Abstract
The capacity of the olfactory epithelium (OE) for lifelong neurogenesis and regeneration depends on the persistence of neurocompetent stem cells, which self-renew as well as generating all of the cell types found within the nasal epithelium. This Review focuses on the types of stem and progenitor cells in the epithelium and their regulation. Both horizontal basal cells (HBCs) and some among the population of globose basal cells (GBCs) are stem cells, but the two types plays vastly different roles. The GBC population includes the basal cells that proliferate in the uninjured OE and is heterogeneous with respect to transcription factor expression. From upstream in the hierarchy to downstream, GBCs encompass 1) Sox2+/Pax6+ stem-like cells that are totipotent and self-renew over the long term, 2) Ascl1+ transit-amplifying progenitors with a limited capacity for expansive proliferation, and 3) Neurog1+/NeuroD1+ immediate precursor cells that make neurons directly. In contrast, the normally quiescent HBCs are activated to multipotency and proliferate when sustentacular cells are killed, but not when only OSNs die, indicating that HBCs are reserve stem cells that respond to severe epithelial injury. The master regulator of HBC activation is the ΔN isoform of the transcription factor p63; eliminating ΔNp63 unleashes HBC multipotency. Notch signaling, via Jagged1 ligand on Sus cells and Notch1 and Notch2 receptors on HBCs, is likely to play a major role in setting the level of p63 expression. Thus, ΔNp63 becomes a potential therapeutic target for reversing the neurogenic exhaustion characteristic of the aged OE.
INDEXING TERMS: active stem cell, reserve stem cell, p63, horizontal basal cell, globose basal cell, dedifferentiation, neurogenesis, aging
As a general rule, the nervous system of mammals recovers poorly after injury, being unable to replace neurons that die and incapable of reinnervating target structures with the same exquisite specificity as during development. The exception to the general rule of limited neural repair is the olfactory epithelium (OE), a specialized neuroepithelium that lines the posterodorsal aspect of the nasal cavity. The OE consists of a handful of cell types including several varieties of nonneuronal cells as well as the olfactory sensory neurons (OSNs), which subserve odorant transduction and project their axons via the olfactory nerve onto the olfactory bulb (Andres, 1966; Graziadei, 1974; Graziadei and Monti Graziadei, 1979; Fig. 1). Unlike other parts of the nervous system, the OE retains a lifetime capacity for wholesale anatomical and functional recovery after injury (Nagahara, 1940; Schultz, 1941, 1960; Graziadei, 1978; Monti Graziadei and Graziadei, 1979; Morrison and Costanzo, 1989; Schwob et al., 1995, 1999; Costanzo, 2000; Cummings et al., 2000; Christensen et al., 2001; Schwob, 2002; Iwema et al., 2004; Schwob and Costanzo, 2008; Cheung et al., 2013; Holbrook et al., 2014). This regenerative capacity implies the lifelong persistence of stem cells, i.e., cells that are able to progress through intermediate progenitor cell stages to generate all of the differentiated cell types of the epithelium, including neurons, and that maintain that tissue totipotency through unlimited self-renewal (Schwob et al., 2012). In contrast to the stem cells, the progenitor cells that are intermediate between the stem cells and the differentiated constituent cells have only a limited capacity for self-renewal and expansion and generally give rise to only a subset of cell types. As described below, the hierarchy of stem cells and progenitors can be defined operationally and by molecular phenotype. The accessibility of olfactory stem cells and their lifelong potency make them attractive subjects for intensive examination and, potentially, therapeutic exploitation. Accordingly, the cellular identity, capacity for differentiation, molecular regulation, and potential exploitation of olfactory stem and progenitor cells of the adult OE are the subjects of this Review.
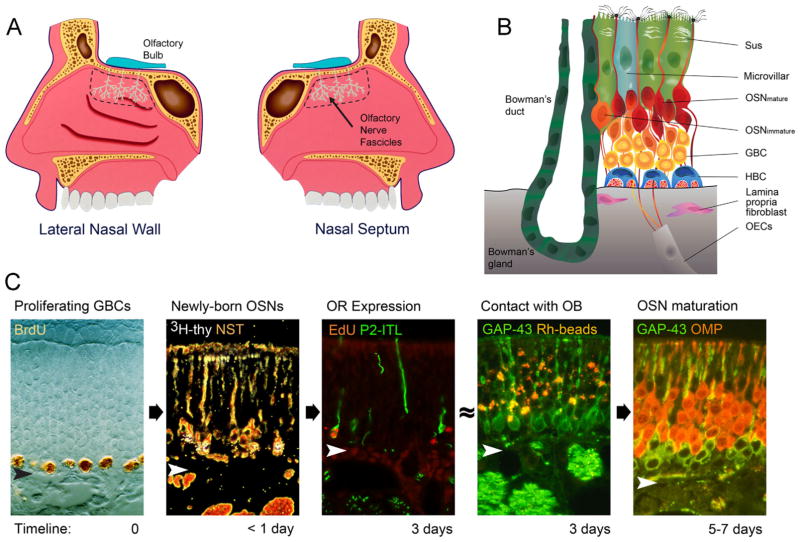
Figure 1.
A: Schematic representation of the olfactory area (outlined by the dashed line) on the lateral nasal wall and nasal septum of the bisected nose along with the fibers of the olfactory nerve (teal). B: The major cellular constituents of the olfactory epithelium (OE). Note particularly the two populations of basal cells, globose basal cells (GBC) and horizontal basal cells (HBC). Sus, sustentacular cells; OSN, olfactory sensory neuron; OECs, olfactory ensheathing cells in fascicles of the olfactory nerve. C: Progression from proliferating GBC to mature OSN as a function of time after terminal S-phase (timeline). Proliferating GBCs are marked by the incorporation of BrdU or other thymidine analogues. Less than 24 hours after injection of 3H-thymidine (thy) or other thymidine analogue, the label is chased into differentiating OSNs marked by neuron-specific tubulin (NST). approximately 3 days after a neuron becomes postmitotic, olfactory receptors (OR) such as P2, which is marked by the labeling with tau-fused beta galactosidase (P2-ITL), are expressed. At about the same time, immature, GAP-43-expressing OSNs can be labeled by retrograde transport of rhodamine-labeled latex microspheres (Rh beads) following injections in the olfactory bulb (OB). Finally, morphological and functional maturation is evident by 5–7 days later, as marked by the transition from expression of GAP-43 to the olfactory marker protein (OMP; Verhaagen et al., 1989; Schwob, 1991; Schwob et al., 1992; Rodriguez-Gil et al., 2015). Arrowheads designate the basal lamina.
CELL TYPES OF THE MAMMALIAN OE
Resident cells of the OE include not only the aforementioned OSNs but also specialized nonneuronal elements (Fig. 1; Andres, 1966; Graziadei, 1974; Graziadei and Monti Graziadei, 1979). These include 1) microvillar-capped sustentacular (Sus) cells (supporting elements that express biotransformation enzymes and cytokeratins 8 and 18; Ding et al., 1991; Chen et al., 1992), 2) microvillar (MV) cells (a heterogeneous population with respect to phenotype and function; Moran et al., 1982; Morrison and Costanzo, 1992; Asan and Drenckhahn, 2005; Montani et al., 2006; Hegg et al., 2010; Jia et al., 2013; Kusumakshi et al., 2015), 3) the Bowman’s duct-gland units (chains of cells extending upward from the acini within the lamina propria to the OE’s surface and sharing some phenotypic characteristics with Sus cells), and 4) at least two populations of basal cells: horizontal or dark basal cells (HBCs) and globose or light basal cells (GBCs; Fig. 2; Graziadei and Monti Graziadei, 1979).
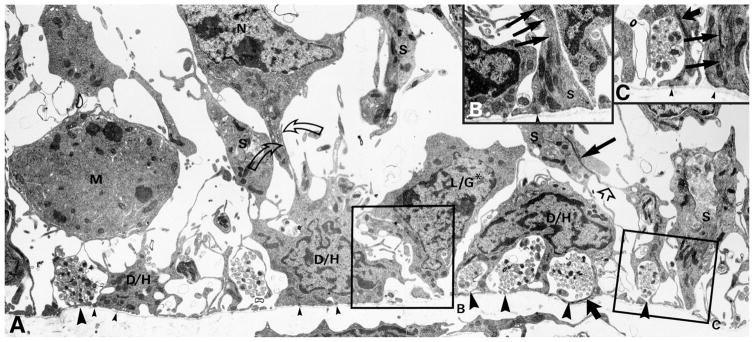
Figure 2.
Electron microscopic investigation illustrates the characteristics of the basal region of the OE. A–C: The HBCs (D/H) tightly adhere to the basal lamina (small arrowheads indicate hemidesmosomes) forming cytoplasmic bridges that enfold clusters of olfactory axons (large arrowheads). The GBCs (L/G* and M) are found at a remove from the basal lamina, including those that are undergoing mitosis (M). The GBC marked by the asterisk (L/G*) has a process that touches the basal lamina (in contrast to the one marked M) and apparently corresponds to a “third type” of basal cell described previously (Graziadei and Monti Graziadei, 1979), the significance of which is unclear. Note the numerous adherens junctions between cells in the basal region: Sus cell (S) and HBCs (straight open arrow), HBCs and neurons (N; curved open arrows). Cellular identification is based on morphology, i.e., the foot process of Sus cells, and the presence of characteristic intermediate filaments: in Sus cells (thin straight solid arrows) and in HBCs (thick straight solid arrows). B,C: Higher magnification electron micrographs taken from the region of the boxed areas in A but from a nearby section. Modified from Holbrook et al. (1995).
The HBCs are morphologically complex and express many morphological and molecular features in common with the basal cells of other epithelia, including the respiratory epithelium (RE; Randell et al., 1991; Shimizu et al., 1991, 1992). Phenotypic similarities include the formation of hemidesmosomes attaching the cell to the basal lamina and paired expression of cytokeratins (K) 5 and 14, ICAM-1, selected integrins, the EGF receptor, the sugar moiety(ies) recognized by the lectin Bandeirea (Griffonia) simplicifolia-I, and the transcription factor p63 (Miragall et al., 1988; Calof and Chikaraishi, 1989; Schwartz Levey et al., 1992; Mahanthappa and Schwarting, 1993; Holbrook et al., 1995; Getchell et al., 2000; Packard et al., 2011b). In addition, the HBCs have a remarkably intimate relationship with fascicles of olfactory axons as they exit the epithelium (Holbrook et al., 1995). In the uninjured OE, HBCs are mitotically quiescent, in contrast to basal cells of epidermis, skin adnexa, and RE.
The GBCs, which seem to be unique to the OE, are grouped in clusters with large gaps between them, and they lie just superficial to the HBC layer (Graziadei and Monti Graziadei, 1979; Huard and Schwob, 1995; Loo et al., 1996; Jang et al., 2003, 2014). In the normal OE, GBCs encompass the population of proliferating basal cells marked by the incorporation of analogues of thymidine or by the expression of proteins associated with mitotic progression (Figs. 1–3; Moulton, 1970, 1974; Graziadei and Metcalf, 1971; Graziadei, 1973; Graziadei and Monti Graziadei, 1979; Schwarz Levey et al., 1991; Huard and Schwob, 1995; Loo et al., 1996; Jang et al., 2003, 2014). GBCs are morphologically unremarkable (round with scant cytoplasm) but are actually a heterogeneous population distinguished by the mix of transcription factors that they express, including Sox2, Pax6, Six1, Ascl1, Neurog1, and NeuroD1 (Fig. 3; Guillemot and Joyner, 1993; Guillemot et al., 1993; Gordon et al., 1995; Cau et al., 1997, 2000, 2002; Manglapus et al., 2004; Guo et al., 2010; Ikeda et al., 2010). The differences in transcription factor profile are established early in development and persist throughout adult life. The pattern of transcription factor expression can be used to construct a hierarchy of GBC subtypes proceeding from more undifferentiated through to the birth of OSNs, based on the timing of first appearance in the embryo and during the recovery from epithelial injury and on the effects of gene knockout (Fig. 3; Guillemot and Joyner, 1993; Guillemot et al., 1993; Cau et al., 1997, 2000, 2002; Manglapus et al., 2004; Guo et al., 2010; Ikeda et al., 2010). For example, most epithelial cells at the olfactory placode/pit stage of embryonic development are marked by the transcription factors Sox2, Pax6, and Six1; expression of these transcription factors carries forward in GBCs after that definitive population is established (Ikeda et al., 2007, 2010; Tucker et al., 2010; Moody and LaMantia, 2015). Other cells of the embryonic epithelium express Ascl1 as well as Sox2, Pax6, and Six1 and are next to emerge at the apical surface of the epithelium at the olfactory pit stage. A bit later in development, Ascl1-expressing cells lie at the base of the epithelium, as mitotically active basal cells appear (Smart, 1971; Cuschieri and Bannister, 1975a,b; Guillemot and Joyner, 1993; Guillemot et al., 1993; Cau et al., 1997). Still other basal cells in the embryonic epithelium express Neurog1, followed by NeuroD1 soon after (Cau et al., 1997). The appearance of cells expressing the aforementioned transcription factors at the base of the epithelium is coincident with the emergence of proliferating basal cells there. In the normal adult epithelium, Neurog1+ and NeuroD1+ GBCs lack detectable expression of Six1, Sox2, and Pax6 (Guo et al., 2010; Packard et al., 2011a; Krolewski et al., 2013). Some investigators have identified a third type of basal cell, distinguished from the other two by location (nestled between HBCs and extending a process downward to touch the basal lamina) and the arrangement of condensed chromatin around the periphery of the nucleus (Fig. 2; Graziadei and Monti Graziadei, 1979; Holbrook et al., 1995; Carter et al., 2004).
