsiveness to the treatment was maintained through the duration of this fMRI study. The result from the longitudinal fMRI study, which shows lasting cortical activations over three years with a one-time subretinal injection, parallels the three-year longitudinal clinical evaluation results recently reported.1 These lasting cortical activations are likely related to structural improvements observed in these subjects, which, in turn, are further strengthened through visual experience.30–32
The fMRI results clearly show that the activation of the visual cortex closely corresponds to the AAV-exposed portion of the retinas. As depicted in Figure 1, fMRI results from the RPE65 subjects after gene therapy showed bilateral occipital cortex activations which correspond to the superior macula subretinal injection site that has spread to the entire naso-temporal retinal area for all subjects except CH10 (see column 1 of Figure 1 in Bennett et al, 2016).1 Activation for CH12, the oldest participant at 46 years of age, resulted in increased bilateral compensatory activations in the extrastriatal cortex, although there was no change in the activation of the primary visual cortex (medial surface of the occipital lobe). The location of subretinal injection is expected to generate bilateral fMRI activation in the cortical distribution as was observed in the majority of RPE65 subjects, with lesser extent in CH10. However, as shown in Figure 2 panels A and B, cortical activations were consistently higher for the right hemisphere at all time points. This asymmetric activation pattern can be attributed to the fact that the majority of the RPE65 participants (6/7) received their first subretinal injections in their right eye in the superior temporal aspect of their retina. Injections administered in this area of the retina cause preferential right visual pathway strengthening because the right temporal projections from the retina stay on the right visual cortex and do not cross to the left hemisphere.32
In general, visual processing starts at the retina, where the light rays that enter the eye are converted by the photoreceptors (rods and cones) to electrical signals. These signals are then transmitted through the retina to the retinal ganglion cells (RGCs), which transfer this information through the optic nerve to the lateral geniculate nucleus (LGN) of the brain. The LGN then proceeds to send signals to the primary visual areas of the occipital lobe.33 Information from these areas is then projected to other areas of the cerebral cortex (extrastriate) that are involved in higher complex visual perceptions. Thus, these primary visual areas (which are located along the medial surfaces of the visual cortex) receive the main visual signals from the photoreceptors in the retina and are responsible for our first sense of visual perception.33 In RPE65 subjects whose photoreceptors are highly non-functional3 the process of light to electrical signal conversion is greatly interrupted. As such, depending on the disease progression little or no light rays are converted by the photoreceptors to be transmitted to RGCs resulting in diminished visual signal transmission to the primary visual areas. Upon subretinal administration of AAV2-hRPE65v2 vector (gene therapy), the missing isomerohydrolase (RPE65) is restored, and portions of the revived photoreceptors regain their processing ability of transferring electrical activities to the RGCs and eventually to the primary visual areas.
We believe the fMRI results from RPE65 subjects, presented in Figure 1, accurately reflect the timeline of changes in the stimulation of the photoreceptor populations from their non-functioning state at baseline to when they reconnect with RGCs and reinstate vision after gene therapy. As shown in the first column of Figure 1, at baseline (before retinal intervention and when photoreceptors are non-functional), there are no or small amounts of activation in and around primary visual areas for all RPE65 subjects except for NP15, who presented with the highest visual functions and cortical activations at baseline. As depicted in columns 2, 3, and 4 of Figure 1, subsequent to receiving retinal gene therapy (when portion of photoreceptors are reinstated by gene therapy), with the exception of CH12, the oldest subject at age 46, the RPE65 subjects’ levels of activation within the primary visual areas (medial surface of visual cortex) increased considerably as compared to baseline and stayed at an elevated level through years 2 and 3 of fMRI examinations. Although levels of activations within the extrastriate cortex also increased, it is important to note that the primary visual areas in fact mediate much of the information to the extrastriate cortex.33, 34 In addition to receiving information from the primary visual areas, the extrastriate cortex is also known to receive direct inputs from the retina through the pulvinar and superior colliculus35–38. This component is particularly dominant in the absence of visual signals to the primary visual areas.39–42 This may explain the reason for higher levels of extrastriate activations before gene therapy (see first column in Figure 1), when little or no visual signals were transmitted to the primary visual areas due to photoreceptor malfunction. Thus, in Figure 1 when comparing column 1 (baseline, before gene therapy) with other columns (after gene therapy), the change in the pattern of cortical activations closely follows the process of gene therapy. Minimal activations when photoreceptors are non-functional and significant amount of activations after the rescue/revival of the photoreceptors, particularly in the primary visual cortex.
