Abstract
Objective
To investigate the effect of anticoagulant treatment on pregnancy outcomes in patients with previous recurrent miscarriages (RM) who carry a methylenetetrahydrofolate reductase (MTHFR) gene mutation.
Methods
In this longitudinal retrospective study, patients with RM were treated during pregnancy with either: (i) 100 mg/day aspirin and 5 mg/day folic acid (group 1); or the same protocol plus 0.4 mg/day enoxaparin (group 2). An age-matched group of triparous women without RM or thrombophilia was used as the control group (group 3).
Results
This study enrolled 246 women with RM (123 per treatment group) and age-matched controls (n = 117). The delivery rate was significantly lower in group 1 than group 2 (46.3% versus 79.7%, respectively). The miscarriage rate was significantly lower in group 2 compared with group 1 (20.3% versus 51.2%, respectively). In the control group 3, the delivery rate was 86.3% and the miscarriage rate was 12.8%.
Conclusion
Treatment with low-dose aspirin, enoxaparin and folic acid was the most effective therapy in women with RM who carried a C677T MTHFR mutation.
Keywords: Recurrent miscarriages, methylenetetrahydrofolate reductase (MTHFR), mutation, aspirin, low-molecular-weight heparin, folic acid
Introduction
Recurrent miscarriage (RM) is defined as three or more consecutive spontaneous fetal losses and it affects 0.3% to 1% of pregnancies.1 After one miscarriage, the mean risk of recurrence is 20%; after two, three or four miscarriages, the rate rises to 28%, 30% and 45%, respectively.2 However, 25–50% of RM cases are idiopathic.3 Several studies have described correlations between RM and thrombophilia,4–6 which comprises of: (i) hereditary conditions linked to deficiencies in natural coagulation inhibitors (antithrombin III, proteins S and C) or mutations in the methylenetetrahydrofolate reductase (MTHFR), prothrombin (mutation G20210A) or factor V Leiden genes; and (ii) acquired conditions such as antiphospholipid antibody syndrome and patients that produce lupus anticoagulants.5
The MTHFR enzyme plays a key role in the conversion of 5,10-methylenetetrahydrofolate to 5-methylenetetrahydrofolate, which provides the single-carbon for homocysteine in methionine synthesis.7 The single nucleotide polymorphism C677T encoded by rs1801133 results in an alanine to valine substitution at amino acid 222 in the MTHFR enzyme.7 The activity of the MTHFR enzyme is reduced by 35% in people who are 677CT carriers (heterozygosity) and by 70% among 677TT carriers (homozygosity).7 A reduction in the enzyme activity decreases the conversion of homocysteine into methionine, subsequently resulting in homocysteine (Hcy) accumulation in the blood.7 Hyperhomocysteinaemia (HHcy) has been shown to be caused by both enzyme deficiencies (MTHFR) and environmental factors related to the lack of cofactors in methionine metabolism (vitamins B6 and B12 and folic acid).7 Folic acid is an essential factor for the growing placental tissue and it acts as a substrate for the metabolism of several amino acids and is involved in the transmethylation pathway.8 MTHFR also maintains the methyl pool for the control of gene expression by DNA methylation during implantation and invasion of the embryo in the first trimester of pregnancy.9,10 A previous study showed that a homozygous MTHFR gene mutation led to a 3.3-fold increase in the risk of miscarriage in a population of 185 Caucasian women relative to 113 mutation-free controls.11
There is little consensus regarding the effect of preventive treatment with low-molecular-weight heparin (LMWH), with some studies reporting a positive effect in patients withRMandthrombophilia,12–14 but with other studies showing no effect.15–17 The aim of this present study was to compare the effect of treatment with a combination of aspirin, LMWH and folic acid versus a combination of aspirin and folic acid in women with consecutive first-trimester recurrent miscarriages who were carrying a C677T MTHFR gene mutation.
Patients and methods
Patient population
This longitudinal retrospective study enrolled consecutive patients with three or more consecutive first-trimester miscarriages who were carrying the C677T MTHFR gene mutation and were treated and followed-up by the Department of Obstetrics, Gynaecology and Reproductive Medicine, Amiens University Medical Centre, Amiens, France between January 2006 and December 2011. The C677CT (heterozygosity) and C677TT (homozygosity) mutations were the only MTHFR gene mutations considered in this study. Patients with the A1298C polymorphism were excluded. All enrolled women were screened for uterine malformations (by hysteroscopy), chromosomal abnormalities (karyotype, also undertaken for their male partner), endocrine disorders (fasting and post-prandial blood glucose, thyroid hormones) and thrombophilia. Table 1 summarizes the thrombophilia criteria screened for each patient and the age-matched control subjects. A fasting blood homocysteine level > 15 µmol/l was considered to be pathological and defined as HHcy. The MTHFR gene mutations of the patient’s male partner were not genotyped.