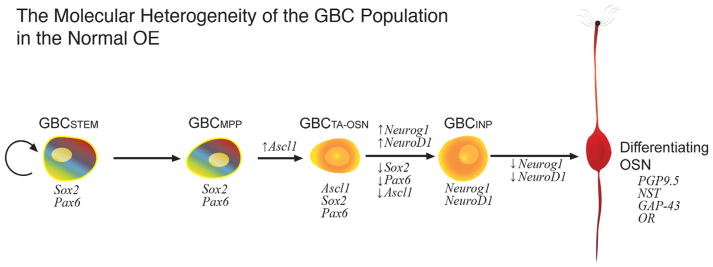
Figure 3.
Diagram illustrating the molecular hetereogeneity of the GBCs in the normal olfactory epithelium (OE) and the progression from stem cell through to differentiating olfactory sensory neuron (OSN). A relatively simple linear sequence proceeds from GBC stem cells through multiple molecularly discrete stages, with marker genes designated in italics and potential multipotency designated by the rainbow gradient; that multipotency is not evident in the normal epithelium in situ but is observed following transplantation from normal OE into the lesioned environment (Goldstein et al., 1998; Chen et al., 2004). HBCs are not shown and do not contribute to the OSN population in the normal OE (Caggiano et al., 1994). GBCSTEM, stem cell-like GBC expressing Sox2 and Pax6 that is mitotically quiescent (thymidine label-retaining); GBCMPP, multipotent GBC expressing Sox2 and Pax6 and first to appear during development and regeneration; GBCTA-OSN, transit-amplifying GBC restricted to a neuronal fate and expressing Sox2, Pax6, and Ascl1; GBCINP, GBC functioning as an immediate neuronal precursor, i.e., giving rise to a small number of OSNs, and expressing Neurog1 and NeuroD1. The evidence that some GBCs are stem cells is described in the text.
GBCs AS PROGENITOR CELLS FOR OSNs
The pulse labeling of GBCs in normal rodent OE by incorporation of a thymidine analogue and the “chasing” of the label into their descendents by delaying tissue harvest suggest that among the population of GBCs are progenitors that give rise to OSNs (Moulton, 1970, 1974; Graziadei and Metcalf, 1971; Graziadei, 1973; Schwartz Levey et al., 1991; Huard and Schwob, 1995). In contrast, the HBCs remain mitotically inactive/quiescent in the normal OE or even in the face of accelerated GBC proliferation and neuronal production following ablation of the olfactory bulb, indicating that they are not functioning as neuronal progenitors in that setting (Graziadei and Monti Graziadei, 1979, Schwartz Levey et al., 1991; Huard and Schwob, 1995). Similarly, proliferation is restricted to GBCs in the human OE as well (Holbrook et al., 2011).
The differentiation of the OSNs after their birth from GBCs progresses from an immature state, in which neurons express molecular markers of axonal growth and differentiation (Schwob et al., 1992), into fully mature OSNs, which express the olfactory marker protein (OMP) and the apparatus of odorant transduction (Graziadei, 1973; Moulton, 1974; Graziadei and Monti Graziadei, 1979; Miragall and Monti Graziadei, 1982; Schwob et al., 1992; Fig. 1C).
Further evidence for the generation of OSNs by GBCs was obtained by using retroviral lineage tracing to transduce dividing GBCs of normal or bulbectomized mice. Retroviral transduction permits progenitors to pass a heritable marker to all their progeny. In the setting of normal or accelerated neurogenesis, GBCs gave rise only to GBCs and neurons (Caggiano et al., 1994; Hunter et al., 1994; Schwob et al., 1994a). In addition, the altered composition of the epithelium after gene mutation also indicates a neurogenic role for GBCs. Thus, functional deletion of Ascl1, which is normally found in GBCs classified as transit-amplifying cells, blocks the formation of almost all OSNs and Neurog1/NeuroD1-expressing GBCs and expands the population of Sox2/Pax6-expressing GBCs. These data establish a hierarchy of GBC types in which Sox2/Pax6-expressing GBCs anticipate and give rise to Ascl1-expressing GBCs, which in turn anticipate and give rise to Neurog1/NeuroD1-expressing GBCs within the heterogeneous population (Guillemot et al., 1993; Cau et al., 1997; Krolewski et al., 2012; Fig. 3); that hierarchy is also suggested by the relative timing of gene expression. Furthermore, because Ascl1 knockout causes neuronal depletion but spares the nonneuronal cell types, Ascl1 expression seems to mark neuronally committed progenitors (Krolewski et al., 2012). Genetic lineage tracing, i.e., confining a marker to the specific classes of GBCs and their descendants, provides the capstone proof of a progenitor–progeny relationship. The genetic approach takes advantage of Cre recombinase, in either its native or its tamoxifen-dependent form, to excise a “stop” moiety upstream of a marker gene inserted into the ROSA26 locus, thereby unblocking its expression. For example, a BAC transgene expressing Cre from the NeuroD1 locus, which is expressed in some GBCs, labels seemingly all OSNs and only OSNs within the OE (Packard et al., 2011a). Likewise, Lgr5+ GBCs (whose place in the hierarchy of GBCs is not fully defined but likely to be well upstream of the NeuroD1+ stage) give rise just to neurons in the uninjured OE (Chen et al., 2014). GBCs that express c-Kit seem to have a somewhat broader potential, at least during development, giving rise to neurons, microvillar cells, and duct/gland cells as well as supporting/facilitating olfactory regeneration (Goldstein et al., 2015; Goss et al., 2015).
All of these approaches demonstrate that the overwhelming majority of the differentiated progeny of GBCs within the normal OE are OSNs. Although these accumulated data have been interpreted as proof that the GBCs are a selective neuronal stem cell, they do not prove that GBCs make only neurons in all settings, which is the criterion for selectivity, nor do these experiments come anywhere near proving that the GBCs are infinitely self-renewing, which is required of a stem cell (although see below, where direct evidence is cited proving their stemness). In retrospect, the lack of multipotency/neuronal selectivity is not surprising, because the only cell types needing substantial replacement, either in uninjured or bulbectomized animals, are the OSNs. Indeed, light and electron microscopic examinations of olfactory epithelium recovering from direct toxin-caused injury were used to put forward alternative candidates for the olfactory stem cells, including HBCs and/or duct/gland cells and/or GBCs (Mulvaney, 1971; Matulionis, 1975, 1976).
REVEALING THE MULTIPOTENCY AND CAPABILITIES OF DIFFERENT KINDS OF GBCS
The pulse-chase and lineage data do provide strong evidence that among the GBCs are cells that act with a high degree of fidelity as progenitors of OSNs within the context of a normal or neuron-depleted epithelium. However, to demonstrate that GBCs can make only neurons, one has to challenge them by depleting or destroying several or all epithelial cell types in order to challenge the full progenitive potency of the GBCs (or any other type of progenitor cell, for that matter). To that end various compounds have been used to injure the OE selectively, directly, and comprehensively, including drugs and toxins that are inhaled (methyl bromide; Schwob et al., 1995), systemically administered (methimazole, dichlobenil; Brittebo, 1995; Genter et al., 1995, 1996; Bergman et al., 2002), or delivered by intranasal irrigation (Triton X-100, zinc sulfate; Smith, 1938; Matulionis, 1975, 1976; Harding et al., 1978; Cancalon, 1982, 1983; Stewart et al., 1983; Kream and Margolis, 1984; Verhaagen et al., 1990).
In particular, exposure to the olfactotoxic gas methyl bromide (MeBr) has been useful for studies of olfactory regeneration and the identity of unipotent vs. multipotent progenitor cells because passive inhalation by unrestrained animals is an easy means of delivering the toxin, only the OE is harmed, the wounding can be limited to one side of the nose by plugging a naris during the exposure period, and active tissue damage essentially terminates when the animal is removed from the gas, because MeBr is both highly volatile and eliminated from the animal quickly (Hurtt et al., 1987, 1988; Schwob et al., 1994b, 1995, 1999). Consequently, onset of repair is prompt and begins just after the end of the exposure period. Most importantly, epithelial regeneration restores normal epithelial composition and structure (as well as the spatially restricted expression of olfactory receptor genes by OSNs) because basal cells can be selectively spared by careful titration of the dose and duration of exposure (Schwob et al., 1995; Iwema et al., 2004). As a result of the abrupt termination of toxin exposure and the synchronization of repair, the passage of time after the injury correlates closely with progressive stages in the hierarchy of progenitors responsible for regenerating the differentiated cells of the epithelium. Thus, analysis of transcription factor expression during the immediate post-MeBr period recapitulates the sequence of markers observed during the early embryonic development of the OE (Fig. 4; Manglapus et al., 2004). In addition, that the damage is limited to the exposure period permits more rapid clearance of debris, which in turn has two further implications. First, spared stem and progenitor cells in the lesioned OE become exposed to the nasal cavity, which permits their easy transduction with viral vectors delivered via intranasal irrigation; the vector can be used to deliver a heritable lineage tracer alone or in combination with exogenous genes designed to alter the behavior of the cells (Schwob et al., 1994a; Huard et al., 1998; Peluso et al., 2012; Schnittke et al., 2015). Second, the MeBr-lesioned epithelium is a receptive substrate for the engraftment of cell suspensions following intranasal infusion, whether the cells are dissociated from the OE immediately before transplantation or maintained and manipulated in tissue culture for a period of time (Goldstein et al., 1998; Chen et al., 2004; Jang et al., 2008; Krolewski et al., 2011).
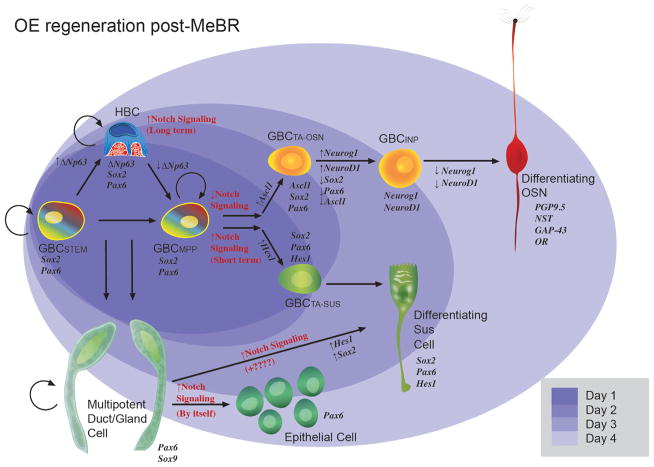
Figure 4.
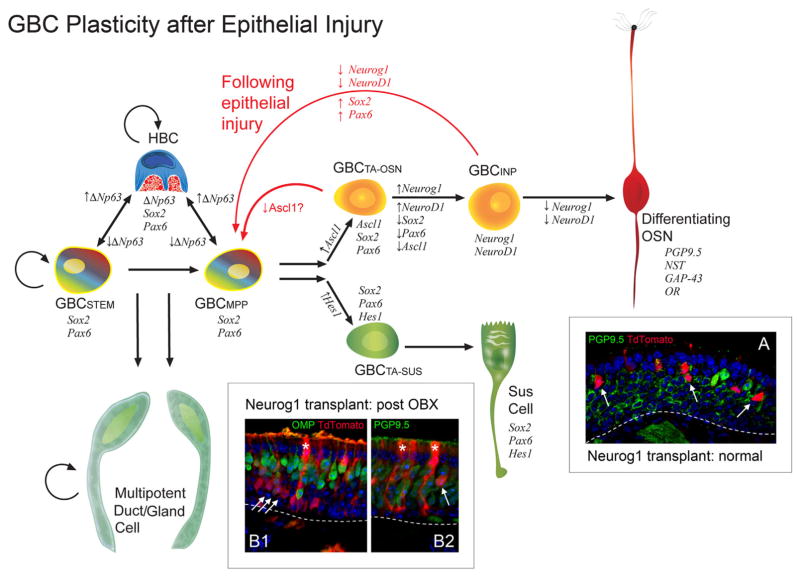
Diagram illustrating the process of epitheliopoiesis in the OE during recovery from methyl bromide (MeBr) injury, which is substantially more complex than the progression observed in the normal OE (cf. Fig. 3). The cellular lineages to which basal cells give rise and the timing of their re-emergence after damage (shaded ovals in the background) are indicated. The rainbow gradient filling the GBCSTEM and GBCMPP symbolizes their active multipotency, i.e., those GBCs that generate, in aggregate, all of the epithelial cell types following epithelial injury, including HBCs. The self-renewal capacity of the GBCSTEM is indicated by the circular arrow; self renewal is strongly suggested by the persistence of neurogenesis when the contribution of HBCs has been eliminated (see Fig. 5 and text for details). Thus, some GBCs satisfy both the multipotency and self-renewal criteria for being classified as stem cells. The retention of thymidine label by some GBCs also suggests stem-like quiescence. As an example of GBC multipotency, 1 day after MeBr (darkest purple oval), the epithelium consists of HBCs and GBCs, characterized by the indicated transcription factor profiles, including the selective HBC marker p63. Some among the GBCs express Hes1, which marks the GBCs that are transitioning directly into Sus cells. Notch signaling is key to the determination of neuronal vs. nonneuronal differentiation (Herrick and Schwob, 2015). Also indicated is the dual origin of the Sus cells from GBCs and from gland/duct cells, which has been demonstrated by retroviral lineage tracing and transplantation experiments, as summarized in the text (Huard et al., 1998). GBCSTEM, stem cell-like GBC expressing Sox2 and Pax6 that is mitotically quiescent (thymidine label-retaining); GBCMPP, multipotent GBC expressing Sox2 and Pax6 and appearing first during development and regeneration; GBCTA-OSN, transit-amplifying GBC restricted to the neuronal lineage and expressing Sox2, Pax6, and Ascl1; GBCINP, GBC functioning as an immediate neuronal precursor, i.e., giving rise to a small number of OSNs, and expressing Neurog1 and NeuroD1.
