Abstract
Objective
Functional magnetic resonance imaging (fMRI) analysis of the effects of acupuncturing the Tongli (HT5) and Tongli (HT5)–Xuanzhong (GB39) acupoints on the normal language areas with a view to providing a theoretical basis for using acupuncture to treat patients with aphasia.
Methods
This study enrolled healthy volunteers. The following acupoints were stimulated: right Tongli (HT5), right Tongli (HT5)–Xuanzhong (GB39), right Tongli (HT5) sham acupuncture, left Tongli (HT5), and left Tongli (HT5)–Xuanzhong (GB39) acupoints. Acupuncture stimulation was delivered whilst fMRI scanning of the brain was undertaken.
Results
Ten healthy volunteers (five males) were included in this study (mean age 44.5 ± 2.5 years; range 40–55 years). Based on the statistical analyses, only acupuncturing the right Tongli (HT5) acupoint resulted in activation of multiple regions of the bilateral cerebral hemisphere that were closely related to the language regions. The right Tongli (HT5) stimulation had a laterality index of 0.0952; with the activated voxels on the left side language-related areas being greater than those on the right side.
Conclusions
Acupuncturing the right Tongli (HT5) acupoint results in activation of the bilateral language-related areas, so this acupoint might be useful for the acupuncture treatment of aphasia caused by cerebral infarction.
Keywords: Tongli (HT5), Xuanzhong (GB39), electroacupuncture, language, blood oxygenation level-dependent functional magnetic resonance imaging (BOLD-fMRI)
Introduction
Traditional Chinese medicine considers that the human body is an organic integrity with meridian acupoints, which consist of main and collateral channels, where the internal organs are in close contact with each other. Acupuncture is an important part of traditional Chinese medicine and the curative effects of acupuncture and moxibustion have been recognized within the medical community worldwide.1–3 Research using blood oxygenation level-dependent functional magnetic resonance imaging (BOLD-fMRI) has confirmed that needling the acupuncture points can adjust cortical and subcortical nerve activity.4–6 Needling language-related acupoints can adjust the normal language network.7
A stroke in the brain is the most common cause of aphasia.8 In recent years, as the population ages and lifestyles change, the number of strokes has increased.9 In patients with acute cerebrovascular disease, 34.2% experience speech disorders and 16.6% experience aphasia, both of which can have a negative impact on a patient’s quality-of-life and result in a considerable burden to the family and society.10 The subsequent anxiety, depression and other mental health issues caused by aphasia have resulted in society paying more attention to the complications of stroke.11 Aphasia rehabilitation takes a long time and the medical treatment is associated with considerable costs, but the outcomes are often limited.12 Therefore, understanding how to effectively treat aphasia and reduce medical costs has become an important subject for clinical and scientific research.
During the treatment of aphasia, several acupoints can be selected and no unified standards exist. Receiving acupuncture at the Tongli (HT5) and Xuanzhong (GB39) acupoints is often used to treat aphasia, although the mechanism of action remains unclear. To the best of our knowledge, there are no reports in the literature describing either acupuncturing single or paired acupoints (right or left side acupoints) that can activate the language-related regions of the brain. Therefore, this present study investigated the effects of receiving acupuncture to the left and right Tongli (HT5) and Tongli (HT5)–Xuanzhong (GB39) acupoints in healthy volunteers using BOLD-fMRI in order to compare the status of various brain regions activated under different acupuncture meridians.
Participants and methods
Healthy volunteers
This observational study enrolled healthy male and female volunteers aged 40–55 years in the Department of Radiology, Taian City Central Hospital, Taian, Shandong Province, China between February 2016 and November 2016. The population of healthy volunteers was recruited using advertising posters placed around Taian City Central Hospital. The inclusion criteria were as follows: (i) in good health with no language or cognitive impairments; (ii) right-hand dominant; (iii) educated to junior high school degree or above; (iv) no prior electroacupuncture treatment; (v) no oral administration or intravenous injection of sedatives or anaesthetics in the week prior to the study.
The study was approved by the Ethics Committee of Taian City Central Hospital, Taian, Shandong Province, China (no. 2016-012). All study participants provided written informed consent.
Electroacupuncture methods
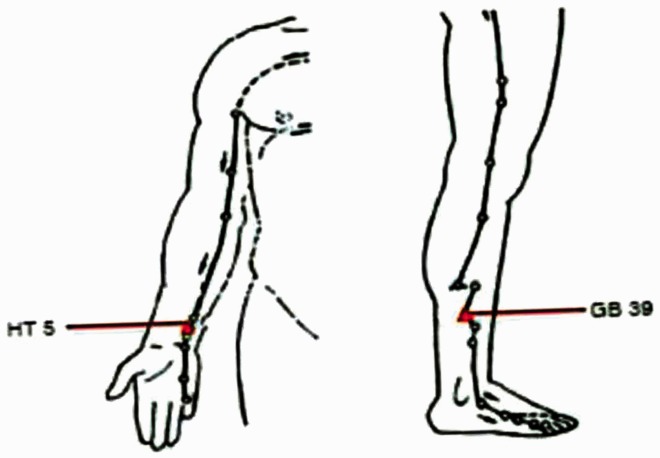
The electroacupuncture technique used 0.40*40 mm sterile silver acupuncture needles (Zhongyan Taihe Company, Beijing, China) with an acusector adaption of the HM6505-1 meridian point therapeutic apparatus (Sichuan Hengming Technology Development Limited Company, Chengdu, China). The two acupoints were located as follows: (i) Tongli (HT5) was 1.5” above the wrist crease; and (ii) Xuanzhong (GB39) was above the ankle joint, 3” superior to the prominence of the lateral malleolus, between the posterior border of the fibula and the tendons of the peroneus longus and brevis (Figure 1). The acupuncture needle depth was 0.5” and the needle angle was 90°.
Figure 1.