Table 1.
Thrombophilia screening panel used for the women (n = 246) with recurrent first-trimester miscarriages and the age-matched control women (n = 117) who participated in this study to evaluate two miscarriage prevention treatment regimens.
| Antiphospholipid antibodiesa |
|---|
| Lupus anticoagulant |
| Factor V Leiden |
| Prothrombin G20210A mutation |
| MTHFR C677 and A1298 |
| Homocysteine |
| Protein C |
| Protein S |
| Antithrombin III |
Anti-phosphatidylserin immunoglobulin (Ig)G, anti-cardiolipin IgM and IgG, and β2 glycoprotein.
MTHFR, methylenetetrahydrofolate reductase.
An age-matched group of triparous women without RM and thrombophilia (screened for thrombophilia as for the two study groups) who became pregnant during the same time period and followed-up in the same hospital was used as the control group.
Baseline demographic and clinical data were collected for all study participants, including previous number of pregnancies, number of first-trimester miscarriages, previous intra-uterine fetal death, previous pre-eclampsia, previous intra-uterine growth restriction, previous successful deliveries, body mass index (kg/m2), HHcy status, alcohol consumption status defined as any alcohol consumed per day and smoking status defined as >five cigarettes per day.
All study participants gave their written, informed consent and the study protocol was approved by the Picardie University Jules Verne Bioethics Committee, Amiens University Medical Centre, Amiens, France.
MTHFR genotyping
Screening for the C677T MTHFR gene mutation was performed according to a method described previously.18 Briefly, genomic DNA was extracted from peripheral blood using a QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s protocol. Blood samples were stored at –80℃ prior to analysis. Genotyping was performed by polymerase chain reaction (PCR)-restriction fragment length polymorphism or real-time PCR. Amplification of the c.677C > T region of the MTHFR gene was performed using the primer pairs 5′-TGAAGGAGAAGGTGTCTGCGGGA-3′ (forward primer) and 5′-AGGACGGTGCGGTGAGAGTG-3′ (reverse primer) as described previously.18 PCR amplifications (25 µl volume) consisted of the following: 5 ng of genomic DNA in the case of patients and controls; 10 pmol each of the c.677C > T forward and reverse primers; 0.625 units of Taq DNA polymerase (5 PRIME, Hamburg, Germany); 0.2 mM of dNTPs (Thermo Scientific, Colchester, UK) in 1 × PCR Buffer (5 PRIME). Amplification of the correct fragment was initially confirmed by unidirectional DNA sequencing followed by comparison and alignment to the NCBI Reference Sequence NG_013351.1.
The PCR was followed by minisequencing as described previously with some modifications.7 Prior to the minisequencing reaction, 0.5 µl of PCR product was purified using ExoSAP-IT® PCR Product Cleanup (Affymetrix UK, High Wycombe, UK) following the manufacturer’s instructions. Minisequencing was then conducted in a final volume of 5 μl, consisting of 0.5 µl of each of the purified MTHFR c.677C > T PCR products, 2.5 µl SNaPshot® Multiplex Ready Reaction Mix (Applied Biosystems, Foster City, CA, USA) and 1 pmol of each of the minisequencing primers for MTHFR c.677C > T: 5′-TGAAGGAGAAGGTGTCTGCGGGA-3′ (forward primer); and 5′- AGGACGGTGCGGGAGAGTC-3′ (reverse primer). The cycling programme involved preliminary denaturation at 95℃ for 10 min, followed by 25 cycles of denaturation at 96℃ for 10 s, annealing at 50℃ for 5 s, and elongation at 60℃ for 30 s, followed by a final elongation step at 72℃ for 7 min. Minisequencing products were analysed by capillary electrophoresis on an Applied Biosystems®3130 Genetic Analyzer (Applied Biosystems). The reaction included 9.25 μl Hi-Di™ Formamide (Applied Biosystems), 0.25 μl GeneScan™ 1200 LIZ® dye Size Standard (Applied Biosystems) and 0.5 μl of minisequencing product. Incubation at 95℃ for 3 min to denature the minisequencing product was performed prior to electrophoresis. Data were subsequently visualized and analysed using the GeneMapper® Software version X3.4 (Applied Biosystems).