When progenitor–progeny relationships are investigated in OE that has been directly injured by exposure to MeBr, clones of cells that originate from a single transduced progenitor are substantially more complex than cells in the exclusively neurogenic epithelium of normal and bulbectomized animals. In aggregate, the progeny derived from the spared cells includes OSNs, Sus cells, MV cells, HBCs, GBCs, and D/G units, and individual clones very often contain multiple cell types (Schwob et al., 1994a; Huard et al., 1998). The clones fall into two general categories, those composed of neurons, MV cells, Sus cells, and basal cells in varying numbers and proportions, and others composed of D/G cells and Sus cells (Huard et al., 1998). At the time of retroviral infection, most residual, mitotically active cells are GBCs; they express GBC markers and not HBC or Bowman’s duct markers (Schwob et al., 1995; Huard et al., 1998). However, substantial numbers of duct cells at the level of the basal lamina are also spared, proliferating, and susceptible to transduction (Schwob et al., 1995). The most likely explanation for the lineage-tracing results posits that the first type of clone derives from spared GBCs, which is also supported by the study of GBC marker expression in the lesioned–recovering OE (Goldstein and Schwob, 1996; Jang et al., 2007). These lineage-tracing data strongly suggest that single GBCs are broadly potent and certainly able to give rise to many more cell types than neurons (Fig. 4). The differentiation of GBCs into Sus cells in the lesioned OE is also demonstrated by observing the transition from one into the other on the basis of marker expression. In this instance, some GBCs are labeled by the expression of Hes1 within 1 day after MeBr injury. Hes 1 is a downstream mediator of canonical Notch signaling and is strongly expressed by Sus cells in the normal OE. Consequently, the stabilization of Hes1-expressing cells at the apical surface of the OE as the epithelium recovers from one or two cell layers thick to its full width is highly suggestive that the Hes1-expressing GBCs differentiate into Sus cells, demonstrating GBC multipotency (Manglapus et al., 2004). Clones of the other type (Sus plus D/G cells) likely come from remaining duct cells (Huard et al., 1998). The latter interpretation is also congruent with morphological indications that duct cells detach and begin to migrate tangentially along the surface of the epithelium to take up residence as Sus cells (Schwob et al., 1995).
One of the most powerful pieces of evidence for the multipotency of GBCs in the lesioned OE follows from transplanting GBCs from normal or bulbectomized epithelium (where selective neurogenesis is their usual fate) into the MeBr-lesioned OE (a necessary precondition for the engraftment of the transplanted GBCs) and determining the cells to which the engrafted GBCs give rise. The outcome of GBC engraftment can be compared with the results following the transplantation of HBCs and D/G cells. As a means of determining progenitor cell capacity, cell transplantation into the lesioned OE is directly analogous to the colony-forming unit (CFU) assay used by McCulloch and Till to unravel the “stem cell-to-progenitor cell-to-terminally differentiated blood cell” hierarchy in the hematopoietic system (Becker et al., 1963).
Two methods have been used to isolate specific types of donor cells from the OE. The first approach used selective GBC proliferation and a powerful molecular biological tactic for determining clonality. Rat epithelium was harvested at short survivals after olfactory bulb ablation, which has the effect of upregulating GBC proliferation and leaving the other cell types quiescent, as reviewed above. Before infusing the dissociated cells into the host nasal cavity, the cells were infected in suspension with a highly complex, bar-coded library of retroviruses encompassing approximately 105 molecularly distinct viral vectors; the complexity of the library can be used to prove that a cluster of engrafted cells is clonally derived by sequencing the recovered retroviral tag (Goldstein et al., 1998). Well in excess of 90% of the proliferating cells in the epithelium of bulbectomized rodents are GBCs and infectable, suggesting that the vast majority, if not all, of the clones that emerged following transplantation derive from GBCs (Schwartz Levey et al., 1991; Carr and Farbman, 1992). As observed with lineage tracing in situ after injury, the clones were composed of a mixture of cells ranging from Sus cell- or OSN-only clones to complex clones with OSNs, Sus cells, GBCs, and HBCs in various combinations and proportions (Goldstein et al., 1998).
The second approach uses fluorescence-activated cell sorting (FACS) on the basis of cell-surface markers to harvest subsets of cells, including GBCs and subtypes of GBCs from transgenic mice that express GFP, TdTomato, or β-galactosidase heritably, constitutively, and universally. Monoclonal antibody GBC-2 was used originally to isolate GBCs (along with immature neurons, which do not engraft) from the dissociated OE of uninjured transgenic mice, which were then infused intranasally (Chen et al., 2004). Clonality of the clusters of labeled cells in the host was demonstrated by infusing a mixture of cells from mice bearing different heritable markers and finding that clusters were usually homogeneous and composed of cells labeled with only one of the markers. Under these conditions, GBCs gave rise, in aggregate, to all of the cell types of the OE: OSNs, Sus cells, MV cells, D/G cells, GBCs, and HBCs (Chen et al., 2004). Columnar, ciliated, respiratory epithelial cells also arose within some clones, indicating that GBCs can differentiate into the vast majority, if not all, of the cell types that derive from the olfactory placode. Individual clones ranged in complexity from OSN-only and Sus cell-only to large, complex clones that include many different epithelial cell types. Moreover, FACS-purified GBCs that were retrovirally transduced ex vivo during the preparation for intranasal infusion also gave rise to a similar breadth of cell types. Only those cells that are in the mitotic cycle at tissue harvest are susceptible to retroviral infection. Thus, at least some of the GBCs that are fated to make neurons if left in situ must be capable of functioning as more broadly potent progenitors in the context of the injured epithelium without requiring any form of preconditioning. In contrast, HBCs isolated from the normal OE using an HBC-selective cell-surface marker did not engraft, and infusion of Sus and D/G cells, harvested using a monoclonal antibody that binds to both, gave rise only to themselves (Chen et al., 2004). These data demonstrate that at least some GBCs satisfy the tissue totipotency criterion required of stem cells (Fig. 4) but still do not address the question of unlimited self-renewal.
Additional data from mice in which the Ascl1 gene (previously known as Mash1) no longer encodes a functional protein also suggest the multipotency of GBCs (Murray et al., 2003). In this setting, Sus cells express the truncated Ascl1 gene, implying that at least some Sus cells derive from an upstream progenitor that is also capable of making OSNs. When OSNs are not generated because of the knockout of Ascl1, that common progenitor is compelled to make Sus cells even though the signals responsible for accelerating neurogenesis continue to drive expression of the nonfunctional Ascl1. In keeping with this interpretation, the population of Sox2/Pax6-expressing GBCs is expanded as a consequence of Ascl1 knockout (Krolewski et al., 2012). The expansion of the Sox2/Pax6-expressing GBCs suggests that they are “upstream,” multipotent cells giving rise to both OSNs and Sus cells, and the increase in the population suggests that the OE is being “pushed” in the attempt to make neurons. These results do not rule out the possibility that the Ascl1-expressing GBCs are themselves multipotent. However, the consequences of genetic mutation along with their kinetic behavior suggests that Ascl1-expressing GBCs are transit-amplifying progenitors (Gordon et al., 1995) that are capable of a limited degree of expansion by symmetric proliferation but fated to make neurons.
Transplantation provides a highly faithful recapitulation of regeneration. Neurons that are generated by the engrafted stem/progenitor cells, which now find themselves in a novel region of the epithelium, extend axons to the region of the bulb to which their new neighbors project (Chen et al., 2004). Once there, these transplant-derived axons innervate glomeruli (Hewitt et al., 2015). Moreover, the transplant-derived neurons adopt the identity of their new neighbors, for the most part, with respect to two spatially regulated features of neuronal differentiation: first, the expression of the dorsomedial marker NQO1 vs. the ventrolateral marker OCAM/mamFasII, and, second, the identity of the olfactory receptor that they express (i.e., typical of dorsomedial vs. ventrolateral OE; Hewitt et al., 2015). As noted above, MeBr lesioned-recovered OE is equivalent to normal OE with respect to its spatial organization (Iwema et al., 2004).
All of the accumulated lines of evidence indicate that at least some among the GBCs are manifestly multipotent, even to the extent of apparently matching the differentiative ability of the cells of the olfactory placode (Fig. 4). Which among the several types of GBCs are likely to have that capacity, and further consideration of whether they satisfy the self-renewal requirement to be classified as stem cells, will be deferred to a later section.
HBCs AS RESERVE STEM CELLS OF THE OE
HBCs have been described as stem cells of the adult OE (Graziadei and Monti Graziadei, 1979; Carter et al., 2004); some authors have nominated them as “the” stem cell of the epithelium on limited evidence (Duggan and Ngai, 2007). Until the advent of genetic lineage tracing, the HBC-as-stem-cell hypothesis had been based on morphological observations following epithelial injury and on results in tissue culture (Mulvaney and Heist, 1971; Carter et al., 2004). In particular, it was reported that a preparation of HBCs from the neonatal epithelium makes a variety of cell types, including neurons in vitro (Carter et al., 2004), although the starting material for these cultures fell well short of the purity achieved with FACS or the selectivity associated with genetic forms of lineage tracing, raising questions about the actual cell type that generated the neurons in vitro. Similar results were reported for cultures of an HBC-like, keratin-expressing, immortalized cell line (Satoh and Yoshida, 2000).
As noted above, HBCs in the normal or bulbectomized adult OE are mitotically quiescent and are ineffective as progenitors in the CFU assay that tests incorporation and cell generation following transplantation (Schwartz Levey et al., 1991; Huard and Schwob, 1995; Chen et al., 2004; Schnittke et al., 2015). Moreover, the HBCs are late to emerge during embryonic development of the OE, long after the other cell types are well established (Holbrook et al., 1995; Packard et al., 2011b), which would be unusual for a cell that is serving as “the” stem cell of an epithelium. In other tissues, K5/K14-expressing basal cells are often the first cell type of the definitive epithelium to emerge, and among that population are cells functioning as stem cells for the tissue (Alonso and Fuchs, 2003). In the case of the OE, HBCs are scattered and scant until late in the first postnatal week in rodents (Holbrook et al., 1995; Packard et al., 2011b). Finally, the HBCs are dispensable for the initial formation of the OE as shown by genetic manipulation of the gene encoding the transcription factor p63, a member of the p53 gene family. The p63 gene encodes two major alternatively spliced classes of transcripts; one class retains an N-terminal transactivating domain (Tap63), and in the other that domain is spliced out and replaced by an alternate exon (ΔNp63). Each group has five additional isoforms with differing lengths of the C-terminal domain designated α, β, δ, γ, ε in order of decreasing length. The ΔNp63α protein isoform is the dominant isoform in the OE (the others are barely detectable), which is typical of most epithelia in which p63 is expressed, and is found at high levels in HBCs and no other olfactory cell type (Packard et al., 2011b). An intact p63 gene is absolutely required for the formation of HBCs during epithelial development as shown by their absence from the OE of homozygous p63-null mutant mice (Fig. 5; Packard et al., 2011b). Moreover, in the context of a different mutation of p63, in which GFP is knocked into the ΔNp63 isoform-specific exon, the cells that express GFP from the mutated p63 locus are diverted to become Sus and D/G cells as shown by the perdurance of GFP labeling in these cell types (Packard et al., 2011b). Despite the absence of HBCs, the epithelium is otherwise normal in appearance, with a robust and normally constituted population of OSNs, Sus cells, and D/G cells (Packard et al., 2011b).
Figure 5.
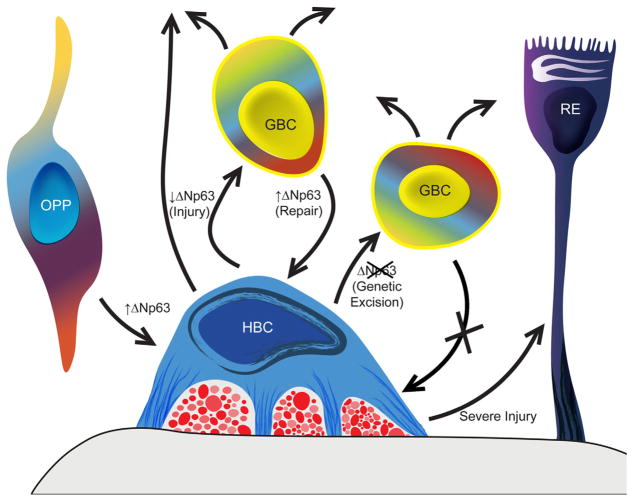
p63 Is the master regulator of the formation and activation–dormancy of the HBCs, the second category of OE stem cells, which are held in reserve normally. HBCs are formed from basally migrating olfactory placodal precursors (OPP) when ΔNp63 is upregulated during perinatal development (Packard et al., 2011b). In the absence of direct epithelial damage, HBCs are dormant (mitotically quiescent and nonparticipatory in the generation of replacement neurons; Huard and Schwob, 1995; Leung et al., 2007). With injury, ΔNp63 levels decline, and many of the HBCs differentiate into GBCs, which in turn give rise to neurons and other epithelial cell types (Packard et al., 2011b; Fletcher et al., 2011; Schnittke et al., 2015). The suggestion that HBCs may directly give rise to other cell types following injury is indicated by the upward-pointing arrow (Schwob et al., 1995). During epithelial repair, GBCs that initiate ΔNp63 expression in response to local cues or as a consequence of retroviral transduction give rise to HBCs, which return to quiescence (Schnittke et al., 2015). Genetic excision of ΔNp63 activates HBCs, generates GBCs that remain active for more than 6 months indicating stem cell capacity, and simultaneously prevents the regeneration of HBCs from GBCs bearing the mutated gene (Schnittke et al., 2015). Thus, expression of ΔNp63 is both necessary and sufficient for the formation of HBCs, and conversely downregulation of ΔNp63 is both necessary and sufficient for HBCs to activate. As a result of very severe injury, in which the population of GBCs and possibly gland/duct cells is completely depleted, the activated HBCs contribute to respiratory metaplasia, i.e., give rise to ciliated columnar cells like those found in respiratory epithelium (RE; red profiles between HBC and basal lamina represent olfactory axons; Xie et al., 2013).
