Abstract
The significance, mechanisms and consequences of coronary microvascular dysfunction associated with diabetes mellitus are topics into which we have insufficient insight at this time. It is widely recognized that endothelial dysfunction that is caused by diabetes in various vascular beds contributes to a wide range of complications and exerts unfavorable effects on microcirculatory regulation. The coronary microcirculation is precisely regulated through a number of interconnected physiological processes with the purpose of matching local blood flow to myocardial metabolic demands. Dysregulation of this network might contribute to varying degrees of pathological consequences. This review discusses the most important findings regarding coronary microvascular dysfunction in diabetes from pre-clinical and clinical perspectives.
Keywords: Diabetes mellitus, coronary microvascular dysfunction, microcirculation, endothelial dysfunction, cardiomyopathy, artery, nitric oxide, arachidonic acid metabolites
Introduction
Diabetes mellitus, a complex metabolic disorder with an immense global health burden, leads to a wide range of vascular and non-vascular complications.1–3 Global diabetes prevalence has increased dramatically from 30 million individuals in 1985 to 382 million in 2014,1 making it a daunting challenge for health care providers all over the world.
Coronary microcirculation dysfunction associated with diabetes, although explored extensively in recent years,3 still represents a poorly understood phenomenon in the clinical setting. Endothelial dysfunction, with its unfavorable consequences in various vascular beds, has been widely recognized to be a result of pathophysiological processes in diabetes,4–6 with less information available in the context of the coronary microvasculature. Functional changes precede morphological changes,3 and therefore timely recognition of this problem and identification of appropriate action strategies will be crucial in successful future care of patients. The purpose of this review is to discuss the most important findings regarding coronary microvascular dysfunction in diabetes, from pre-clinical and clinical perspectives, because this topic has become of greater interest to researchers and clinicians in recent years. This field of research is especially relevant when considering the increasing morbidity, as well as the relatively high mortality, as discussed later. Additional knowledge and a greater awareness of the issue among professionals in a wide range of fields are needed to facilitate earlier diagnosis and development of effective treatment strategies. Thus, a review of the most essential information is useful.
Endothelial dysfunction in diabetes mellitus
Diabetes mellitus and cardiovascular disease
In individuals with type 2 diabetes, there is accelerated development and an increased incidence of atherosclerotic vascular lesions. Furthermore, cardiovascular disease, such as myocardial infarction and stroke, is 2 to 4 times more prevalent in patients with type 2 diabetes than in non-diabetic individuals. Even with impaired glucose tolerance only, there is an increased risk of atherosclerosis.7–9
Diabetic complications affect many organs and are responsible for most illnesses and deaths associated with diabetes. They can be categorized as vascular or non-vascular complications. Vascular complications can be microvascular (microangiopathy), such as retinopathy or nephropathy, or macrovascular (macroangiopathy), including cerebrovascular disease, coronary artery disease (CAD) and peripheral vascular disease. Non-vascular complications include forms of chronic neuropathy. In addition to hyperglycaemia, other factors, including hypertension, dyslipidemia, genetic factors, glucose oscillations, hypoglycaemia, obesity, coagulopathy and smoking, contribute to the development of chronic complications.7,10–12
The leading cause of death in people with diabetes is heart and blood vessel disease (70–80%). The risk of heart and blood vessel disease is 8 times higher in people with diabetes. Patients with diabetes without previous heart attacks are at higher risk of a heart attack as compared with non-diabetic patients who have previously had a heart attack. Diabetes is the most common risk factor for stroke, especially in women (5.4 times higher risk). Diabetic retinopathy is a significant cause of blindness, nephropathy is the most important cause of kidney failure, and diabetic foot is the primary cause of lower extremity amputations and the most important cause of disability in patients.7–11,13,14
Endothelial dysfunction
Normal cells can adapt to changes in the environment in multiple ways, such as through changes in the amount and effectiveness of enzymes, receptors and other proteins, hypertrophy, hyperplasia, atrophy and metaplasia. However, when the capacity of cells to adapt is exceeded and homeostasis is disturbed, there is damage that can initially manifest as dysfunction and later as morphological changes. Cell dysfunction can become evident as an increase, a reduction or an interruption of normal function in a tissue. Irreversible disorders can cause cell death (necrosis or apoptosis).15,16
The endothelium is the monolayer of cells that covers the inner vascular wall. It serves multiple functions that are indispensable for the maintenance of vascular homeostasis, including regulation of vessel integrity, vascular tone and blood flow, vascular growth and remodeling, cell adhesion, angiogenesis, tissue growth and metabolism, immune responses, hemostasis and vascular permeability.17 Damage to endothelial cell function, or “endothelial dysfunction,” leads to leukocyte adhesion and migration and loss of endothelial antiaggregation properties, resulting in platelet aggregation and prevailing of vasoconstrictive substances. Inflammatory mediators, including tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1) and endotoxin, modify and activate endothelial cell functions. This leads to adhesion molecule expression on the surface of endothelial cells (ICAM-1, VCAM-1, E-selectin and P-selectin), cytokine and chemokine secretion and reactive oxygen species production, which causes LDL-cholesterol oxidation in the subendothelial layer. Inflammatory stimuli reinforce the otherwise minimal connection between leukocytes and endothelial cells, and this process is regulated by membrane expression of adhesion molecules. Thus, the endothelium actively participates in various stages of the inflammatory response.18–24
Damaged endothelium produces less nitric oxide (NO) with a consequently reduced or paradoxical effect of vasodilators, enhanced activity of vasoconstrictors and increased platelet aggregation. Endothelial dysfunction in diabetes mellitus leads to reduced NO production through decreased endothelial NO synthase (eNOS) expression, eNOS uncoupling (a process where eNOS is converted from an NO-producing enzyme to one generating superoxide anions) as a result of oxidative stress and accelerated NO breakdown (mostly caused by reactive oxygen species).17 In addition to the reduced NO bioavailability, endothelial dysfunction in diabetes also manifests through a number of other mechanisms, including decreased endothelial prostacyclin secretion, altered endothelium-dependent hyperpolarizing factor-mediated responses, impaired fibrinolytic ability, increased procoagulant activity, overproduction of growth factors and extracellular matrix proteins, increased endothelial permeability and increased oxidative stress.17,25 The described mechanisms are also involved in the pathogenesis of atherosclerotic cardiovascular disease.26–28 Table 1 summarizes the primary endothelial dysfunction traits.
Table 1.
| Normal endothelial function | Endothelial dysfunction | Mechanisms |
|---|---|---|
| Normal NO bioavailability and vasodilation | ↓NO bioavailability and vasodilation | ↓eNOS, eNOS uncoupling, ↑NO breakdown (ROS) |
| ↑Prostacyclin | ↓Prostacyclin | ↓Prostacyclin synthase |
| ↑EDHF-mediated responses | ↓EDHF-mediated responses | |
| Normal vasoconstrictor levels | ↑Vasoconstriction | ↑ET-1, PGH2 and other endothelium-derived factors |
| Anti-inflammatory properties | Pro-inflammatory properties | ↑Adhesion molecules, ↑cytokines |
| ↓Endothelial permeability | ↑Endothelial permeability | |
| Anti-thrombotic properties | Pro-thrombotic properties | ↑PAI-1, vWF, P-selectin, ↑platelet activity |
| Normal fibrinolytic activity | ↓Fibrinolytic activity | |
| ↓Oxidative stress | ↑Oxidative stress | ↑NADPH oxidase, eNOS uncoupling, other enzymes, ↓antioxidant defense |
| Anti-atherogenic properties | Pro-atherogenic properties | ↑FFA, ↑LDL oxidation, ↑AGE + above described mechanisms |
| Normal quantity and quality of EPC | ↓Quantity and quality of EPC | Weak bone marrow mobilization, ↓proliferation, ↓survival, ↑ROS |
| Normal vascular structure and angiogenesis | Remodelling | Changes in growth factors, inflammation, matrix proteins, etc. |
EDHF: endothelium-derived hyperpolarizing factor; ROS: reactive oxygen species; PAI-1: plasminogen activator inhibitor-1; vWF: von Willebrand factor; eNOS: endothelial nitric oxide synthase; PGH2: prostaglandin H2; EPC: endothelial progenitor cells; NADPH: nicotinamide adenine dinucleotide phosphate; AGE: advanced glycation end products; LDL: low-density lipoprotein; FFA: free fatty acids.
The effect of hyperglycaemia
An important consequence of hyperglycaemia is increased non-enzymatic protein glycation. Through a series of reactions, stable glycated proteins form within the cell, on the cell surface, in the extracellular matrix and in the blood stream. The consequences are changes in enzyme activity, regulatory molecule binding, protein cross linking, proteolysis susceptibility, macromolecular recognition, endocytosis and immunogenicity, resulting in alterations in different physiological processes and the development of chronic diabetic complications. Haemoglobin glycation is the earliest and best-studied example of protein glycation. Determination of haemoglobin glycation has practical applications, because it indicates the degree of chronic hyperglycaemia.11,20,29,30 In addition to augmented formation of advanced glycation end products, hyperglycaemia leads to vascular damage in various cells of the vessel wall. This is mediated through mechanisms, including increased flux of glucose and other sugars through the polyol pathway, elevated expression of the receptor for advanced glycation end products and its activating ligands, protein kinase C isoform activation, hexosamine pathway overactivation and increased reactive oxygen species production.17
Insulin and vascular dysfunction
Insulin resistance, the defining mechanism underlying type 2 diabetes, exists when insulin levels are higher than expected relative to glucose levels. Thus, in vivo insulin resistance is tethered to hyperinsulinaemia.31 Insulin leads to increased eNOS activation (and NO production) through the phosphatidylinositol 3-kinase (PI3K)/Akt pathway. Insulin resistance in diabetes attenuates this physiological process and suppresses normal NO secretion.5 Insulin infusion in healthy human subjects stimulates vasodilation and increases peripheral tissue blood flow, whereas this effect is blunted in patients with diabetes and insulin resistance.4 Furthermore, insulin activates the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase pathway and promotes endothelin-1 expression and cellular proliferation.32 Under physiological conditions, the PI3K/Akt pathway prevails in vasomotor regulation. Under insulin resistant conditions, there is a selective deficiency in this pathway (leading to endothelial dysfunction), while MAPK pathway signaling is unaffected, leading to unfavorable effects, including further promotion of hyperinsulinaemia and atherogenesis.17,32 The hyperinsulinaemic state is associated with a number of important pathophysiological processes, such as elevated free fatty acid and pro-inflammatory mediator levels.31 The link between endothelial dysfunction and insulin resistance is also evident in intervention studies that demonstrate improved endothelial function following treatments that improve insulin sensitivity. For example, metformin, rosiglitazone, troglitazone and pioglitazone treatment have been demonstrated to improve endothelial dysfunction in various vascular beds.4
Blood glucose control and vascular complications
Several large epidemiological studies have attempted to examine the connection between diabetes management and the emergence of a number of diabetic complications.33–35 For example, the Diabetes Control and Complications Trial, a large multicenter investigation conducted in 1,400 patients with type 1 diabetes, determined that improved glycaemic control reduced nonproliferative and proliferative retinopathy by 47%, microalbuminuria by 39%, clinical nephropathy by 54% and neuropathy by 60%. The improvement in glycaemic control also slowed the deterioration of existing complications. The study results indicated no significant changes in macrovascular complications. Similar to the Diabetes Control and Complications Trial study, the United Kingdom Prospective Diabetes Study, which was conducted in more than 5,000 patients with type 2 diabetes and lasted more than 10 years, suggested an interrelation between glycaemic control and the development of diabetic complications. Although no statistically significant effect of blood glucose levels on cardiovascular complications was demonstrated, there was a 16% reduction in myocardial infarctions. Improved blood glucose control did not consequently reduce nor increase mortality from macrovascular complications. Similar results regarding reduced microvascular complication risk were also determined in a small study on thin Japanese patients with type 2 diabetes (Kumamoto study).33–35 Firm evidence that improved glycaemic control in type 2 diabetes mellitus can reverse or prevent cardiovascular disease remains incomplete and requires large, long-term clinical trials in patients with diabetes at low risk utilizing modern treatment strategies.36
Physiology of coronary microcirculation
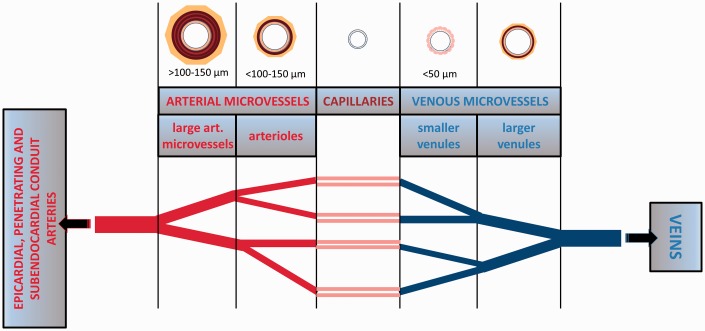
Coronary microvascular networks are crucial for matching local blood flow to tissue metabolic demands by precise regulation of smooth muscle tone, vessel diameter and substance transport.37,38 Although coronary microvessels can broadly be divided into “arterial microvessels” (through which blood flows towards capillaries) and “venous microvessels” or “venules” (those that drain blood from capillaries), there are no uniform term definitions for the designation of microvascular segments, such as small arteries, arterioles and venules.39 Classification of microvessels based on structural characteristics is somewhat arbitrary, with no abrupt demarcation in the transition between small artery and arteriole. Some studies define “microvessels” as vessels <300 µm in internal diameter.37,39 Figure 1 illustrates the different microvessel categories.
