BACKGROUND
Beyond the effects of sex hormones, differences in male and female atherogenesis are poorly understood. Neglecting to study basic pathophysiology in male and female animals separately may have negative consequences for drug development in the era of personalized medicine.
The purpose of this study is to examine how atherosclerosis develops differently between male and female mice using the well-established low density lipoprotein receptor knockout (LDLR KO) mouse model of atherosclerosis. We hypothesize that atherosclerosis develops differently between male and female LDLR KO mice.
AIMS
The aim of this study was to examine the differences in atherogenesis between male and female LDLR KO mice.
METHODS
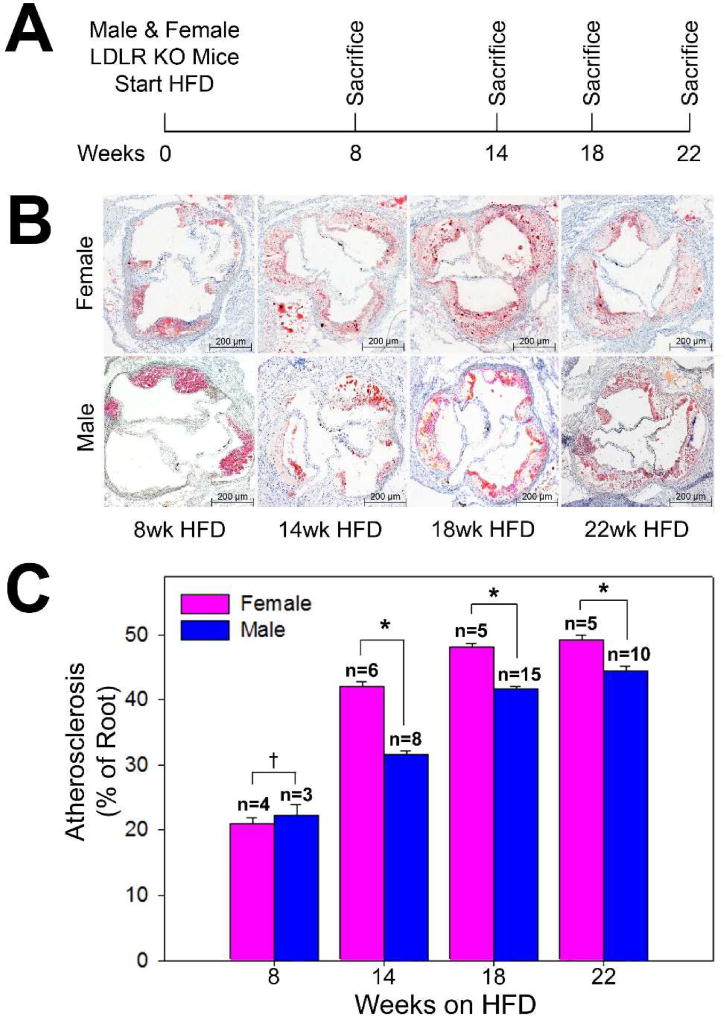
Procedures were performed with Northwestern University Institutional Animal Care and Use Committee. LDLR-KO mice (The Jackson Laboratory, Bar Harbor, ME) were fed a high-fat “Western” diet (HFD) (Tekland TD.88137) consisting of 20% fat, 0.2% cholesterol, and 34% high sucrose starting at 4 weeks of age. Male and female mice were fed the HFD for 8, 14, 18, and 22 weeks prior to sacrifice (n=3–15/group; Figure 1A). Prior to euthanasia of mice at the 14 weeks high fat diet time point, blood was obtained through direct right ventricular cardiac puncture and used for serum lipid analysis by IDEXX (IDEXX laboratories, Westbrook, ME). Due to inadequate blood volume collection, serum lipid analysis was not performed on all included subjects. Following in situ perfusion with PBS then 10% sucrose, the heart and aortic roots were flash frozen in Optimal Cutting Temperature embedding medium 4583 (Tissue-Tek®, Sakura Finetek USA, Torrance, CA) with liquid nitrogen. Frozen specimens were cryosectioned through the aortic valve as evident by the three aortic valve cusps, as previously described.(1) Aortic root sections were stained with Oil-red-O to visualize atherosclerosis. To normalize the effect of variable aortic root size over time and between the sexes, atherosclerosis was quantified as plaque area as a percentage of total aortic root area using ImageJ software (National Institutes of Health). Statistical analysis including ANOVA (for atherosclerosis as percentage of aortic root comparisons) and the rank sum test (for serum lipid analysis) was performed in STATA version 14.0 (Statacorp, College Station, TX) and SigmaPlot version 10.0 (Systat Software Inc., San Jose, CA). Atherosclerosis as percentage of the aortic root is expressed as group means with standard deviations, and serum lipid analyses and weights are expressed as group medians with standard deviations.