However, in the MeBr-lesioned OE of both mice and rats, HBCs do begin to proliferate and accumulate as multiple layers of K5/K14+ cells that eventually regress to a single layer (Schwob et al., 1995; Chen et al., 2004; Packard et al., 2011b). Lineage tracing using the promoter(K5)-CreERT2 transgene and tamoxifen administration drives and limits heritable expression of β-galactosidase or some other marker protein to HBCs and their progeny. In the normal or bulbectomized epithelium, the overwhelming majority of cells labeled following survivals of days, weeks, or months are HBCs, indicating that they are truly dormant when only OSNs are being replaced (Leung et al., 2007; Fletcher et al., 2011; Schnittke et al., 2015). However, if the epithelium is lesioned by MeBr inhalation or methimazole injection, HBCs give rise to a panoply of progeny that includes all of the cell types to which GBCs give rise, including OSNs, Sus cells, D/G cells, and even ciliated respiratory epithelial cells (Leung et al., 2007; Fig. 5). A large percentage of HBCs do not appear to be activated fully despite epithelial lesion, because substantial swathes of the OE contain marked HBCs by themselves, suggesting that spared GBCs may suppress the activation of the HBCs there (Leung et al., 2007). (A proviso should be offered: as a consequence of unregulated activity [in other words, leakiness] of a mutant Cre–progesterone receptor fusion recombinase in HBCs from about birth onward, HBCs are known to make some contribution to other cell types in the developing undamaged OE, as opposed to the mature OE [Iwai et al., 2008]. The enhanced formation of non-HBCs in this mutant line may reflect a limited period of enhanced HBC progenitor activity and HBC plasticity in the developing OE.)
These data indicate that HBCs are roused from their dormancy when the OE is injured directly by toxin. Their activation is accompanied by a more-or-less universal downregulation of ΔNp63 expression by HBCs; protein levels fall first, followed slightly later by a decrease in p63 mRNA, suggesting that there is active proteolytic digestion of ΔNp63 following injury (Packard et al., 2011b; Schnittke et al., 2015). An early morphological correlate of that downregulation is the change in shape of the HBCs from spiky to smooth as their processes retract (Schnittke et al., 2015; Fig. 6). After injury-induced downregulation of p63, some of the HBCs differentiate into GBCs and give rise to the array of normal epithelial cell types (Leung et al., 2007; Fletcher et al., 2011; Schnittke et al., 2015). In activated HBCs, ΔNp63 levels are restored within 1 or 2 days in those K5/K14-expressing cells that are immediately adjacent to the basal lamina (Packard et al., 2011b). Indeed, direct manipulation of p63 expression demonstrates that the downregulation of p63 is both necessary and sufficient to achieve activation of HBCs. As a demonstration of sufficiency, excision of p63 by tamoxifen-dependent, K5-driven recombination activates HBCs to make OSNs and MV cells within the otherwise normal OE (Fig. 4; Fletcher et al., 2011; Schnittke et al., 2015). Recombined, p63-excised HBCs are broadly multipotent when the animal is subject to epithelial injury after tamoxifen injection or when the p63− HBCs are transplanted from uninjured mice into the OE of an MeBr-lesioned host (Schnittke et al., 2015). As a demonstration of necessity, overexpression of ΔNp63 by retroviral infection at 1 day post-MeBr lesion prevents activation as the transduced cells remain or become HBCs, with only a few escaping quiescence to make very limited numbers of non-HBCs (Schnittke et al., 2015). In sum, p63 apparently functions as the master switch whose level controls the transition of HBCs between activation and dormancy.
Figure 6.
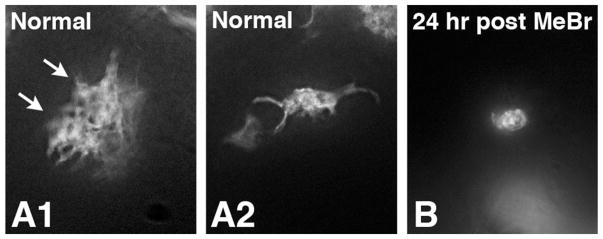
TdTomato labeling of HBCs in normal OE (A1,2) or in OE harvested 24 hours after MeBr lesion (B) from mice in which the K5-CreERT2 transgene excises a floxed(stop) motif in the ROSA26 locus in response to tamoxifen, thereby driving expression of TdTomato exclusively in HBCs. The shape of the marked HBCs is illustrated in whole mounts of the OE subjected to processing using the CLARITY technique (for details see Schnittke et al., 2015). Note the retraction of processes following lesion. The arrows in A1 indicate two adjacent HBCs.
Given that HBCs downregulate ΔNp63 in the lesioned OE and become multipotent when they remain in situ following epithelial injury, why then are HBCs harvested from the normal OE largely incapable of engrafting, activating, and differentiating when transplanted into the lesioned epithelium? In this limitation on their potency, HBCs are unlike GBCs, which immediately begin to function as multipotent progenitors when they encounter the injured OE (Chen et al., 2004). The discrepant outcomes suggest that HBCs must experience the lesioned environment in situ before they are isolated from the donor and transplanted if they are to become activated and multipotent in the damaged epithelial environment of the host (Schnittke et al., 2015). As proof, HBCs marked by the heritable expression of the fluorescent protein TdTomato underwent transplantation 18 hours after the completion of MeBr exposure, at which time ΔNp63 protein levels are at their nadir (Schnittke et al., 2015). Unlike HBCs from normal OE, the HBCs that experienced the epithelial injury while in situ were capable of robust engraftment and of functioning as multipotent progenitors in the lesioned host environment following transplantation. These data suggest that the absence vs. presence of a time-limited cue in the OE of normal vs. toxin-treated mice, respectively, is somehow responsible for the difference between the outcomes.
Some progress has been made toward identifying the signals that regulate ΔNp63 expression. Selective death of Sus and D/G cells, accomplished by Sus cell-limited expression of the A subunit of diphtheria toxin, is sufficient to cause activation of HBCs to multipotency (Fig. 7; Herrick and Schwob, 2015). Gene expression analysis during the initial response to MeBr exposure implicates Notch signaling as a regulator of ΔNp63 levels in OE (Herrick and Schwob, 2015). Notch1 and Notch2 are expressed by HBCs, as is Hes1, the usual downstream transcription factor mediator of Notch action (Herrick and Schwob, 2015). Indeed, genetic excision of Notch1 enhances the very low rate of spontaneous HBC activation in the uninjured OE and causes marked levels of activation after bulbectomy as compared with intact Notch1 (Herrick and Schwob, 2015). Notch ligands are expressed by both HBCs (Delta-like1 and Jagged1) and Sus cells (Jagged1), which may explain the activating effect of Sus cell death and Notch1 knockout on HBCs (Fig. 7; Herrick and Schwob, 2015). Notch signaling has also been linked to regulation of p63 in multiple other tissues (Tadeu and Horsley, 2013; Mori et al., 2015; Yoh and Prywes, 2015).
Figure 7.
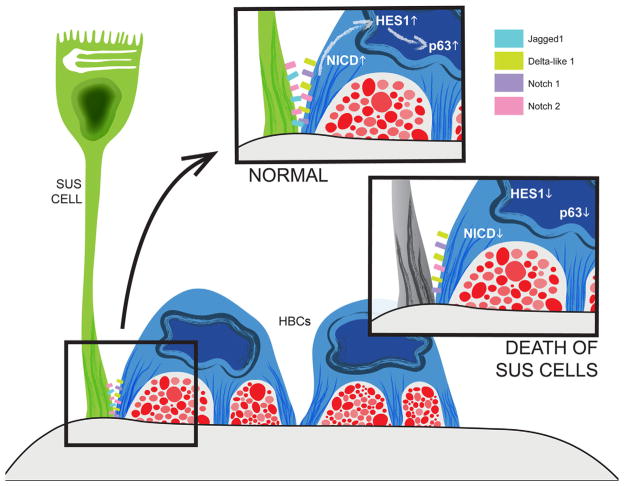
Death of Sus cells (as a result of the Cyp2G1-driven expression of diphtheria toxin A) causes downregulation of ΔNp63 in HBCs and, thereby, HBC proliferation and activation to multipotency (Herrick and Schwob, 2015). The effect on ΔNp63 is mediated, at least in part, by changes in Notch signaling. HBCs express both Notch1 and Notch2 receptors as well as the Delta-like1 ligand, whereas Sus cells express the Jagged1 ligand as well as the Notch2 receptor. Constitutive activation of Notch signaling by overexpression of the Notch1 intracellular domain selectively in HBCs elevates the downstream factor Hes1 as well as the levels of ΔNp63 expression. Conversely, excision of Notch1 from HBCs decreases their expression of ΔNp63 and enhances the rate of HBC spontaneous activation (Herrick and Schwob, 2015).
Downstream of activation, HBCs are susceptible to tissue-derived signals as shown by tracing their progeny following dichlobenil lesion of the OE (Xie et al., 2013). Damage caused by dichlobenil is so severe in the dorsomedial OE that all GBCs are destroyed, and the epithelium undergoes respiratory metaplasia while attempting to repair the damage (Genter et al., 1996; Bergman et al., 2002; Xie et al., 2013). (The ventrolateral OE is much less severely affected by dichlobenil intoxication, and substantial neuron sparing is seen there [Xie et al., 2013]). In the context of the devastation of the dorsal OE caused by dichlobenil, spared HBCs give rise to ciliated columnar epithelial cells (Fig. 5; Xie et al., 2013). Thus, HBCs by themselves are incapable of restoring neurogenesis. Interestingly, intravenous infusion of human adipose stem cells apparently has the potential to restore some degree of neurogenesis to dichlobenil-desolated dorsal OE, despite the severity of the initial injury (Franceschini et al., 2014). The nature of the interaction between the adipose stem cells and the residual HBCs may be a fruitful area for future investigation. For example, HBCs elaborate primary cilia, and disruption of the cilia by genetic recombination and knockout of Ift88 impairs recovery after epithelial injury (Joiner et al., 2015). Primary cilia on other cells in other tissues are known to be critical for signal transduction and may be serving that function in the OE as well (Irigoin and Badano, 2011).
GBCs AS ACTIVE STEM CELLS OF THE OE
The data described above suggest that both HBCs and GBCs are capable of functioning as neurocompetent, broadly multipotent progenitors. HBCs can be classified as a kind of reserve stem cell, but they are not intrinsically compelled to make neurons, whereas GBCs are a more active progenitor population responsible for day-to-day replacement of neurons as well as reconstituting both neuronal and nonneuronal populations of the epithelium following injury. Are GBCs tissue stem cells; i.e., do they have a capacity for substantial or infinite self-renewal? The question of self-renewal has not been easy to answer directly for either GBCs or HBCs. In some tissues, such as the bone marrow, serial transplantation or long-term repopulation provides an in vivo assessment of stem cell self-renewal (Spangrude, 1991). Serial transplantation is not a feasible strategy in the OE. For other tissues, including the neural stem cells of the CNS, the capacity for self-renewal has been assayed in vitro, using serial passaging of neurospheres as an operational definition for stem cell self-renewal (Reynolds and Weiss, 1996). That strategy, too, is not currently applicable to the OE because protocols for culturing GBCs and HBCs are still in their infancy.
However, if HBCs are activated via the excision of p63, the capacity of GBCs can be assayed in isolation by tracing the self-renewal of HBC-derived GBCs in vivo. Because p63 is absolutely required both to form HBCs and to hold them as quiescent and noncontributory during epithelial reconstitution, GBCs that are created following elimination of p63 cannot regenerate HBCs (Packard et al., 2011b; Schnittke et al., 2015). Thus, the time at which the GBCs were generated can be pinpointed by the tamoxifen-dependent excision of p63 (Schnittke et al., 2015). The multipotency of these GBCs, summarized above (Fletcher et al., 2011; Schnittke et al., 2015), satisfies one of the criteria for stemness. In addition, the GBCs continue to generate neurons for periods of time exceeding 6 months, which satisfies the other stem cell requirement for self-renewal. Assaying neurogenesis long after ΔNp63 excision and unilateral bulbectomy offers proof of prolonged neurogenesis because neurons found within the OE following bulb ablation are necessarily born 2 weeks or less before tissue harvest; their abbreviated life span is a consequence of removing the trophic support that normally supports long-term neuronal survival (Schwob et al., 1992). Moreover, on the unoperated side, labeled neurons reached the bulb and entered the glomerular layer, indicating that the HBC-derived GBCs generated neurons that were capable of full differentiation (Schnittke et al., 2015).
A subsidiary feature usually associated with self-renewing stem cells is extended mitotic quiescence, as demonstrated by the retention of thymidine label (Cotsarelis et al., 1989; Potten, 2004; Takeda et al., 2011; Tian et al., 2011). Some within the GBC population satisfy that criterion, since EM-verified, EdU- or BrdU-labeled GBCs are present for at least 1 month after analogue injection (Jang et al., 2014). Label-retaining GBCs resume mitosis immediately after MeBr lesion but then return to quiescence shortly after the early phase of epithelial reconstitution (Jang et al., 2014). Likewise, GBCs that have remained quiescent for at least multiple days are present in mice among the GBCs that emerge following the excision of p63 (Schnittke et al., 2015).
WHICH AMONG THE HETEROGENEOUS POPULATION OF GBCs ARE STEM CELLS?
