Abstract
Study Objectives
The time-on-task (TOT) effect and total sleep deprivation (TSD) have similar effects on neurobehavioral functioning, including increased performance instability during tasks requiring sustained attention. The TOT effect is exacerbated by TSD, suggesting potentially overlapping mechanisms. We probed these mechanisms by investigating genotype–phenotype relationships on psychomotor vigilance test (PVT) performance for 3 a-priori selected genes previously linked to the TOT effect and/or TSD: dopamine active transporter 1 (DAT1), catechol-O-methyltransferase (COMT), and tumor necrosis factor alpha (TNFα).
Methods
N = 82 healthy adults participated in 1 of 3 laboratory studies. A 10-min PVT was administered repeatedly during 38 h of TSD. We assessed changes in response time (RT) across each minute of the PVT as a function of time awake and genotype. Additionally, cumulative relative RT frequency distributions were constructed to examine changes in performance from the first to the second 5 min of the PVT as a function of genotype.
Results
DAT1, COMT, and TNFα were associated with differences in the build-up of the TOT effect across the 10-min PVT. DAT1 additionally modulated the interaction between TSD and the TOT effect. Subjects homozygous for the DAT1 10-repeat allele were relatively protected against TOT deficits on the PVT during TSD compared to carriers of the 9-repeat allele.
Conclusions
DAT1 is known to regulate dopamine reuptake and is highly expressed in the striatum. Our results implicate striatal dopamine in mechanisms involved in performance instability that appear to be common to TSD and the TOT effect. Furthermore, DAT1 may be a candidate biomarker of resilience to the build-up of performance impairment across TOT due to TSD.
Keywords: cognitive performance, fatigue, vigilance decrement, mental workload, dopamine active transporter 1 (DAT1), catechol-O-methyltransferase (COMT), tumor necrosis factor alpha (TNFα), striatum, psychomotor vigilance test (PVT), total sleep deprivation
Statement of Significance
The time-on-task effect—the performance decrement across the duration of a performance task—is substantially amplified by sleep deprivation. Both the time-on-task effect and sleep deprivation are characterized by performance instability, separately and in interaction, suggesting overlapping mechanisms. Previous work implicated dopamine active transporter 1 (DAT1) genotype in the magnitude of the time-on-task effect. We showed that DAT1 is also associated with the amplification of performance instability due to interaction between the time-on-task effect and sleep deprivation, implicating DAT1 in shared underlying mechanisms. DAT1 is highly expressed in the striatum, where dopaminergic cognition-related mechanisms intertwine with adenosinergic mechanisms of sleep/wake homeostasis, thus suggesting that the striatum is involved in performance instability from time-on-task and sleep deprivation.
INTRODUCTION
The “time-on-task effect” or “vigilance decrement” refers to a progressive decrement of performance across the duration of a cognitive task. The phenomenon tends to be particularly pronounced during sustained engagement in a vigilance task,1 and a rest break provides recuperation.2 The time-on-task (TOT) effect is a critical determinant of productivity and safety in systems monitoring, transportation, and security operations.3–9 Yet, the neurobiological mechanisms underlying the TOT effect remain unclear.10
Importantly, the TOT effect entails an increase in performance “variability” over the duration of a task.11 On the psychomotor vigilance test (PVT)—a vigilance task with a well-documented TOT effect12—this is observed as a steady increase in the standard deviation of response times (RTs) across the 10-min duration of the task.13 Interestingly, increased RT variability is also a hallmark of how sleep deprivation affects performance on the PVT.14 Moreover, the TOT effect interacts with sleep deprivation, such that prior sleep loss amplifies the increase in RT variability from the TOT effect.13 This interaction has been observed for sleep loss from total sleep deprivation (TSD) as well as sustained sleep restriction15,16 and suggests that the effects of TOT and sleep loss on vigilance task performance may have shared underlying mechanisms.13,15,17,18
It has been posited that the effects on vigilance performance due to TOT, sleep loss, and their interaction may collectively be the result of sleep-regulatory processes induced by sustained use of neuronal networks subserving task performance.15 Specifically, it has been speculated that these effects are caused by failure in cognitive pathways to adequately process information due to local, use-dependent expression of a neuronal, sleep-like state.19 This “local sleep”20 would lead to cognitive instability during otherwise functional wakefulness, thus presumably giving rise to the observed performance variability.21 Neuroimaging studies have provided findings that appear to be consistent with this view,19,22,23 but more conclusive evidence is needed.
We set out to further examine the idea that the effects of TOT and sleep loss on vigilance task performance have shared underlying mechanisms, by investigating genetic polymorphisms implicated in the TOT effect or the performance variability associated with sleep deprivation. A priori we selected 3 genes of interest:
DAT1: The dopamine transporter 1 gene (DAT1, also known as the dopamine active transporter or SLC6A3) has a polymorphism involving a variable number tandem repeat (VNTR) of 40 base pairs in the 3′ untranslated region, of which the 9- and 10-repeat alleles are the most common in the population.24 Under well-rested (baseline) laboratory conditions, this polymorphism has been found to moderate the magnitude of the TOT effect on PVT performance.25 DAT1 has also been implicated in homeostatic sleep–wake regulation26,27 and the mechanisms of action of the wake-promoting compound modafinil.26,28 Modafinil has been found to mitigate the effect of TSD on the TOT effect.29 Taken together, these findings suggest that the VNTR polymorphism of DAT1 may be involved in a shared mechanism underlying the effects of TOT and sleep loss on performance.
COMT: The catechol-O-methyltransferase gene (COMT) has a functional single-nucleotide polymorphism (SNP) involving a valine (Val) to methionine (Met) substitution at codon 158 (COMT Val158Met).30 Under well-rested laboratory conditions, this polymorphism has been shown to moderate the magnitude of the TOT effect on PVT performance.25 COMT has also been implicated in homeostatic sleep–wake regulation31 and the effect of the wake-promoting compound modafinil.31,32 Therefore, like the VNTR polymorphism of DAT1, the Val158Met polymorphism of COMT may be involved in a shared mechanism underlying the effects of TOT and sleep loss on performance.