Study treatment groups
This study undertook retrospective analyses of two groups of patients with the C677T MTHFR gene mutation who had been treated with two different protocols during two different time periods. The first group of patients (group 1) was treated between January 2006 and December 2008 with protocol P1 that consisted of the following: low-dose aspirin (Aspegic®, 100 mg/day; Sanofi, Gentilly, France) prior to conception and up until 35 weeks of amenorrhoea; and folic acid (SpeciaFoldine®, 5 mg/day; Theraplix, Paris, France) as soon as pregnancy was confirmed and up until delivery. Because of the unsatisfactory results with the P1 protocol, the P2 protocol was introduced for patients having the same characteristics as group 1. Patients in group 2 were treated between January 2009 and December 2011 with protocol P2 that consisted of the following: low-dose aspirin (Aspegic®, 100 mg/day; Sanofi) prior to conception and up until 35 weeks of amenorrhoea; and folic acid (SpeciaFoldine®, 5 mg/day; Theraplix) as soon as pregnancy was confirmed and up until delivery; combined with a LMWH (enoxaparin, subcutaneous injection; Lovenox®, 0.4 mg/day; Aventis, Paris, France) as soon as pregnancy was confirmed (by a plasma β-human chorionic gonadotropin assay) and up until delivery. The two active treatment groups were compared with an age-matched control group (group 3). The control group was permitted to receive any antithrombotic and vitamin treatments that were considered necessary by their physicians. Fetal development was routinely monitored and no differences were observed between the three groups. During pregnancy, each woman in the three groups received the same standard obstetric care and pregnancy monitoring, with a platelet count control if heparin was prescribed.
Study outcomes
The following pregnancy outcomes in the three groups were analysed: maternal age at start of pregnancy, number of successful deliveries, duration of pregnancy (weeks of amenorrhoea), infant birth weight, and pathological outcomes including first-trimester miscarriage, intra-uterine fetal death, pre-eclampsia and/or intra-uterine growth restriction (<fifth percentile), and premature deliveries (under 37 weeks of amenorrhoea).
Statistical analyses
All statistical analyses were performed using the StatView statistical package, version 5.0.1 (SAS Institute Inc., Cary, NC, USA) for Windows®. Comparisons between the three groups of patients were undertaken using Student’s t-test or Mann–Whitney U-test for continuous variables (mean ± SD) and χ2-test or Fisher’s exact test for the first-trimester miscarriage and delivery rates. A P-value < 0.05 was considered to be statistically significant.
Results
This longitudinal retrospective study enrolled 246 women with three or more consecutive first-trimester recurrent miscarriages who were carrying the C677T MTHFR gene mutation. Of these, 171 (69.5%) women were 677CT heterozygotes and 75 (30.5%) women were 677TT homozygotes. None of the 677CT women was classified as having HHcy compared with 22 (29.3%) of the 75 677TT women who were classified as having HHcy (12 of 123 [9.8%] in group 1 compared with 10 of 123 [8.1%] in group 2; no significant between-group difference). The 246 women who were carrying the C677T MTHFR gene mutation formed the two treatment groups (groups 1 and 2; n = 123 per group). The age-matched control group consisted of 117 pregnant women. The baseline characteristics, obstetric antecedents and C677T MTHFR gene mutation status for the three groups of patients are presented in Table 2. There were no significant differences between the baseline characteristics of the three groups including body mass index, cigarette consumption and alcohol consumption. In the 246 women with the C677T MTHFR gene mutation, before any preventive treatment, 941 first-trimester miscarriages were reported (mean 3.8 per woman; range 3–15), with no significant difference being observed in the distribution of miscarriages between the two treatment groups (Table 2).
Table 2.
Baseline characteristics and obstetric antecedents in women carrying a methylenetetrahydrofolate reductase (MTHFR) gene mutation (n = 246) compared with age-matched control women (n = 117).
| Group 1 n = 123 | Group 2 n = 123 | Group 3 n = 117 | |
|---|---|---|---|
| MTHFR gene mutation | |||
| 677CT | 84 (68.3) | 87 (70.7) | 0 |
| 677TT | 39 (31.7) | 36 (29.3) | 0 |
| Hyperhomocysteinaemiaa | 12 (9.8) | 10 (8.1) | 0 |
| Body mass index, kg/m2 | 24.2 ± 2.1 | 23.9 ± 2.4 | 24.0 ± 1.9 |
| Alcohol consumption, yes | 6 (4.9) | 7 (5.7) | 6 (5.1) |
| Smoking, yes | 35 (28.5) | 34 (27.6) | 33 (28.2) |
| Total pregnancies | 519 | 549 | 373 |
| First-trimester miscarriage (n per woman) | 458 (3.7) | 483 (3.9) | 20 (0.2) |
| Intra-uterine fetal death | 16 (13.0) | 15 (12.2) | 2 (1.7) |
| Pre-eclampsia and/or intra-uterine growth restriction | 11 (8.9) | 9 (7.3) | 2 (1.7) |
| Number of previous successful deliveries |
n = 40 |
n = 41 |
n = 117 |
| 45 | 51 | 351 |
Data presented as n of patients (%) or mean ± SD.