On the basis of the timing of their emergence during early development and during the initial stages in the recovery of the OE following MeBr lesion, GBCs that express Sox2 and Pax6 but not the neurogenic basic helix-loop-helix transcription factors (Ascl1, Neurog1, and NeuroD1) are multipotent and thus are stem cell candidates (Fig. 3; Cau et al., 1997; Manglapus et al., 2004; Chen et al., 2009; Guo et al., 2010). A marker (or set of markers) that is unique to the stem cell population has not yet been conclusively identified. Lgr5 is one candidate, because Lgr5-expressing GBCs are able to give rise to multiple cell types when the OE is directly lesioned (Chen et al., 2014). The pattern of Lgr5 expression in the OE, the cell types to which Lgr5+ GBCs give rise after epithelial damage, and the functions that Lgr5 and Wnt signaling (to which Lgr5 contributes as an R-spondin receptor) have in active stem cell populations elsewhere are all consistent with that role (Sancho et al., 2004; Wang et al., 2011; Chen et al., 2014).
However, this implicitly conceptualizes the relationship of stem cell–progenitor cell-differentiated progeny within the OE as a unidirectional hierarchy, with a stem cell at its apex (Figs. 3 and 4). This may be a gross oversimplification arising from an overly broad generalization of the unidirectional cellular flow that is true of hematopoiesis (Spangrude, 1991). Perhaps the most striking general example of plasticity among progenitors is the reprogramming of somatic cells to induced pluripotent stem cells, notwithstanding that the conditions under which pluripotency is elicited in vitro are artificial and operate with very low efficiency (Takahashi and Yamanaka, 2006). Recently, in vivo examples of reprogramming or dedifferentiation or transdifferentiation (the descriptor used depending on one’s preferred terminology and the extent to which intermediate steps in the transition between the initial and subsequent cell states have been documented) have been identified in several tissues: liver (Michalopoulos, 2011), pancreas (Jurczyk et al., 2014), and small intestine (Sipos and Muzes, 2015), among others. Indeed, it is possible that the apparently strict unidirectionality of the hierarchy observed during hematopoiesis may be the exception to the fluidity of the flow from progenitor to progeny and back again rather than the rule.
In the OE, as well, it appears that marker-defined types of GBCs that are thought to be “downstream” display substantially more flexibility with respect to progenitor capacity than might be expected on the basis of the consequences observed following genetic mutation (Lin et al., 2015). For example, Neurog1-CreERT2 and Ascl1-CreERT2 strains have been used to demonstrate that differentiative capacity is broader following injury compared with the normal OE.
In the normal OE in situ, both Neurog1+ and Ascl1+ GBCs give rise almost exclusively to OSNs, and, in both cases, their progeny are promoted fully out of the GBC compartment within a few days following tamoxifen injection (Lin et al., 2015). Interestingly, rare duct/gland cells are observed downstream of Ascl1, even in the absence of injury. However, as noted repeatedly here, the true test of differentiative potency requires that the progenitors be challenged by an environment in which multiple cell types have been depleted, for example, following toxic damage to the epithelium.
In the case of the Neurog1-expressing progenitors, OSNs constitute the vast majority of the progeny emerging after transplantation from the normal OE into the MeBr-lesioned OE (Fig. 8). However, a much different result is obtained if the Neurog1+ GBCs are isolated following either the administration of methimazole, with its killing of multiple cell types within the OE, or the ablation of the olfactory bulb, with the selective neuronal death that it produces (Fig. 8; Lin et al., 2015). With either form of lesion, Neurog1-expressing GBCs generate Sus cells, MV cells, and other nonneuronal cells following transplantation and to a greater extent following methimazole lesion than after bulbectomy. Similarly, when tamoxifen is administered immediately before methimazole and the outcome is assessed in situ, the progeny include multiple types of nonneuronal cells. Thus, some aspect of the lesioned environment, which is not yet known, causes a broadening of the progenitor capacity of this cell type. Candidate mediators for the assumption of multipotency have been identified. For example, immediately after olfactory bulbectomy, GFP+ GBCs from Neurog1-GFP BAC transgenic mice or Neurog1-CreERT2 lineage-traced cells begin to express Sox2 and Pax6, which they lack in the normal adult OE (Fig. 3 and 4), and genetic excision of Sox2 prevents multipotency after lesion (Fig. 8; Lin et al., 2015). As noted above, Sox2- and Pax6-expression is characteristic of the multipotent GBCs of the MeBr-lesioned and of the embryonic OE, indicating that the downstream GBCs are assuming the molecular phenotype of more upstream and multipotent progenitors.
Figure 8.
The hierarchy of stem cell progressing to progenitor cell among the GBC population can be reversed following direct injury to the OE or depletion of the OSN population by massive retrograde degeneration. In the normal OE, Neurog1+ GBCs, whose progeny are labeled genetically and permanently by the expression of CreERT2, give rise only to OSNs in situ and when they engraft in the MeBr-lesioned host (A, normal) and are considered to be immediate neuronal progenitors (GBCINP); arrows indicate PGP9.5+/TdTomato+ OSNs derived from the transplanted Neurog1-CreERT2-expressing progenitors. In contrast, following ablation of the olfactory bulb (OBX) or methimazole-induced damage to the OE, Neurog1+ GBCs initiate the expression of Sox2 and Pax6, which are expressed by more upstream GBCs and evince multipotency either in situ or after transplantation (B1,2; post-OBX). B1,2: Asterisks mark Sus cells derived from the Neurog1+ progenitors, whereas arrows indicate OSNs identified on the basis of immunostaining or morphology. Likewise, Ascl1+ GBCs, which precede Neurog1-expressing GBCs during embryonic development or recovery from MeBr lesion, express Sox2 and Pax6 and are considered to be GBCTA-OSN, are also multipotent in situ following methimazole lesion and to a greater extent than the Neurog1+ GBCs (as indicated by the reverse arrows). Thus, the stem cell–progenitor cell “hierarchy” within the OE is not strictly unidirectional and is much more fluid than previously imagined (Lin et al., 2015).
Ascl1+, putative transit-amplifying GBCs, which are immediately upstream of Neurog1+ GBCs, also evince an enhanced plasticity following epithelial injury and to a greater extent than is true of the Neurog1 expressors. Recombination 1 day before methimazole administration demonstrates that Ascl1-expressing GBCs robustly give rise to nonneuronal cells as well as OSNs following direct lesion and to a more limited extent after bulbectomy (Fig. 8; Lin et al., 2015). Ascl1 is normally coexpressed with Sox2, consistent with a role for the transcription factor in the broadening of progenitor capacity.
These observations demonstrate that multipotency is a fluid property but do not address the self-renewal criterion needed for classifying the newly multipotent GBCs as stem cells. For the question of self-renewal, we find that the duration over which the newly multipotent progenitors remain active is prolonged when the epithelium is damaged. With methimazole lesion, Ascl1-derived GBCs persist in substantial numbers for more than 1 month, which is not true of the normal OE, where they undergo terminal mitoses and neuronal differentiation within 1 week after being marked by recombination (Lin et al., 2015). Thus, the data are consistent with the hypothesis that the environment of the lesioned epithelium drives Ascl1+ GBCs to become stem cells, but we do not have sufficient lengths of survival to conclude that definitively yet. The multipotent Neurog1-derived progenitors do not exhibit the same degree of persistence; they are sparse when sought more than 1 week after lesion (Lin et al., 2015). On that basis, Neurog1+ GBCs, though multipotent, lack the self-renewing capabilities of true stem cells. In sum, downstream progenitor cells of the OE are not completely and equivalently plastic in response to epithelial damage but rather are biased by their previous cell state.
IMPLICATIONS FOR HUMAN OLFACTORY DYSFUNCTION
Despite the ostensibly lifelong capacity for neurogenesis, olfactory dysfunction in humans is a frequent consequence of head trauma, upper respiratory infection by viruses or bacteria, or toxin exposure and, perhaps most frequently, with advancing age (Doty, 1979, 1989, 1997; Murphy et al., 2002; Schubert et al., 2009). The deterioration of the OE among the elderly and other kinds of patients may be a result, at least in part, of dysfunction or destruction of the GBC/active stem cell population and inappropriate quiescence/loss of neuro-competency of the HBC/reserve stem cell population. To an increasing extent as we age, two forms of pathology emerge in the human OE. First, OSNs and GBCs disappear from the epithelium, but Sus cells and dormant HBCs remain (Douek et al., 1975; Holbrook et al., 2005, 2011). OE with this appearance is classified as aneuronal and nonneurogenic, or neurogenically exhausted. Second, OE can be replaced by metaplastic respiratory epithelium (Nakashima et al., 1984, 1985a,b; Holbrook et al., 2005, 2011). As noted above when discussing the context dependence of the neuro-competency of HBCs, respiratory metaplasia not uncommonly follows severe toxin-mediated epithelial injury, and ciliated columnar epithelial cells can arise from spared HBCs (Schwob et al., 1994b; Jang et al., 2003; Xie et al., 2013).
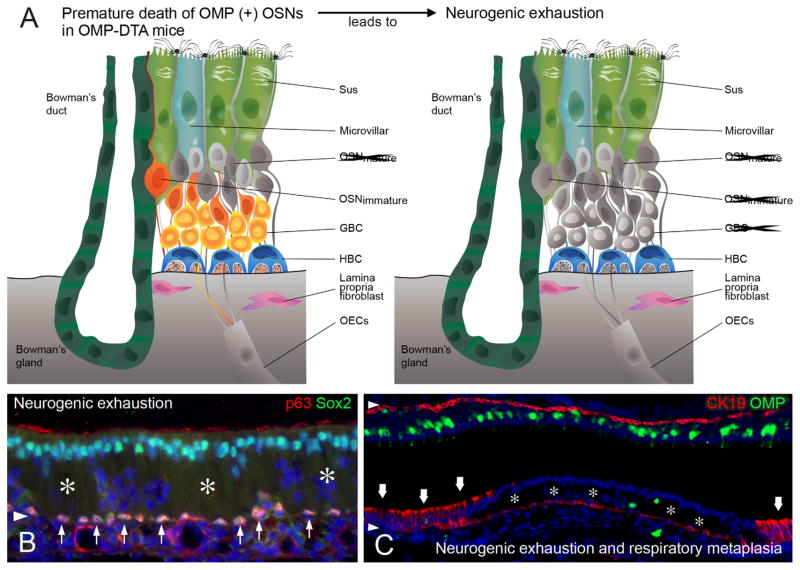
With respect to neurogenic exhaustion, accelerated turnover of OSNs in rodents, for example, following OB ablation, can result in an OE that closely resembles the pathological change noted in aged humans, a neurogenically exhausted epithelium that lacks all OSNs and GBCs (Largent et al., 1993; Kondo et al., 2009, 2010; Suzukawa et al., 2011). Thus, the results suggest that the GBC population may experience “burn out.” However, there is a substantial lag between the insult and the exhaustion when using bulbectomy as the experimental manipulation. A more aggressive form of accelerated turnover accompanies the transgenic expression of subunit A of diptheria toxin (DTA) in mature olfactory neurons, accomplished by using a Tet-off driver from the OMP locus to activate expression of TetO-DTA (Fig. 9; Jang et al., 2015). In this case, the depletion of OMP neurons begins as soon as they begin to appear perinatally. Within a few months of age, substantial swathes of the epithelium lack neurons and GBCs, just as in aneuronal human OE (Fig. 9; Jang et al., 2015). Surprisingly, areas of the OMP-DTA epithelium also undergo metaplasia and become respiratory-like in character, even though the OE is not lesioned directly by an exogenous agent. In these areas an inflammatory infiltrate is prominent and may be responsible for the onset of metaplasia.
Figure 9.
The depletion and disappearance of OSNs and GBCs that is characteristic of the OE in the elderly also emerges in a mouse model of accelerated aging. A: In that model, the premature death of OSNs follows the expression of diphtheria toxin subunit A (DTA) driven by the expression of OMP as the OSNs reach maturity. The dead neurons are “grayed out” in the schematic. Over time, the reduction in the neuronal population progresses to neurogenic exhaustion, in which all neurons and GBCs have disappeared (gray), whereas the HBCs remain dormant. B: Areas of neurogenic exhaustion (asterisks) are evident by the absence of Sox2+ GBCs and the gaps in the neuronal layer as shown by DAPI staining. The dormant HBCs remain strongly positive for p63 and Sox2 expression (thin arrows). C: Areas of neurogenic exhaustion (asterisks) can progress to respiratory metaplasia, where the epithelium consists of CK19+ columnar respiratory epithelial cells (thick arrows; Jang et al., 2015).
Potential therapeutic interventions are suggested by the histopathology. In particular, the persistence of dormant HBCs in the neurogenically exhausted OE suggests that reducing or eliminating ΔNp63 and the consequent activation of the HBCs might thereby regenerate GBCs and rejuvenate neurogenesis. The OMP-DTA model for aging offers the opportunity to test whether manipulating p63 to activate the dormant HBCs is potentially efficacious. Likewise, that some aspect of the adipose stem cell secretome might alleviate respiratory metaplasia after severe injury leads to potential manipulations designed to exploit that particular kind of signaling (Franceschini et al., 2014). However, rapid identification of the means and methods for targeting p63 and/or restoring neurogenic competency will depend on a more facile means of studying HBC biology, for example, in tissue culture. Although the field is in its relative infancy, both 3-D and adherent tissue culture models for the study of olfactory stem cells are beginning to emerge (Jang et al., 2008; Krolewski et al., 2011; Peterson and Schwob, 2015).
CONCLUSIONS, UNANSWERED QUESTIONS, AND FUTURE DIRECTIONS
The current state of our knowledge of the stem and progenitor cells of the mammalian OE can be encapsulated by saying that both HBCs and some among the molecularly heterogeneous population of GBCs seem to satisfy the criteria that are commonly used to identify tissue stem cells. With respect to the several molecularly and functionally defined GBC types, those GBCs that express Sox2 and Pax6 without Ascl1 are likely candidates for being broadly multipotent progenitor cells and that some among them are also capable of self-renewal and maintained potency over the long term, implying that they are true tissue stem cells. At present, we cannot conclude that the label-retaining GBCs, which are Sox2- and Pax6-expressing, are those stem cells, although they are strong candidates. In other GBCs, the expression of the neurogenic basic helix-loop-helix factors is closely correlated with a contraction in their potency with respect to cell types, making only neurons, and cell numbers to which they give rise in the context of the uninjured epithelium. However, a kind of “indeterminancy” is evident when probing the capacity of different types of GBCs that emerge in response to epithelial injury. The fluidity in the functional capacity of the different types of GBC progenitors is perhaps frustrating at an analytic level although potentially encouraging when contemplating epithelial repair. As a result, at present we cannot be more definitive in saying what features of the molecular phenotype make a GBC behave as a stem cell.