TNFα: The tumor necrosis factor alpha gene (TNFα) has a functional SNP in the promoter region involving a guanine (G) to adenine (A) substitution at position 308 (TNFα G308A).33 This polymorphism has been shown to moderate the magnitude of the effect of TSD on PVT performance.34 TNFα has been implicated in local sleep,35 which is also a hypothesized mechanism for the effect of the G308A polymorphism of TNFα on PVT performance during sleep deprivation.34 If local sleep is involved in the TOT effect as well, then the G308A polymorphism of TNFα may be linked to a shared mechanism underlying the effects of TOT and sleep loss on performance.
For each of the 3 genes of interest, we investigated whether and how their respective polymorphisms affect PVT performance, with a particular focus on the interaction between the TOT effect and the effect of sleep deprivation.
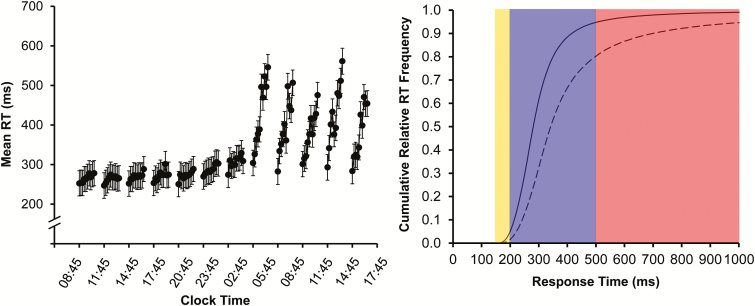
We investigated this interaction in 2 complementary ways. First, we analyzed changes in RT across 1-min bins of the 10-min task duration of the PVT, as a function of time awake. See Figure 1 (left) for results from an earlier study36 to illustrate this approach. Second, we analyzed differences in cumulative relative frequency distributions of the RTs14 in the first versus second 5 min on the PVT, comparing sleep deprivation against baseline. See Figure 1 (right) for an illustration of this procedure.
Figure 1.
Illustration of analysis approaches. Left: mean RT (±standard error) in 1-min bins for each of twelve 10-min PVTs administered at 3-h intervals across 38 h of total sleep deprivation in an earlier study (16 healthy subjects).36 The graph shows the increase in mean RT across the 1-min bins in each test bout, with the rate of change increasing substantially with progressing time awake. Data are plotted against the start times of the PVT bouts; placement of the 1-min bins in each test bout is not to scale on the clock time axis. Graph modified from Grant et al.36 with permission from Springer Science+Business Media. Right: cumulative relative RT frequency distributions, showing on the ordinate the number of responses (expressed relative to the grand total number of responses) that is equal to or faster than a given RT on the abscissa. The curves shown here represent cumulative relative RT frequency distributions in the first 5 min (solid curve) versus second 5 min (dashed curve) of the 10-min PVT under conditions of sleep deprivation (simulated based on the diffusion model for one-choice reaction-time tasks43 using exaggerated parameter values for illustration purposes). The yellow-shaded area contains the fastest RTs, where the 2 curves begin to separate (notice they start at the same point on the RT axis in this illustration). The purple-shaded portion shows the heart of the cumulative relative frequency distributions, where the curves are separating progressively. Notice that the vertical separation is maximal at ~500 ms, which coincides with the cut-off traditionally used to define lapses of attention (ie, RTs ≥ 500 ms) on the PVT.14 The red-shaded area shows the slower RTs or lapse domain, where the curves begin to approach each other again (ultimately asymptoting on 1 if the abscissa were extended). PVT = psychomotor vigilance test; RT = response time.
METHODS
Overview
We analyzed data from N = 82 subjects who each participated in 1 of 3 in-laboratory TSD studies. The 10-min PVT14 was administered every 2–5 h across 38 h of continuous wakefulness common to all 3 studies; see Figure 2. For each test bout, performance across TOT was quantified based on the raw RT data for every 1-min interval of the PVT (see Figure 1, left). Subjects were grouped by genotype for DAT1, COMT, and TNFα to investigate polymorphism-specific performance degradation due to the TOT effect, sleep deprivation, and their interaction.
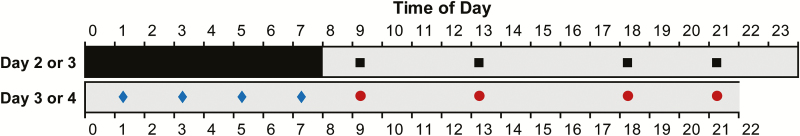
Figure 2.
Simplified schematic of the laboratory study design. After 1 (studies 1 and 2) or 2 (study 3) baseline days with 10 h sleep opportunities (22:00–08:00), subjects were kept awake for at least 38 h under constant behavioral monitoring. The PVT was administered at 2–5 h intervals throughout scheduled wakefulness. Test bouts serving as baseline (hours 1–14 of wakefulness) are indicated with a black square; test bouts capturing nighttime sleep deprivation (hours 15–24 of wakefulness) are indicated with a blue diamond; and test bouts capturing daytime sleep deprivation (hours 25–38 of wakefulness) are indicated with a red circle. Gray denotes scheduled wakefulness, and black indicates a baseline sleep opportunity (starting at 22:00 on the preceding day). Subjects went to bed for recovery sleep at 22:00 on day 3 (studies 1 and 2) or were kept awake for an additional 24 h (study 3) (not shown). PVT = psychomotor vigilance test.
Subjects
N = 82 healthy young adults (ages 27.0 ± 4.8 y; 43 females) participated in 1 of 3 laboratory studies conducted in the Sleep and Performance Research Center at Washington State University Spokane. The studies were approved by the Institutional Review Board (IRB) of Washington State University. Subjects gave written informed consent and were compensated for their time.