Figure 1.
Study design and aortic root atherosclerosis. (A) Low density lipoprotein cholesterol receptor knockout (LDLR KO) mice of both sexes were fed a high fat diet HFD) for 8, 14, 18, and 22 weeks prior to sacrifice. (B) Representative histologic cross sections of aortic roots from female and male LDLR KO mice at 8, 14, 18, and 22 weeks on the high fat diet. (C) Quantification of atherosclerosis in the aortic root of LDLR KO mice (†P=0.52, *P<0.0001).
RESULTS
After being fed the HFD for 14 weeks females had significantly higher levels of total cholesterol (1909+/−168 mg/dL vs. 958+/−443 mg/dL, P=0.025) and low density lipoprotein cholesterol (541+/−227 mg/dL vs. 235+/−201 mg/dL, P=0.025) compared to males. There were no significant differences between male and female mice in HDL cholesterol (66+/−3 mg/dL females vs. 87+/−8 mg/dL males) or triglycerides (846+/−207 mg/dL females vs. 584+/−412 mg/dL males). Males had a significantly higher body weight (33.9+/−1.5 g) compared to females (25.6+/−1.4 g) after 14 weeks on the HFD (P=0.034) (Table 1). Atherosclerosis developed progressively over time in the aortic roots in both male and female mice fed the HFD. While both sexes displayed a similar degree of atherosclerosis after 8 weeks on the HFD (female 21.0+/−5.2%, male 22.2+/−8.3%, p=0.52), females developed significantly more atherosclerosis compared to males after 14 weeks (female 42.1+/−6.4%, male 31.6+/−7.2%, p<0.05), 18 weeks (female 48.2+/−3.1%, male 41.6+/−6.5%, p<0.05), and 22 weeks (female 49.2+/−5.8%, male 44.5+/−8.5%, p<0.05) on the HFD (Figure 1B–C). Furthermore, the interaction between sex and time was significant (P<0.0001), confirming that the increase in atherosclerosis over time differs by sex, and sex modifies the time-dependent increase in atherosclerosis.
Table 1.
Serum lipid and body weight analysis.
| N | LDLR KO - 14wk High Fat Diet | ||
|---|---|---|---|
| Male | Female | P | |
| 3 | 5 | ||
| Cholesterol (mg/dL) | 958 (+/− 443) | 1909 (+/− 168) | 0.025 |
| Triglycerides (mg/dL) | 584 (+/− 412) | 846 (+/− 207) | 0.456 |
| HDL Cholesterol (mg/dL) | 87 (+/− 8) | 66 (+/− 3) | 0.180 |
| LDL Cholesterol (mg/dL) | 235 (+/− 201) | 541 (+/− 227) | 0.025 |
| Body Weight (g)* | 33.9 (+/− 1.5) | 25.6 (+/− 1.4) | .0339 |
Body weight measurements 1 n=8 male and n=6 female.
CONCLUSION
These data demonstrate that the LDLR KO mouse develops progressive atherosclerosis in the aortic root over time, consistent with prior studies.(2, 3) These data confirm our hypothesis that atherosclerosis develops differently between hormonally intact male and female LDLR KO mice.
Historically, biomedical research has been primarily conducted in one sex of animal models.(4) We recently reported that 22% of published animal research studies fail to specify the sex of animals used, and when the sex was reported, both sexes were studied only 3% of the time.(5) Although our results conflicted with our preconceived notions of the pathophysiology of atherosclerosis, they underscore the importance of conducting sex-specific research at the most fundamental level.
An important limitation of our study is we show a difference in atherosclerosis without investigation regarding the mechanism of this disparity. Additionally, it should be noted that the size of male and female aortic roots are different. To account for this variable, we measured atherosclerosis as a percentage of the aortic root area, which normalizes the data for variation in vessel size between sexes and over time.