With respect to the HBCs, these basal cells also satisfy criteria for being tissue stem cells, although of a reserve type. Furthermore, HBCs are intractably dormant in the absence of direct injury to the OE that kills nonneuronal cells, specifically Sus cells, which is problematic when aging leads to neurogenic exhaustion. Moreover, HBCs are not sufficient by themselves to repair all forms of olfactory pathology, as witnessed by the respiratory metaplasia for which the HBCs are responsible after very severe injury.
Thus, even with all that has been learned we are left with a multitude of unanswered questions, several of which are particularly key to any therapeutic intervention, especially given the finding that the outcome of HBC activation and the plasticity in the GBC population depend on the status of the tissue environment. The key areas in need of additional investigation include 1) the need to identify precisely and characterize those GBCs of the OE that have the capacity to behave as stem cells (are they only the label-retaining GBCs, or are some mitotically active GBCs also stem cells, much like the Lgr5+ crypt base stem cells of the GI tract?); 2) the molecular regulation of stem cell activation and function; 3) the nature of the cues that direct activated stem and progenitor cells to alternative fates (for example, the generation of respiratory columnar epithelial cells by both GBCs and HBCs in what was OE); 4) how and why the GBC population establishes and maintains clustering across the epithelial sheet; 5) the molecular mechanism by which so-called downstream progenitors (expressing Ascl1, Neurog1, or NeuroD1) can paddle back upstream and assume multipotency and perhaps gain the capacity for self-renewal that would define them as stem cells; 6) in light of that plasticity, determining what is or is not a definitive stem cell in this tissue; and, 7) finally, the conundrum of how and why stem and progenitor cell capacities become exhausted and contribute to a decline in function, as seen particularly as the human ages. Although many of these types of questions bedevil our understanding of stem cells in any tissue, including the CNS, finding answers to them for the OE will likely benefit from many of the technical advantages attending study of this tissue: its large size, easy accessibility, suitable animal models for human pathophysiology, capacity for subtle and straightforward manipulation in vivo (and increasingly in vitro), and a nearly unparalleled aptitude for accepting transplanted cell types for purposes of assessing differentiative potency by means of colony-forming unit assays. Answers to these and other emerging questions will inform our ability to alleviate olfactory dysfunction and to exploit neurocompetent olfactory stem cells in both an olfactory and a nonolfactory setting.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant numbers: R01 DC002167 (to J.E.S.); Grant number: R01 DC014217 (to J.E.S.); Grant number: F31 DC014637 (to B.L.); Grant number: F30 DC013962 (to D.B.H.); Grant number: F31 DC014398 (to J.H.C.).
The authors thank the many past and present members of the Schwob laboratory for their innumerable and exceptional contributions to the study of olfactory stem and progenitor cells. All of them were bright, careful, precise, thoughtful, insightful, and forward-looking during their tenure in the laboratory, and their efforts are gratefully acknowledged.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest.
ROLE OF AUTHORS
All authors are responsible for defining the scope of the review and for writing it.
LITERATURE CITED
- Alonso L, Fuchs E. Stem cells of the skin epithelium. Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11830–11835. doi: 10.1073/pnas.1734203100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres KH. The fine structure of the olfactory region of macrosmatic animals. Z Zellforsch Mikrosk Anat. 1966;69:140–154. [PubMed] [Google Scholar]
- Asan E, Drenckhahn D. Immunocytochemical characterization of two types of microvillar cells in rodent olfactory epithelium. Histochem Cell Biol. 2005;123:157–168. doi: 10.1007/s00418-005-0759-4. [DOI] [PubMed] [Google Scholar]
- Becker AJ, McCulloch EA, Till JE. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature. 1963;197:452–454. doi: 10.1038/197452a0. [DOI] [PubMed] [Google Scholar]
- Bergman U, Ostergren A, Gustafson AL, Brittebo B. Differential effects of olfactory toxicants on olfactory regeneration. Arch Toxicol. 2002;76:104–112. doi: 10.1007/s00204-002-0321-2. [DOI] [PubMed] [Google Scholar]
- Brittebo EB. Metabolism-dependent toxicity of methimazole in the olfactory nasal mucosa. Pharmacol Toxicol. 1995;76:76–79. doi: 10.1111/j.1600-0773.1995.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Caggiano M, Kauer JS, Hunter DD. Globose basal cells are neuronal progenitors in the olfactory epithelium: a lineage analysis using a replication-incompetent retrovirus. Neuron. 1994;13:339–352. doi: 10.1016/0896-6273(94)90351-4. [DOI] [PubMed] [Google Scholar]
- Calof AL, Chikaraishi DM. Analysis of neurogenesis in a mammalian neuroepithelium: proliferation and differentiation of an olfactory neuron precursor in vitro. Neuron. 1989;3:115–127. doi: 10.1016/0896-6273(89)90120-7. [DOI] [PubMed] [Google Scholar]
- Cancalon P. Degeneration and regeneration of olfactory cells induced by ZnSO4 and other chemicals. Tissue Cell. 1982;14:717–733. doi: 10.1016/0040-8166(82)90061-1. [DOI] [PubMed] [Google Scholar]
- Cancalon P. Influence of a detergent on the catfish olfactory mucosa. Tissue Cell. 1983;15:245–258. doi: 10.1016/0040-8166(83)90020-4. [DOI] [PubMed] [Google Scholar]
- Carr VM, Farbman AI. Ablation of the olfactory bulb up-regulates the rate of neurogenesis and induces precocious cell death in olfactory epithelium. Exp Neurol. 1992;115:55–59. doi: 10.1016/0014-4886(92)90221-b. [DOI] [PubMed] [Google Scholar]
- Carter LA, MacDonald JL, Roskams AJ. Olfactory horizontal basal cells demonstrate a conserved multipotent progenitor phenotype. J Neurosci. 2004;24:5670–5683. doi: 10.1523/JNEUROSCI.0330-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Fode C, Guillemot F. Mash1 activates a cascade of bHLH regulators in olfactory neuron progenitors. Development. 1997;124:1611–1621. doi: 10.1242/dev.124.8.1611. [DOI] [PubMed] [Google Scholar]
- Cau E, Gradwohl G, Casarosa S, Kageyama R, Guillemot F. Hes genes regulate sequential stages of neurogenesis in the olfactory epithelium. Development. 2000;127:2323–2332. doi: 10.1242/dev.127.11.2323. [DOI] [PubMed] [Google Scholar]
- Cau E, Casarosa S, Guillemot F. Mash1 and Ngn1 control distinct steps of determination and differentiation in the olfactory sensory neuron lineage. Development. 2002;129:1871–1880. doi: 10.1242/dev.129.8.1871. [DOI] [PubMed] [Google Scholar]
- Chen B, Kim EH, Xu PX. Initiation of olfactory placode development and neurogenesis is blocked in mice lacking both Six1 and Six4. Dev Biol. 2009;326:75–85. doi: 10.1016/j.ydbio.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Tian S, Yang X, Lane AP, Reed RR, Liu H. Wnt-responsive Lgr5+ globose basal cells function as multi-potent olfactory epithelium progenitor cells. J Neurosci. 2014;34:8268–8276. doi: 10.1523/JNEUROSCI.0240-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Fang H, Schwob JE. Multipotency of purified, transplanted globose basal cells in olfactory epithelium. J Comp Neurol. 2004;469:457–474. doi: 10.1002/cne.11031. [DOI] [PubMed] [Google Scholar]
- Chen Y, Getchell ML, Ding X, Getchell TV. Immunolocalization of two cytochrome P450 isozymes in rat nasal chemosensory tissue. Neuroreport. 1992;3:749–752. doi: 10.1097/00001756-199209000-00007. [DOI] [PubMed] [Google Scholar]
- Cheung MC, Jang W, Schwob JE, Wachowiak M. Functional recovery of odor representations in regenerated sensory inputs to the olfactory bulb. Front Neural Circuits. 2013;7:207. doi: 10.3389/fncir.2013.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MD, Holbrook EH, Costanzo RM, Schwob JE. Rhinotopy is disrupted during the reinnervation of the olfactory bulb that follows transection of the olfactory nerve. Chem Senses. 2001;26:359–369. doi: 10.1093/chemse/26.4.359. [DOI] [PubMed] [Google Scholar]
- Costanzo RM. Rewiring the olfactory bulb: changes in odor maps following recovery from nerve transection. Chem Senses. 2000;25:199–205. doi: 10.1093/chemse/25.2.199. [DOI] [PubMed] [Google Scholar]
- Cotsarelis G, Cheng SZ, Dong G, Sun TT, Lavker RM. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. doi: 10.1016/0092-8674(89)90958-6. [DOI] [PubMed] [Google Scholar]
- Cummings DM, Emge DK, Small SL, Margolis FL. Pattern of olfactory bulb innervation returns after recovery from reversible peripheral deafferentation. J Comp Neurol. 2000;421:362–373. [PubMed] [Google Scholar]
- Cuschieri A, Bannister LH. The development of the olfactory mucosa in the mouse: electron microscopy. J Anat. 1975a;119:471–498. [PMC free article] [PubMed] [Google Scholar]
- Cuschieri A, Bannister LH. The development of the olfactory mucosa in the mouse: light microscopy. J Anat. 1975b;119:277–286. [PMC free article] [PubMed] [Google Scholar]
- Ding XX, Porter TD, Peng HM, Coon MJ. cDNA and derived amino acid sequence of rabbit nasal cytochrome P450NMb (P450IIG1), a unique isozyme possibly involved in olfaction. Arch Biochem Biophys. 1991;285:120–125. doi: 10.1016/0003-9861(91)90337-i. [DOI] [PubMed] [Google Scholar]
- Doty RL. A review of olfactory dysfunctions in man. Am J Otolaryngol. 1979;1:57–79. doi: 10.1016/s0196-0709(79)80010-1. [DOI] [PubMed] [Google Scholar]
- Doty RL. Influence of age and age-related diseases on olfactory function. Ann N Y Acad Sci. 1989;561:76–86. doi: 10.1111/j.1749-6632.1989.tb20971.x. [DOI] [PubMed] [Google Scholar]
- Doty RL. Studies of human olfaction from the University of Pennsylvania Smell and Taste Center. Chem Senses. 1997;22:565–586. doi: 10.1093/chemse/22.5.565. [DOI] [PubMed] [Google Scholar]
- Douek E, Bannister LH, Dodson HC. Recent advances in the pathology of olfaction. Proc R Soc Med. 1975;68:467–470. [PMC free article] [PubMed] [Google Scholar]
- Duggan CD, Ngai J. Scent of a stem cell. Nat Neurosci. 2007;10:673–674. doi: 10.1038/nn0607-673. [DOI] [PubMed] [Google Scholar]
- Fletcher RB, Prasol MS, Estrada J, Baudhuin A, Vranizan K, Choi YG, Ngai J. p63 regulates olfactory stem cell self-renewal and differentiation. Neuron. 2011;72:748–759. doi: 10.1016/j.neuron.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini V, Bettini S, Pifferi S, Menini A, Siciliano G, Ognio E, Brini AT, Di Oto E, Revoltella RP. Transplanted human adipose tissue-derived stem cells engraft and induce regeneration in mice olfactory neuroepithelium in response to dichlobenil subministration. Chem Senses. 2014;39:617–629. doi: 10.1093/chemse/bju035. [DOI] [PubMed] [Google Scholar]
- Genter MB, Deamer NJ, Blake BL, Wesley DS, Levi PE. Olfactory toxicity of methimazole: dose–response and structure–activity studies and characterization of flavin-containing monooxygenase activity in the Long-Evans rat olfactory mucosa. Toxicol Pathol. 1995;23:477–486. doi: 10.1177/019262339502300404. [DOI] [PubMed] [Google Scholar]
- Genter MB, Owens DM, Carlone HB, Crofton KM. Characterization of olfactory deficits in the rat following administration of 2,6-dichlorobenzonitrile (dichlobenil), 3,3′-iminodipropionitrile, or methimazole. Fundam Appl Toxicol. 1996;29:71–77. doi: 10.1006/faat.1996.0007. [DOI] [PubMed] [Google Scholar]
- Getchell TV, Narla RK, Little S, Hyde JF, Getchell ML. Horizontal basal cell proliferation in the olfactory epithelium of transforming growth factor-alpha transgenic mice. Cell Tissue Res. 2000;299:185–192. doi: 10.1007/s004419900149. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Schwob JE. Analysis of the globose basal cell compartment in rat olfactory epithelium using GBC-1, a new monoclonal antibody against globose basal cells. J Neurosci. 1996;16:4005–4016. doi: 10.1523/JNEUROSCI.16-12-04005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BJ, Fang H, Youngentob SL, Schwob JE. Transplantation of multipotent progenitors from the adult olfactory epithelium. Neuroreport. 1998;9:1611–1617. doi: 10.1097/00001756-199805110-00065. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Goss GM, Hatzistergos KE, Rangel EB, Seidler B, Saur D, Hare JM. Adult c-Kit+ progenitor cells are necessary for maintenance and regeneration of olfactory neurons. J Comp Neurol. 2015;523:15–31. doi: 10.1002/cne.23653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MK, Mumm JS, Davis RA, Holcomb JD, Calof AL. Dynamics of MASH1 expression in vitro and in vivo suggest a non-stem cell site of MASH1 action in the olfactory receptor neuron lineage. Mol Cell Neurosci. 1995;6:363–379. doi: 10.1006/mcne.1995.1028. [DOI] [PubMed] [Google Scholar]