The anatomical location of the Tongli (HT5) and Xuanzhong (GB39) acupoints. Tongli (HT5) was 1.5” above the wrist crease; and Xuanzhong (GB39) was above the ankle joint, 3” superior to the prominence of the lateral malleolus, between the posterior border of the fibula and the tendons of the peroneus longus and brevis. The colour version of this figure is available at: http://imr.sagepub.com.
The healthy volunteers laid on their backs on the MRI scanner bed and a silver acupuncture needle was applied to the Tongli (HT5) and Xuanzhong (GB39) acupoints after routine disinfection of the skin. The acupuncture needle was twisted to cause the ‘de qi’ sensation without pain as previously described.13,14 One end of the acusector wire was connected to the acupuncture needle handle and the other end was connected to the HM6505-1 meridian point therapeutic apparatus. The electroacupuncture frequency was 2 Hz and the electric current was 1–2 mA as previously described.15,16 The stimulation waveform was a continuous constant amplitude pulse wave.
Functional MRI scanning
The study used a 3.0 T MAGNETOM Skyra MRI scanner (Siemens Healthcare Limited, Erlangen, Germany) with a 16-channel coil. The scan sequence and parameters were as follows for the structural image scanning: fast spin echo (TSE) sequence to acquire transverse structure T2-weighted images; horizontal position; repetition time/echo time (TR/TE) =6540 ms/99ms; slice thickness 4 mm; interval 0 mm; resolution ratio 288*384; field of view (FOV) = 220 × 220 mm; and 35 layers of anatomical images were acquired.
The scan sequence and parameters were as follows for the BOLD-fMRI scanning: gradient echo sequence (Epfid); copy T1-weighted image set; slice thickness 4 mm; interval 0 mm; TR = 3000 ms; TE = 30 ms; resolution ratio 64*64; and FOV = 220 × 220 mm. Five series of BOLD-fMRI scanning were undertaken during electroacupuncture of the two acupoints as follows: right Tongli (HT5), right Tongli (HT5)–Xuanzhong (GB39), right Tongli (HT5) sham acupuncture, left Tongli (HT5), and left Tongli (HT5)–Xuanzhong (GB39). The stimulus mode alternated between 45 s of electrical stimulation and 45 s of rest for three cycles in total. Each sequence interval was 10 mins.
A fast flash (TFL) sequence was used to gather 3-dimensional images using the following parameters: slice thickness 1 mm; interval 0 mm; TR/TE = 2300 ms/3 ms; TI = 900 ms; bandwidth = 240HZ/Px; resolution ratio 248*256; FOV = 248 × 248 mm; for 192 layers of high resolution anatomical images.
Data processing
The DICOM raw data from each of the study participants were converted into NIfTI format using MRI conversion software (Version 2.0; Lewis Center for Neuroimaging, University of Oregon, Eugene, OR, USA). NIfTI data in the MATLAB® version R2012a platform (Licence Number :161052; MathWorks®, Natick, MA, USA) were analysed using Statistical Parametric Mapping software, version 8 (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK), following the steps as described in brief: (i) undertake head correction with Realign by registering all functional images and the first phase functional images with the application of a 3-dimensional sync interpolation algorithm, correct for head movement, and generate corrected images and the average image; (ii) adopt a coregister by taking the average functional image generated by Realign as a reference and register the structural phase with it; (iii) the registered structural images were divided into three parts (grey matter, white matter and cerebrospinal fluid) with Segment, at the same time taking an East Asian brain as the template for the structural phase’s space standardized parameters; (iv) normalizing the functional image after head movement correction and registered structural images were space standardized with space standardized parameters acquired by Segment, the former voxel size interpolation was 2*2*2 mm and the latter interpolation was 1*1*1 mm; and (v) spatial smoothing of the functional image using Gaussian smoothing with the full width at half maximum of 6 mm.
The laterality index (LI) was calculated using the following formula: LI = (activated voxels in the left – activated voxels in the right)/(activated voxels in the left + activated voxels in the right). If the number was positive, it suggested an opposite relationship between the acupuncture side and the side of the brain that was activated.
Statistical analyses
Statistical analyses of individual study participants were undertaken as follows. Using the stimulation mode function combined with the haemodynamic response function as the design matrix, the general linear model was used to estimate the parameters of the time series images and to obtain statistical parametric mapping under different acupuncture stimulations by single-sample t-test. The activation map of the group analysis was obtained with the space threshold < 10 voxels as the statistical difference threshold. An uncorrected P-value < 0.005 was considered statistically significant.
Statistical analyses of the study group were undertaken as follows. Statistical parametric maps obtained for the group for right Tongli (HT5) sham acupuncture, left Tongli (HT5), right Tongli (HT5), left Tongli (HT5)–Xuanzhong (GB39) and right Tongli (HT5)–Xuanzhong (GB39) were analysed using single-sample t-test. The activation map of the group analysis was obtained with the space threshold less than 10 voxels as the statistical difference threshold. An uncorrected P-value < 0.005 was considered statistically significant.
The SPM8 plug-in, which is Anatomical Automatic Labelling, was used to obtain the spatial coordinates of each activated area; which were then used to localize and calculate the size of the activated voxels corresponding to the Montreal Neurological Institute (MNI) standard brain. Overlapping the activated parametric mapping that was larger than the selected threshold value to the T1-weighted image of the MNI resulted in 2-dimensional and 3-dimensional mapping of activated brain regions. The results were visualized using xjView toolbox version 8 software.17
Results
The study included 10 healthy volunteers (five males/five females) with a mean age of 44.5 ± 2.5 years (range 40–55 years).