Homocysteine level > 15 µmol/l.
Group 1 were treated with low-dose aspirin and folic acid; group 2 were treated with low-dose aspirin, enoxaparin and folic acid; and group 3 were the age-matched control women without recurrent miscarriages and thrombophilia.
The pregnancy-related outcomes of the three groups are summarized in Table 3. There were no significant differences between the three groups in terms of age at the start of the pregnancy. In group 1, 57 women gave birth (including six premature deliveries) after a mean ± SD pregnancy duration of 37.4 ± 3.2 weeks of amenorrhoea. The mean ± SD infant birth weight was 2820 ± 585 g. In group 2, 98 births occurred (including seven premature deliveries), with a mean ± SD pregnancy duration of 39.1 ± 3.7 weeks of amenorrhoea (group 1 versus group 2; P < 0.001) and a mean ± SD infant birth weight of 3372 ± 167 g (group 1 versus group 2; P < 0.001). The delivery rate was significantly higher in group 2 versus group 1 (79.7% versus 46.3%; P < 0.001). The first-trimester miscarriage rate was significantly higher in group 1 compared with group 2 (51.2% versus 20.3%, respectively; P < 0.001). There was no significant difference in the first-trimester miscarriage rates between the two treatment groups (group 1 versus group 2) in relation to the HHcy status (6/12 [50.0%] in group 1 versus 2/10 [20.0%] in group 2). No heparin-induced thrombopenia or iatrogenic bleeding occurred during administration of the P2 protocol. In the control group 3, 101 live births (including eight premature deliveries) were recorded. The mean ± SD infant birth weight and pregnancy duration were 3230 ± 565 g (group 3 versus group 2: no significant difference; group 3 versus group 1: P < 0.001) and 38.2 ± 2.3 weeks of amenorrhoea (group 3 versus group 2 and group 3 versus group 1: P < 0.05), respectively. There was no significant difference in the delivery rates between women carrying the C677T MTHFR gene mutation and treated with the P2 protocol and the women of the control group 3 (79.7% and 86.3%, respectively).
Table 3.
Pregnancy-related outcomes of women carrying a methylenetetrahydrofolate reductase (MTHFR) gene mutation (n = 246) treated with either low-dose aspirin and folic acid or low-dose aspirin, enoxaparin and folic acid compared with age-matched control women (n = 117).
| Characteristic | Group 1 n = 123 | Group 2 n = 123 | Group 3 n = 117 |
|---|---|---|---|
| Age at start of pregnancy, years | 32.2 ± 3.9 | 33.5 ± 5.2 | 32.8 ± 3.1 |
| First-trimester miscarriage | 63 (51.2)a | 25 (20.3)b | 15 (12.8)c |
| Intra-uterine fetal death | 3 (2.4) | 0 | 1 (0.9) |
| Pre-eclampsia and/or intra-uterine growth restriction | 6 (4.9) | 3 (2.4) | 3 (2.6) |
| Delivery | 57 (46.3)d | 98 (79.7)e | 101 (86.3)f |
Data presented as mean ± SD or n of patients (%).
Group 1 were treated with low-dose aspirin and folic acid; group 2 were treated with low-dose aspirin, enoxaparin and folic acid; and group 3 were the age-matched control women without recurrent miscarriages and thrombophilia.
P < 0.001 for comparisons between a–b, a–c, d–e, d–f; Student’s t-test or Mann–Whitney U-test for continuous variables and χ2-test or Fisher’s exact test categorical data.
No significant between-group difference for b–c and e–f (P ≥ 0.05); Student’s t-test or Mann–Whitney U-test for continuous variables and χ2-test or Fisher’s exact test categorical data.