- Goss GM, Chaudhari N, Hare JM, Nwojo R, Seidler B, Saur D, Goldstein BJ. Differentiation potential of individual olfactory c-Kit+ progenitors determined via multicolor lineage tracing. Dev Neurobiol. 2015;76:241–251. doi: 10.1002/dneu.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei PP. Cell dynamics in the olfactory mucosa. Tissue Cell. 1973;5:113–131. doi: 10.1016/s0040-8166(73)80010-2. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC. The olfactory organ of vertebrates: a survey. In: Bellairs R, Gray EG, editors. Essays on structure and function in the nervous system. London: Clarendon; 1974. pp. 191–222. [Google Scholar]
- Graziadei PP, Metcalf JF. Autoradiographic and ultra-structural observations on the frog’s olfactory mucosa. Z Zellforsch Mikrosk Anat. 1971;116:305–318. doi: 10.1007/BF00330630. [DOI] [PubMed] [Google Scholar]
- Graziadei PPC, Monti Graziadei GA. Continuous nerve cell renewal in the olfactory system. In: Jacobson M, editor. Handbook of sensory physiology. IX. Berlin: Springer-Verlag; 1978. pp. 55–82. [Google Scholar]
- Graziadei PP, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Joyner AL. Dynamic expression of the murine Achaete-Scute homologue Mash-1 in the developing nervous system. Mech Dev. 1993;42:171–185. doi: 10.1016/0925-4773(93)90006-j. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Lo LC, Johnson JE, Auerbach A, Anderson DJ, Joyner AL. Mammalian achaetescute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- Guo Z, Packard A, Krolewski RC, Harris MT, Manglapus GL, Schwob JE. Expression of pax6 and sox2 in adult olfactory epithelium. J Comp Neurol. 2010;518:4395–4418. doi: 10.1002/cne.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding JW, Getchell TV, Margolis FL. Denervation of the primary olfactory pathway in mice. V. Long-term effect of intranasal ZnSO4 irrigation on behavior, biochemistry and morphology. Brain Res. 1978;140:271–285. doi: 10.1016/0006-8993(78)90460-2. [DOI] [PubMed] [Google Scholar]
- Hegg CC, Jia C, Chick WS, Restrepo D, Hansen A. Micro-villous cells expressing IP3 receptor type 3 in the olfactory epithelium of mice. Eur J Neurosci. 2010;32:1632–1645. doi: 10.1111/j.1460-9568.2010.07449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick DB, Schwob JE. Program No. 291.06, 2015 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2015. Notch signaling helps maintain reserve neural stem cell quiescence in the setting of neuronal injury. Online. [Google Scholar]
- Hewitt JC, Lane RP, Schwob JE. Program No. 291.02, 2015 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2015. Location, location, location: neuronal diversification in the olfactory epithelium. Online. [Google Scholar]
- Holbrook EH, Szumowski KE, Schwob JE. An immuno-chemical, ultrastructural, and developmental characterization of the horizontal basal cells of rat olfactory epithelium. J Comp Neurol. 1995;363:129–146. doi: 10.1002/cne.903630111. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Leopold DA, Schwob JE. Abnormalities of axon growth in human olfactory mucosa. Laryngoscope. 2005;115:2144–2154. doi: 10.1097/01.MLG.0000181493.83661.CE. [DOI] [PubMed] [Google Scholar]
- Holbrook EH, Wu E, Curry WT, Lin DT, Schwob JE. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121:1687–1701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook EH, Iwema CL, Peluso CE, Schwob JE. The regeneration of P2 olfactory sensory neurons is selectively impaired following methyl bromide lesion. Chem Senses. 2014;39:601–616. doi: 10.1093/chemse/bju033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard JM, Schwob JE. Cell cycle of globose basal cells in rat olfactory epithelium. Dev Dyn. 1995;203:17–26. doi: 10.1002/aja.1002030103. [DOI] [PubMed] [Google Scholar]
- Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400:469–486. [PubMed] [Google Scholar]
- Hunter DD, Caggiano M, Kauer JS. Lineage analysis of the olfactory epithelium using a replication-incompetent retrovirus. Chem Senses. 1994;19:683–693. doi: 10.1093/chemse/19.6.683. [DOI] [PubMed] [Google Scholar]
- Hurtt ME, Morgan KT, Working PK. Histopathology of acute toxic responses in selected tissues from rats exposed by inhalation to methyl bromide. Fundam Appl Toxicol. 1987;9:352–365. doi: 10.1016/0272-0590(87)90057-1. [DOI] [PubMed] [Google Scholar]
- Hurtt ME, Thomas DA, Working PK, Monticello TM, Morgan KT. Degeneration and regeneration of the olfactory epithelium following inhalation exposure to methyl bromide: pathology, cell kinetics, and olfactory function. Toxicol Appl Pharmacol. 1988;94:311–328. doi: 10.1016/0041-008x(88)90273-6. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Ookawara S, Sato S, Ando Z, Kageyama R, Kawakami K. Six1 is essential for early neurogenesis in the development of olfactory epithelium. Dev Biol. 2007;311:53–68. doi: 10.1016/j.ydbio.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Kageyama R, Suzuki Y, Kawakami K. Six1 is indispensable for production of functional progenitor cells during olfactory epithelial development. Int J Dev Biol. 2010;54:1453–1464. doi: 10.1387/ijdb.093041ki. [DOI] [PubMed] [Google Scholar]
- Irigoin F, Badano JL. Keeping the balance between proliferation and differentiation: the primary cilium. Curr Genomics. 2011;12:285–297. doi: 10.2174/138920211795860134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai N, Zhou Z, Roop DR, Behringer RR. Horizontal basal cells are multipotent progenitors in normal and injured adult olfactory epithelium. Stem Cells. 2008;26:1298–1306. doi: 10.1634/stemcells.2007-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwema CL, Fang H, Kurtz DB, Youngentob SL, Schwob JE. Odorant receptor expression patterns are restored in lesion-recovered rat olfactory epithelium. J Neurosci. 2004;24:356–369. doi: 10.1523/JNEUROSCI.1219-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Youngentob SL, Schwob JE. Globose basal cells are required for reconstitution of olfactory epithelium after methyl bromide lesion. J Comp Neurol. 2003;460:123–140. doi: 10.1002/cne.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Kim KP, Schwob JE. Nonintegrin laminin receptor precursor protein is expressed on olfactory stem and progenitor cells. J Comp Neurol. 2007;502:367–381. doi: 10.1002/cne.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Lambropoulos J, Woo JK, Peluso CE, Schwob JE. Maintaining epitheliopoietic potency when culturing olfactory progenitors. Exp Neurol. 2008;214:25–36. doi: 10.1016/j.expneurol.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Chen X, Flis D, Harris M, Schwob JE. Label-retaining, quiescent globose basal cells are found in the olfactory epithelium. J Comp Neurol. 2014;522:731–749. doi: 10.1002/cne.23470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang W, Holbrook EH, Child K, Schwob JE. Program 291.09, 2015 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2015. A transgenic mouse model for accelerated olfactory aging. Online. [Google Scholar]
- Jia C, Hayoz S, Hutch CR, Iqbal TR, Pooley AE, Hegg CC. An IP3R3- and NPY-expressing microvillous cell mediates tissue homeostasis and regeneration in the mouse olfactory epithelium. PLoS One. 2013;8(3):e58668. doi: 10.1371/journal.pone.0058668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner AM, Green WW, McIntyre JC, Allen BL, Schwob JE, Martens JR. Primary cilia on horizontal basal cells regulate regeneration of the olfactory epithelium. J Neurosci. 2015;35:13761–13772. doi: 10.1523/JNEUROSCI.1708-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurczyk A, Bortell R, Alonso LC. Human beta-cell regeneration: progress, hurdles, and controversy. Curr Opin Endocrinol Diabetes Obes. 2014;21:102–108. doi: 10.1097/MED.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Watanabe K, Sakamoto T, Suzukawa K, Nibu K, Kaga K, Yamasoba T. Distribution and severity of spontaneous lesions in the neuroepithelium and Bowman’s glands in mouse olfactory mucosa: age-related progression. Cell Tissue Res. 2009;335:489–503. doi: 10.1007/s00441-008-0739-9. [DOI] [PubMed] [Google Scholar]
- Kondo K, Suzukawa K, Sakamoto T, Watanabe K, Kanaya K, Ushio M, Yamaguchi T, Nibu K, Kaga K, Yamasoba T. Age-related changes in cell dynamics of the post-natal mouse olfactory neuroepithelium: cell proliferation, neuronal differentiation, and cell death. J Comp Neurol. 2010;518:1962–1975. doi: 10.1002/cne.22316. [DOI] [PubMed] [Google Scholar]
- Kream RM, Margolis FL. Olfactory marker protein: turnover and transport in normal and regenerating neurons. J Neurosci. 1984;4:868–879. doi: 10.1523/JNEUROSCI.04-03-00868.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewski RC, Jang W, Schwob JE. The generation of olfactory epithelial neurospheres in vitro predicts engraftment capacity following transplantation in vivo. Exp Neurol. 2011;229:308–323. doi: 10.1016/j.expneurol.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewski RC, Packard A, Jang W, Wildner H, Schwob JE. Ascl1 (Mash1) knockout perturbs differentiation of nonneuronal cells in olfactory epithelium. PLoS One. 2012;7(12):e51737. doi: 10.1371/journal.pone.0051737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krolewski RC, Packard A, Schwob JE. Global expression profiling of globose basal cells and neurogenic progression within the olfactory epithelium. J Comp Neurol. 2013;521:833–859. doi: 10.1002/cne.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusumakshi S, Voigt A, Hubner S, Hermans-Borgmeyer I, Ortalli A, Pyrski M, Dorr J, Zufall F, Flockerzi V, Meyerhof W, Montmayeur JP, Boehm U. A binary genetic approach to characterize TRPM5 cells in mice. Chem Senses. 2015;40:413–425. doi: 10.1093/chemse/bjv023. [DOI] [PubMed] [Google Scholar]
- Largent BL, Sosnowski RG, Reed RR. Directed expression of an oncogene to the olfactory neuronal lineage in transgenic mice. J Neurosci. 1993;13:300–312. doi: 10.1523/JNEUROSCI.13-01-00300.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Lin B, Hewitt J, Peterson J, Schwob JE. Program no: 291.11, 2015 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience; 2015. Injury can induce neuronally committed Neurog1+ progenitors to become multi-potent. Online. [Google Scholar]
- Loo AT, Youngentob SL, Kent PF, Schwob JE. The aging olfactory epithelium: neurogenesis, response to damage, and odorant-induced activity. Int J Dev Neurosci. 1996;14:881–900. doi: 10.1016/s0736-5748(96)00046-9. [DOI] [PubMed] [Google Scholar]
- Mahanthappa NK, Schwarting GA. Peptide growth factor control of olfactory neurogenesis and neuron survival in vitro: roles of EGF and TGF-betas. Neuron. 1993;10:293–305. doi: 10.1016/0896-6273(93)90319-m. [DOI] [PubMed] [Google Scholar]
- Manglapus GL, Youngentob SL, Schwob JE. Expression patterns of basic helix-loop-helix transcription factors define subsets of olfactory progenitor cells. J Comp Neurol. 2004;479:216–233. doi: 10.1002/cne.20316. [DOI] [PubMed] [Google Scholar]
- Matulionis DH. Ultrastructural study of mouse olfactory epithelium following destruction by ZnSO4 and its subsequent regeneration. Am J Anat. 1975;142:67–89. doi: 10.1002/aja.1001420106. [DOI] [PubMed] [Google Scholar]
- Matulionis DH. Light and electron microscopic study of the degeneration and early regeneration of olfactory epithelium in the mouse. Am J Anat. 1976;145:79–99. doi: 10.1002/aja.1001450106. [DOI] [PubMed] [Google Scholar]
- Michalopoulos GK. Liver regeneration: alternative epithelial pathways. Int J Biochem Cell Biol. 2011;43:173–179. doi: 10.1016/j.biocel.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miragall F, Monti Graziadei GA. Experimental studies on the olfactory marker protein. II. Appearance of the olfactory marker protein during differentiation of the olfactory sensory neurons of mouse: an immunohistochemical and autoradiographic study. Brain Res. 1982;239:245–250. doi: 10.1016/0006-8993(82)90846-0. [DOI] [PubMed] [Google Scholar]
- Miragall F, Kadmon G, Husmann M, Schachner M. Expression of cell adhesion molecules in the olfactory system of the adult mouse: presence of the embryonic form of N-CAM. Dev Biol. 1988;129:516–531. doi: 10.1016/0012-1606(88)90397-1. [DOI] [PubMed] [Google Scholar]
- Montani G, Tonelli S, Elsaesser R, Paysan J, Tirindelli R. Neuropeptide Y in the olfactory microvillar cells. Eur J Neurosci. 2006;24:20–24. doi: 10.1111/j.1460-9568.2006.04878.x. [DOI] [PubMed] [Google Scholar]
- Monti Graziadei GA, Graziadei PP. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979;8:197–213. doi: 10.1007/BF01175561. [DOI] [PubMed] [Google Scholar]
- Moody SA, LaMantia AS. Transcriptional regulation of cranial sensory placode development. Curr Top Dev Biol. 2015;111:301–350. doi: 10.1016/bs.ctdb.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran DT, Rowley JC, 3rd, Jafek BW. Electron microscopy of human olfactory epithelium reveals a new cell type: the microvillar cell. Brain Res. 1982;253:39–46. doi: 10.1016/0006-8993(82)90671-0. [DOI] [PubMed] [Google Scholar]