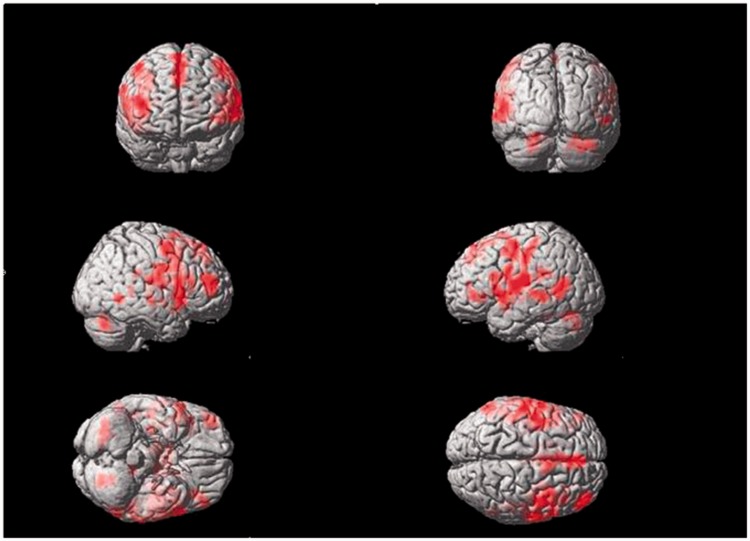
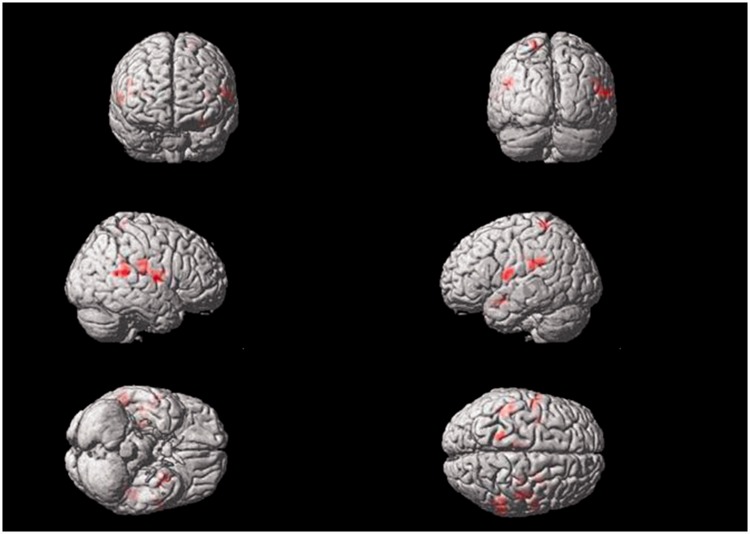
Electrical stimulation of the right Tongli (HT5) acupoint activated the following brain areas (Table 1, Figure 2): the left insula, left inferior frontal gyrus triangle and operculum, left middle temporal gyrus, left postcentral gyrus, right superior temporal gyrus, right middle temporal gyrus, right inferior frontal operculum, right middle frontal gyrus, bilateral precentral gyrus, bilateral frontal superior medial gyrus, bilateral supplementary motor area, bilateral postcentral gyrus, bilateral central rolandic operculum region, and bilateral cerebellar superior gyrus.
Table 1.
Areas of brain activation induced by acupuncture stimulation of the right Tongli (HT5) acupoint.
| Coordinates of local maxima |
Brain region | % cluster | Number of voxels cluster % | Label | Number of voxels label | T value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| –42 | –10 | 10 | Postcentral_L | 26.27 | 731 | 16.65 | 3892 | 9.94 |
| Rolandic_ Oper_L | 16.28 | 731 | 40.57 | 990 | ||||
| Insula_L | 10.26 | 731 | 13.62 | 1858 | ||||
| 24 | –1 | 12 | Rolandic_ Oper_R | 33.69 | 187 | 15.97 | 1331 | 7.91 |
| Postcentral_R | 28.34 | 187 | 4.68 | 3823 | ||||
| 48 | –40 | 1 | Temporal_Mid_R | 50.00 | 10 | 0.38 | 4409 | 6.43 |
| Temporal_Sup_R | 30.00 | 10 | 0.32 | 3141 | ||||
| 33 | 8 | 34 | Frontal_Inf_Oper_R | 35.53 | 349 | 29.91 | 1399 | 6.32 |
| 30 | –70 | –35 | Cerebelum_Crus1_R | 80.65 | 93 | 9.56 | 2648 | 6.28 |
| 45 | –4 | 52 | Frontal_Mid_R | 62.86 | 70 | 2.91 | 5104 | 6.05 |
| Precentral_R | 34.29 | 70 | 2.40 | 3381 | ||||
| –48 | –7 | 49 | Precentral_L | 76.52 | 115 | 8.42 | 3526 | 5.85 |
| 6 | 8 | 70 | Frontal_Sup_Medial_L | 41.62 | 185 | 8.69 | 2992 | 5.60 |
| Supp_Motor_Area_L | 20.54 | 185 | 5.97 | 2147 | ||||
| Supp_Motor_Area_R | 16.76 | 185 | 4.41 | 2371 | ||||
| Frontal_Sup_Medial_R | 16.22 | 185 | 4.74 | 2134 | ||||
| –66 | –37 | –2 | Temporal_Mid_L | 94.05 | 168 | 10.79 | 4942 | 5.52 |
| –21 | –61 | –26 | Cerebelum_6_L | 68.00 | 75 | 10.16 | 1694 | 5.31 |
| –42 | 38 | –2 | Frontal_Inf_Tri_L | 82.69 | 52 | 5.74 | 2529 | 4.69 |
| Frontal_Inf_Orb_L | 15.38 | 52 | 1.60 | 1690 | ||||
Table shows the first local maximum per cluster. The activation map of the group analysis was obtained with the space threshold < 10 voxels as the statistical difference threshold. An uncorrected P-value < 0.005 was considered statistically significant.
Figure 2.
Areas of brain activation induced by acupuncture stimulation of the right Tongli (HT5) acupoint. The results from functional magnetic resonance imaging were surface-rendered onto a canonical brain. The red areas represent all voxels that were significant at P < 0.005. The colour version of this figure is available at: http://imr.sagepub.com.
Electrical stimulation of the right Tongli (HT5)–Xuanzhong (GB39) acupoints activated the following brain areas (Table 2, Figure 3): the left middle cingulate gyrus, left superior temporal gyrus, left supramarginal gyrus, left postcentral gyrus, right inferior frontal operculum, right precentral gyrus, bilateral supplementary motor area, and bilateral central rolandic operculum region.