Figure 1.
Overview of different microvessel categories in the heart.
Arterial microvessels consist of a thin tunica intima, a thick tunica media composed of one to several layers of smooth muscle cells (“arterioles” possess 1–4 layers and are typically defined as <100–150 µm in diameter, whereas “large arterial microvessels” possess 4–6 layers, can be >100–150 µm in diameter and can also be called “small arteries”) and a tunica adventitia.39 Venous microvessels have thinner vascular walls compared with those of arterial microvessels, with venules <50 µm in diameter and not possessing smooth muscle cell layers. Smaller venules possess only endothelial cells and pericytes.39 From epicardial conduit arteries, numerous arterial branches arise that contribute to perfusion of the outer two-thirds of the myocardium. Penetrating arteries pass through the outer myocardial layers without branching. Ultimately, in the inner one-third of the myocardium, small branches arise from penetrating arteries to form the arterial plexus that perfuses the subendocardium.39
Oxygen extraction is almost maximal in the coronary circulation, and therefore blood flow must increase proportionally to increased oxygen demand. Myocardial oxygen consumption increases by up to four times during severe exercise compared with consumption at rest.40 When oxygen consumption is kept constant, coronary blood flow is constant and independent of coronary artery pressure variations. In contrast, when pressure is kept constant, variations in oxygen consumption lead to linear flow variations.40 However, when tissue regions smaller than 1 g are considered, flow distribution is heterogenous.40 Precise regulation of functionally distinct microvascular categories (for example, capillaries and venules where water and solute are primarily exchanged, or arterioles where vascular resistance is predominantly adjusted) is achieved with specialized mechanisms.41 Neurogenic, hormonal, metabolic, myogenic and flow (shear stress-induced) endothelial mechanisms control coronary vascular resistance at specific microvascular sites.40–42 Segments most sensitive to flow are coronary arterioles with diameters of 120–150 µm. The greatest myogenic responsiveness was observed in small arterioles with diameters of 30–60 µm. Arterioles <30 µm appear to be most sensitive to metabolic stimuli. Flow-induced dilatation is also a regulating mechanism in isolated coronary venules and could be important during arteriolar dilatation, when it would limit the rise in capillary pressure that occurs with decreased arteriolar resistance.41 Integration of multiple mechanisms could hypothetically form a system matching coronary blood flow to myocardial metabolic demands. However, there are many unknown or incompletely understood factors involved, including unclear precise mediators of the metabolic mechanisms that have traditionally been attributed as important in this system.40,41 Reactive oxygen species, which are metabolites produced in proportion to oxidative metabolism, may also control coronary blood flow,42 a phenomenon that is incompletely understood.
In addition to adaptation to acute physiological stresses, such as increased myocardial metabolism, the coronary microcirculation can also adapt to chronic stresses. For example, exercise training induces numerous adaptive changes that may be responsible for a reduction in coronary heart disease.41 Exercise leads to increased blood flow capacity and transvascular exchange capacity, possibly because of remodeling of the coronary vascular bed and alterations in vascular control. Exercise can alter microvascular responses to mechanical stimuli, such as stretch and flow, increase NO synthase levels in the coronary microvasculature and possibly alter coronary vascular permeability regulation.41 Structural remodeling and angiogenesis are important vascular regulatory mechanisms in the coronary microcirculation,42 with structural changes ultimately affecting function.
It should be noted that the coronary vascular system is also impacted by cardiac contraction forces. The average coronary per beat blood volume is not only dependent on perfusion conditions, such as the level of vasodilation and perfusion pressure, but it also depends on changes due to cardiac contraction and is lower in systole compared with diastole. Because coronary inflow occurs primarily in diastole, time-averaged intramural blood volume lessens with increasing heart rate (which causes shortening of diastole).40 The contraction process dynamics include consequent variations in compressive forces, coronary artery pressure and intramural blood volume. Furthermore, vessels are stretched and shortened during the heart cycle, and the diameter of the elastic vessels changes as a function of the transmural pressure difference across the vascular wall. This makes the coronary vascular system very complex40 and analyses of functional regulation of the microcirculation more challenging.
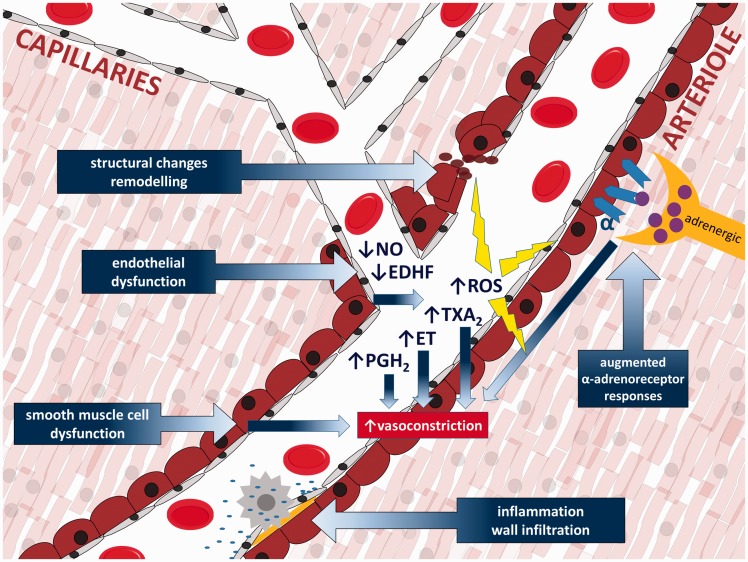
Pathophysiologic mechanisms (schematically illustrated in Figure 2) possibly implicated in coronary microvascular function disorders include: 1. endothelial dysfunction (reduced bioavailability of NO, increased oxidative stress and increased endothelial release of endothelin, thromboxane A2 and prostaglandin H2), 2. smooth muscle cell dysfunction (primary impairment of smooth muscle cell relaxation), 3. microvascular spasm/sympathetic dysfunction (augmented responses to α-adrenoceptor activation) and 4. altered microvascular remodelling. Additional pathophysiological hypotheses include vascular rarefaction, extramural compression, vascular wall infiltration, luminal obstruction, degraded endothelial surface layer (for instance through damage caused by reactive oxygen species, ischemia, inflammation or altered plasma composition), compromised conduction of signals along the vessel wall and impaired metabolic feedback.37
Figure 2.
Primary pathophysiologic mechanisms of coronary microvascular dysfunction.
EDHF: endothelium-derived hyperpolarizing factor; ROS: reactive oxygen species; ET: endothelin; PGH2: prostaglandin H2; TXA2: thromboxane A2.
Basic aspects of diabetic coronary microvascular dysfunction
NO and arachidonic acid metabolites in diabetic coronary microvascular dysfunction
Impaired endothelium-dependent vasodilatation has been demonstrated in various microvascular beds in animal models43 and in the microcirculation of humans with diabetes.44 Diabetes mellitus is associated with coronary microvascular dysfunction, attenuated vasodilation to various agonists and reduced coronary venous pO2.45 In humans with type 2 diabetes mellitus, reduced NO production, increased reactive oxygen species generation and enhanced vasoconstrictor tone were related to impaired endothelium-dependent vasodilation.46 Although baseline myogenic tone and resting membrane potential of coronary arterioles were not affected by diabetes mellitus, both type 1 and type 2 diabetes mellitus impair human coronary arteriolar dilation to KATP opening, leading to reduced dilation to hypoxia.47 Several models of genetically altered mice have been utilized to elucidate the mechanisms underlying altered endothelium-dependent vasodilation of coronary microcirculation. The majority of the studies pointed to a role for reactive oxygen species and their interaction with vascular cell dilatory pathways. For example, recovered acetylcholine-stimulated coronary artery vasodilation, increased manganese-dependent superoxide dismutase expression and decreased nitrotyrosine levels in hearts have been observed in diabetic db/db mice that were moderately exercised. These observations suggest that increased reactive oxygen species production and nitrotyrosine levels in the heart are associated with impaired endothelium-dependent coronary artery vasodilation.48 Attenuated dilation to acetylcholine in type 2 diabetic Lepr(db) mice appears to occur because of the interaction between Nuclear factor-kappa B (NF-κB) and TNF-α signaling, which induces inhibitor of nuclear factor kappa-B kinase subunit beta (IKK-β) activation and increases oxidative stress.43 Similarly, in coronary microvessels from diabetic dogs, bradykinin and acetylcholine caused lesser increases in NO production than that in non-diabetic dogs.49 Functional studies of small coronary arteries in a preatherosclerotic porcine model with type 2 diabetes characteristics indicated coronary microvascular dysfunction, including impaired bradykinin-induced vasodilatation because of NO loss and reduced vasoconstriction to endothelin-1 because of decreased endothelin ETA receptor dominance.50
Although NO is the most important vasodilatory metabolite in the coronary circulation, NO loss or reduction has been shown to be associated with increased activity of endothelium-derived hyperpolarizing factors, such as cyclooxygenase (COX)-2-mediated production of vasoactive prostaglandins. This may present an adaptive mechanism for reduction of the debilitating effects of diabetes on coronary blood flow, as demonstrated in human coronary arterioles.45 However, impaired endothelium-dependent dilation in diabetes might also be related to the release of vasoconstrictor mediators, such as increased 20-hydroxyeicosatetraenoic acid (20-HETE) production. This may lead to the activation of reactive oxygen species through an NADPH-dependent pathway,51 increased oxidative stress and overexpression of both COX-1 and COX-2,52 TXA2-mediated contraction in diabetic arteries53 and low-grade inflammation.45 Hyperglycaemia increases COX-2 expression,54 arachidonic acid release and prostanoid production, all of which may modify vasomotor tone.55
Peroxisome proliferator-activated receptor-γ, a regulator of lipid and glucose metabolism, may play a role in diabetic coronary microvascular dysfunction.3 It was demonstrated that treatment of type 2 diabetic mice with rosiglitazone (peroxisome proliferator-activated receptor-γ activator) increased NO-mediated flow-dependent dilations of coronary arterioles by reducing vascular superoxide production. These changes were associated with a favorable alteration of oxidant/antioxidant enzyme activities.3
Diabetes upregulates cytochrome P450 (CYP) 4A isoforms and leads to elevated 20-HETE levels,56 while the 20-HETE inhibitor HET0016 attenuates the development of vascular dysfunction induced by diabetes.57 In addition, recent findings suggest that insulin-stimulated vasodilator effects that are mediated by the insulin receptor substrate 1 (IRS-1)/PI3K/Akt/eNOS pathway are impaired by 20-HETE.57 Elevated CYP-derived 20-HETE levels in patients with diabetes and cardiac ischemia are associated with dysfunction of circulating endothelial progenitor cells and angiogenic capacity.58 In a glucose intolerance model, elevated soluble epoxide hydrolase expression might contribute to endothelial dysfunction through increased degradation of epoxeicosatrienoic acids.59 Data from a diabetic model reveal a different mechanism, specifically a shift in the balance between 20-HETE and epoxeicosatrienoic acid production caused by changes in CYP4A3 and CYP2J4 expression.59–61
Reactive oxygen species and transient receptor potential vanilloid type 1 (TRPV1) receptor–mediated responses
The toxic effect of a chronic hyperglycaemic state, as in diabetes mellitus, is a well-known generator of oxidative stress in all vascular beds. A number of animal studies have demonstrated deleterious effects of diabetes mellitus related to a hyperoxidative status on cardiac endothelial function.62–64 While the role of different potassium channels in vascular tone regulation in the coronary vasculature is well known,62,65,66 the roles of other pathways, including the TRPV1 receptor-activated pathway, are under investigation. The TRPV1 protein is highly expressed in canine coronary arteries.67
To elucidate whether TRPV1 expression and/or function is altered by metabolic syndrome, Bratz et al. used coronary arteries from lean and obese Ossabaw miniature swine. Capsaicin, a TRPV1 agonist, caused dose-dependent relaxation, which was inhibited by capsazepine (a TRPV1 antagonist), endothelial denudation, potassium channel inhibitors and NOS inhibition. The capsaicin effect was blunted in pigs with metabolic syndrome. TRPV1 expression and capsaicin-mediated divalent cation influx was significantly higher in the lean swine group. This observation suggests that TRPV1 channels are functionally expressed in the coronary circulation and mediate endothelium-dependent vasodilation with mechanisms that include NO and potassium channels.68
In db/db diabetic mice, capsaicin-mediated increased coronary blood flow was attenuated, while control C57BKS/J mice displayed an increased vasodilator response in a capsaicin dose-dependent manner. Mice lacking TRPV1 [TRPV1(-/-)] did not display changes in coronary flow. Capsazepine and the NOS inhibitor L-NAME blocked the response in control mice. In addition, pH changes (from pH 7.6 to pH 6.0) caused relaxation of previously contracted coronary arteries with a thromboxane mimetic, but this effect was significantly less in the TRPV1(-/-) and db/db groups. The TRPV1 inhibitor SB366791 l-NAME and the large conductance calcium-sensitive potassium channel inhibitor iberiotoxin inhibited capsaicin-dependent relaxation in pressurized coronary vasculature. This observation indicates that TRPV1 couples metabolic needs of the myocardium with coronary blood flow via large conductance calcium-sensitive potassium channels and the NO pathway.69
DelloStritto et al.70 analyzed the role of TRPV1 in coronary vascular reactivity in an H2O2-regulated environment in a mouse model of diabetes. The wild type C57BKS/J (WT) mice responded with coronary blood flow vasodilatation in an H2O2 dose-dependent manner, while this response was blocked by the TRPV1 antagonist SB366791. In TRPV1(-/-) and db/db diabetic mice, H2O2 vasodilative responses were significantly inhibited. WT, but not db/db or TRPV1(-/-), mice displayed TRPV1 agonist SB366791-sensitive dilation in the presence of H2O2. Acute H2O2 exposure potentiated capsaicin-mediated vasodilative responses in WT mice, while extended H2O2 exposure attenuated this effect. Electrophysiological experiments that included bovine aortic cells and isolated mouse coronary endothelial cells confirmed the role of TRPV1 in coronary microcirculation. Considering the results of this study, it appears that prolonged H2O2 exposure impairs TRPV1-dependent coronary vascular signaling and can be related to coronary endothelial dysfunction in diabetes.