Discussion
This present study observed a significant effect on miscarriage prevention in patients with a C677T MTHFR gene mutation when a treatment combination of aspirin, LMWH and folic acid was used. To increase the power of this present study, a control group of triparous women without RM and thrombophilia were used and they demonstrated a similar delivery rate compared with the group of C677T women treated with aspirin, LMWH and folic acid (86.3% versus 79.7%, respectively). A previous study showed a pregnancy salvage rate of 43.1% with aspirin alone in women with RM and a rescue of 66.8% pregnancies with aspirin-LMWH therapy in the aspirin-failed cases.19 This previous result was in relation to the HHcy status in women with polycystic ovary syndrome, insulin resistance and obesity.19 In the present study, only 8.9% of women had HHcy (22 women with the 677TT mutation and none of those with the 677CT mutation), so this status was not the explanation for the preventive effect of the combined therapy of aspirin, LMWH and folic acid. However, the administration of 5 mg/day folic acid could have normalized the homocysteine level, but this treatment was undertaken in both groups 1 and 2. HHcy increases the monocyte secretion of procoagulants and cytokines (e.g. interleukin-8 and monocyte chemoattractant protein-1), which attract white blood cells and contribute to an alteration of the endothelium that results in venous thrombosis and placental insufficiency.20 In a review of the literature on HHcy that included 35 studies with a total of 7167 patients, first-trimester miscarriages were shown to be associated with HHcy with a relative risk (RR) of 6.25 (95% confidence interval [CI] 1.37, 28.42).21 In contrast, this hypothesis was not supported by two other studies that showed that: (i) homocysteine levels did not appear to be related to the MTHFR genotype; and (ii) first-trimester RM also occurred in patients with normal plasma homocysteine levels.22,23 In a study of 200 Tunisian women with three or more previous miscarriages (n = 726, between 7 and 32 weeks of amenorrhoea) and 200 control women free of fetal loss or complications of pregnancy, the frequency of CT or TT MTHFR gene mutations was significantly higher in the RM group than in the control group (P < 0.001).24
The negative effect of MTHFR gene mutations on RM remains the subject of considerable debate. Different studies have demonstrated higher frequencies of MTHFR mutations in cases of RM,25–28 with a RR for 677TT between 1.4 (95% CI 1, 2)10 and 3.7 (95% CI 1.2, 11.8). In contrast, two other studies failed to find a relationship between the C677T MTHFR gene polymorphism and RM.29,30 Recently, a large meta-analysis (3559 RM versus 5097 controls) reported an odds ratio of 1.68 for 677TT versus total genotypes (95% CI 1.32, 2.13; P < 0.0001), 1.35 for 677CT and 677TT genotypes combined versus total genotypes (95% CI 1.04, 1.76; P = 0.0224) and 1.34 for T versus total alleles (95% CI 1.13, 1.58; P = 0.0008).31
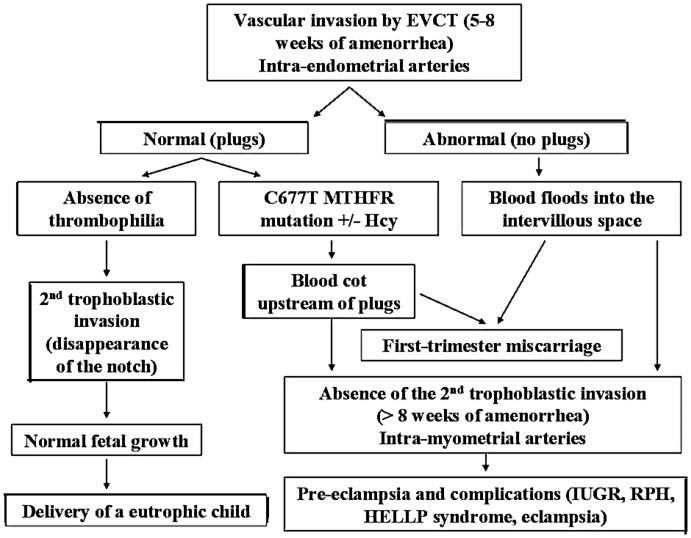
This present study hypothesized that only C677T MTHFR gene mutations without HHcy (91% of the women did have not HHcy) could be linked to RM by defective utero-placental vascularization and trophoblast apoptosis. At the start of pregnancy (between 5 and 8 weeks of amenorrhoea), the uterine spiral arteries are obstructed by plugs of trophoblasts, which are essential for subsequent development of the pregnancy. From 8 to 13 weeks of amenorrhoea onwards, disaggregation of the plugs prompts the second migration of trophoblasts into the maternal arteries and the invasion of the myometrial spiral uterine artery wall (disappearance of the notch).32 In cases of thrombotic factors such as C677T MTHFR gene mutations, clots form upstream of the plugs and persist after plug disaggregation, leading to a blockage of the chorionic villous vascularization and the absence of the second trophoblast migration (Figure 1).32 These anomalies are associated with an increase of pregnancy-associated vascular pathologies, such as pre-eclampsia, vascular intra-uterine growth restriction and placenta abruption;33,34 and a higher frequency of C677T MTHFR gene mutations was found in patients presenting with these pathologies.35
Figure 1.