Subjects eligible for study participation met the following criteria: age 22–40 y; physically and psychologically healthy; no current medical or drug treatment (except contraceptives); no current or relevant history of psychiatric illness; no clinically significant irregularities in blood or urine; no sleep or circadian disorders; no history of moderate to severe brain injury; no history of learning disabilities; proficient in English; not a current smoker; free from traces of drugs and alcohol; no history of drug or alcohol abuse in the last year; no history of methamphetamine abuse; not pregnant; no past adverse reactions to sleep deprivation; no vision impairment, unless corrected to normal; no hearing impairment, unless corrected to normal (for studies 1 and 2 only); no travel across time zones 1 month prior to the study; no shift work 3 months prior to the study; habitual sleep duration between 6 h and 10 h per night; and usual wake times between 06:00 and 09:00. Study 3 included performance testing on a high-fidelity driving simulator and intravenous (IV) blood draws (from hours 12 to 36 during sleep deprivation). This required the following additional eligibility criteria: valid driver’s license; not susceptible to simulator adaptation syndrome; suitable veins for IV insertion; no history of complications with blood draws or blood donations; and not having donated blood within 2 months of entering the study. Subjects’ eligibility was confirmed with physical examination, history, blood and urine chemistry, breathalyzer, baseline polysomnography, and a battery of questionnaires.
For the 7 days prior to entering the laboratory, subjects were instructed to refrain from caffeine, alcohol, tobacco, and drug use. Subjects were also instructed to avoid napping, and to maintain their habitual sleep/wake times, which was verified by means of wrist actigraphy (studies 1 and 2: Actiwatch-2, Philips Respironics, Bend, OR; study 3: MicroMini Motionlogger, Ambulatory Monitoring, Ardsley, NY) and sleep diary, along with twice-daily phone calls to a time-stamped voice recorder to report bedtimes and wake-up times. Actigraph records were scored for sleep using computer software (studies 1 and 2: Actiware, Philips Respironics; study 3: Act Millennium, Ambulatory Monitoring), and average sleep duration across the 7 days was calculated as an index of habitual sleep duration. Due to equipment failure, actigraphy data were missing for 1 subject; for this individual, the sleep diary data were used to calculate sleep duration.
Prior to admission into the laboratory, subjects were confirmed to be free of traces of alcohol and drugs by means of breathalyzer test and urinalysis.
Subject demographics and genotypes (see below) are shown in the Supplementary Material (Table S1).
Experimental Design
Studies 1 and 2
These 2 studies were similar in design. In study 1, n = 34 healthy young adults (ages 27.7 ± 5.0 y; 14 females) lived in the laboratory for 4 days (3 nights). In study 2, n = 36 healthy young adults (ages 26.6 ± 4.8 y; 22 females) likewise lived in the laboratory for 4 days (3 nights). During both studies, subjects had a baseline day with a 10-h sleep opportunity (22:00–08:00), subsequently underwent 38 h of TSD, and then had a 10-h recovery sleep opportunity (22:00–08:00). During the 38 h of TSD, the PVT was administered 12 times at 2–5 h intervals (see Figure 2). Studies 1 and 2 have previously been described elsewhere.34,37
Study 3
In study 3, n = 12 healthy young adults (ages 26.6 ± 4.4 y; 7 females) lived in the laboratory for 7 days (6 nights). Subjects had 2 baseline days, each with a 10-h sleep opportunity (22:00–08:00), subsequently underwent 62 h of TSD—of which only the first 38 h were used for analysis here—and then had 2 recovery days with 10-h sleep opportunities (22:00–08:00). During the first 38 h of TSD, the PVT was administered 12 times at 2–5 h intervals (see Figure 2). Study 3 has previously been described elsewhere.38
For each of the 3 studies, the laboratory conditions were strictly controlled. Light levels were fixed below 100 lux during scheduled wakefulness and lights were off during scheduled sleep periods. The ambient temperature was maintained at 21°C (±1°C). Subjects were not allowed to engage in strenuous physical activity while in the laboratory. They did not have contact with individuals outside the laboratory, and did not have access to live television or radio, phones, personal computers, internet, or video games. Subjects participated in groups of up to 4 and were each assigned their own room for performance testing and for baseline and recovery sleep. Trained research assistants monitored subjects’ behavior around the clock.
Genotyping
A venous whole blood sample was collected from each subject during a prestudy screening session. Blood was collected in Vacutainer tubes coated with ethylenediaminetetraacetic dipotassium dihydrate (K2EDTA). Samples were immediately aliquoted and stored at –80°C until analysis.
The whole blood samples were red-cell depleted and genomic DNA (gDNA) was extracted. The DNA samples were assayed for the VNTR of the DAT1 gene (rs28363170, chromosome 5) and the SNPs COMT Val158Met (rs4680, chromosome 22) and TNFα G308A (rs1800629, chromosome 6).
DAT1 genotyping was performed using standard polymerase chain reaction (PCR) procedures described in the literature.39 Samples were amplified with 20 µM of forward primer 5′-TGTGGTGTAGGGAACGGCCTGAG-3′ and 20 µM of reverse primer 5′-CTTCCTGGAGGTCACGGCTCAAGG-3′. PCR procedures were carried out in a final reaction volume of 20 µl containing the following: 10 µl of Go-Taq Hot Start Green Master Mix (Promega, Madison, WI), 1 µl of each primer (forward and reverse), 6 µl of nuclease-free water, and 2 µl of gDNA. PCR conditions involved initial denaturation at 94°C for 3 min, followed by 39 cycles of: denaturation at 94°C for 45 s, annealing at 69°C for 30 s, and extension at 72°C for 30 s. Final extension was at 72°C for 5 min. Amplified products were electrophoresed on a 3% agarose gel stained with ethidium bromide and visualized under UV light. DAT1 fragment sizes for 8-, 9-, 10-, and 11-repeats were 360 bp, 400 bp, 440 bp, and 480 bp, respectively.39
Three subjects were not included in the overall analysis of DAT1 due to the rarity of their genotypes in the general population (8/8 for 1 subject and 10/11 for 2 subjects), leaving 79 subjects for DAT1 statistical analyses. Seven homozygous and 27 heterozygous subjects carrying the 9-repeat allele were grouped together and labeled as 9R.
COMT genotyping was performed using the Taqman Drug Metabolism Assay (Assay ID: C__25746809_50; ThermoFisher Scientific, Waltham, MA). Real-time PCR was carried out per the manufacturer protocol using VIC/FAM context sequence CCAGCGGATGGTGGATTTCGCTGGC[A/G]GAAGGACAAGGTGTGCATGCCTGA. Wet DNA was used, and reactions were carried out on a 96-well plate with a final reaction volume of 25 µl. All samples were assayed in duplicate, and a no-DNA negative control was included. Allelic discrimination analysis was performed using MJ Opticon Monitor Analysis Software v3.1 (Bio-Rad Laboratories, Hercules, CA).