Based on these studies, it is apparent that hypertension, hyperlipidemia and diabetes, which occur combined in metabolic syndrome, can underlie pathologic changes in the coronary circulation.
Clinical aspects of coronary microvascular changes in patients with diabetes
Diabetes mellitus is a metabolic disease with rapidly increasing prevalence. Sedentary lifestyle, ageing, overfeeding and obesity lead to diabetes mellitus and result in over 80% of cardiovascular deaths among these patients.71 Clinical and experimental studies have demonstrated compromised cardiac function as a result of altered myocardial metabolism caused by diabetes, with a mismatch of myocardial supply and demand.72 Cardiovascular consequences of diabetes are atherosclerotic epicardial CAD and cardiomyocyte and myocardium changes. Diabetes mellitus is a risk factor for cardiovascular disease, independent of age, hypertension, obesity and hyperlipidemia.73 The term diabetes-associated cardiovascular dysfunction includes a number of myocardial changes, including functional, metabolic and structural changes.74,75
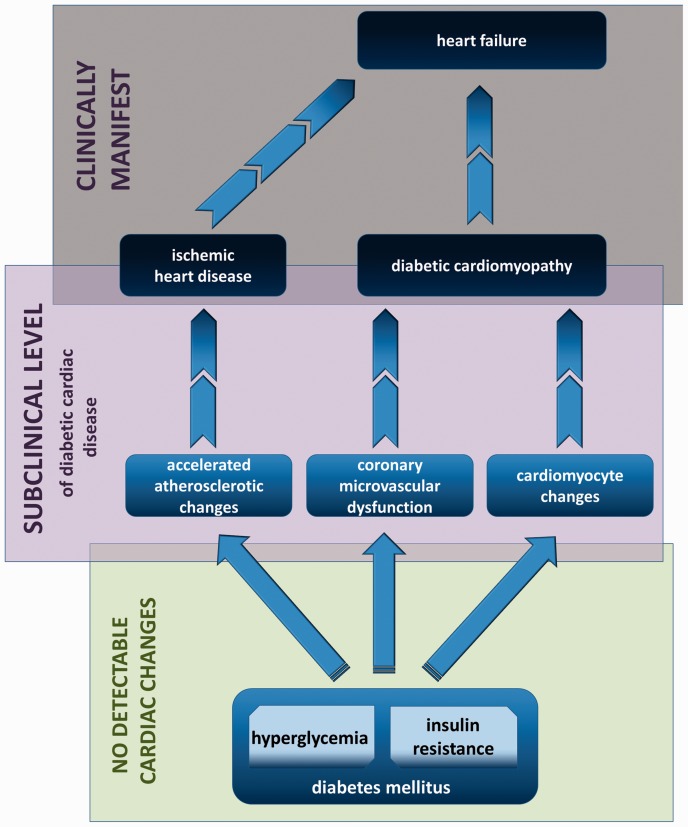
Subclinical level of disease
The subclinical level of diabetes-induced myocardial changes can be categorized as atherosclerotic changes of epicardial coronary arteries or microvascular coronary endothelial dysfunction with cardiomyocyte changes (schematically illustrated in Figure 3). The endothelium is a central regulator of vascular homeostasis. This includes hormone transport and distribution, metabolic waste product disposal and regulation of regional blood flow by synthesis and release of different mediators with opposing vascular properties (NO, prostacyclin and endothelial-derived hyperpolarizing factor are balanced by Endothelin 1 (ET-1), angiotensin II and thromboxane A2 to assure maintenance of nutrient and oxygen delivery to tissues by controlling vascular tone, vascular permeability and hemostasis).76 The key pathophysiologic process of diabetes-induced coronary microvascular dysfunction is impaired coronary arteriole vasomotion, including impaired endothelial-mediated vasodilation, hypoxia-induced vasodilation and myogenic response.72 Vasomotor dysfunction, affecting both smooth muscle- and endothelium-mediated mechanisms, is an early change, with long-term structural changes and diabetic microangiopathy development. Hyperglycaemia, as a primary pathogenic mechanism together with insulin resistance, hyperinsulinaemia, TNF-α overexpression and inflammation, supresses flow-mediated endothelial-dependent vasodilatation via decreased NO levels and increased ET-1 levels, causing acute intracellular changes.72,76,77
Figure 3.
Clinical progression of diabetes-induced adverse cardiac changes.
One of the primary physiological stimuli involved in local regulation of arterial diameter is intraluminal blood flow, predominantly via NO endothelium-dependent vasodilation. NO production and vasodilation is reduced in diabetic animals and patients with diabetes, with reduced response to pharmacological and mechanical stimuli.72,78 Structural changes in microvessels are a sign of chronicity, manifested as a thickened capillary basement membrane, microaneurisms, spiral deformation, medial arteriole thickening, perivascular fibrosis and constriction, reduced vascular density as a result of vascular endothelial cell growth factor disturbances, reduced angiogenesis and perivascular fibrosis.79–81 Additional mechanisms of diabetes-related cardiomyocyte changes in contractility and stiffness include: altered Ca2+ handling with decreased myofilament function; increased mitochondrial and non-mitochondrial reactive oxygen species formation and impaired antioxidant defenses that contribute to oxidative stress in type 1 and type 2 diabetes mellitus (with increased DNA damage and loss of activity DNA repair pathways promoting abnormal cardiac remodeling and cell death); decreased mitochondrial dysfunction as a result of functional and structural changes with reduced ATP production and contractility; decreased glucose oxidation and increased fatty acid oxidation; increased lipotoxicity; accumulation of ceramides; increased renin-angiotensin-aldosterone system activation with oxidative stress damage; endothelial/cardiomyocyte cell apoptosis and necrosis; and myocardial fibrosis, collagen formation, increased thrombosis, decreased fibrinolysis and autonomic dysfunction with sympathetic overdrive.72,77,82–84
Elucidation of the molecular mechanisms underlying endothelial physiology is crucial for a better understanding of the pathophysiology involved in endothelial dysfunction during diabetes. Diabetic endothelial dysfunction involves a complex set of changes that includes metabolic and functional alterations, microvascular and neurohormonal disturbances and autonomic dysfunction. A better understanding of these processes may help in the development of novel approaches for early detection of resulting cardiovascular complications and eventual diabetic cardiomyopathy (DMCMP).76,84
Coronary microvascular disease diagnosis requires the exclusion of abnormalities and significant stenosis of major epicardial vessels by coronary angiography. In patients with suspected coronary microvascular disease, attempts should not be made to rely on this exclusion, but rather to obtain objective evidence of coronary microvascular disease.
Non-invasive diagnostic methods
Microvascular disease should be suspected when chest pain persists after physical effort and shows slow or no response to short-acting nitrates.85,86 Transthoracic Doppler echocardiography (TTDE) is an initial screening method to detect significant microcirculation impairment. It allows the measurement of coronary blood flow (CBF) velocity in the left anterior descending artery. With this method, coronary microvascular dilatator function is calculated as the ratio of diastolic CBF velocity at peak vasodilatation to CBF velocity at rest.87 A ratio < 2.0 in response to adenosine or dipyridamole strongly suggests coronary microvascular dysfunction. Mild coronary microvascular dysfunction may not be identified by TTDE, and microvascular dysfunction can be assessed only in the left anterior descending artery perfusion area, because other coronary arteries are not well visualized.87
Contrast stress echocardiography is promising for coronary microvascular disease detection and may be used when TTDE results are inconclusive or unreliable.87 Yağmur et al.88 demonstrated impaired longitudinal systolic strain on speckle tracking echocardiography despite normal systolic parameters. 2-D-speckle tracking echocardiography is a feasible technique that allows the evaluation of left ventricular regional systolic function in patients with microvascular dysfunction. This study contributed to our understanding of the relationship between fiber architecture, myocardial function and coronary microvascular disease. Leung et al.89 demonstrated that left ventricle contractile reserve with low dose dobutamine echocardiography can be used to non-invasively estimate the index of microcirculatory resistance. Impaired contractile reserve in this study indicated coronary microvascular dysfunction.