Because of the primary visual cortex’s key role in the hierarchy of the visual system, the fMRI correlations with patients’ clinical measures were performed separately for the total cortical activations distributed across the entire surface of the visual cortex (see Figure S1) and the activations limited to the medial surface of the visual cortex (see Figures S2). Results for the association of the clinical measures and fMRI cortical activations over time are presented in Figures 3 and 4 for the whole visual cortex activations and the activations restricted to the medial surface of the visual cortex for each hemisphere respectively. As expected, values for the primary visual cortex activations were stronger predictors of the change in the subjects’ clinical measure over time, particularly for the visual field measure (see Tables 2 and 3 and Figures 3&4). We observed similar associations for the change in cortical activations over time and the subjects’ clinical measures for the left and the right visual cortex separately (see Supplementary Figures S3–S6). However, comparing the results from the left and right hemispheres, the right hemisphere’s medial surface activation values showed higher significance in association and predictive power for the subjects’ clinical outcome (see Tables 2 and 3; Figures S3–S6). We hypothesize that the observed left-right asymmetry in the correlation results may be due to the fact that 6/7 subjects received gene therapy to their right eyes with a subretinal injection in the superior aspect of their temporal retina, which has cortical projections to the brain that remain on the ipsilateral (right) cortex.32
Neither of the two cortical activation measures (medial surface or whole visual cortex) showed significant association with the changes in RPE65 subjects’ visual acuity. Most importantly, this lack of association could be due to the fact that the three-year follow up results from the clinical assessment of these RPE65 subjects showed no improvement in VA over time.1 This may also be due to the fact that the cortical activation areas of the most posterior pole of the visual cortex - where the foveal region of the retina projects to - was not separately measured for corrections with visual acuity. Smaller checkerboard patterns than the one used in our experiments are reported to preferentially stimulate the foveal region responsible for visual acuity (Kothari et al, 2014), however larger checkerboard size is used because most individuals with retinal disease lack the visual acuity to detect smaller size checkerboard stimuli (Donnell, 2015). Additionally, only 5 of the RPE65 subjects received full foveal exposure and 3 of them did not participate in the neuroimaging study. Furthermore, the foveal cones may have severely degenerated by the time the vector was administered, and it may not have been possible to successfully recover them (unless treatment is applied earlier in life).
The mechanism by which vision changes affect neuronal circuitry is complex. Much of our knowledge of visual system plasticity after visual restoration comes from animal studies. Here we studied this phenomenon in-vivo, through noninvasive brain imaging before and longitudinally after retinal intervention in a group of subjects with autosomal recessive mutations in RPE65, who experienced improved vision after retinal gene therapy. To our knowledge, this is the first demonstration of long-term temporal/spatial changes in retinal/cortical activations in humans, as reflected by the response of the visual cortex. It is the first demonstration of long term improved retinio-cortical responses after gene therapy administration to RPE65 subjects’ contralateral eyes. The longitudinal fMRI results unequivocally show the efficacy of one-time gene therapy by demonstrating the absence of cortical responses from affected retinal cells before gene therapy and significant and wide spread cortical activation after gene therapy with durability of up to 3 years, with observation ongoing. More importantly, the long lasting visual cortex activations, particularly those restricted to the primary visual areas showed significant association with the changes in the subjects’ clinical measures, such as full-field stimulus threshold (FST) response to the white, blue and red lights, pupillary light response (PLR), and to a lesser extent the changes in the subjects visual field (VF).
As such, fMRI may have the potential to provide quantitative neuroimaging biomarkers to serve as an outcome or predictive measure to potentially augment clinical evaluations of future gene and cell therapy subjects before and after retinal intervention.
Supplementary Material
Acknowledgments
Financial Support: This study was supported by R01EY025287-01A1, R21EY020662 from the National Eye Institute. This study was also funded in part by the Foundation Fighting Blindness–sponsored CHOP-PENN Pediatric Center for Retinal Degenerations, Clinical Translational Science Award NIH/NCRR UL1-RR-024134, 1R01EY019014-01A2, Research to Prevent Blindness, the Paul and Evanina Mackall Foundation Trust at Scheie Eye Institute and the F. M. Kirby Foundation and Spark Therapeutics. The sponsors and funding organizations had no role in the design or conduct of this research.