TNFα308 genotyping was performed using standard PCR and restriction enzyme digestion procedures described in the literature,40 and as described in detail previously.34
A χ2 goodness-of-fit test was used to test for deviations from Hardy–Weinberg equilibrium in the subject sample for each of the 3 genes examined. Furthermore, Fisher’s exact testing was used to determine if any of the genotypes were associated with one another. One-way analysis of variance (ANOVA) was used to test for differences in age between genotypes, and logistic regression was used to test for differences in gender and race/ethnicity between genotypes.
Performance Testing
The 10-min PVT was administered on a desktop computer. Subjects were presented with a visual stimulus in the form of a millisecond counter. The stimulus was presented at random intervals between 2 and 10 s. Subjects were instructed to respond to the stimulus as quickly as possible, without making false starts, by pressing a button on a response box.
The raw RT data recorded during the 12 test bouts across the 38-h TSD period (see Figure 2) were used to assess the TOT effect. For each 10-min test bout of each individual subject, the RT data were grouped into ten 1-min bins. On average, the number of RTs per bin was 9.3 (see Table S2 in the Supplemental Material for descriptive statistics by bin). False starts, including RTs <100 ms, were not included.
Additionally, cumulative relative RT frequency distributions (see Figure 1, right) were constructed from the combined data in the first five 1-min bins (first half of task duration) and from the combined data in the second five 1-min bins (second half of task duration). This was done separately for baseline test bouts (bouts 1–4) and daytime sleep deprivation bouts (bouts 9–12), so as to be able to compare the TOT effect (first versus second half of task duration) between well-rested and sleep-deprived states while controlling for time of day (see Figure 2). To construct the cumulative relative RT frequency distributions, the RT data sets were aggregated across test bouts and pooled over subjects by genotype, and then divided into 28 logarithmically sized RT bins. (To obtain RT counts of the same order of magnitude across RT bins, the bins were bounded by [in ms]: 175, 188, 203, 222, 246, 277, 315, 363, 424, 500, 597, 718, 871, 1063, 1304, 1609, 1992, 2475, 3082, 3847, 4809, 6021, 7547, 9468, 11886, 14931, 18763, 23588, 30000. Here, RTs <175 ms [which were rare, constituting less than 0.5% of the data set] were counted as false starts and were not included.)
For 7 of the 82 subjects, 1 or 2 PVT bouts were potentially confounded by a microsleep event, a distraction, or failure to put effort into the task, as documented by our trained research assistants during the experiments. These test bouts were removed from the data set, leaving a total of 974 test bouts comprising 90494 RTs (98.9% of the original total) for analysis.
Statistical Analyses
For each of the 12 PVT test bouts of each individual subject, the RTs were grouped into 1-min bins across the 10-min task duration. The raw RTs thus binned were analyzed using a mixed-effects ANOVA with fixed effects of time awake (test bouts 1–12), TOT (1-min bins 1–10), genotype, and their interactions, controlling for study and with a random effect over subject on the intercept. The analysis was carried out separately for DAT1 (9R, 10/10), COMT (Met/Met, Val/Met, Val/Val), and TNFα (A/G, G/G). The primary effect of interest was the interaction between time awake, TOT, and genotype. For graphical representation, test bouts were also divided into 3 phases of the 38-h TSD period (see Figure 2): baseline (bouts 1–4), nighttime sleep deprivation (bouts 5–8), and daytime sleep deprivation (bouts 9–12). Secondary analyses repeated the mixed-effects ANOVA controlling for age, gender, race/ethnicity, and habitual sleep duration, in addition to study.
The cumulative relative frequency distributions of the PVT RTs were analyzed using a mixed-effects ANOVA with fixed effects of RT bin (1–28) alone and in interaction with TOT (first, second half of task duration) and genotype and their interaction. This analysis was controlled for study, included a random effect over subjects on the intercept (with a Toeplitz covariance structure to account for autocorrelation in the cumulative frequency data), and was weighted by total number of RTs contributed by each subject. The analysis was carried out separately for baseline (aggregated over PVT bouts 1–4; hours 1–14 of wakefulness) and daytime sleep deprivation 24 h later (aggregated over bouts 9–12; hours 25–38 of wakefulness).
RESULTS
Table 1 shows the genotype counts and frequencies for our sample. The relative allele frequencies were as follows: DAT1, 9-repeat allele 0.26, 10-repeat allele 0.74; COMT, Met allele 0.49, Val allele 0.51; TNFα, A allele 0.15, G allele 0.85. The allele frequencies were found to be in Hardy–Weinberg equilibrium for each gene (DAT1: χ21 = 0.97, p = .33; COMT: χ21 = 2.19, p = .14; TNFα: χ21 = 2.41, p = .12). Our observed genotype frequencies were comparable to those in the published literature.41,42 Fisher’s exact tests showed that in our sample, the DAT1, COMT, and TNFα genotypes did not co-segregate. DAT1 was not significantly associated with COMT (p = .18) or TNFα (p = .81). COMT and TNFα were also not significantly associated (p = .54).
Table 1.
Genotype counts and frequencies for each gene.
| Genotype Count | Genotype Frequency | ||||
|---|---|---|---|---|---|
| Observed | Expecteda | Observed | Expecteda | Publishedb | |
| DAT1 | |||||
| 9/9c | 7 | 5.32 | 0.09 | 0.07 | 0.06 |
| 9/10c | 27 | 30.36 | 0.34 | 0.38 | 0.37 |
| 10/10 | 45 | 43.32 | 0.57 | 0.55 | 0.56 |
| COMT | |||||
| Met/Met | 17 | 19.51 | 0.21 | 0.24 | 0.33 |
| Val/Met | 46 | 40.98 | 0.56 | 0.50 | 0.46 |
| Val/Val | 19 | 21.51 | 0.23 | 0.26 | 0.22 |
| TNFα | |||||
| A/A | 0 | 1.76 | 0.00 | 0.02 | 0.04 |
| A/G | 24 | 20.49 | 0.29 | 0.25 | 0.26 |
| G/G | 58 | 59.76 | 0.71 | 0.73 | 0.69 |
aCalculated based on Hardy–Weinberg equilibrium.
bDAT1 and COMT: Valomon et al.,39 115 healthy subjects; TNFα: Almpanidou et al.,40 318 healthy subjects.
cThe 9-repeat homozygous and heterozygous individuals were combined for analysis purposes as 9R.