Regional left ventricle myocardial perfusion reserve (ratio between maximal hyperemic and basal myocardial blood flow) can be estimated in patients with diabetes with single photon technology, which is broadly available. Myocardial blood flow index can be determined from gated Single-photon emission computed tomography (SPECT) images using 99 mTc-labelled tracers by measuring first transit counts in the pulmonary artery and myocardial count rate.90
Coronary magnetic resonance imaging, with a pharmacological stress test and gadolinium as a flow tracer, is a relatively new noninvasive method to detect microvascular angina. Currently, this is still a complex, expensive and time-consuming technique for widespread utilization.91,92 Positron emission tomography (PET) allows measurement of CBF per gram of myocardial tissue and can be used to identify coronary microvascular dysfunction.93 However, coronary flow reserve (ratio of maximal hyperemic to basal CBF) may be preserved in mild forms of coronary microvascular disease. PET can also detect coronary vasomotor abnormalities caused by microvascular disease.94,95 Recent studies suggest that 82Rb PET/CT is emerging as an important as well as rapid non-invasive assessment method of microvascular function and structure in asymptomatic patients with type 2 diabetes.96 Some authors consider PET as the gold standard for myocardial flow reserve measurements and myocardial viability assessments.97
The objective documentation of myocardial ischemia in patients with microvascular dysfunction can be obtained with the use of specific tests. For example, the assessment of lipid peroxidation products in the coronary sinus after stress tests represents a sensitive method to detect myocardial ischemia in patients with microvascular dysfunction.87 Unfortunately, this method is invasive and cannot be proposed for routine application. Coronary magnetic resonance spectroscopy may detect phosphorus metabolism abnormalities after a stress test,98 but this method is very expensive and can only detect anterior heart wall defects.
Invasive diagnostic methods
In patients with unstable microvascular angina, a standard ECG record can detect newly developing abnormalities, including ST depression, T-wave inversion and elevation of cardio-sensitive markers, especially troponin. Unstable microvascular angina diagnosis requires exclusion of coronary spasm and coronary thrombosis. Patients with coronary spasm typically have chest pain after physical effort and display ST elevation in an ECG. Thrombosis is unlikely when there is no evidence of atherosclerosis or coronary abnormalities, but some conditions can be associated with thrombus formation in vessels without atherosclerosis.99 Coronary microvascular disease diagnosis can be confirmed with vasoconstrictor agents, such as ergonovine and acetylcholine, and should be performed during coronary angiography. An induction of angina and ST changes without epicardial vasoconstriction would be diagnostic for coronary microvascular disease.
Coronary angiography allows us to assess and analyze coronary microvascular function with several different methods. Some of these methods include thermodilution, the gas washout method and intracoronary Doppler wire flow. All of these methods allow CBF and coronary flow reserve quantification.
Intravascular Doppler ultrasonography (IVUS) allows us to visualize the state of arterial walls and to detect atherosclerotic plaques that cannot be adequately observed on angiograms. IVUS is a method that is primarily applied in the intracoronary recording of CBF using Doppler and pressure wires and allows exact measurement of CBF velocity. Using intracoronary Doppler and pressure sensors we can determine the index of microvascular resistance. This is defined as the distal coronary pressure multiplied by the exact transit time of reactive hyperemia.100
It should not be forgotten that a “normal” coronary angiogram does not necessarily rule out an atherosclerotic plaque. With IVUS we can detect non-obstructive atherosclerotic plaques in coronary arteries. Often in diffuse CAD, the wall thickens outward without forming notches in the lumen. In patients who have diffuse epicardial artery disease but without significant proximal stenosis, fractional flow reserve analysis can be performed. Fractional flow reserve is the ratio between the distal coronary pressure and the aortic pressure during maximal coronary vasodilatation. A value <0.8 is suggestive of hemodynamically significant plaques that are perhaps not yet obstructive. Thus, we can avoid erroneously attributing patients’ symptoms to microvascular dysfunction.101
Coronary angiography allows indirect evaluation of microvascular function by measurement of angiographic indexes. Myocardial blush is the myocardial opacification that results from injection of dye into the coronary circulation. We can determine the myocardial blush grade by counting the number of heart cycles required for the dye to fade out, which depends on the microcirculatory resistance to contrast passage and venous drainage efficiency.100,102 The total myocardial blush score is the sum of the myocardial blush grade of each coronary territory and defines the overall microvascular functionality.102
The TIMI frame count (TFC) can be calculated according to the number of frames that are required for contrast to reach the standardized distal coronary artery landmark and using a correction factor that depends on vessel length. TFC is related to the velocity of the contrast filling the epicardial vessel and the microvascular district resistance index.103 TFC, similar to total myocardial blush score, represents the sum of three major epicardial artery scores and is useful for coronary microcirculation assessment.104
Studies linking diabetes to coronary microvascular dysfunction in human patients
There is a high prevalence of coronary microvascular dysfunction in patients with type 2 diabetes who are free of overt cardiovascular disease.96 Von Scholten et al. measured coronary flow reserve with cardiac 82Rb PET/computed tomography in a cross-sectional study. Reduced coronary flow reserve was significantly more common in patients with diabetes, particularly those with concomitant albuminuria, than in control subjects, suggesting that a common microvascular impairment is occurring in multiple microvascular beds. Di Carli and coworkers measured myocardial blood flow (using PET with N-13 ammonia as the flow tracer) at rest during adenosine-induced hyperaemia and in response to a cold pressor test in healthy control subjects and patients with type 1 and type 2 diabetes who were free of overt cardiovascular complications. They confirmed markedly reduced endothelium-dependent (adenosine-induced hyperaemia) and -independent (cold pressor test) coronary vasodilator function in subjects with diabetes, which were similarly reduced in both type 1 and type 2 diabetes. The differences compared with control subjects persisted after adjusting for diabetes mellitus duration, insulin treatment, metabolic abnormalities and autonomic neuropathy. The data suggest an important pathogenetic role of chronic hyperglycaemia in diabetic vascular dysfunction.105 An impaired vasodilator response of coronary resistance vessels to increased sympathetic stimulation was associated with diabetic autonomic neuropathy and related to the degree of sympathetic nerve dysfunction.106 Separate studies in young patients with type 1 diabetes without coronary heart disease suggested impairment of coronary vascular reactivity, as determined by PET and H2O measurements of myocardial blood flow at rest and after dipyridamole administration.107 In patients with type 2 diabetes, similar measurements (but using PET and N-13 ammonia) revealed reduced myocardial flow reserve.108 Myocardial blood flow at rest was comparable between healthy controls and patients with type 2 diabetes, whereas myocardial blood flow after dipyridamole administration was significantly lower in patients with diabetes.108 Moreover, myocardial flow reserve was inversely correlated with average haemoglobin A1C for 5 years and fasting plasma glucose levels, implying that glycaemic control is related to coronary microvascular function.108 In patients with type 2 diabetes, application of the SPECT method also documented the presence of microvascular dysfunction, which was homogeneously distributed throughout the walls of the left ventricle and most frequently not associated with reversible perfusion defects.90 Invasive studies in patients with diabetes with angiographically normal coronary arteries and normal left ventricular systolic function demonstrated impaired coronary vascular reserve (measured by intracoronary Doppler with an intracoronary maximally vasodilating dose of papaverine) and acetylcholine-induced coronary vasodilation.109 These coronary microcirculatory alterations in diabetes may contribute to progressive myocardial deterioration and DMCMP pathogenesis.109
DMCMP
DMCMP is a multifactorial disease and a result of complex pathophysiologic processes.74 The impact of diabetes on atherosclerotic vascular disease has been well established, but non-ischemic heart failure in patients with diabetes has received less attention.81 DMCMP is defined as structural and functional abnormalities of the myocardium in patients with diabetes who have no coronary disease or hypertension.110
From a histological perspective, DMCMP is characterised by the loss of normal microvessels, extracellular matrix remodelling, capillary basement membrane thickening, interstitial and perivascular fibrosis, cardiomyocyte hypertrophy independent of hypertension and cardiomyocyte atrophy.81,111 The Strong Heart Study reported that both men and women with type 2 diabetes mellitus have increased left ventricle mass and wall thickness,112 because hyperinsulinaemia as a result of insulin resistance can act as a growth factor.75 Obesity is an additional risk factor in type 2 diabetes mellitus, where increased intramyocardial lipid levels act as lipotoxic deposits that can contribute to cell death via as of yet unexplained mechanisms and, from a clinical point of view, lead to diastolic dysfunction.113,114
After myocardial infarction, the diabetic myocardium possesses reduced compensatory mechanisms to restore cardiac function because of a complex set of intra- and extra-myocardial factors.115 From a clinical perspective, type 1 and type 2 diabetes mellitus differ in aetiology, clinical presentation and metabolic profiles with adverse clinical presentation. However, the two types share many features of cardiomyopathy.81 Systolic dysfunction is usually predominant in type 1 diabetes, where autoimmunity predisposes to a dilative phenotype and heart failure with reduced ejection fraction, or is related to atherosclerotic coronary and chronic ischemic heart diseases.116 Coronary microvascular dysfunction in type 2 diabetes is predominantly the result of hyperglycaemia, lipotoxicity and hyperinsulinaemia. This occurs independently or in combination with other risk factors and leads primarily to diastolic dysfunction, concentric left ventricle remodelling and clinical presentation of heart failure with preserved ejection fraction. Furthermore, there is a progressive problem manifesting in myocardial stiffness (impaired relaxation and passive filling) and restrictive pattern development, with or without subclinical levels of depressed left ventricle systolic function.71,116–118 Both types of diabetes have the same myocardial results, including coronary microvascular rarefaction and advanced glycation end-product depositions.116
DMCMP is highly prevalent in asymptomatic patients with type 2 diabetes. Screening and early detection of myocardial changes may be crucial for slowing heart failure development and progression. Thus, early determination of myocardial manifestations can have major implications for prognosis in patients with diabetes. The most sensitive test for cardiac systolic and diastolic function is transthoracic echocardiography.119 Schwanell and coworkers demonstrated that diastolic left ventricular dysfunction is a first sign of myocardial changes even in younger patients with normal systolic function detected by transthoracic echocardiography.120 Poirer and coworkers confirmed this finding in male patients with well-controlled type 2 diabetes. The authors used the Valsava manoeuvre and pulmonary venous recordings, together with a standard echocardiographic transmitral left ventricular filling pattern of abnormal relaxation and/or pseudonormal filling, to unmask pseudonormal patterns of ventricular filling.121 Tissue Doppler imaging, which can measure myocardial tissue velocities in the longitudinal direction, is more sensitive for the detection of left ventricular dysfunction. The peak early diastolic myocardial velocity reflects global left ventricular diastolic dysfunction.81 Additional echocardiographic tools include strain echocardiography, in which measurement of global longitudinal strain can detect myocardial abnormalities before left ventricular ejection fraction declines.122
Prognosis
A clear strategy for prevention or treatment of diabetes-induced/related cardiomyopathy and, consequently, a prognosis have not yet been established.81 The increasing number of both basic research and clinical studies, particularly those that develop novel imaging techniques, are promising for detection of early microvascular functional changes and early changes in the myocardium in diabetes. Interestingly, there is evidence that patients with diabetes with a preserved coronary flow reserve have cardiac event rates similar to those of non-diabetic patients.\123 Additionally, among diabetic patients without evident CAD, individuals with impaired coronary flow reserve (below the median) have mortality rates comparable to non-diabetic patients with prior CAD (cardiac death rate 2.8 vs. 2.0% per year).123 This strongly implies that it is crucial to focus on the development and utilization of therapeutic strategies that will preserve normal coronary microvascular function in patients with diabetes. Cortigani et al.124 demonstrated that coronary microvascular dysfunction before the occurrence of coronary artery involvement is an independent and strong predictor of adverse outcomes in patients with type 2 diabetes. They investigated Doppler-derived coronary flow velocity reserve of the left anterior descending coronary artery in 144 patients with type 2 diabetes who had chest pain or angina-like symptoms, with preserved left ventricular systolic function and without flow-limiting stenoses on angiography. During 29 months of follow-up, 17 hard events (five deaths and twelve nonfatal myocardial infarctions) occurred, with a 13.9% annual hard-event rate in patients with a reduced coronary flow velocity reserve and 2.0% in patients with a higher coronary flow velocity reserve. This finding illustrated the adverse effect of microvascular dysfunction on prognosis, while nonobstructive CAD was not an independent predictor.124 Impaired coronary microvascular function was also implicated in a poor prognosis in the setting of acute myocardial infarction, where patients with diabetes have higher mortality rates.125 The lack of microvascular reperfusion following revascularization in patients with diabetes with myocardial infarction appears to be one mechanism underlying a poor clinical outcome with major adverse cardiac events (death, reinfarction and congestive heart failure) that occurs more frequently in patients with a lack of microvascular reperfusion (30.8% vs. 15.9%).126
Early detection of diastolic dysfunction in young patients with diabetes is also useful for detecting increased arrhythmia risk.120 Subclinical levels of left ventricular dysfunction, detected by strain echo and global longitudinal strain, is another important parameter in asymptomatic patients with diabetes, because it is associated with adverse outcomes122 and correlated with coronary microvascular dysfunction.127 A DMCMP diagnosis requires impaired glucose metabolism as well as the exclusion of coronary, congenital, familial, infiltrative, viral, valvular and hypertensive heart disease.116 It should be clarified that heart failure in patients with diabetes is not simply an advanced stage of DMCMP but results from a constellation of pathophysiologic processes.128 Many unanswered questions need to be resolved, such as the issue of pathophysiologic and clinical differences between type 1 and type 2 diabetes mellitus and the lack of any pathognomonic histologic changes or imaging characteristics associated with the diagnosis.128 Additionally, explanations of clinical manifestations of diabetes with respect to atherosclerotic coronary disease development or diabetic coronary microangiopathy/cardiomyopathy development are required. The big challenge in DMCMP diagnosis is how to exclude hypertension/hypertensive heart disease in patients with type 2 diabetes and metabolic syndrome.