Table 2.
Areas of brain activation induced by acupuncture stimulation of the right Tongli (HT5)–Xuanzhong (GB39) acupoints.
| Coordinates of local maxima |
Brain region | % cluster | Number of voxels cluster% | Label | Number of voxels label | T value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| –9 | 5 | 40 | Cingulum_Mid_L | 74.07 | 27 | 3.48 | 1941 | 8.23 |
| Supp_Motor_Area_L | 14.81 | 27 | 0.63 | 2147 | ||||
| 9 | 11 | 49 | Supp_Motor_Area_R | 94.59 | 37 | 4.98 | 2371 | 4.97 |
| 63 | 11 | 22 | Rolandic_Oper_R | 42.42 | 33 | 3.55 | 1331 | 4.80 |
| Frontal_Inf_Oper_R | 27.27 | 33 | 2.17 | 1399 | ||||
| Precentral_R | 24.24 | 33 | 0.80 | 3381 | ||||
| –54 | –34 | 25 | SupraMarginal_L | 72.00 | 25 | 4.84 | 1256 | 4.77 |
| Temporal_Sup_L | 28.00 | 25 | 1.03 | 2296 | ||||
| –3 | –7 | 52 | Supp_Motor_Area_L | 100.00 | 21 | 3.30 | 2147 | 4.44 |
| –51 | –16 | 19 | Postcentral_L | 58.06 | 31 | 1.56 | 3892 | 3.97 |
| Rolandic_Oper_L | 41.94 | 31 | 4.43 | 990 | ||||
Table shows the first local maximum per cluster. The activation map of the group analysis was obtained with the space threshold < 10 voxels as the statistical difference threshold. An uncorrected P-value < 0.005 was considered statistically significant.
Figure 3.
Areas of brain activation induced by acupuncture stimulation of the right Tongli (HT5)–Xuanzhong (GB39) acupoints. The results from functional magnetic resonance imaging were surface-rendered onto a canonical brain. The red areas represent all voxels that were significant at P < 0.005. The colour version of this figure is available at: http://imr.sagepub.com.
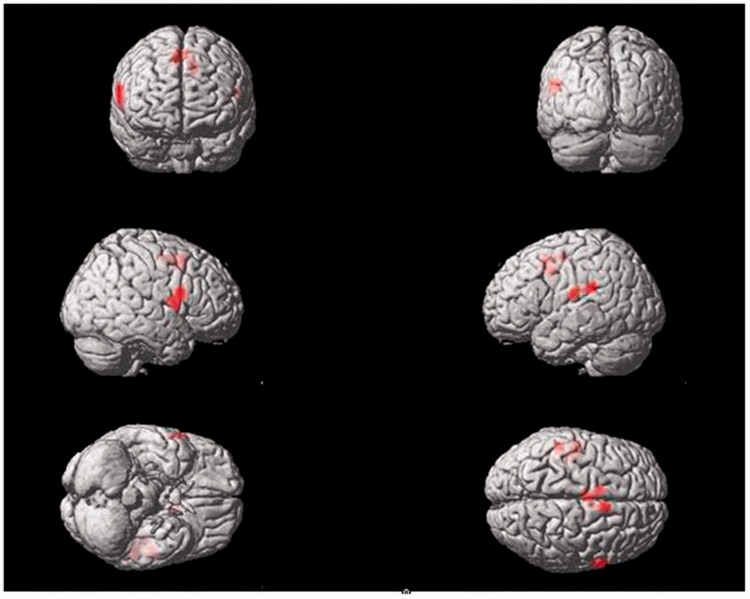
Sham acupuncture of the right Tongli (HT5) acupoint activated the following brain areas (Table 3, Figure 4): the left postcentral gyrus, left inferior parietal lobule, left middle frontal gyrus, left precuneus, right insula, right inferior frontal operculum and triangle, right middle cingulate gyrus, bilateral supramarginal gyrus, bilateral superior parietal lobule, bilateral superior frontal gyrus, occipital lobe, and cerebellum.
Table 3.
Areas of brain activation induced by sham acupuncture stimulation of the right Tongli (HT5) acupoint.
| Coordinates of local maxima |
Brain region | % cluster | Number of voxels cluster % | Label | Number of voxels label | T value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| –27 | –67 | 19 | Occipital_Mid_L | 63.67 | 311 | 20.44 | 3270 | 7.57 |
| 24 | –61 | –47 | Cerebelum_8_R | 100.00 | 15 | 2.19 | 2308 | 6.48 |
| 51 | –13 | 28 | Postcentral_R | 58.06 | 62 | 3.18 | 3823 | 6.35 |
| SupraMarginal_R | 27.42 | 62 | 2.91 | 1974 | ||||
| 39 | –73 | 28 | Occipital_Mid_R | 47.41 | 270 | 20.59 | 2098 | 5.74 |
| 66 | –40 | 37 | SupraMarginal_R | 95.65 | 23 | 3.76 | 1974 | 4.89 |
| 15 | –61 | 67 | Parietal_Sup_R | 65.22 | 23 | 2.28 | 2222 | 4.57 |
| –66 | –34 | 34 | SupraMarginal_L | 55.56 | 27 | 4.03 | 1256 | 4.57 |
| 24 | –4 | 64 | Frontal_Sup_R | 100.00 | 22 | 1.83 | 4056 | 4.56 |
| –36 | –28 | 37 | Postcentral_L | 51.11 | 90 | 3.99 | 3892 | 4.47 |
| –24 | –1 | 64 | Frontal_Sup_L | 64.00 | 25 | 1.50 | 3599 | 4.35 |
| Frontal_Mid_L | 28.00 | 25 | 0.49 | 4863 | ||||
| 42 | 14 | 4 | Frontal_Inf_Oper_R | 50.00 | 12 | 1.45 | 1399 | 4.30 |
| Insula_R | 33.33 | 12 | 0.76 | 1770 | ||||
| Frontal_Inf_Tri_R | 16.67 | 12 | 0.31 | 2151 | ||||
| –12 | –79 | 46 | Parietal_Sup_L | 56.41 | 39 | 3.60 | 2065 | 4.24 |
| 9 | –37 | 43 | Cingulum_Mid_R | 92.31 | 13 | 1.84 | 2203 | 4.15 |
| –33 | 44 | 34 | Frontal_Mid_L | 100.00 | 18 | 1.25 | 4863 | 3.80 |
| –27 | –49 | 49 | Parietal_Inf_L | 90.00 | 10 | 1.24 | 2447 | 3.78 |
Table shows the first local maximum per cluster. The activation map of the group analysis was obtained with the space threshold < 10 voxels as the statistical difference threshold. An uncorrected P-value < 0.005 was considered statistically significant.