Subjects’ habitual sleep duration, as estimated by the average sleep duration assessed using wrist actigraphy in the week before the experiment, was 7.3 ± 0.8 h. Habitual sleep duration did not differ by genotype for any of the 3 genes considered here (see Table S3 in the Supplemental Material).
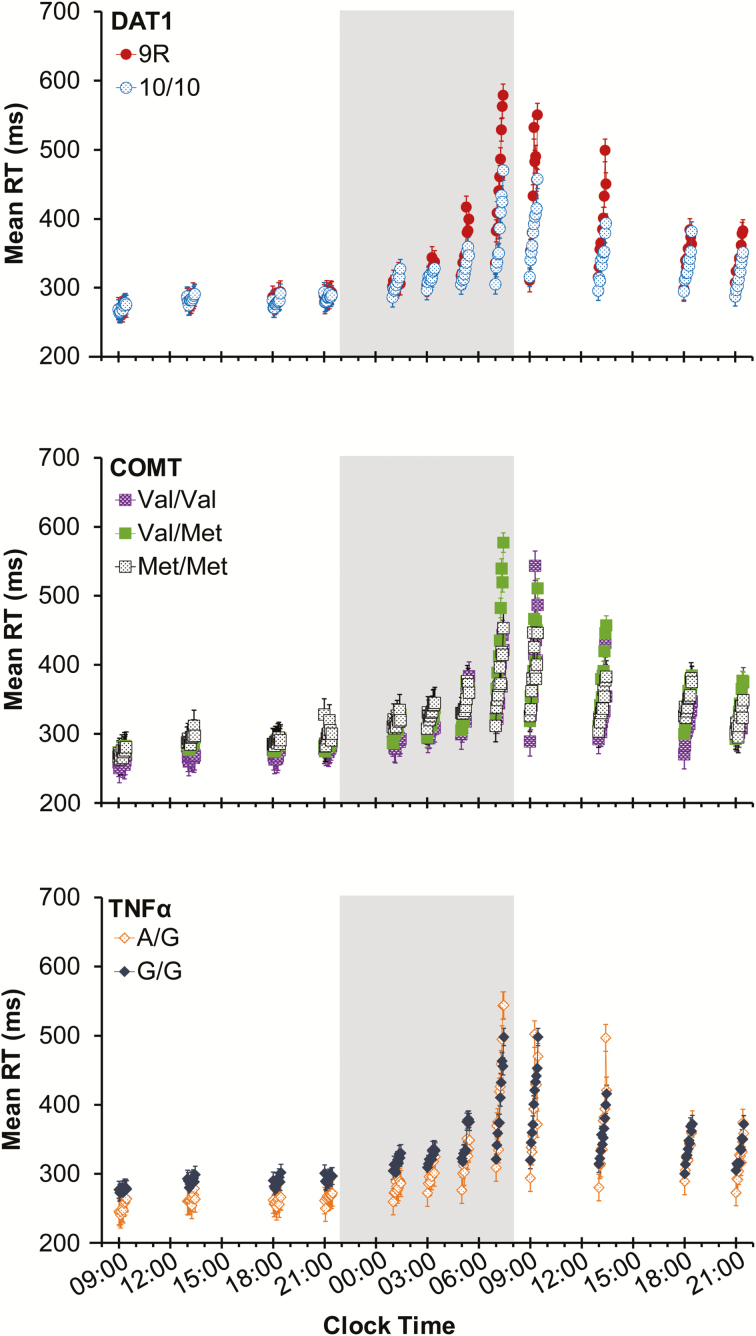
Figure 3 shows TOT performance data from each of the 12 PVT test bouts across 38 h of continuous wakefulness for the different genotypes of DAT1, COMT, and TNFα. The full set of statistical results from each mixed-effects ANOVA is reported in the Supplemental Material (Table S3). Each of the graphs in Figure 3 shows the well-established effects of time awake (F > 223, p < .001), TOT (F > 54, p < .001), and their interaction (F ≥ 4.9, p < .001) on RT for each of the genotypes. Furthermore, the data replicated previously observed interactions of time awake with genotype27,32,34 (F ≥ 9.2, p < .001); see the Supplementary Material (Figure S1) for graphs depicting this interaction independent of TOT.
Figure 3.
Mean RT (±standard error) in 1-min bins on the 10-min PVT across test bouts during 38 h of TSD, for each of the 3 genes. Data are plotted against the start times of the PVT bouts; placement of the 1-min bins in each test bout is not to scale on the clock time axis. Shaded area: nighttime test bouts during TSD. PVT = psychomotor vigilance test; RT = response time; TSD = total sleep deprivation.
In what follows, we focus on our novel findings pertaining to the interaction between TSD, TOT, and genotype. For comparison, results from a well-rested control group are shown in the Supplementary Material (Figure S2).
For DAT1 (Figure 3, top), performance diverged across TOT between the 9R and 10/10 genotypes as TSD progressed, especially during the early and late morning hours. There was a significant interaction between time awake, TOT, and DAT1 genotype (F99,87000 = 1.35, p = .011), which captured 23.2% of the overall variance explained by DAT1 genotype. Subjects homozygous for the 10-repeat allele showed a substantially reduced TOT effect during TSD compared to subjects homozygous or heterozygous for the 9-repeat allele. Subjects in the well-rested control group did not show any performance impairment or any differences by DAT1 genotype (see Supplementary Material, Figure S2). This confirms that the divergence across TOT between genotypes in Figure 3 (top) was due to the interaction with TSD.
For COMT and TNFα (Figure 3, middle and bottom, respectively), there was no divergence across TOT by genotype as TSD progressed. There was no significant interaction between time awake, TOT, and COMT genotype (F198,90000 = 1.13, p = .10). There was a trend for the interaction between time awake, TOT, and TNFα genotype (F99,90000 = 1.21, p = .080). However, both TNFα genotypes (A/G and G/G) showed similar TOT effects across the entire 38-h TSD period, but the G/G subjects tended to have slower RTs (Figure 3, bottom). Subjects in the well-rested control group did not show any performance impairment or any differences by COMT or TNFα genotype (see Supplemental Material, Figure S2).