Physiopathology of the methylenetetrahydrofolate reductase (MTHFR) gene mutation’s thrombotic effects during pregnancy.32 EVCT, extra-villous cytotrophoblast; Hcy, homocysteine; IUGR, intra-uterine growth restriction; RPH, retro-placental haemorrhage; HELLP, haemolysis–elevated liver enzymes–low platelet count.
With regard to preventing RM, because antithrombotic treatment in thrombophilic pregnant women with previous RM has been shown to improve their pregnancy-related prognosis,36–38 the possibility of starting early antithrombotic treatment has been studied. However, the results of several studies were very different when comparing enoxaparin versus placebo (live birth rate: 81% versus 41%, respectively)14 and versus aspirin (86% versus 29%, respectively);13 or, as in others studies, showed no difference (95% versus 88% for enoxaparin versus placebo, respectively;16 and 81% versus 84% for enoxaparin versus aspirin, respectively).15 In these studies, many different types of thrombophilia (V and II mutations, anti-thrombin III, S and C protein deficiencies) were included and could have explained the variations of the results. In contrast, two previous studies failed to report an effect of heparin treatment in women with unexplained recurrent miscarriages.39,40
In the present study, when only women with the C677T MTHFR gene polymorphism were included, a significant effect of LMWH was reported, as observed in other studies.41,42 In a study of 351 women with RM (12% of whom carried C677T MTHFR gene mutations) and treated with aspirin and LMWH until live birth term, a normal pregnancy outcome was reported in 94% of cases.41 During the follow-up of eight patients who had experienced 20 first-trimester abortions and had normal homocysteine levels, preventive treatment with LMWH from the beginning of pregnancy onwards was evaluated.42 Three pregnancies of the eight treated patients had been achieved, with two full-term births at the time of publication.42 The effect of combining treatment with folic acid was previously evaluated in women with HHcy and three or four previous miscarriages.43 The study demonstrated that 1 month of treatment with 15 mg/day folic acid and 750 mg/day vitamin B6 led to a significant decrease (>90%) in the risk of first-trimester miscarriage.43 In contrast, folic acid supplementation (0.4 mg/day) in a population of 23 806 primiparous Chinese women did not have a positive effect on miscarriage.44 In this study, the miscarriage rate was the same regardless of whether the patients were supplemented or not (9.0% versus 9.3%, respectively; RR 0.97; 95% CI 0.84, 1.12),44 which was possibly due to the low dose of folic acid used. In the present study, where all the women received the same dose of folic acid (5 mg/day), the results showed that folic acid was not sufficient to explain the difference in the rate of RM.
In conclusion, the present study has demonstrated that in women with recurrent miscarriages who are carrying a C677T MTHFR gene mutation, preventive treatment with aspirin, LMWH and folic acid was effective at increasing the delivery rate. The benefit of anticoagulation did not appear to be related to the thrombogenic effect of homocysteine.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Quenby SM, Farquharson RG. Predicting recurring miscarriage: what is important? Obstet Gynecol 1993; 82: 132–138. [PubMed] [Google Scholar]
- 2.Regan L, Braude PR, Trembath PL. Influence of past reproductive performance on risk of spontaneous abortion. BMJ 1989; 299: 541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril 1996; 66: 24–29. [PubMed] [Google Scholar]
- 4.Mahjoub T, Mtiraoui N, Tamim H, et al. Association between adverse pregnancy outcomes and maternal factor V G1691A (Leiden) and prothrombin G20210A genotypes in women with a history of recurrent idiopathic miscarriages. Am J Hematol 2005; 80: 12–19. [DOI] [PubMed] [Google Scholar]
- 5.Kovalevsky G, Gracia CR, Berlin JA, et al. Evaluation of the association between hereditary thrombophilias and recurrent pregnancy loss: a meta-analysis. Arch Intern Med 2004; 164: 558–563. [DOI] [PubMed] [Google Scholar]