The LDLR KO mouse was first developed and described by Ishibashi et al in 1993.(6) In the original description, serum lipid disparities were noted between males and females; however, differences in quantified atherosclerosis were not measured and reported.(6) Contrary to our data, Tangirala and colleagues measured the extent of lesions between male and female LDLR KO mice and reported increased atherosclerotic lesion area in male mice.(7) While we used mice of the same strain and from the same vendor as Tangirala et al, the diets from our two studies were quite different and may account for the differences in results. We used a HFD (21% fat) with 0.2% cholesterol whereas Tangirala et al used a standard cholate-free diet with high cholesterol (1%). In support of our data, Teupser and colleagues measured lipid levels and atherosclerotic lesion area in male and female LDLR KO mice. Interestingly, on a C57BL/6 background, female LDLR KO mice expressed more atherosclerosis than males on diets containing increasing amounts of cholesterol (0.0–0.6%).(8) Unfortunately, a direct statistical comparison between the sexes was not performed. Thus, while prior studies have established the LDLR KO mouse model in male and female mice, most studies have not been conducted or statistically analyzed in animals of both sexes.
The purpose of this work is not to explain why there is a difference between the sexes, but to highlight that there is a difference between the sexes. In this current era of personalized medicine, in order to properly perform investigation into novel therapies for atherosclerosis, we must acknowledge that male and female model organisms express atherosclerosis differently. Once we accept this, we can begin to develop therapies that can be applied to either males or females, or can be safely and effectively used in the entire population.
In conclusion, male and female LDLR KO mice express atherosclerosis differently. Surprisingly, atherosclerosis was more severe in the female model. The underlying mechanism of differential expression of atherosclerosis is yet to be understood. Since evidence exists supporting a difference between male and female models of atherosclerosis, we suggest that investigators use animals of both sexes and normalize the variation in vessel sizes in performing basic and translational research on atherosclerosis in murine models.
Highlights.
-
-
Differences in male and female atherogenesis are poorly understood.
-
-
Female LDLR KO mice develop more severe hyperlipidemia compared to males.
-
-
Female LDLR KO mice develop more severe atherosclerosis compared to males.
Acknowledgments
The authors would like to acknowledge Mrs. Lynnette Dangerfield and Mrs. Elizabeth Gorsuch for their administrative and editorial assistance, and Dr. Irene B. Helenowski for assistance with statistical analysis.
Funding Acknowledgement: This research was supported, in part, by funding from a NIH/NHLBI Bioengineering Research Partnership grant (5R01HL116577), a NIH/NHLBI grant (1R01HL108118-01), a Northwestern Memorial Foundation Dixon Translational Research Innovation Award, a NIH/NHLBI Training Grant (2T32HL094293), an American Medical Association Foundation 2016 Seed Grant, the National Center for Advancing Translational Sciences (UL1TR001422 NUCATS), and NCI CCSGP30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors declare that there are no conflicts of interest.
References
- 1.Baglione J, Smith JD. Quantitative assay for mouse atherosclerosis in the aortic root. Methods Mol Med. 2006;129:83–95. doi: 10.1385/1-59745-213-0:83. [DOI] [PubMed] [Google Scholar]
- 2.Ma Y, Wang W, Zhang J, Lu Y, Wu W, Yan H, et al. Hyperlipidemia and atherosclerotic lesion development in Ldlr-deficient mice on a long-term high-fat diet. PloS one. 2012;7(4):e35835. doi: 10.1371/journal.pone.0035835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. The Journal of clinical investigation. 1994;93(5):1885–93. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neuroscience and biobehavioral reviews. 2011;35(3):565–72. doi: 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon DY, Mansukhani NA, Stubbs VC, Helenowski IB, Woodruff TK, Kibbe MR. Sex bias exists in basic science and translational surgical research. Surgery. 2014;156(3):508–16. doi: 10.1016/j.surg.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. The Journal of clinical investigation. 1993;92(2):883–93. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tangirala RK, Rubin EM, Palinski W. Quantitation of atherosclerosis in murine models: correlation between lesions in the aortic origin and in the entire aorta, and differences in the extent of lesions between sexes in LDL receptor-deficient and apolipoprotein E-deficient mice. Journal of lipid research. 1995;36(11):2320–8. [PubMed] [Google Scholar]
- 8.Teupser D, Persky AD, Breslow JL. Induction of atherosclerosis by low-fat, semisynthetic diets in LDL receptor-deficient C57BL/6J and FVB/NJ mice: comparison of lesions of the aortic root, brachiocephalic artery, and whole aorta (en face measurement) Arteriosclerosis, thrombosis, and vascular biology. 2003;23(10):1907–13. doi: 10.1161/01.ATV.0000090126.34881.B1. [DOI] [PubMed] [Google Scholar]