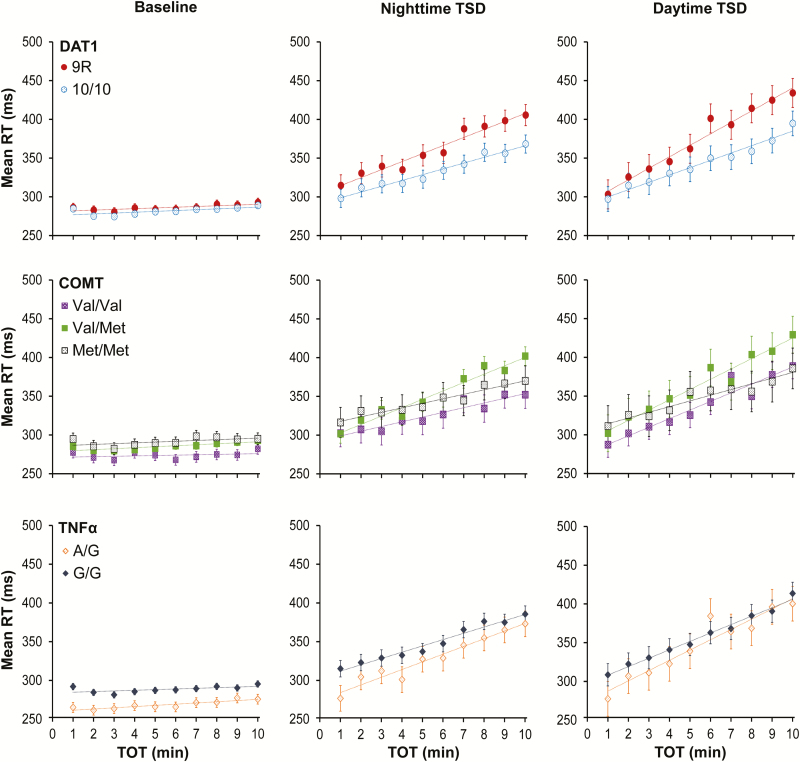
To further clarify the results regarding TOT, Figure 4 shows the build-up of PVT performance impairment across TOT for DAT1, COMT, and TNFα, collapsed across test bouts for well-rested baseline (left), nighttime TSD (middle), and daytime TSD (right). For DAT1 (Figure 4, top), performance across TOT was nearly identical for the 9R and 10/10 genotypes at baseline. However, in line with the significant interaction between time awake, TOT, and DAT1 genotype discussed above, clear differences in the rate of change across TOT appeared during TSD. Subjects homozygous for the 10-repeat allele showed less performance impairment build-up over the 10-min PVT than the 9-repeat allele carriers.
Figure 4.
Mean RT (±standard error) in 1-min bins on the 10-min PVT, collapsed over intervals of time awake, for each of the 3 genes. Left panels correspond to the baseline period (09:00–21:59; from 1 up to 14 h wakefulness); middle panels correspond to the nighttime TSD period (23:00–07:59; from 15 up to 23 h wakefulness); right panels correspond to the daytime TSD period (09:00–21:59; from 25 up to 38 h wakefulness). Trend lines are included to depict the general rate of change in RTs across the 10-min PVT. PVT = psychomotor vigilance test; RT = response time; TOT = time-on-task; TSD = total sleep deprivation.
Consistent with the absence of a 3-way interaction for COMT and TNFα (see above), no such clear picture emerged for these 2 genes. For COMT (Figure 4, middle), subjects homozygous for the Val allele exhibited overall faster RTs, especially at baseline. Likewise, for TNFα (Figure 4, bottom), carriers of the A allele had overall faster RTs, especially at baseline. For both genes, TSD increased the rate of change across TOT; however, there were no pronounced differences in the rate of impairment build-up between genotypes. This shows that divergence across TOT between genotypes in interaction with time awake was a property specific to DAT1.
There were no significant differences between DAT1, COMT, and TNFα genotypes based on age, gender, race/ethnicity, and habitual sleep duration (see Supplemental Material, Table S3). All significant effects described above held true in secondary analyses controlling for age, gender, race/ethnicity, and habitual sleep duration.
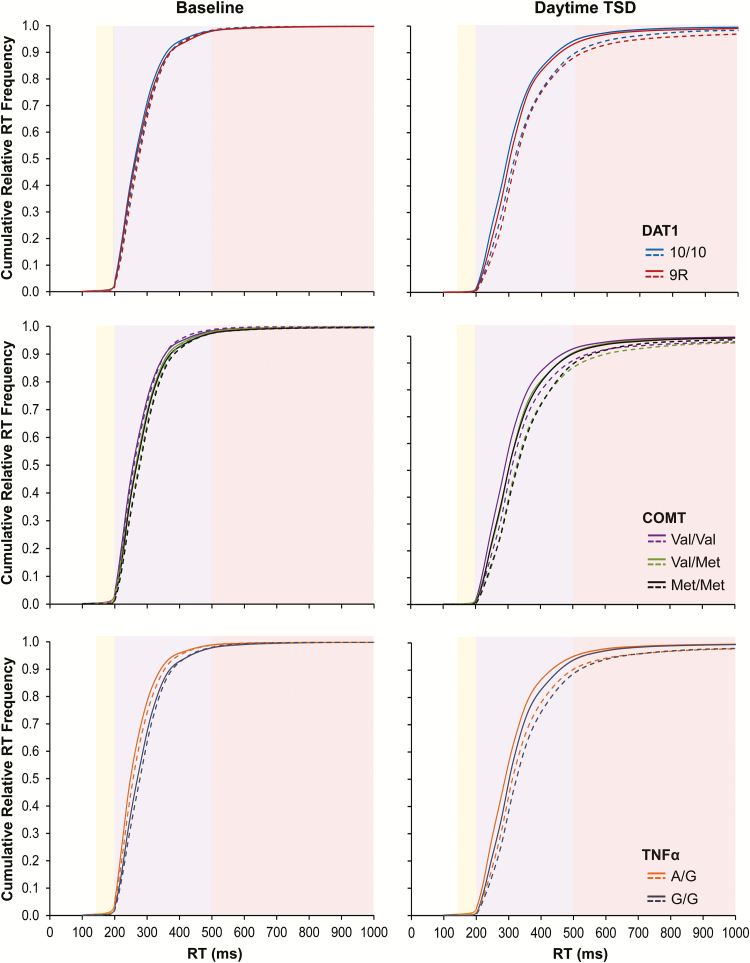
Figure 5 shows the cumulative relative RT frequency distributions for PVT test bouts performed during well-rested baseline and during daytime TSD (at the same times of day). Each panel depicts the relative RT frequency distributions during the first 5 min versus the second 5 min of the PVT. During TSD (Figure 5, right), as compared to baseline (Figure 5, left), the distributions displayed the expected skewing to the right that is characteristic of sleep loss.14 Furthermore, during baseline—and especially during TSD—the distributions showed the expected skewing to the right from the first 5 min (solid curves) to the second 5 min (dashed curves) of the PVT, congruent with the previously reported increase in performance variability across TOT11 (cf. illustration in Figure 1, right). However, the patterns of change were not the same for the 3 genes considered. The full set of statistical results pertaining to the cumulative relative RT frequency distributions is reported in the Supplemental Material (Table S3); here, we only describe the effects involving interactions with genotype.