Figure 4.
Areas of brain activation induced by sham acupuncture stimulation of the right Tongli (HT5) acupoint. The results from functional magnetic resonance imaging were surface-rendered onto a canonical brain. The red areas represent all voxels that were significant at P < 0.005. The colour version of this figure is available at: http://imr.sagepub.com.
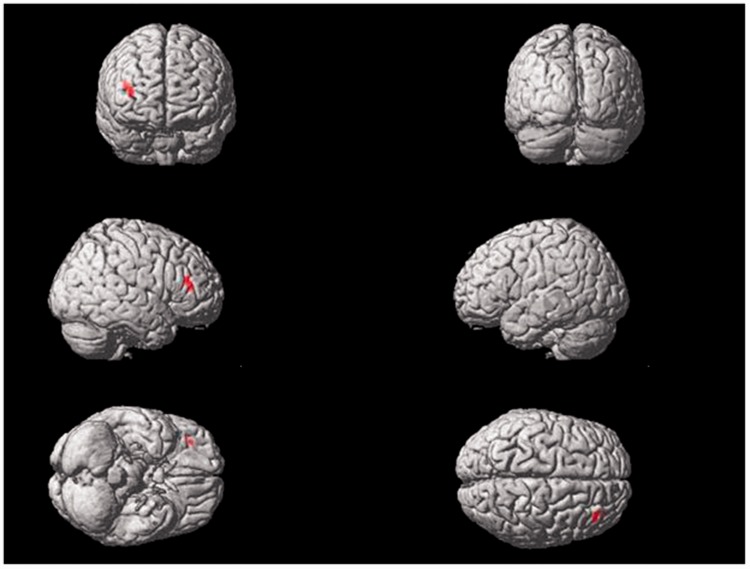
Electrical stimulation of the left Tongli (HT5) acupoint activated the following brain area (Table 4, Figure 5): the right middle frontal gyrus.
Table 4.
Areas of brain activation induced by acupuncture stimulation of the left Tongli (HT5) acupoint.
| Coordinates of local maxima |
Brain region | % cluster | Number of voxels cluster % | Label | Number of voxels label | T value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| 36 | 38 | 10 | Frontal_Mid_R | 100.00 | 11 | 0.73 | 5104 | 4.15 |
Table shows the first local maximum per cluster. The activation map of the group analysis was obtained with the space threshold < 10 voxels as the statistical difference threshold. An uncorrected P-value < 0.005 was considered statistically significant.
Figure 5.
Areas of brain activation induced by acupuncture stimulation of the left Tongli (HT5) acupoint. The results from functional magnetic resonance imaging were surface-rendered onto a canonical brain. The red areas represent all voxels that were significant at P < 0.005. The colour version of this figure is available at: http://imr.sagepub.com.
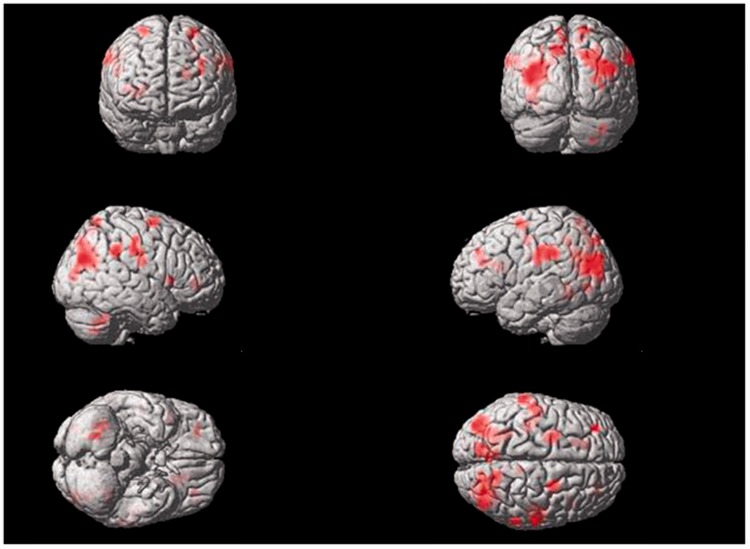
Electrical stimulation of the left Tongli (HT5)–Xuanzhong (GB39) acupoints activated the following brain areas (Table 5, Figure 6): the left supramarginal gyrus, left postcentral gyrus, left precentral gyrus, left precuneus, left superior parietal lobule, right supramarginal gyrus, right superior temporal gyrus, bilateral insula, and bilateral central rolandic operculum region.
Table 5.