- Moran DT, Jafek BW, Eller PM, Rowley JC., 3rd Ultra-structural histopathology of human olfactory dysfunction. Microsc Res Techniq. 1992;23:103–110. doi: 10.1002/jemt.1070230202. [DOI] [PubMed] [Google Scholar]
- Mori M, Mahoney JE, Stupnikov MR, Paez-Cortez JR, Szymaniak AD, Herrick DB, Schwob JE, Zhang H, Cardoso WV. Notch3-Jagged signaling controls the pool of undifferentiated airway progenitors. Development. 2015;142:258–267. doi: 10.1242/dev.116855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison EE, Costanzo RM. Scanning electron microscopic study of degeneration and regeneration in the olfactory epithelium after axotomy. J Neurocytol. 1989;18:393–405. doi: 10.1007/BF01190842. [DOI] [PubMed] [Google Scholar]
- Morrison EE, Costanzo RM. Morphology of olfactory epithelium in humans and other vertebrates. Microsc Res Techniq. 1992;23:49–61. doi: 10.1002/jemt.1070230105. [DOI] [PubMed] [Google Scholar]
- Moulton DG. Dynamics of cell populations in the olfactory epithelium. Ann N Y Acad Sci. 1974;237:52–61. doi: 10.1111/j.1749-6632.1974.tb49843.x. [DOI] [PubMed] [Google Scholar]
- Moulton DG, Celebi G, Fink RP. Olfaction in mammals—two aspects: proliferation of cells in the olfactory epithelium and sensitivity to odours. In: Wolstenholme GEW, Knight J, editors. Ciba Foundation Symposium on taste and smell in vertebrates. London: Churchill; 1970. pp. 227–250. [Google Scholar]
- Mulvaney BD, Heist HE. Regeneration of rabbit olfactory epithelium. Am J Anat. 1971;131:241–252. doi: 10.1002/aja.1001310208. [DOI] [PubMed] [Google Scholar]
- Murphy C, Schubert CR, Cruickshanks KJ, Klein BE, Klein R, Nondahl DM. Prevalence of olfactory impairment in older adults. JAMA. 2002;288:2307–2312. doi: 10.1001/jama.288.18.2307. [DOI] [PubMed] [Google Scholar]
- Murray RC, Navi D, Fesenko J, Lander AD, Calof AL. Widespread defects in the primary olfactory pathway caused by loss of Mash1 function. J Neurosci. 2003;23:1769–1780. doi: 10.1523/JNEUROSCI.23-05-01769.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naessen R. An enquiry on the morphological characteristics and possible changes with age in the olfactory region of man. Acta Otolaryngol. 1971;71:49–62. doi: 10.3109/00016487109125332. [DOI] [PubMed] [Google Scholar]
- Nagahara Y. Experimentelle Studien uber die histologiischen Veranderungen des Geruchsorgan nach der Olfactoriusdurschneidung. Beitrage zur Kenntnis des feineren Baus des Geruchsorgans. Jpn J Med Sci V Pathol. 1940;5:165–169. [Google Scholar]
- Nakashima T, Kimmelman CP, Snow JB., Jr Structure of human fetal and adult olfactory neuroepithelium. Arch Otolaryngol. 1984;110:641–646. doi: 10.1001/archotol.1984.00800360013003. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Kimmelman CP, Snow JB., Jr Immunohistopathology of human olfactory epithelium, nerve and bulb. Laryngoscope. 1985a;95:391–396. doi: 10.1288/00005537-198504000-00004. [DOI] [PubMed] [Google Scholar]
- Nakashima T, Kimmelman CP, Snow JB., Jr Olfactory marker protein in the human olfactory pathway. Arch Otolaryngol. 1985b;111:294–297. doi: 10.1001/archotol.1985.00800070046004. [DOI] [PubMed] [Google Scholar]
- Packard A, Giel-Moloney M, Leiter A, Schwob JE. Progenitor cell capacity of NeuroD1-expressing globose basal cells in the mouse olfactory epithelium. J Comp Neurol. 2011a;519:3580–3596. doi: 10.1002/cne.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard A, Schnittke N, Romano RA, Sinha S, Schwob JE. DeltaNp63 regulates stem cell dynamics in the mammalian olfactory epithelium. J Neurosci. 2011b;31:8748–8759. doi: 10.1523/JNEUROSCI.0681-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso CE, Jang W, Drager UC, Schwob JE. Differential expression of components of the retinoic acid signaling pathway in the adult mouse olfactory epithelium. J Comp Neurol. 2012;520:3707–3726. doi: 10.1002/cne.23124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson JN, Schwob JE. Program No. 291.01, 2015 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience. Online; 2015. Potency and neurogenesis in the basal stem cells of the olfactory and respiratory epithelia. [Google Scholar]
- Potten CS. Keratinocyte stem cells, label-retaining cells and possible genome protection mechanisms. J Invest Dermatol Symp Proc. 2004;9:183–195. doi: 10.1111/j.1087-0024.2004.09305.x. [DOI] [PubMed] [Google Scholar]
- Randell SH, Comment CE, Ramaekers FC, Nettesheim P. Properties of rat tracheal epithelial cells separated based on expression of cell surface alphagalactosyl end groups. Am J Respir Cell Mol Biol. 1991;4:544–554. doi: 10.1165/ajrcmb/4.6.544. [DOI] [PubMed] [Google Scholar]
- Reynolds BA, Weiss S. Clonal and population analyses demonstrate that an EGF-responsive mammalian embryonic CNS precursor is a stem cell. Dev Biol. 1996;175:1–13. doi: 10.1006/dbio.1996.0090. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Gil DJ, Bartel DL, Jaspers AW, Mobley AS, Imamura F, Greer CA. Odorant receptors regulate the final glomerular coalescence of olfactory sensory neuron axons. Proc Natl Acad Sci U S A. 2015;112:5821–5826. doi: 10.1073/pnas.1417955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. doi: 10.1146/annurev.cellbio.20.010403.092805. [DOI] [PubMed] [Google Scholar]
- Satoh M, Yoshida T. Expression of neural properties in olfactory cytokeratin-positive basal cell line. Brain Res Dev Brain Res. 2000;121:219–222. doi: 10.1016/s0165-3806(00)00040-7. [DOI] [PubMed] [Google Scholar]
- Schnittke N, Herrick DB, Lin B, Peterson J, Coleman JH, Packard AI, Jang W, Schwob JE. Transcription factor p63 controls the reserve status but not the stemness of horizontal basal cells in the olfactory epithelium. Proc Natl Acad Sci U S A. 2015;112:E5068–E5077. doi: 10.1073/pnas.1512272112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert CR, Cruickshanks KJ, Murphy C, Huang GH, Klein BE, Klein R, Nieto FJ, Pankow JS, Tweed TS. Olfactory impairment in adults. Ann N Y Acad Sci. 2009;1170:531–536. doi: 10.1111/j.1749-6632.2009.04102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz EW. Regeneration of olfactory cells. Proc Soc Exp Biol Med. 1941;46:41–43. [Google Scholar]
- Schultz EW. Repair of the olfactory mucosa with special reference to regeneration of olfactory cells (sensory neurons) Am J Pathol. 1960;37:1–19. [PMC free article] [PubMed] [Google Scholar]
- Schwartz Levey M, Chikaraishi DM, Kauer JS. Characterization of potential precursor populations in the mouse olfactory epithelium using immunocytochemistry and autoradiography. J Neurosci. 1991;11:3556–3564. doi: 10.1523/JNEUROSCI.11-11-03556.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz Levey M, Cinelli AR, Kauer JS. Intracellular injection of vital dyes into single cells in the salamander olfactory epithelium. Neurosci Lett. 1992;140:265–269. doi: 10.1016/0304-3940(92)90117-p. [DOI] [PubMed] [Google Scholar]
- Schwob JE. Stages in the differentiation of olfactory sensory neurons. Chem Senses. 1991;16:578. [Google Scholar]
- Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269:33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Costanzo RM. Regeneration of the olfactory epithelium. In: Basbaum AI, Kaneko A, Shepherd GM, Westheimer G, Albright TD, Masland RH, Dallos P, Oertel D, Firestein S, Beauchamp GK, Bushnell MC, Kaas JH, Gardner E, editors. The senses: a comprehensive reference. Vol. 4. New York: Academic Press; 2008. pp. 591–612. [Google Scholar]
- Schwob JE, Huard JM, Luskin MB, Youngentob SL. Retroviral lineage studies of the rat olfactory epithelium. Chem Senses. 1994a;19:671–682. doi: 10.1093/chemse/19.6.671. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Meiri KF. On the formation of neuromata in the primary olfactory projection. J Comp Neurol. 1994b;340:361–380. doi: 10.1002/cne.903400307. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Mezza RC. Reconstitution of the rat olfactory epithelium after methyl bromide-induced lesion. J Comp Neurol. 1995;359:15–37. doi: 10.1002/cne.903590103. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Youngentob SL, Ring G, Iwema CL, Mezza RC. Reinnervation of the rat olfactory bulb after methyl bromide-induced lesion: timing and extent of reinnervation. J Comp Neurol. 1999;412:439–457. doi: 10.1002/(sici)1096-9861(19990927)412:3<439::aid-cne5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Jang W, Holbrook EH. Stem cells of the olfactory epithelium. In: Rao MS, editor. Neural development and stem cells. 3. New York: Springer Science+Business Media; 2012. pp. 201–222. [Google Scholar]
- Shimizu T, Nettesheim P, Mahler JF, Randell SH. Cell type-specific lectin staining of the tracheobronchial epithelium of the rat: quantitative studies with Griffonia simplicifolia I isolectin B4. J Histochem Cytochem. 1991;39:7–14. doi: 10.1177/39.1.1701188. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Nettesheim P, Eddy EM, Randell SH. Monoclonal antibody (Mab) markers for subpopulations of rat tracheal epithelial (RTE) cells. Exp Lung Res. 1992;18:323–342. doi: 10.3109/01902149209031688. [DOI] [PubMed] [Google Scholar]
- Sipos F, Muzes G. Injury-associated reacquiring of intestinal stem cell function. World J Gastroenterol. 2015;21:2005–2010. doi: 10.3748/wjg.v21.i7.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart IH. Location and orientation of mitotic figures in the developing mouse olfactory epithelium. J Anat. 1971;109(Part 2):243–251. [PMC free article] [PubMed] [Google Scholar]
- Smith CG. Changes in the olfactory mucosa and the olfactory nerves following intranasal treatment with one percent zinc sulphate. Can Med Assoc J. 1938;39:138–140. [PMC free article] [PubMed] [Google Scholar]
- Spangrude GJ. Hematopoietic stem-cell differentiation. Curr Opin Immunol. 1991;3:171–178. doi: 10.1016/0952-7915(91)90046-4. [DOI] [PubMed] [Google Scholar]
- Stewart WB, Greer CA, Teicher MH. The effect of intra-nasal zinc sulfate treatment on odor-mediated behavior and on odor-induced metabolic activity in the olfactory bulbs of neonatal rats. Brain Res. 1983;284:247–259. doi: 10.1016/0165-3806(83)90009-3. [DOI] [PubMed] [Google Scholar]
- Suzukawa K, Kondo K, Kanaya K, Sakamoto T, Watanabe K, Ushio M, Kaga K, Yamasoba T. Age-related changes of the regeneration mode in the mouse peripheral olfactory system following olfactotoxic drug methimazole-induced damage. J Comp Neurol. 2011;519:2154–2174. doi: 10.1002/cne.22611. [DOI] [PubMed] [Google Scholar]
- Tadeu AM, Horsley V. Notch signaling represses p63 expression in the developing surface ectoderm. Development. 2013;140:3777–3786. doi: 10.1242/dev.093948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker ES, Lehtinen MK, Maynard T, Zirlinger M, Dulac C, Rawson N, Pevny L, Lamantia AS. Proliferative and transcriptional identity of distinct classes of neural precursors in the mammalian olfactory epithelium. Development. 2010;137:2471–2481. doi: 10.1242/dev.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaagen J, Oestreicher AB, Grillo M, Khew-Goodall YS, Gispen WH, Margolis FL. The expression of the growth-associated protein B-50/GAP43 in the olfactory system of neonatal and adult rats. J Neurosci. 1989;9:683–691. doi: 10.1523/JNEUROSCI.09-02-00683.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaagen J, Oestreicher AB, Grillo M, Khew-Goodall YS, Gispen WH, Margolis FL. Neuroplasticity in the olfactory system: differential effects of central and peripheral lesions of the primary olfactory pathway on the expression of B-50/GAP43 and the olfactory marker protein. J Neurosci Res. 1990;26:31–44. doi: 10.1002/jnr.490260105. [DOI] [PubMed] [Google Scholar]
- Wang YZ, Yamagami T, Gan Q, Wang Y, Zhao T, Hamad S, Lott P, Schnittke N, Schwob JE, Zhou CJ. Canonical Wnt signaling promotes the proliferation and neurogenesis of peripheral olfactory stem cells during postnatal development and adult regeneration. J Cell Sci. 2011;124(Part 9):1553–1563. doi: 10.1242/jcs.080580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, Fang C, Schnittke N, Schwob JE, Ding X. Mechanisms of permanent loss of olfactory receptor neurons induced by the herbicide 2,6-dichlorobenzonitrile: effects on stem cells and noninvolvement of acute induction of the inflammatory cytokine IL-6. Toxicol Appl Pharmacol. 2013;272:598–607. doi: 10.1016/j.taap.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi M, Nakano Y. A re-evaluation of the classification of olfactory epithelia in patients with olfactory disorders. Eur Arch Otorhinolaryngol. 1992;249:393–399. doi: 10.1007/BF00192261. [DOI] [PubMed] [Google Scholar]
- Yoh K, Prywes R. Pathway regulation of p63, a director of epithelial cell fate. Front Endocrinol. 2015;6:51. doi: 10.3389/fendo.2015.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]