Figure 5.
Cumulative relative RT frequency distributions for each gene during the baseline period (left panels) and the daytime TSD period (right panels). Solid curves correspond to performance during the first 5 min (1–5) of the 10-min PVT; dashed curves correspond to performance during the second 5 min (6–10) of the 10-min PVT. The yellow-shaded area contains the fastest RTs; the purple-shaded area shows the heart of the cumulative relative frequency distributions; and the red-shaded area shows the slower RTs or lapse domain (cf. Figure 1, right). PVT = psychomotor vigilance test; RT = response time; TSD = total sleep deprivation.
For DAT1, the 2 genotypes had similar RT distributions during well-rested baseline (Figure 5, top left), with no significant genotype interactions. During TSD, however, the distribution curves for the DAT1 genotypes diverged (Figure 5, top right). There was a significant interaction of RT bin by genotype (F28,4235 = 1.61, p = .022). The RT distributions diverged in the lapse domain (Figure 5, top right, red-shaded area). In the 9R group, longer RTs occurred more frequently during the second 5 min (dashed red line) than during the first 5 min (solid red line), whereas this was not seen in the 10/10 group (dashed versus solid blue line). This is consistent with the results of Figure 3 (top) and Figure 4 (top) indicating that subjects homozygous for the 10-repeat allele exhibited a significantly reduced TOT effect during TSD compared to subjects homozygous or heterozygous for the 9-repeat allele. Figure 5 (top right) elucidates that this group difference was due to relatively greater skewing of the RT distribution due to interaction of TSD with the TOT effect in the subjects homozygous or heterozygous for the 9-repeat allele of DAT1.
For COMT and TNFα, in contrast to DAT1, a significant genotype difference in the cumulative relative RT frequency distribution was already apparent during well-rested baseline. For COMT (Figure 5, middle left), there was a significant interaction of RT bin by genotype (F56,4345 = 2.24, p < .001). For TNFα (Figure 5, bottom left), there was also a significant interaction of RT bin by genotype (F28,4400 = 15.76, p < .001). Subjects homozygous for the Val allele of the Val158Met polymorphism of COMT, and especially subjects carrying the A allele of the G308A polymorphism of TNFα, produced fast RTs more frequently at baseline.
TSD further amplified these genotype differences for COMT and TNFα. For COMT (Figure 5, middle right), there was again a significant interaction of RT bin by genotype (F56,4345 = 1.73, p = .001). For TNFα (Figure 5, bottom right), there was also again a significant interaction of RT bin by genotype (F28,4400 = 3.37, p < .001). After TSD, as during baseline, subjects homozygous for the COMT Val allele and carriers of the TNFα A allele produced fast RTs more often relative to the other COMT and TNFα genotypes, during both the first 5 min and the second 5 min of the PVT. In contrast to DAT1, the differences between the COMT and TNFα genotypes were most prominent in the heart of the RT distribution (Figure 5, middle and bottom right, purple-shaded area) and vanished in the lapse domain. This is consistent with the lack of significant interactions between TSD, TOT, and COMT or TNFα genotype in Figure 3 (middle and bottom, respectively) and Figure 4 (middle and bottom, respectively).
DISCUSSION
In this study, we observed systematic interaction of the TOT effect with TSD. Matching earlier findings,12,13,15–17 both TOT and TSD increased RT mean and RT variability on the PVT; moreover, TSD amplified the changes across TOT. The similarities of the TOT and TSD effects on PVT performance, as well as their interplay, suggest that there may be shared underlying mechanisms.15,17 To examine this possibility, we investigated 3 genetic polymorphisms, selected a priori because of previously documented associations with TOT or TSD25,34: DAT1 (VNTR), COMT (Val158Met), and TNFα (G308A). For all 3 genes, we observed effects of polymorphisms on the RT distribution, especially during TSD (Figure 5). However, only DAT1 genotype affected the interaction between TOT and TSD (Figures 3 and 4). Specifically, compared to carriers of the DAT1 9-repeat allele, subjects homozygous for the 10-repeat allele were relatively protected against TOT-induced performance deficits during TSD.
In line with our findings, Holst and colleagues27 recently reported that the DAT1 10-repeat allele confers resilience to PVT performance impairment across 40 h of TSD. They did not report DAT1 genotype modulation of the interaction between TSD and TOT. To investigate the TOT effect, however, they divided the task duration into quintiles and characterized each quintile by the number of responses in the lapse domain or by the variability in RTs. This approach yields considerably less statistical power than using the individual RTs in 1-min bins, and may have obscured a TSD by TOT by DAT1 genotype interaction in their study. In contrast, Lim and colleagues25 found that subjects homozygous for the 10-repeat allele of DAT1 and subjects homozygous for the Val allele of COMT Val158Met are relatively resilient to the decline of performance across TOT on a 20-min PVT under well-rested baseline conditions. These results are consistent with our findings, in that increasing the task duration from 10 to 20 min would be expected to enhance the TOT effect similar to what TSD did in our study. Thus, the present study extends and integrates the work of Holst and colleagues27 and that of Lim and colleagues.25
Whereas COMT and TNFα were associated with genotype differences in the heart of the RT distribution (Figure 5, middle and bottom right, purple-shaded area), DAT1 was associated with genotype differences in the lapse domain of the RT distribution (Figure 5, top right, red-shaded area). This distinction may be interpreted by means of a cognitive model called the diffusion model of one-choice reaction-time tests,43 which abstractly describes performance on the PVT as a one-boundary diffusion process symbolizing the accumulation of stimulus information (evidence) until a decision criterion is reached and a response is initiated. The rate of evidence accumulation is represented by a drift ratio parameter, while the decision criterion is represented by a boundary separation parameter. Differences in the boundary separation parameter impact the heart of the RT distribution, and differences in the drift ratio parameter impact the lapse domain of the RT distribution (see Supplemental Material, Figure S3).