Patients with comorbid heart failure and diabetes mellitus represent a growing patient population. Future clinical studies in both heart failure and diabetes are encouraged to refocus and differentially investigate the efficacy, safety and outcomes in patients with heart failure and concomitant diabetes mellitus.84
Treatment options
There is a little research evaluating treatment options in patients with microvascular dysfunction. All patients should achieve optimal coronary risk factor control. Treatment options are empirical and arise from the experiences obtained from treatment of “classical” CAD.
Angiotensin is a potent vasoconstrictor. Inhibition of its action, by angiotensin-converting enzyme (ACE) inhibition or angiotensin receptor 1 (AT1) receptor blockade, counteracts its influence and promotes vessel dilation. Pauly et al.129, in their study investigating quinapril in women with chest pain without obstructive coronary disease and reduced coronary flow reserve <2.5, identified significant changes in coronary flow reserve measured by intra IC Doppler. Other authors found similar results in their studies, demonstrating improved stress test parameters and coronary flow reserve.130,131 Combination of eplerenone with angiotensin II inhibition did not improve endothelial dysfunction in a study by Bavry on women with signs and symptoms of ischemia in the setting of nonobstructive CAD.132 However, investigation of microvascular dysfunction in patients with diabetes revealed that mineralocorticoid receptors might indeed be an appealing target to treat microvascular coronary dysfunction in diabetes.133 It was demonstrated that interruption of the renin-angiotensin-aldosterone system with the use of ACE inhibitors improved coronary flow reserve (measured by quantitative PET) in patients with diabetes without ischemic heart disease. This effect was further enhanced by spironolactone.133,134
Calcium channel blockade decreases microvascular spasm and tone, potentially improving coronary flow reserve in patients with coronary microvascular dysfunction.135 Verapamil, nifedipine and lidoflazine have been demonstrated to improve exercise stress parameters.136,137 Sütsch and colleagues did not determine that diltiazem improves coronary flow reserve.138
Statins have anti-inflammatory and anti-atherogenic effects. Zhang et al.139 demonstrated an improvement in coronary flow reserve and symptoms following fluvastatin treatment. Eshtehardi et al.140 revealed improved coronary flow reserve in patients treated with atorvastatin. Additionally, some studies have indicated that an inability to reach target cholesterol values with statin therapy is associated with impaired coronary flow reserve and a worse prognosis.141
NO is a crucial player in endothelium-dependent mediation of coronary microvasculature tone.142 In one study, the authors evaluated the role of sildenafil in symptomatic patients with coronary flow reserve <2.5 compared with patients with coronary flow reserve >2.5. Patients with reduced coronary flow reserve displayed a significant increase in coronary flow reserve.143 Considering the fact that L-arginine is a precursor of NO, Bottcher et al.101 analysed a one-time infusion of L-arginine in 25 patients with chest pain without obstructive CAD or coronary microvascular dysfunction by PET. They noted no improvement in symptoms after infusion. In contrast, Egashira and Gellman noticed improved coronary flow reserve after a single L-arginine infusion.144,145 However, Lerman identified improved symptoms but no change in coronary flow reserve after 6 months of supplementation.146
Alpha-blockers reduce sympathetic activity and can thus potentially decrease microvascular tone. Botker, in his double-blind, placebo-controlled, crossover study, did not observe a change in exercise duration, time to angina onset or exercise time to > 0.1 mV ST segment depression between patients administered doxazosin and those receiving a placebo.147
It has been theorized that an oestrogen deficiency could play a role in microvascular dysfunction. Bairey Merz et al.148 demonstrated an improvement in anginal symptoms but without improvements in myocardial ischemia or brachial artery flow-mediated dilatation. In contrast, Knuuti et al.149 revealed improved average myocardial perfusion reserve after oestrogen use.
Spinal cord stimulation modulates pain-related nerve signals and increases myocardial blood flow. Jessurun et al.150 demonstrated an improvement in symptoms after the use of transcutaneous spinal cord stimulator therapy. Some other studies revealed similar results with angina symptom relief as well as improved coronary flow reserve.151
Beta-blockers are the gold standard for the treatment of stable angina and CAD. They reduce myocardial oxygen demand and increase diastolic perfusion. However, there do not appear to be any studies investigating beta-blocker treatment in patients with diabetic microvascular dysfunction.152 The first and second generation of beta-blockers (propranolol, atenolol, metoprolol and bisoprolol) reduce coronary flow at rest, but their action on hyperaemic coronary flow is controversial and variable. Third generation beta-blockers (carvedilol and nebivolol) have vasodilatatory effects through NO synthesis and alpha-adrenergic receptor blockade, ameliorating maximal hyperaemia of CBF.153
Fukomoto and coworkers investigated the effect of the rho-kinase inhibitor, fasudil. Intracoronary fasudil (300 µg) administration reduced coronary sinus lactate production and improved myocardial ischemia parameters (ST segment changes and angina symptoms).152
There are several types of antianginal drugs (ivabradine, ranolazine, mibefradil, nicorandil and trimetazidine) that have been investigated in patients with microvascular dysfunction. These drugs work through several different mechanisms to reduce myocardial oxygen demand and ischemia. Ranolazine has been extensively studied and was confirmed to improve myocardial perfusion reserve, as well as other symptomatic and stress test metrics.155,156 Several studies have examined the role of nitrates in chest pain without obstructive CAD and have found no benefits. Russo et al.157 observed no significant changes in stress test parameters following isosorbide dinitrate use, and some authors have observed worsened angina and reduced CBF with rapid atrial pacing.158
Teophilline, an adenosine receptor blocker, was assessed in several small studies. The majority of these reports demonstrated improved exercise capacity.152 Six-week exercise training versus a low cholesterol diet and relaxation techniques were also analysed. An improvement in coronary flow reserve in the exercise group was observed.159 Some psychiatric therapy, such as the tricyclic antidepressant imipramine, can also improve symptoms.
Selective intracoronary endothelin A-receptor blockade (with the selective blocker BQ123) reversed the coronary microvascular dysfunction that is present during coronary stenting in patients with type 2 diabetes. Thus, therapeutic targeting of the endothelin system might be useful in protecting the myocardium against ischemic events during elective percutaneous coronary intervention in patients with diabetes.160
Jadhav et al. compared metformin treatment (500 mg twice a day; n = 16) with placebo administration (n = 17) in women with normal results from a coronary angiogram, with two consecutive positive ergometry tests (positive defined as ST-segment depression ≥ 1 mm). Metformin treatment was associated with a significant reduction in weight and in homeostatic model assessment of insulin resistance.161 Metformin treatment also improved the microvascular endothelial-dependent response to acetylcholine using laser Doppler devices, while a positive response was absent in the placebo group. Maximum ischemic ST denivelation, Duke score and reduced chest pain were also present in the metformin-treated group compared with those receiving the placebo.161 Metformin has been demonstrated to have direct vascular effects. Treatment improves vascular function and dramatically reduces cardiovascular end points and mortality in patients with type 2 diabetes mellitus in large-scale clinical trials, but with incompletely elucidated mechanisms. Recently, it was determined that metformin treatment leads to increased NO production, which is induced by AMP protein kinase activation.162
However, it is inconclusive whether glycaemic control can contribute to prevention or reversal of diabetic coronary microvascular dysfunction. Valenzuela-Garcia et al. analysed 100 patients with type 2 diabetes and 214 patients without diabetes and observed a lack of correlation between optimal glycaemic control and coronary microvascular dysfunction.163 Furthermore, more intensive glycaemic control in patients with type 2 diabetes did not reduce the occurrence of heart failure.164
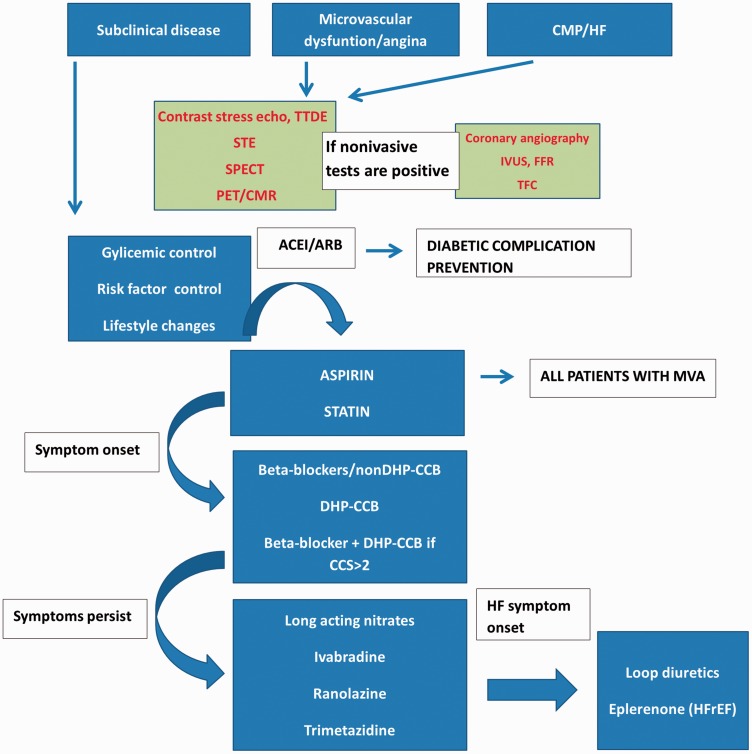
A proposed diagnostic-therapeutic algorithm, based on the information provided earlier, is displayed in Figure 4. The first steps are widely known preventive measures for type 2 diabetes. Patients with diabetic coronary microvascular dysfunction should minimize risk factors. It is recommended that all patients receive secondary prevention therapy, including aspirin and statins. Beta-blockers are recommended as a first-line therapy, and calcium channel blockers are recommended if beta-blockers do not achieve sufficient symptom control or are not tolerated. Other therapy options have lower level of recommendation. In patients with microvascular angina, the therapeutic response is highly variable and challenging, even without concomitant diabetes mellitus. Treatment often requires a combination of medications to control symptoms. There is still a lack of research evaluating therapies to relieve angina symptoms and reduce risk in populations suffering from microvascular dysfunction and, in particular, effective therapies targeting coronary microvascular dysfunction in diabetes. Future research should be focused on symptom improvement, improvement in quality of life and on the use of drugs that act directly on the pathophysiological processes.
Figure 4.
Proposed diagnostic-therapeutic algorithm.
There is no consensus or specific clinical guidelines for diabetic coronary microvascular dysfunction, because studies are limited and known information is scarce. Therapeutic strategies can, at this point, be based on preventive measures, treatment of diabetes (although intensive glycaemic control has not been proven to improve coronary microvascular dysfunction, it is important for preventing diabetic complications) and treatment of microvascular angina and/or cardiomyopathy/heart failure. Early detection/diagnosis and monitoring of disease progression are advisable and can contribute to timely initiation of therapeutic interventions.