Areas of brain activation induced by acupuncture stimulation of the left Tongli (HT5)–Xuanzhong (GB39) acupoints.
| Coordinates of local maxima |
Brain region | % cluster | Number of voxels cluster % | Label | Number of voxels label | T value | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| –45 | –40 | 28 | SupraMarginal_L | 78.12 | 32 | 6.72 | 1256 | 4.68 |
| 30 | –22 | 16 | Insula_R | 53.33 | 15 | 1.53 | 1770 | 4.64 |
| 48 | –10 | 13 | Rolandic_Oper_R | 51.61 | 31 | 4.06 | 1331 | 4.47 |
| SupraMarginal_R | 22.58 | 31 | 1.20 | 1974 | ||||
| 57 | –43 | 16 | Temporal_Sup_R | 82.86 | 35 | 3.12 | 3141 | 4.37 |
| SupraMarginal_R | 11.43 | 35 | 0.68 | 1974 | ||||
| –54 | –4 | 16 | Postcentral_L | 72.22 | 18 | 1.13 | 3892 | 4.00 |
| Rolandic_Oper_L | 16.67 | 18 | 1.02 | 990 | ||||
| Precentral_L | 11.11 | 18 | 0.19 | 3526 | ||||
| –33 | –1 | 13 | Insula_L | 84.62 | 13 | 2.00 | 1858 | 3.99 |
| 60 | –7 | 10 | Rolandic_Oper_R | 100.00 | 11 | 2.79 | 1331 | 3.53 |
| –18 | –46 | 67 | Postcentral_L | 70.00 | 10 | 0.61 | 3892 | 3.50 |
| Precuneus_L | 20.00 | 10 | 0.19 | 3528 | ||||
| Parietal_Sup_L | 10.00 | 10 | 0.16 | 2065 | ||||
Table shows the first local maximum per cluster. The activation map of the group analysis was obtained with the space threshold < 10 voxels as the statistical difference threshold. An uncorrected P-value < 0.005 was considered statistically significant.
Figure 6.
Areas of brain activation induced by acupuncture stimulation of the left Tongli (HT5)–Xuanzhong (GB39) acupoints. The results from functional magnetic resonance imaging were surface-rendered onto a canonical brain. The red areas represent all voxels that were significant at P < 0.005. The colour version of this figure is available at: http://imr.sagepub.com.
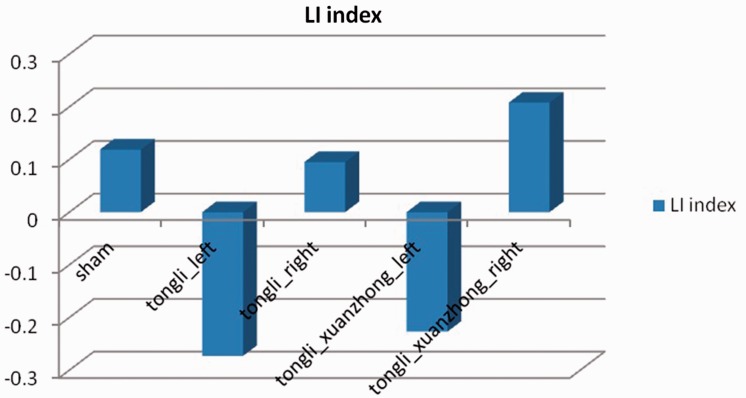
In terms of the LI, the highest number of activated voxels occurred when the right Tongli (HT5) acupoint was stimulated (data not shown), and the left hemisphere of the brain had more activated voxels than the right side (data not shown). These findings suggest that there was a partial lateralization tendency on the left side when acupuncturing the right Tongli (HT5) acupoint (LI = 0.0952) (Figure 7).
Figure 7.
Laterality index (LI) for each of the acupuncture points that were stimulated during the study: sham right Tongli (HT5) (LI = 0.119), left Tongli (HT5) (LI = –0.273), right Tongli (HT5) (LI = 0.0952), left Tongli (HT5)–Xuanzhong (GB39) (LI = –0.227) and right Tongli (HT5)–Xuanzhong (GB39) (LI = 0.208) acupoints.
Discussion
Aphasia is the most common complication of cerebral stroke and recovery of language function is limited.18,19 Speech therapy is the most useful method for treating aphasia.20 Speech rehabilitation therapy is twice as effective as unaided speech recovery,21 but the effectiveness of speech therapy remains clinically unsatisfactory. Therefore, other treatments such as acupuncture have been applied to speech therapy rehabilitation.22,23 Scalp, tongue and body acupuncture can be used to treat aphasia.24,25 After experiencing a cerebral infarction, patients with aphasia have different degrees of hemiplegia. As it can be difficult to move the patient, scalp and tongue acupuncture are difficult to apply during the early phase of stroke. In addition, these methods are not readily accepted by most patients. As a result, there is limited use of scalp and tongue acupuncture in Western medicine-based general hospitals.
Although acupuncture is used widely all over the world, its biological mechanism of action is not well understood. From a neurophysiological point of view, acupuncture can be regarded as a complex somatosensory stimulation.26 According to clinical and experimental research, body acupuncture is an effective therapy for aphasia, but the selection of the most appropriate acupoints is not standardized. For example, a previous study demonstrated that the electrical stimulation of the Tongli (HT5) acupoint could improve speech function.27 A case report of a patient with subcortical aphasia found that acupuncturing the Tongli (HT5) and Xuanzhong (GB39) acupoints activated language-related regions in the brain on fMRI.28 A report of seven cases of chronic aphasia described significant improvements in the language behaviour index after acupuncturing the Hegu (LI4), Neiguan (P6), Taichong (LIV3) and Zusanli (ST36) acupoints.29 The authors also observed that activation of the left middle temporal gyrus and left superior temporal gyrus was positively correlated with the improvements in the language behaviour index.29 Another report of seven cases of aphasia caused by vascular disease found that the left insula and lateral fissure areas were significantly activated after acupuncturing the Sanyangluo (SJ8) acupoint and the authors concluded that acupuncture may have therapeutic benefits in patients with post-stroke aphasia.30
In traditional Chinese medicine rehabilitation work, the Tongli (HT5) and Xuanzhong (GB39) acupoints are mostly applied to treat aphasia. Tongli (HT5) is a contact point for the heart meridian, its content was first mentioned in the ‘Pivot Channels’: ‘The name of hand of Shaoyin is the meridian that circulates in the heart and belongs to the department of the tongue and eye system’. Acupuncturing Tongli (HT5) can promote the mind, wake the patient from unconsciousness by clearing away heart fire, and cure the symptoms of sudden speech loss. The Xuanzhong (GB39) acupoint belongs to the foot sanyang meridians and the foot shaoyang gallbladder lines. Marrow gathers in the xuanzhong and the brain is the sea of the marrow. Therefore, the Tongli (HT5) and Xuanzhong (GB39) acupoints can be useful for treating language dysfunction.