- 6.Bates SM. Consultative hematology: the pregnant patient pregnancy loss. Hematology Am Soc Hematol Educ Program 2010; 2010: 166–172. [DOI] [PubMed] [Google Scholar]
- 7.Zetterberg H. Methylenetetrahydrofolate reductase and transcobalamin genetic polymorphisms in human spontaneous abortion: biological and clinical implications. Reprod Biol Endocrinol 2004; 2: 7–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dasarathy J, Gruca LL, Bennett C, et al. Methionione metabolism in human pregnancy. Am J Clin Nutr 2010; 91: 357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munro SK, Farquhar CM, Mitchell MD, et al. Epigenetic regulation of endometrium during the menstrual cycle. Mol Hum Reprod 2010; 16: 297–310. [DOI] [PubMed] [Google Scholar]
- 10.Pozharny Y, Lambertini L, Ma Y, et al. Genomic loss of imprinting in first-trimester human placenta. Am J Obstet Gynecol 2010; 202: e1–e8. [DOI] [PubMed] [Google Scholar]
- 11.Nelen WL, Steegers EA, Eskes TK, et al. Genetic risk factor for unexplained recurrent early pregnancy loss. Lancet 1997; 350: 861–861. [DOI] [PubMed] [Google Scholar]
- 12.Derksen RH, Khamashta MA, Branch DW. Management of the obstetric antiphospholipid syndrome. Arthritis Rheum 2004; 50: 1028–1039. [DOI] [PubMed] [Google Scholar]
- 13.Gris JC, Mercier E, Quere I, et al. Low-molecular-weight heparin versus low-dose aspirin in women with one fetal loss and a constitutional thrombophilic disorder. Blood 2004; 103: 3695–3699. [DOI] [PubMed] [Google Scholar]
- 14.Fawzy M, Shokeir T, El-Tatongy M, et al. Treatment options and pregnancy outcome in women with idiopathic recurrent miscarriage: a randomized placebo-controlled study. Arch Gynecol Obstet 2008; 278: 33–38. [DOI] [PubMed] [Google Scholar]
- 15.Dolitzky M, Inbal A, Segal Y, et al. A randomized study of thromboprophylaxis in women with unexplained consecutive recurrent miscarriages. Fertil Steril 2006; 86: 362–366. [DOI] [PubMed] [Google Scholar]
- 16.Badawy AM, Khiary M, Sherif LS, et al. Low-molecular weight heparin in patients with recurrent early miscarriages of unknown aetiology. J Obstet Gynaecol 2008; 28: 280–284. [DOI] [PubMed] [Google Scholar]
- 17.Laskin CA, Spitzer KA, Clark CA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA trial. J Rheumatol 2009; 36: 279–287. [DOI] [PubMed] [Google Scholar]
- 18.Frosst P, Blom HJ, Milos R, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 1995; 10: 111–113. [DOI] [PubMed] [Google Scholar]
- 19.Chakraborty P, Banerjee S, Saha P, et al. Aspirin and low-molecular weight heparin combination therapy effectively prevents recurrent miscarriage in hyperhomocysteinemic women. PLoS One 2013; 8: e74155–e74155. doi: 10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poddar R, Sivasubramanian N, DiBello PM, et al. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: implications for vascular disease. Circulation 2001; 103: 2717–2723. [DOI] [PubMed] [Google Scholar]
- 21.Robertson L, Wu O, Langhorne P, et al. Thrombophilia in pregnancy: a systematic review. Br J Haematol 2006; 132: 171–196. [DOI] [PubMed] [Google Scholar]
- 22.Powers RW, Dunbar MS, Gallaher MJ, et al. The 677C-T methylenetetrahydrofolate reductase mutation does not predict increased maternal homocysteine during pregnancy. Obstet Gynecol 2003; 101: 762–766. [DOI] [PubMed] [Google Scholar]
- 23.Nadir Y, Hoffman R, Brenner B. Association of homocysteine, vitamin B12, folic acid, and MTHFR C677T in patients with a thrombotic event or recurrent fetal loss. Ann Hematol 2007; 86: 35–40. [DOI] [PubMed] [Google Scholar]
- 24.Mtiraoui N, Zammiti W, Ghazouani L, et al. Methylenetetrahydrofolate reductase C677T and A1298C polymorphism and changes in homocysteine concentrations in women with idiopathic recurrent pregnancy losses. Reproduction 2006; 131: 395–401. [DOI] [PubMed] [Google Scholar]
- 25.Behjati R, Modarressi MH, Jeddi-Tehrani M, et al. Thrombophilic mutations in Iranian patients with infertility and recurrent spontaneous abortion. Ann Hematol 2006; 85: 268–271. [DOI] [PubMed] [Google Scholar]