TTDE: transthoracic Doppler echocardiography; STE: SPECT-single photon emission tomography; PET: positron emission tomography; CMR: cardiac magnetic resonance; IVUS: intravascular ultrasound; FFR: fractional flow reserve; TFC: TIMI frame count; MVA: microvascular angina; BB: beta-blockers; CCB: calcium channel blockers; ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin receptor blocker; CCS: Canadian Cardiac Society; CMP: cardiomyopathy; HF: heart failure; HFREF: heart failure with reduced ejection fraction.
Conclusion
The coronary microcirculation is a tightly regulated network, with several interrelated physiological processes acting to match myocardial perfusion to metabolic demands. Dysfunction of this important system occurs in the context of diabetes mellitus, as a result of complex metabolic disorders and pathophysiological mechanisms, as determined in animal models and in human diabetic patients using non-invasive and invasive diagnostic and research procedures. A reduced coronary flow reserve, consequent to a number of damages to normal endothelium-dependent and endothelium-independent vasomotion, in response to metabolic, hemodynamic, neural, hormonal and other stimuli, is a hallmark of diabetes-induced microvascular dysfunction. Failure of a normally functioning microvasculature, which precedes morphological changes, significantly increases mortality. It ultimately culminates in the development of DMCMP and associated consequences. Unfortunately, our present therapeutic strategies for treatment of coronary microvascular dysfunction induced by diabetes are very limited. Additional research in this area will provide us with more effective treatment approaches and thereby lead to improved care of patients with diabetes.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1.Leon BM, Maddox TM. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes 2015; 6: 1246–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kibel A. Could angiotensin-(1–7) be connected with improvement of microvascular function in diabetic patients? Angiotensin-(1–7) iontophoresis may provide the answer. Med Hypotheses 2016; 93: 16–20. 10.1016/j.mehy.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Picchi A, Capobianco S, Qiu T, et al. Coronary microvascular dysfunction in diabetes mellitus: a review. World J Cardiol 2010; 2: 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tabit CE, Chung WB, Hamburg NM, et al. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord 2010; 11: 61–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tousoulis D, Papageorgiou N, Androulakis E, et al. Diabetes mellitus-associated vascular impairment: novel circulating biomarkers and therapeutic approaches. J Am Coll Cardiol 2013; 62: 667–676. [DOI] [PubMed] [Google Scholar]
- 6.Avogaro A, Albiero M, Menegazzo L, et al. Endothelial dysfunction in diabetes: the role of reparatory mechanisms. Diabetes Care 2011; 34(Suppl 2): S285–S290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haffner SJ, Cassells H. Hyperglycemia as a cardiovascular risk factor. Am J Med 2003; 115(Suppl 8A): 6S–11S. [DOI] [PubMed] [Google Scholar]
- 8.Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229–234. [DOI] [PubMed] [Google Scholar]
- 9.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 1979; 241: 2035–2038. [DOI] [PubMed] [Google Scholar]
- 10.Gan D. Diabetes atlas. 2nd ed. Brussels: International Diabetes Federation, 2003.
- 11.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001; 414: 813–820. [DOI] [PubMed] [Google Scholar]
- 12.Ceriello A. Coagulation activation in diabetes mellitus: the role of hyperglycaemia and therapeutic prospects. Diabetologia 1993; 3: 1119–1125. [DOI] [PubMed] [Google Scholar]
- 13.Wild S, Roglic G, Green A, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047–1053. [DOI] [PubMed] [Google Scholar]
- 14.Howard BV, Rodriguez BL, Bennett PH, et al. Diabetes and cardiovascular disease. Circulation 2002; 105: 132–7. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez MA, Selwyn AP. Endothelial function, inflammation, and prognosis in cardiovascular disease. Am J Med 2003; 115: 99–106. [DOI] [PubMed] [Google Scholar]
- 16.Baumgartner-Parzer SM, Waldhäusl WK. The endothelium as a metabolic and endocrine organ: its relation with insulin resistance. Exp Clin Endocrinol Diabetes 2001; 109(Suppl 2): S166–S179. [DOI] [PubMed] [Google Scholar]
- 17.Sena CM, Pereira AM, Seiça R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim Biophys Acta 2013; 1832: 2216–2231. [DOI] [PubMed] [Google Scholar]
- 18.Schram MT, Stehouwer CD. Endothelial dysfunction, cellular adhesion molecules and the metabolic syndrome. Horm Metab Res 2005; 37(Suppl 1): 49–55. [DOI] [PubMed] [Google Scholar]
- 19.Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis 2003; 170: 191–203. [DOI] [PubMed] [Google Scholar]
- 20.Ross R. Atherosclerosis-an inflammatory disease. N Engl J Med 1999; 340: 115–126. [DOI] [PubMed] [Google Scholar]
- 21.Rask-Madsen C, King GL. Mechanisms of Disease: endothelial dysfunction in insulin resistance and diabetes. Nat Clin Pract Endocrinol Metab 2007; 3: 46–56. [DOI] [PubMed] [Google Scholar]
- 22.Matsumoto K, Sera Y, Ueki Y, et al. Comparison of serum concentrations of soluble adhesion molecules in diabetic microangiopathy and macroangiopathy. Diabet Med 2002; 19: 822–826. [DOI] [PubMed] [Google Scholar]
- 23.Price D, Loscalzo J. Cellular adhesion molecules and atherogenesis. Am J Med 1999; 107: 85–97. [DOI] [PubMed] [Google Scholar]
- 24.Kado S, Nagata N. Circulating intercellular adhesion molecule-1, vascular cell adhesion molecule-1, and E-selectin in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract 1999; 46: 143–148. [DOI] [PubMed] [Google Scholar]
- 25.Cooke CL, Davidge ST. Peroxynitrite increases iNOS through NF-kappaB and decreases prostacyclin synthase in endothelial cells. Am J Physiol Cell Physiol 2002; 282: C395–C402. [DOI] [PubMed] [Google Scholar]
- 26.Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol 2004; 15: 1983–1992. [DOI] [PubMed] [Google Scholar]
- 27.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 2000; 87: 840–844. [DOI] [PubMed] [Google Scholar]
- 28.De Caterina R. Endothelial dysfunctions: common denominators in vascular disease. Curr Opin Clin Nutr Metab Care 2000; 3: 453–467. [DOI] [PubMed] [Google Scholar]
- 29.Jafar N, Edriss H, Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am J Med Sci 2016; 351: 201–211. [DOI] [PubMed] [Google Scholar]
- 30.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005; 54: 1–7. [DOI] [PubMed] [Google Scholar]
- 31.Shanik MH, Xu Y, Skrha J, et al. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care 2008; 31(Suppl 2): S262–S268. [DOI] [PubMed] [Google Scholar]
- 32.Roberts AC, Porter KE. Cellular and molecular mechanisms of endothelial dysfunction in diabetes. Diab Vasc Dis Res 2013; 10: 472–482. [DOI] [PubMed] [Google Scholar]
- 33.Hadden DR. The diabetes control and complications trial (DCCT): what every endocrinologist needs to know. Clin Endocrinol (Oxf) 1994; 40: 293–294. [DOI] [PubMed] [Google Scholar]
- 34.Intensive blood glucose control with sulfonylureas or insulin compared with conventional treatment and risk for complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352: 837–853. [PubMed] [Google Scholar]
- 35.Shichiri M, Kishikawa H, Ohkubo Y, et al. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000; 23(Suppl 2): B21–B29. [PubMed] [Google Scholar]
- 36.Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J 2015; 36: 2288–2296. [DOI] [PubMed] [Google Scholar]
- 37.Pries AR, Reglin B. Coronary microcirculatory pathophysiology: can we afford it to remain a black box? Eur Heart J 2016. pii: ehv760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deussen A, Ohanyan V, Jannasch A, Yet, et al. Mechanisms of metabolic coronary flow regulation. J Mol Cell Cardiol 2012; 52: 794–801. [DOI] [PubMed] [Google Scholar]
- 39.Komaru T, Kanatsuka H, Shirato K. Coronary microcirculation: physiology and pharmacology. Pharmacol Ther 2000; 86: 217–261. [DOI] [PubMed] [Google Scholar]
- 40.Spaan J, Kolyva C, van den Wijngaard J, et al. Coronary structure and perfusion in health and disease. Philos Trans A Math Phys Eng Sci 2008; 366: 3137–3153. [DOI] [PubMed] [Google Scholar]
- 41.Chilian WM. Coronary microcirculation in health and disease. Summary of an NHLBI workshop. Circulation 1997; 95: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pries AR, Badimon L, Bugiardini R, et al. Coronary vascular regulation, remodelling, and collateralization: mechanisms and clinical implications on behalf of the working group on coronary pathophysiology and microcirculation. Eur Heart J 2015; 36: 3134–3146. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Park Y, Zhang H, et al. Feed-forward signaling of TNF-alpha and NF-kappaB via IKK-beta pathway contributes to insulin resistance and coronary arteriolar dysfunction in type 2 diabetic mice. Am J Physiol Heart Circ Physiol 2009; 296: H1850–H1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Woodman RJ, Watts GF, Playford DA, et al. Oxidized LDL and small LDL particle size are independently predictive of a selective defect in microcirculatory endothelial function in type 2 diabetes. Diabetes Obes Metab 2005; 7: 612–617. [DOI] [PubMed] [Google Scholar]
- 45.Koller A, Balasko M, Bagi Z. Endothelial regulation of coronary microcirculation in health and cardiometabolic diseases. Intern Emerg Med 2013; 8(Suppl 1): S51–S54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Okon EB, Chung AW, Rauniyar P, et al. Compromised arterial function in human type 2 diabetic patients. Diabetes 2005; 54: 2415–2423. [DOI] [PubMed] [Google Scholar]
- 47.Miura H, Wachtel RE, Loberiza FR, Jr, et al. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res 2003; 92: 151–158. [DOI] [PubMed] [Google Scholar]
- 48.Moien-Afshari F, Ghosh S, Elmi S, et al. Exercise restores coronary vascular function independent of myogenic tone or hyperglycemic status in db/db mice. Am J Physiol Heart Circ Physiol 2008; 295: H1470–H1480. [DOI] [PubMed] [Google Scholar]
- 49.Zhao G, Zhang X, Xu X, et al. Depressed modulation of oxygen consumption by endogenous nitric oxide in cardiac muscle from diabetic dogs. Am J Physiol Heart Circ Physiol 2000; 279: H520–H527. [DOI] [PubMed] [Google Scholar]
- 50.van den Heuvel M, Sorop O, Koopmans SJ, et al. Coronary microvascular dysfunction in a porcine model of early atherosclerosis and diabetes. Am J Physiol Heart Circ Physiol 2012; 302: H85–H94. [DOI] [PubMed] [Google Scholar]
- 51.Eid S, Abou-Kheir W, Sabra R, et al. Involvement of renal cytochromes P450 and arachidonic acid metabolites in diabetic nephropathy. J Biol Regul Homeost Agents 2013; 27: 693–703. [PubMed] [Google Scholar]
- 52.Vanhoutte PM, Shimokawa H, Tang EH, et al. Endothelial dysfunction and vascular disease. Acta Physiol (Oxf) 2009; 196: 193–222. [DOI] [PubMed] [Google Scholar]
- 53.Gimeno AL, Sterin-Borda L, Borda ES, et al. Arachidonate evokes constriction of coronary and mesenteric arteries isolated from diabetic dogs. Adv Prostaglandin Thromboxane Leukot Res 1983; 12: 235–240. [PubMed] [Google Scholar]
- 54.Bagi Z, Erdei N, Papp Z, et al. Up-regulation of vascular cyclooxygenase-2 in diabetes mellitus. Pharmacol Rep 2006; 58(Suppl): 52–56. [PubMed] [Google Scholar]
- 55.Davel AP, Wenceslau CF, Akamine EH, et al. Endothelial dysfunction in cardiovascular and endocrine-metabolic diseases: an update. Braz J Med Biol Res 2011; 44: 920–932. [DOI] [PubMed] [Google Scholar]