Based on the published literature,31 the left side of the brain is positively activated when acupuncturing right body acupoints. However, a case report of a patient with subcortical aphasia found that acupuncturing the Tongli (HT5) and Xuanzhong (GB39) acupoints on the left side resulted in better bilateral temporal cortical activation than acupuncturing the right side.28 The levels of activated voxels in the left side of the cerebrum were higher than the ones of the right side, suggesting a consistent relationship between the acupuncture side and the side of the brain that was activated. In order to further clarify the specificity of particular acupoints, this present study compared the effects of acupuncturing the left Tongli (HT5), right Tongli (HT5), left Tongli (HT5)–Xuanzhong (GB39), right Tongli (HT5)–Xuanzhong (GB39) acupoints and sham acupuncturing the right Tongli (HT5).
The results of the present study demonstrated that multiple brain regions closely related to language processing were activated when acupuncturing the right Tongli (HT5), such as the left insula, left inferior frontal gyrus triangle and opercula, left middle temporal gyrus, right superior temporal gyrus, right middle temporal gyrus, right inferior frontal operculum, right middle frontal gyrus, and bilateral central rolandic operculum. Combined with the analysis of laterality index, acupuncturing the right Tongli (HT5) acupoint activated bilateral brain regions related to language processing, but the activated voxels were greater on the left side than the right side, suggesting an opposite relationship between the acupuncture side and the side of the brain that was activated. Future experiments will investigate the effects of acupuncturing the right Tongli (HT5) acupoint in patients with motor aphasia caused by cerebral infarction.
The results of the present study demonstrated that acupuncturing the right Tongli (HT5)–Xuanzhong (GB39) acupoints activated the left middle cingulate gyrus, left superior temporal gyrus, left supramarginal gyrus, left postcentral gyrus, right inferior frontal operculum, right precentral gyrus, bilateral supplementary motor area, and bilateral central rolandic operculum region. The level of activated voxels and brain areas were significantly lower than those when the right Tongli (HT5) acupoint alone was stimulated; the reason perhaps being that the two acupoints come from different channels, so they might inhibit each other, although both them are language-associated body acupoints.
When sham acupuncture was undertaken on the right Tongli (HT5) acupoint, the main regions of activation were in the bilateral occipital lobes, central posterior gyrus, and the parietal lobe; the reason perhaps being that sham acupuncture did not properly stimulate the acupoint, so any affects were mainly due to the sense of touch. Bilateral language regions were not obviously activated.
The results of the present study demonstrated that acupuncturing the left Tongli (HT5) resulted in only a small amount of activation in the right middle frontal gyrus. The differences between the areas of the brain that were activated by acupuncturing the left and right Tongli (HT5) acupoints were obvious: the left inferior frontal triangle and operculum, left insula, left middle temporal gyrus, left central rolandic operculum region, left superior temporal gyrus, right middle frontal gyrus, right inferior frontal operculum area, right superior temporal gyrus, right middle temporal gyrus, right calcarine cortex, right lateral lingual gyrus, right middle cingulate gyrus, bilateral precentral gyrus, and bilateral postcentral gyrus. These results highlight the value of the right Tongli (HT5) acupoint in activating language-related areas of the brain.
The results of the present study demonstrated that acupuncturing the left Tongli (HT5)–Xuanzhong (GB39) acupoints activated the left supramarginal gyrus, left postcentral gyrus, left precentral gyrus, left precuneus, left superior parietal lobule, right supramarginal gyrus, right superior temporal gyrus, bilateral insula, and bilateral central rolandic operculum region. Acupuncturing the left Tongli (HT5)–Xuanzhong (GB39) acupoints resulted in fewer areas of brain activation related to language and lower levels of activated voxels, suggesting that these combined acupoints were of little value in activating language-related areas of the brain.
The present study had several limitations. First, the number of volunteers was small. Secondly, as a result of individual differences in sensitivity to electrical stimulation, the stimulation current was not the same in each volunteer. In order to standardize the treatment, each volunteer confirmed that they felt the ‘de qi’ sensation, but it should be noted that this is a subjective feeling. Thirdly, all of the volunteers were acupunctured at the right Tongli (HT5) acupoint first, so they may have been more sensitive to electrical stimulation during the first contact, which might have affected which parts of the brain were activated.
In conclusion, acupuncturing the right Tongli (HT5) acupoint activated bilateral language-related brain regions, so it may be used as a target acupoint in the treatment of aphasia caused by cerebral infarction, which warrants further research.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This research received funding from the Foundation for Outstanding Young Scientist in Shandong Province, China (no. BS2012YY033).