Footnotes
Meeting Presentation: Presented at the XXII Biennial Meeting of the International Society for Eye Research, September 25–29, 2016 Tokyo, Japan
Conflict of Interests: Only the following authors have conflict of interest: JB and AMM are co-inventors of patent for a method to treat or slow the development of blindness (US Patent number 8147823), but both waived any financial interest in this technology in 2002. JB served on scientific advisory boards for Sanofi-Aventis and Avalanche Technologies. JB also reports she is a co-founder of Gensight Biologics. JB is a coauthor of a provisional patent describing the mobility test used in the Spark study. The other authors declare that they have no competing interests.
This article contains additional online material. The following should appear online in the Supplementary Information: Supplementary Methods, fMRI Pre-processing, Figure S1, Figure S2, Figure S3, Figure S4, Figure S5, Figure S6, Table S1, and Table S2.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bennett J, Wellman J, Marshall KA, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388(10045):661–72. doi: 10.1016/S0140-6736(16)30371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone EM. Leber congenital amaurosis - a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2007;144(6):791–811. doi: 10.1016/j.ajo.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 3.den Hollander AI, Roepman R, Koenekoop RK, Cremers FP. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27(4):391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 4.den Hollander AI, Black A, Bennett J, Cremers FP. Lighting a candle in the dark: advances in genetics and gene therapy of recessive retinal dystrophies. J Clin Invest. 2010;120(9):3042–53. doi: 10.1172/JCI42258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marlhens F, Bareil C, Friffoin J-M, et al. Mutations in RPE65 cause Leber’s congenital amaurosis. Nature Genetics. 1997;17:139–41. doi: 10.1038/ng1097-139. [DOI] [PubMed] [Google Scholar]
- 6.Gu SM, Thompson DA, Srikumari CR, et al. Mutations in RPE65 cause autosomal recessive childhood-onset severe retinal dystrophy. Nat Genet. 1997;17(2):194–7. doi: 10.1038/ng1097-194. [DOI] [PubMed] [Google Scholar]
- 7.Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12(6):1072–82. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28(1):92–5. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 9.Dejneka NS, Surace EM, Aleman TS, et al. In utero gene therapy rescues vision in a murine model of congenital blindness. Mol Ther. 2004;9(2):182–8. doi: 10.1016/j.ymthe.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 10.Bennicelli J, Wright JF, Komaromy A, et al. Reversal of blindness in animal models of leber congenital amaurosis using optimized AAV2-mediated gene transfer. Mol Ther. 2008;16(3):458–65. doi: 10.1038/sj.mt.6300389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narfstrom K, Ehinger B, Bruun A. Immunohistochemical studies of cone photoreceptors and cells of the inner retina in feline rod-cone degeneration. Vet Ophthalmol. 2001;4(2):141–5. doi: 10.1046/j.1463-5224.2001.00191.x. [DOI] [PubMed] [Google Scholar]
- 12.Banin E, Bandah-Rozenfeld D, Obolensky A, et al. Molecular anthropology meets genetic medicine to treat blindness in the North African Jewish population: human gene therapy initiated in Israel. Hum Gene Ther. 2010;21(12):1749–57. doi: 10.1089/hum.2010.047. [DOI] [PubMed] [Google Scholar]
- 13.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–90. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105(39):15112–7. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 16.Simonelli F, Maguire AM, Testa F, et al. Gene therapy for Leber’s congenital amaurosis is safe and effective through 1. 5 years after vector administration. Mol Ther. 2010;18(3):643–50. doi: 10.1038/mt.2009.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374(9701):1597–605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Health USNIo. 2016 http://www.ClinicalTrials.govv.