- 56.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 2002; 82: 131–185. [DOI] [PubMed] [Google Scholar]
- 57.Li X, Zhao G, Ma B, et al. 20-Hydroxyeicosatetraenoic acid impairs endothelial insulin signaling by inducing phosphorylation of the insulin receptor substrate-1 at Ser616. PLoS One 2014; 9: e95841–e95841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Issan Y, Hochhauser E, Guo A, et al. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins Other Lipid Mediat 2013; 100–101: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pivackova L, Mensikova L, Gajdacova B, et al. 6D.05: Endothelial dysfunction in animal models of glucose intolerance and diabetes is accompanied by different expression of key enzymes of epoxyeicosatrienoic acids pathway. J Hypertens 2015; 33(Suppl 1): e83–e84. [Google Scholar]
- 60.Kibel A, Novak S, Cosic A, et al. Hyperbaric oxygenation modulates vascular reactivity to angiotensin-(1–7) in diabetic rats: potential role of epoxyeicosatrienoic acids. Diab Vasc Dis Res 2015; 12: 33–45. [DOI] [PubMed] [Google Scholar]
- 61.Kibel A, Cavka A, Cosic A, et al. Effects of hyperbaric oxygenation on vascular reactivity to angiotensin II and angiotensin-(1–7) in rats. Undersea Hyperb Med 2012; 39: 1053–1066. [PubMed] [Google Scholar]
- 62.Liu Y, Gutterman DD. The coronary circulation in diabetes: influence of reactive oxygen species on K+ channel-mediated vasodilation. Vascul Pharmacol 2002; 38: 43–49. [DOI] [PubMed] [Google Scholar]
- 63.Gupte SA, Arshad M, Viola S, et al. Pentose phosphate pathway coordinates multiple redox-controlled relaxing mechanisms in bovine coronary arteries. Am J Physiol Heart Circ Physiol 2003; 285: H2316–H2326. [DOI] [PubMed] [Google Scholar]
- 64.Ammar RF, Jr, Gutterman DD, Brooks LA, et al. Free radicals mediate endothelial dysfunction of coronary arterioles in diabetes. Cardiovasc Res 2000; 47: 595–601. [DOI] [PubMed] [Google Scholar]
- 65.Grainger J, Boachie-Ansah G. Anandamide-induced relaxation of sheep coronary arteries: the role of the vascular endothelium, arachidonic acid metabolites and potassium channels. Br J Pharmacol 2001; 134: 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Korovkina VP, England SK. Detection and implications of potassium channel alterations. Vascul Pharmacol 2002; 38: 3–12. [DOI] [PubMed] [Google Scholar]
- 67.Hiett SC, Owen MK, Li W, et al. Mechanisms underlying capsaicin effects in canine coronary artery: implications for coronary spasm. Cardiovasc Res 2014; 103: 607–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bratz IN, Dick GM, Tune JD, et al. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol 2008; 294: H2489–H2496. [DOI] [PubMed] [Google Scholar]
- 69.Guarini G, Ohanyan VA, Kmetz JG, et al. Disruption of TRPV1-mediated coupling of coronary blood flow to cardiac metabolism in diabetic mice: role of nitric oxide and BK channels. Am J Physiol Heart Circ Physiol 2012; 303: H216–H223. [DOI] [PubMed] [Google Scholar]
- 70.DelloStritto DJ, Connell PJ, Dick GM, et al. Differential regulation of TRPV1 channels by H2O2: implications for diabetic microvascular dysfunction. Basic Res Cardiol 2016; 111: 21–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hayat SA, Patel B, Khattar RS. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (London) 2004; 107: 539–557. [DOI] [PubMed] [Google Scholar]
- 72.Picchi A, Capobianco S, Qiu T, et al. Coronary microvascular dysfunction in diabetes mellitus: A review. World J Cardiol 2010; 2: 377–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol 1974; 34: 29–34. [DOI] [PubMed] [Google Scholar]
- 74.Boudina S, Abel ED. Basic science for clinicians. Diabetic cardiomyopathy revisited. Circ 2007; 115: 3213–3223. [DOI] [PubMed] [Google Scholar]
- 75.Boudina S, Abel ED. Diabetic cardiomyopathy, causes and effects. Rev Endocr Metab Disord 2010; 11: 31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nacci C, Tarquinio M, Montagnani M. Molecular and clinical aspects of endothelial dysfunction in diabetes. Intern Emerg Med 2009; 4: 107–116. [DOI] [PubMed] [Google Scholar]
- 77.Laakso M. Heart in diabetes: a microvascular disease. Diabetes Care 2011; 34(Suppl 2): S145–S149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sjögren A, Edvinsson L. Vasomotor changes in isolated coronary arteries from diabetic rats. Acta Physiol Scand 1988; 134: 429–436. [DOI] [PubMed] [Google Scholar]
- 79.Factor SM, Okun EM, Minase T. Capillary microaneurysms in the human diabetic heart. N Engl J Med 1980; 302: 384–388. [DOI] [PubMed] [Google Scholar]
- 80.Yoon YS, Uchida S, Masuo O, et al. Progressive attenuation of myocardial vascular endothelial growth factor expression is a seminal event in diabetic cardiomyopathy: restoration of microvascular homeostasis and recovery of cardiac function in diabetic cardiomyopathy after replenishment of local vascular endothelial growth factor. Circulation 2005; 111: 2073–2085. [DOI] [PubMed] [Google Scholar]
- 81.Miki T, Yuda S, Kouzu H, et al. Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 2013; 18: 149–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Frustaci A, Kajstura J, Chimenti C, et al. Myocardial cell death in human diabetes. Circ Res 2000; 87: 1123–1132. [DOI] [PubMed] [Google Scholar]
- 83.Joshi M, Kotha SR, Malireddy S, et al. Conundrum of pathogenesis of diabetic cardiomyopathy: role of vascular endothelial dysfunction, reactive oxygen species, and mitochondria. Mol Cell Biochem 2014; 386: 233–249. [DOI] [PubMed] [Google Scholar]
- 84.Dei Cas A, Khan SS, Butler J, et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail 2015; 3: 136–145. [DOI] [PubMed] [Google Scholar]
- 85.Kaski JC, Rosano GM, Collins P, et al. Cardiac syndrome X: clinical characteristics and left ventricular function: long term follow-up study. J Am Coll Cardiol 1995; 25: 807–814. [DOI] [PubMed] [Google Scholar]
- 86.Lamendola P, Lanza GA, Spinelli A, et al. Long-term prognosis of patients with cardiac syndrome X. Int J Cardiol 2010; 140: 197–199. [DOI] [PubMed] [Google Scholar]
- 87.Lanza GA, Crea F. Primary coronary microvascular dysfunction, clinical presentation, pathophysiology and management. Circulation 2010; 121: 2317–2325. [DOI] [PubMed] [Google Scholar]
- 88.Yağmur J, Açuköz N, Cansel M, et al. Assessment of the left ventricular systolic function in cardiac syndrome X using speckle tracking echocardiography. Anatol J Cardiol 2015; 16: 419–423. DOI: 10.5152/AnatolJCardiol.2015.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Leung M, Juergens CP, Lo ST, et al. Evaluation of coronary microvascular function by left ventricular contractile reserve with low-dose dobutamine echocardiography. EuroIntervention 2014; 9: 1202–1219. [DOI] [PubMed] [Google Scholar]
- 90.Marini C, Bezante G, Gandolfo P, et al. Optimization of flow reserve measurement using SPECT technology to evaluate the determinants of coronary microvascular dysfunction in diabetes. Eur J Nucl Med Mol Imaging 2010; 37: 357–367. [DOI] [PubMed] [Google Scholar]
- 91.Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance. N Eng J Med 2002; 346: 1948–1953. [DOI] [PubMed] [Google Scholar]
- 92.Lanza GA, Buffon A, Sestito A, et al. Relation between stress-induced myocardial perfusion defects on cardiovascular magnetic resonance and coronary microvascular dysfunction in patients with cardiac syndrome X. J Am Coll Cardiol 2008; 51: 466–472. [DOI] [PubMed] [Google Scholar]
- 93.Bøttcher M, Botker HE, Sonne H, et al. Endothelium-dependent and -independent perfusion reserve and the effect of L-arginine on myocardial perfusion in patients with syndrome X. Circulation 1999; 99: 1795–1801. [DOI] [PubMed] [Google Scholar]
- 94.Ziadi MC, Dekemp RA, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011; 58: 740–748. [DOI] [PubMed] [Google Scholar]
- 95.Schelbert HR. Positron emission tomography measurements of myocardial blood flow: assessing coronary circulatory function and clinical implications. Heart 2012; 98: 592–600. [DOI] [PubMed] [Google Scholar]
- 96.von Scholten BJ, Hasbak P, Christensen TE, et al. Cardiac (82)Rb PET/CT for fast and non-invasive assessment of microvascular function and structure in asymptomatic patients with type 2 diabetes. Diabetologia 2016; 59: 371–378. [DOI] [PubMed] [Google Scholar]
- 97.Slomka P, Berman DS, Alexanderson E, et al. The role of PET quantification in cardiovascular imaging. Clin Transl Imaging 2014; 2: 343–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buchthal SD, den Hollander JA, Merz CN, et al. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N Engl J Med 2000; 342: 829–835. [DOI] [PubMed] [Google Scholar]
- 99.Bosnjak I, Selthofer-Relatic K, Glasnovic M. Antiphospholipid syndrome- predictor of coronary heart disease. Exp Clin Cardiol 2014; 20: 931–931. [Google Scholar]
- 100.Atmaca Y, Ozdemir AO, Ozdol C, et al. Angiographic evaluation of myocardial perfusion in patients with syndrome X. Am J Cardiol 2005; 96: 803–805. [DOI] [PubMed] [Google Scholar]
- 101.Rodés-Cabau J, Gutiérrez M, Courtis J, et al. Importance of diffuse atherosclerosis in the functional evaluation of coronary stenosis in the proximal-mid segment of a coronary artery by myocardial fractional flow reserve measurements. Am J Cardiol 2011; 108: 483–490. [DOI] [PubMed] [Google Scholar]
- 102.Atmaca Y, Duzen V, Ozdol C, et al. Total blush score: a new index for the assessment of microvascular perfusion in idiopathic dilated cardiomyopathy. Coron Artery Dis 2008; 19: 181–185. [DOI] [PubMed] [Google Scholar]
- 103.Gibson CM, Cannon CP, Daley WL, et al. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation 1996; 93: 879–888. [DOI] [PubMed] [Google Scholar]
- 104.Sucato V, Evola S, Novo G, et al. Stable microvascular angina: instrumental evaluation of coronary microvascular dysfunction with coronary angiography and myocardial scintigraphy. Int J Cardiol 2014; 171: e127–e128. [DOI] [PubMed] [Google Scholar]
- 105.Di Carli MF, Janisse J, Grunberger G, et al. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol 2003; 41: 1387–1393. [DOI] [PubMed] [Google Scholar]
- 106.Di Carli MF, Bianco-Batlles D, Landa ME, et al. Effects of autonomic neuropathy on coronary blood flow in patients with diabetes mellitus. Circulation 1999; 100: 813–819. [DOI] [PubMed] [Google Scholar]
- 107.Pitkänen OP, Nuutila P, Raitakari OT, et al. Coronary flow reserve is reduced in young men with IDDM. Diabetes 1998; 47: 248–254. [DOI] [PubMed] [Google Scholar]
- 108.Yokoyama I, Momomura S, Ohtake T, et al. Reduced myocardial flow reserve in non-insulin-dependent diabetes mellitus. J Am Coll Cardiol 1997; 30: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 109.Nitenberg A, Valensi P, Sachs R, et al. Impairment of coronary vascular reserve and ACh-induced coronary vasodilation in diabetic patients with angiographically normal coronary arteries and normal left ventricular systolic function. Diabetes 1993; 42: 1017–1025. [DOI] [PubMed] [Google Scholar]
- 110.Aneja A, Tang WH, Bansilal S, et al. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 2008; 121: 748–757. [DOI] [PubMed] [Google Scholar]
- 111.Van Hooven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation 1990; 82: 848–855. [DOI] [PubMed] [Google Scholar]
- 112.Devereux RB, Roman MJ, Paranicas M, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation 2000; 101: 2271–2276. [DOI] [PubMed] [Google Scholar]
- 113.Wende AR, Abel ED. Lipotoxicity in the heart. Biochim Biophys Acta 2010; 1801: 311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation 2007; 116: 1170–1175. [DOI] [PubMed] [Google Scholar]