- 26.Nelen WL, Blom HJ, Steegers EA, et al. Homocysteinemia and recurrent early pregnancy loss: a meta-analysis. Fertil Steril 2000; 74: 1196–1199. [DOI] [PubMed] [Google Scholar]
- 27.Unfried G, Griesmacher A, Weismüller W, et al. The C677T polymorphism of the methylenetetrahydrofolate reductase gene and idiopathic recurrent miscarriage. Obstet Gynecol 2002; 99: 614–619. [DOI] [PubMed] [Google Scholar]
- 28.Ren A, Wang JW. Methylenetetrahydrofolate reductase C677T polymorphism and the risk of unexplained recurrent pregnancy loss: a met-analysis. Fertil Steril 2006; 86: 1716–1722. [DOI] [PubMed] [Google Scholar]
- 29.Morales-Machin A, Borjas-Fajardo L, Quintero JM, et al. C677T polymorphism of the methylenetetrahydrofolate reductase gene as a risk factor in women with recurrent abortion. Invest Clin 2009; 50: 327–333. [in Spanish, English Abstract]. [PubMed] [Google Scholar]
- 30.Wiwanitkit V. Roles of methylenetetrahydrofolate reductase C677T polymorphism in repeated pregnancy loss. Clin Appl Thromb Hemost 2005; 11: 343–345. [DOI] [PubMed] [Google Scholar]
- 31.Cao Y, Xu J, Zhang Z, et al. Association study between methylenetetrahydrofolate reductase polymorphisms and unexplained recurrent pregnancy loss: a meta-analysis. Gene 2013; 514: 105–111. [DOI] [PubMed] [Google Scholar]
- 32.Merviel P, Carbillon L, Challier JC, et al. Pathophysiology of preeclampsia: links with implantation disorders. Eur J Obstet Gynecol Reprod Biol 2004; 115: 134–147. [DOI] [PubMed] [Google Scholar]
- 33.Reginald PW, Beard RW, Chapple J, et al. Outcome of pregnancies progressing beyond 28 weeks gestation in women with a history of recurrent miscarriage. Br J Obstet Gynaecol 1987; 94: 643–648. [DOI] [PubMed] [Google Scholar]
- 34.Jivraj S, Anstie B, Cheong YC, et al. Obstetric and neonatal outcome in women with a history of recurrent miscarriage: a cohort study. Hum Reprod 2001; 16: 102–106. [DOI] [PubMed] [Google Scholar]
- 35.Kosmas IP, Tatsioni A, Ioannidis JP. Association of C677T polymorphism in the methylenetetrahydrofolate reductase gene with hypertension in pregnancy and pre-eclampsia: a met-analysis. J Hypertens 2004; 22: 1655–1662. [DOI] [PubMed] [Google Scholar]
- 36.Stephenson MD. Management of recurrent early pregnancy loss. J Reprod Med 2006; 51: 303–310. [PubMed] [Google Scholar]
- 37.Brenner B, Bar J, Ellis M, et al. Effects of enoxaparin on late pregnancy complicated and neonatal outcome in women with recurrent pregnancy loss and thrombophilia: results from the Live-Enox study. Fertil Steril 2005; 84: 770–773. [DOI] [PubMed] [Google Scholar]
- 38.Deruelle P, Denervaud M, Hachulla E, et al. Use of low-molecular-weight heparin from the first trimester of pregnancy: a retrospective study of 111 consecutive pregnancies. Eur J Obstet Gynecol Reprod Biol 2006; 127: 73–78. [DOI] [PubMed] [Google Scholar]
- 39.Visser J, Ulander VM, Helmerhorst FM, et al. Thromboprophylaxis for recurrent miscarriage in women with or without thrombophilia. HABENOX: a randomised multicentre trial. Thromb Haemost 2011; 105: 295–301. [DOI] [PubMed] [Google Scholar]
- 40.Kaandorp SP, Goddijn M, van der Post JA, et al. Aspirin plus heparin or aspirin alone in women with recurrent miscarriage. N Engl J Med 2010; 362: 1586–1596. [DOI] [PubMed] [Google Scholar]
- 41.Bick RL, Hoppensteadt D. Recurrent miscarriage syndrome and infertility due to blood coagulation protein/platelet defects: a review and update. Clin Appl Thromb Hemost 2005; 11: 1–13. [DOI] [PubMed] [Google Scholar]
- 42.Altomare I, Adler A, Aledort LM. The 5,10 methylenetetrahydrofolate reductase C677T mutation and risk of fetal loss: a case series and review of the literature. Thromb J 2007; 5: 17–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quere I, Mercier E, Bellet H, et al. Vitamin supplementation and pregnancy outcome in women with recurrent early pregnancy loss and hyperhomocysteinemia. Fertil Steril 2001; 75: 823–825. [DOI] [PubMed] [Google Scholar]
- 44.Gindler J, Li Z, Berry RJ, et al. Folic acid supplements during pregnancy and risk of miscarriage. Lancet 2001; 358: 796–800. [DOI] [PubMed] [Google Scholar]