- 19.Ashtari M, Cyckowski LL, Monroe JF, et al. The human visual cortex responds to gene therapy-mediated recovery of retinal function. J Clin Invest. 2011;121(6):2160–8. doi: 10.1172/JCI57377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galvin JA, Fishman GA, Stone EM, Koenekoop RK. Evaluation of genotype-phenotype associations in leber congenital amaurosis. Retina. 2005;25(7):919–29. doi: 10.1097/00006982-200510000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz B, Gyurus P, Preising M, et al. Early-onset severe rod-cone dystrophy in young children with RPE65 mutations. Invest Ophthalmol Vis Sci. 2000;41(9):2735–42. [PubMed] [Google Scholar]
- 22.Jacobson SG, Aleman TS, Cideciyan AV, et al. Defining the residual vision in leber congenital amaurosis caused by RPE65 mutations. Invest Ophthalmol Vis Sci. 2009;50(5):2368–75. doi: 10.1167/iovs.08-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett J, Ashtari M, Wellman J, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4(120):120ra15. doi: 10.1126/scitranslmed.3002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman L, Turner JA, Stern H, et al. Chronic smoking and the BOLD response to a visual activation task and a breath hold task in patients with schizophrenia and healthy controls. Neuroimage. 2008;40(3):1181–94. doi: 10.1016/j.neuroimage.2007.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ashtari M, Cyckowski L, Yazdi A, et al. fMRI of Retina-Originated Phosphenes Experienced by Patients with Leber Congenital Amaurosis. PloS one. 2014;9(1):e86068. doi: 10.1371/journal.pone.0086068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Resonance Technology I. VisuaStime XGA.
- 28.Schneider W, Eschman A, Zuccolotto A. E-Prime: User’s guide. Psychology Software Incorporated; 2002. [Google Scholar]
- 29.Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27(5):392–401. doi: 10.1002/hbm.20249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valverde F. Apical dendritic spines of the visual cortex and light deprivation in the mouse. Exp Brain Res. 1967;3(4):337–52. doi: 10.1007/BF00237559. [DOI] [PubMed] [Google Scholar]
- 31.Maya-Vetencourt JF, Origlia N. Visual cortex plasticity: a complex interplay of genetic and environmental influences. Neural Plast. 2012;2012:631965. doi: 10.1155/2012/631965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashtari M, Zhang H, Cook PA, et al. Plasticity of the human visual system after retinal gene therapy in patients with Leber’s congenital amaurosis. Sci Transl Med. 2015;7(296):296ra110. doi: 10.1126/scitranslmed.aaa8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erskine L, Herrera E. Connecting the retina to the brain. ASN Neuro. 2014;6(6) doi: 10.1177/1759091414562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt TM, Do MT, Dacey D, et al. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci. 2011;31(45):16094–101. doi: 10.1523/JNEUROSCI.4132-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spatz WB. Thalamic and other subcortical projections to area MT (visual area of superior temporal sulcus) in the marmoset Callithrix jacchus. Brain Res. 1975;99(1):129–34. doi: 10.1016/0006-8993(75)90614-9. [DOI] [PubMed] [Google Scholar]
- 36.Benevento LA, Standage GP. The organization of projections of the retinorecipient and nonretinorecipient nuclei of the pretectal complex and layers of the superior colliculus to the lateral pulvinar and medial pulvinar in the macaque monkey. J Comp Neurol. 1983;217(3):307–36. doi: 10.1002/cne.902170307. [DOI] [PubMed] [Google Scholar]
- 37.Stepniewska I, Qi HX, Kaas JH. Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? Eur J Neurosci. 1999;11(2):469–80. doi: 10.1046/j.1460-9568.1999.00461.x. [DOI] [PubMed] [Google Scholar]
- 38.Stepniewska I, Qi HX, Kaas JH. Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Visual Neuroscience. 2000;17(4):529–49. doi: 10.1017/s0952523800174048. [DOI] [PubMed] [Google Scholar]
- 39.Cowey A, Stoerig P, Perry VH. Transneuronal retrograde degeneration of retinal ganglion cells after damage to striate cortex in macaque monkeys: selective loss of P beta cells. Neuroscience. 1989;29(1):65–80. doi: 10.1016/0306-4522(89)90333-3. [DOI] [PubMed] [Google Scholar]
- 40.Cowey A, Stoerig P. The neurobiology of blindsight. Trends Neurosci. 1991;14(4):140–5. doi: 10.1016/0166-2236(91)90085-9. [DOI] [PubMed] [Google Scholar]
- 41.Baseler HA, Morland AB, Wandell BA. Topographic organization of human visual areas in the absence of input from primary cortex. J Neurosci. 1999;19(7):2619–27. doi: 10.1523/JNEUROSCI.19-07-02619.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warner CE, Kwan WC, Wright D, et al. Preservation of Vision by the Pulvinar following Early-Life Primary Visual Cortex Lesions. Curr Biol. 2015;25(4):424–34. doi: 10.1016/j.cub.2014.12.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.