- 115.Nahser PJ, Jr, Brown RE, Oskarsson H, et al. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation 1995; 91: 635–640. [DOI] [PubMed] [Google Scholar]
- 116.Seferović PM, Paulus WJ. Clinical diabetic cardiomyopathy: a two faced disease with restrictive and dilated phenotypes. Eur Heart J 2015; 36: 1718–1727. 1727a-1727c. [DOI] [PubMed] [Google Scholar]
- 117.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure: abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med 2004; 350: 1953–1959. [DOI] [PubMed] [Google Scholar]
- 118.Muranaka A, Yuda S, Tsuchihashi K, et al. Quantitative assessment of left ventricular and left atrial functions by strain rate imaging in diabetic patients with and without hypertension. Echocardiography 2009; 26: 262–271. [DOI] [PubMed] [Google Scholar]
- 119.Tarquini R, Lazzeri C, Pala L, et al. The diabetic cardiomyopathy. Acta Diabetol 2011; 48: 173–181. [DOI] [PubMed] [Google Scholar]
- 120.Schwanell CM, Schneppenheim M, Perings S, et al. Left ventricular diastolic dysfunction as an early manifestation of diabetic cardiomyopathy. Cardiology 2002; 98: 33–39. [DOI] [PubMed] [Google Scholar]
- 121.Poirer P, Bogathy P, Garneau C, et al. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes. Diabetes Care 2001; 24: 5–10. [DOI] [PubMed] [Google Scholar]
- 122.Flores-Ramirez R, Azpiri-López JR, González-González JG, et al. Global longitudinal strain as a biomarker in diabetic cardiomypopathy. A comparative study with Gal-3 in patients with preserved ejection fraction. Arch Cardiol Mex 2016. pii: S1405–9940(16)30046–5. doi: 10.1016/j.acmx.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 123.Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012; 126: 1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cortigiani L, Rigo F, Gherardi S, et al. Prognostic meaning of coronary microvascular disease in type 2 diabetes mellitus: a transthoracic Doppler echocardiographic study. J Am Soc Echocardiogr 2014; 27: 742–748. [DOI] [PubMed] [Google Scholar]
- 125.Ishikawa K. Poor prognosis of patients with acute myocardial infarction complicated by diabetes mellitus; possible role of impaired microvascular function. Intern Med 2003; 42: 543–544. [DOI] [PubMed] [Google Scholar]
- 126.Kurisu S, Inoue I, Kawagoe T, et al. Diabetes mellitus is associated with insufficient microvascular reperfusion following revascularization for anterior acute myocardial infarction. Intern Med 2003; 42: 554–559. [DOI] [PubMed] [Google Scholar]
- 127.Kawata T, Daimon M, Miyazaki S, et al. Coronary microvascular function is independently associated with left ventricular filling pressure in patients with type 2 diabetes mellitus. Cardiovasc Diabetol 2015; 14: 98–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Aneja A, Tang WH, Bansilal S, et al. Diabetic Cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med 2008; 121: 748–757. [DOI] [PubMed] [Google Scholar]
- 129.Pauly DF, Johnson BD, Anderson RD, et al. In women with symptoms of cardiac ischemia, nonobstructive coronary arteries, and microvascular dysfunction, angiotensin-converting enzyme inhibition is associated with improved microvascular function: A double-blind randomized study from the National Heart, Lung and Blood Institute Women’s Ischemia Syndrome Evaluation (WISE). Am Heart J 2011; 162: 678–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kaski JC, Rosano G, Gavrielides S, et al. Effects of angiotensin-converting enzyme inhibition on exercise-induced angina and ST segment depression in patients with microvascular angina. J Am Coll Cardiol 1994; 23: 652–657. [DOI] [PubMed] [Google Scholar]
- 131.Motz W, Strauer BE. Improvement of coronary flow reserve after long-term therapy with enalapril. Hypertension 1996; 27: 1031–1308. [DOI] [PubMed] [Google Scholar]
- 132.Bavry AA, Handberg EM, Huo T, et al. Aldosterone inhibition and coronary endothelial function in women without obstructive coronary artery disease: an ancillary study of the national heart, lung, and blood institute-sponsored women’s ischemia syndrome evaluation. Am Heart J 2014; 167: 826–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bender SB, Jia G, Sowers JR. Mineralocorticoid receptors: an appealing target to treat coronary microvascular dysfunction in diabetes. Diabetes 2015; 64: 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Garg R, Rao AD, Baimas-George M, et al. Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes 2015; 64: 236−242–236−242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.McIvor ME, Undemir C, Lawson J, et al. Clinical effects and utility of intracoronary diltiazem. Cathet Cardiovasc Diagn 1995; 35: 287–291. [DOI] [PubMed] [Google Scholar]
- 136.Cannon RO, 3rd, Watson RM, Rosing DR, et al. Efficacy of calcium channel blocker therapy for angina pectoris resulting from small-vessel coronary artery disease and abnormal vasodilator reserve. Am J Cardiol 1985; 56: 242–246. [DOI] [PubMed] [Google Scholar]
- 137.Cannon RO, 3rd, Brush JE, Jr, Schenke WH, et al. Beneficial and detrimental effects of lidoflazine in microvascular angina. Am J Cardiol 1990; 66: 37–41. [DOI] [PubMed] [Google Scholar]
- 138.Sütsch G, Oechslin E, Mayer I, et al. Effect of diltiazem on coronary flow reserve in patients with microvascular angina. Int J Cardiol 1995; 52: 135–143. [DOI] [PubMed] [Google Scholar]
- 139.Zhang X, Li Q, Zhao J, et al. Effects of combination of statin and calcium channel blocker in patients with cardiac syndrome X. Coron Artery Dis 2014; 25: 40–44. [DOI] [PubMed] [Google Scholar]
- 140.Eshtehardi P, McDaniel MC, Dhawan SS, et al. Effect of intensive atorvastatin therapy on coronary atherosclerosis progression, composition, arterial remodeling, and microvascular function. J Invasive Cardiol 2012; 24: 522–529. [PubMed] [Google Scholar]
- 141.Nemes A, Forster T, Gruber N, et al. Coronary flow velocity reserve and indices describing aortic distensibility in patients after coronary angiography. Int J Cardiol 2004; 96: 29–33. [DOI] [PubMed] [Google Scholar]
- 142.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov 2008; 7: 156–167. [DOI] [PubMed] [Google Scholar]
- 143.Denardo SJ, Wen X, Handberg EM, et al. Effect of phosphodiesterase type 5 inhibition on microvascular coronary dysfunction in women: a women’s ischemia syndrome evaluation (WISE) ancillary study. Clin Cardiol 2011; 34: 483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Egashira K, Hirooka Y, Kuga T, et al. Effects of L-arginine supplementation on endothelium-dependent coronary vasodilation in patients with angina pectoris and normal coronary arteriograms. Circulation 1996; 94: 130–134. [DOI] [PubMed] [Google Scholar]
- 145.Gellman J, Hare JM, Lowenstein CJ, et al. L-arginine ameliorates the abnormal sympathetic response of the dysfunctional human coronary microvasculature. Angiology 2004; 55: 1–8. [DOI] [PubMed] [Google Scholar]
- 146.Lerman A, Burnett JC, Jr, Higano ST, et al. Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation 1998; 97: 2123–2128. [DOI] [PubMed] [Google Scholar]
- 147.Bøtker HE, Sonne HS, Schmitz O, et al. Effects of doxazosin on exercise-induced angina pectoris, ST-segment depression, and insulin sensitivity in patients with syndrome X. Am J Cardiol 1998; 82: 1352–1356. [DOI] [PubMed] [Google Scholar]
- 148.Merz CN, Olson MB, McClure C, et al. A randomized controlled trial of low-dose hormone therapy on myocardial ischemia in postmenopausal women with no obstructive coronary artery disease: results from the national institutes of health/national heart, lung, and blood institute-sponsored women’s ischemia syndrome evaluation (WISE). Am Heart J 2010; 159: 987.e1–e7–987.e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Knuuti J, Kalliokoski R, Janatuinen T, et al. Effect of estradiol-drospirenone hormone treatment on myocardial perfusion reserve in postmenopausal women with angina pectoris. Am J Cardiol 2007; 99: 1648–1652. [DOI] [PubMed] [Google Scholar]
- 150.Jessurun GA, Hautvast RW, Tio RA, et al. Electrical neuromodulation improves myocardial perfusion and ameliorates refractory angina pectoris in patients with syndrome X: fad or future? Eur J Pain 2003; 7: 507–512. [DOI] [PubMed] [Google Scholar]
- 151.Luo C, Liu D, Wu G, et al. Effect of enhanced external counterpulsation on coronary slow flow and its relation with endothelial function and inflammation: a mid-term follow-up study. Cardiology 2012; 122: 260–268. [DOI] [PubMed] [Google Scholar]
- 152.Marinescu MA, Löffler AI, Ouellette M, et al. Coronary microvascular dysfunction, microvascular angina, and treatment strategies. JACC Cardiovasc Imaging 2015; 8: 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maurizio G. Beta-Blockers and Coronary Flow Reserve. Prof. Federico Piscione (Ed.) Angina pectoris. ISBN: 978–953-307–359-0, InTech. Available from: http://www.intechopen.com/books/anginapectoris/beta-blockers-and-coronary-flow-reserve.
- 154.Fukomoto Y, Mohri M, Inokuchi K, et al. Anti-ischemic effect of fasudil, specific Rho-kinase inhibitor, in patients with stabile effort angina. J Cardiovascular Pharmacol 2007; 49: 117–121. [DOI] [PubMed] [Google Scholar]
- 155.Mehta PK, Goykhman P, Thomson LE, et al. Ranolazine improves angina in women with evidence of myocardial ischemia but no obstructive coronary artery disease. JACC Cardiovasc Imaging 2011; 4: 514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Villano A, Di Franco A, Nerla R, et al. Effects of ivabradine and ranolazine in patients with microvascular angina pectoris. Am J Cardiol 2013; 112: 8–13. [DOI] [PubMed] [Google Scholar]
- 157.Russo G, Di Franco A, Lamendola P, et al. Lack of effect of nitrates on exercise stress test results in patients with microvascular angina. Cardiovasc Drugs Ther. 27: 229–234. [DOI] [PubMed] [Google Scholar]
- 158.Bugiardini R, Borghi A, Pozzati A, et al. The paradox of nitrates in patients with angina pectoris and angiographically normal coronary arteries. Am J Cardiol 1993; 72: 343–347. patients with microvascular angina. Cardiovasc Drugs Ther 2013; 27(3): 229–34. [DOI] [PubMed] [Google Scholar]
- 159.Nerla R, Tarzia P, Sestito A, et al. Effect of bariatric surgery on peripheral flow-mediated dilation and coronary microvascular function. Nutr Metab Cardiovasc Dis 2012; 22: 626–634. [DOI] [PubMed] [Google Scholar]
- 160.Papadogeorgos NO, Bengtsson M, Kalani M. Selective endothelin A-receptor blockade attenuates coronary microvascular dysfunction after coronary stenting in patients with type 2 diabetes. Vasc Health Risk Manag 2009; 5: 893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jadhav S, Ferrell W, Greer IA, et al. Effects of metformin on microvascular function and exercise tolerance in women with angina and normal coronary arteries: a randomized, double-blind, placebo-controlled study. J Am Coll Cardiol 2006; 48: 956–963. [DOI] [PubMed] [Google Scholar]
- 162.Davis BJ, Xie Z, Viollet B, et al. Activation of the AMP activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes 2006; 55: 496–505. [DOI] [PubMed] [Google Scholar]
- 163.Valenzuela-Garcia LF, Matsuzawa Y, Sara JD, et al. Lack of correlation between the optimal glycaemic control and coronary micro vascular dysfunction in patients with diabetes mellitus: a cross sectional study. Cardiovasc Diabetol 2015; 14: 106–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Castagno D, Baird-Gunning J, Jhund PS, et al. Intensive glycemic control has no impact on the risk of heart failure in type 2 diabetic patients: evidence from a 37,229 patient meta-analysis. Am Heart J 2011; 162: 938–948, e2. [DOI] [PubMed] [Google Scholar]