References
- 1.Kong JC, Lee MS, Shin BC, et al. Acupuncture for functional recovery after stroke: a systematic review of sham-controlled randomized clinical trials. CMAJ 2010; 182: 1723–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu P, Mills E, Moher D, et al. Acupuncture in poststroke rehabilitation: a systematic review and meta-analysis of randomized trials. Stroke 2010; 41: e171–e179. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Jin H, Ma D, et al. Efficacy of integrated rehabilitation techniques of traditional Chinese medicine for ischemic stroke: a randomized controlled trial. Am J Chin Med 2013; 41: 971–981. [DOI] [PubMed] [Google Scholar]
- 4.Bai L, Qin W, Tian J, et al. Time-varied characteristics of acupuncture effects in fMRI studies. Hum Brain Mapp 2009; 30: 3445–3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bai L, Tian J, Zhong C, et al. Acupuncture modulates temporal neural responses in wide brain networks: evidence from fMRI study. Mol Pain 2010; 6: 73–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang W, Pach D, Napadow V, et al. Characterizing acupuncture stimuli using brain imaging with fMRI – A systematic review and meta-analysis of the literature. PLoS One 2012; 7: e32960–e32960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G, Liu HL, Cheung RT, et al. An fMRI study comparing brain activation between word generation and electrical stimulation of language implicated acupoints. Hum Brain Mapp 2003; 18: 233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inatomi Y, Yonehara T, Omiya S, et al. Aphasia during the acute phase in ischemic stroke. Cerebrovasc Dis 2008; 25: 316–323. [DOI] [PubMed] [Google Scholar]
- 9.Liu M, Wu B, Wang WZ, et al. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol 2007; 6: 456–464. [DOI] [PubMed] [Google Scholar]
- 10.Donnan GA, Fisher M, Macleod M, et al. Stroke. Lancet 2008; 371: 1612–1623. [DOI] [PubMed] [Google Scholar]
- 11.Hilari K, Northcott S, Roy P, et al. Psychological distress after stroke and aphasia: the first six months. Clin Rehabil 2010; 24: 181–190. [DOI] [PubMed] [Google Scholar]
- 12.Hillis AE. Aphasia: progress in the last quarter of a century. Neurology 2007; 69: 200–213. [DOI] [PubMed] [Google Scholar]
- 13.Park J, Park H, Lee H, et al. Deqi sensation between the acupuncture-experienced and the naïve: a Korean study II. Am J Chin Med 2005; 33: 329–337. [DOI] [PubMed] [Google Scholar]
- 14.Witt C, Brinkhaus B, Jena S, et al. Acupuncture in patients with osteoarthritis of the knee: a randomised trial. Lancet 2005; 366: 136–143. [DOI] [PubMed] [Google Scholar]
- 15.Li G, Cheung RT, Ma QY, et al. Visual cortical activations on fMRI upon stimulation of the vision-implicated acupoints. Neuroreport 2003; 14: 669–673. [DOI] [PubMed] [Google Scholar]
- 16.Li G, Huang L, Cheung R, et al. Cortical activations upon stimulation of the sensorimotor-implicated acupoints. Magn Reson Imaging 2004; 22: 639––644. [DOI] [PubMed] [Google Scholar]
- 17.xjView: A viewing program for SPM, http://www.alivelearn.net/xjview/ (10 Jan 2016).
- 18.Tilling K, Sterne JA, Rudd AG, et al. A new method for predicting recovery after stroke. Stroke 2001; 32: 2867–2873. [DOI] [PubMed] [Google Scholar]
- 19.Paolucci S, Antonucci G, Pratesi L, et al. Functional outcome in stroke inpatient rehabilitation: predicting no, low and high response patients. Cerebrovasc Dis 1998; 8: 228–234. [DOI] [PubMed] [Google Scholar]
- 20.Martins IP, Leal G, Fonseca I, et al. A randomized, rater-blinded, parallel trial of intensive speech therapy in sub-acute post-stroke aphasia: the SP-I-R-IT study. Int J Lang Commun Disord 2013; 48: 421–431. [DOI] [PubMed] [Google Scholar]
- 21.Nouwens F, Dippel DW, de Jong-Hagelstein M, et al. Rotterdam Aphasia Therapy Study (RATS)-3: “The efficacy of intensive cognitive-linguistic therapy in the acute stage of aphasia”; design of a randomised controlled trial. Trials 2013; 14: 24–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elsner B, Kugler J, Pohl M, et al. Transcranial direct current stimulation (tDCS) for improving aphasia in patients after stroke. Cochrane Database Syst Rev 2013; 6: CD009760–CD009760. [DOI] [PubMed] [Google Scholar]
- 23.Li JA. Clinical observation on acupuncture for treatment of aphasia due to ischemic stroke at the early stage. Chinese Acupuncture & Moxibustion 2005; 25: 760–762. [Article in Chinese, English abstract]. [PubMed] [Google Scholar]
- 24.Chen X, Wang Y, Xu W. Clinical observation on treatment of aphasia due to apoplexy with acupuncture of governor vessel and tongue. Chinese Acupuncture & Moxibustion 2001; 21: 519–521. [Article in Chinese, English abstract]. [Google Scholar]
- 25.Rang M. Observation on therapeutic effect of acupuncture on aphasia due to cerebral infarction. Chinese Acupuncture & Moxibustion 2003; 23: 19–20. [Article in Chinese, English abstract]. [Google Scholar]
- 26.Bäcker M, Hammes M, Sander D, et al. Changes of cerebrovascular response to visual stimulation in migraineurs after repetitive sessions of somatosensory stimulation (acupuncture): a pilot study. Headache 2004; 44: 95–101. [DOI] [PubMed] [Google Scholar]
- 27.Fang W, Yang W, Zhao N, et al. Influence of Tongli acupuncture combined with speech function rehabilitation training on speech functionon in patients with motor aphasia after cerebral infarction. Chinese Journal of Integrative Medicine on Cardio-/Cerebrovascular Disease 2010; 8: 290–292. [Google Scholar]
- 28.Chang J, Gao Y, Zhang H, et al. A Preliminary discussion of the effect of electroacupuncture at acupoints HT5 and GB39 on lingual function and fMRI changes in a case of subcortical aphasia. Chinese Journal of Rehabilitation Medicine 2007; 22: 13–17. [Google Scholar]
- 29.Chau AC, Fai Cheung RT, Jiang X, et al. An fMRI study showing the effect of acupuncture in chronic stage stroke patients with aphasia. J Acupunct Meridian Stud 2010; 3: 53–57. [DOI] [PubMed] [Google Scholar]
- 30.Li G, Yang ES. An fMRI study of acupuncture-induced brain activation of aphasia stroke patients. Complement Ther Med 2011; 19(Suppl 1): S49–S59. [DOI] [PubMed] [Google Scholar]
- 31.Li J, Zhang Y. The fMRI study of cerebral speech cortex activation by electric stimulating Tongli and Shangqiu. Chinese Clinical Radiology 2005; 24: 1039–1043. [Google Scholar]