In the context of our study, this cognitive modeling perspective implies that the COMT and TNFα polymorphisms may be associated with differences in the propensity to respond earliest after the detection of a stimulus.44 This suggests that even though subjects were instructed to respond to PVT stimuli as quickly as possible, without making false starts, there may have been systematic differences among them in the level of effective cognitive control. In line with this interpretation is the observation that the COMT and TNFα genotypes influenced the RT distribution both at baseline and after TSD (Figure 5, middle and bottom). Also in line with this interpretation is that TNFα G308A has been linked with cognitive control45; and, more indirectly, that COMT activity is localized predominantly in the prefrontal cortex,46 which has been linked with cognitive control as well.47
The DAT1 polymorphism, on the other hand, appears to be associated with a differential rate of evidence accumulation (Figure 5, top; cf. Supplemental Material, Figure S3, left). The rate of evidence accumulation during PVT performance is a strong correlate of the fidelity of information processing.37 Both the rate of evidence accumulation and the fidelity of information processing on the PVT have been shown to be affected substantially by TSD.37,43 Our finding that DAT1 genotype affected the interaction between TOT and TSD (Figures 3 and 4)—and may thus be involved in shared mechanisms underlying both TSD and TOT—leads to 2 central hypotheses: (1) the TOT effect, like TSD, involves degradation of the fidelity of information processing; and (2) the degree to which TSD, TOT, and their interaction degrade the fidelity of information processing is modulated by dopaminergic mechanisms.
These hypotheses are compatible with results from studies of sleep deprivation and striatal dopamine.48,49 DAT1, the dopamine transporter 1, is highly localized to the striatum, where it regulates dopamine levels via reuptake from the synaptic cleft to the presynaptic terminal.50 Neuroimaging studies have shown that sleep deprivation does not change synaptic dopamine or dopamine transporter expression within the striatum over time.48 However, the DAT1 VNTR polymorphism modulates the expression of the transporter between subjects, and although the literature is mixed on whether the 9- and 10-repeat alleles increase or decrease the transporter’s expression, a meta-analysis concluded that the 9-repeat allele increases it.51 The implication is that subjects homozygous for the DAT1 10-repeat allele are expected to have increased synaptic dopamine levels in the striatum compared to carriers of the 9-repeat allele. DAT1 knock-out studies in rodents28 and pharmacologic blocking studies in humans52 have shown that increased synaptic dopamine levels promote wakefulness. A recent neuroimaging study found that DAT1 genotype modulates neural responses during sleep loss and alters cognitive functioning.53 Taken together, the literature suggests that increased dopamine availability in the striatum may be causally related to the relatively improved PVT performance in subjects homozygous for the DAT1 10-repeat allele, as compared to carriers of the 9-repeat allele, during TSD and across TOT.
In the striatum, dopamine D2 receptors are co-localized and functionally interact with adenosine A2A receptors (ADORA2A).54 Adenosine has been implicated in mediating the effects of sleep deprivation,55 and has been hypothesized to be involved in the effects of TOT as well.19 Activation of the A2A receptor modulates dopaminergic neurotransmission in an antagonistic manner.54 As such, increased dopamine levels in subjects homozygous for the DAT1 10-repeat allele could potentially provide relative resilience to the interacting effects of TSD and TOT on performance simply by counteracting the antagonistic effect of adenosine. This would suggest that a striatal adenosinergic/dopaminergic mechanism underlies the cognitive impact of TSD interacting with TOT. A previously reported effect of polymorphisms of the ADORA2A gene on psychomotor vigilance performance during sleep loss56 is congruent with this idea.
It should be noted that the subject sample of our study consisted only of young adult women and men (ages 22–40) with limited racial and ethnic diversity. We do not know to what extent our results may generalize to other populations. Additionally, we note that our sample size was modest, which is of concern when effect sizes are small (as if often the case in studies of cognition57). However, the effects of time awake and time-on-task, alone and in interaction, on PVT performance produce robust phenotypes (see Figures 3 and 4). DAT1 is therefore a promising candidate biomarker of resilience to psychomotor vigilance performance impairment due to TSD, TOT, and their interaction.
SUPPLEMENTARY MATERIAL
Supplementary data are available at SLEEP online.
FUNDING
The research was supported by ONR grant N00014-13-1-0302 to Hans Van Dongen (study 1), NIH grant R21CA167691 to John Hinson (study 2), NIH grant R01HL105768 to Melinda Jackson and FAA grant DTFAAC-11-A-00003 to Hans Van Dongen (study 3), and CDMRP grant W81XWH-16-1-0319 to Hans Van Dongen (analyses).
ALL WORK CONDUCTED AT
Sleep and Performance Research Center, Washington State University Spokane, PO Box 1495, Spokane, WA, 99210-1495
DISCLOSURE STATEMENT
The experiments were conducted at Washington State University Spokane. This work was supported by the Office of Naval Research, National Institutes of Health, Federal Aviation Administration, and Congressionally Directed Medical Research Program. The authors have no conflicts of interest to disclose.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge the contributions of Matthew Layton, Gregory Belenky, John Hinson, Paul Whitney, Melinda Jackson, Devon Grant, and the staff of the Human Sleep and Cognition Laboratory in the Sleep and Performance Research Center at Washington State University Spokane.
REFERENCES
- 1. Davies DR, Parasuraman R.. The Psychology of Vigilance. London: Academic Press; 1982. [Google Scholar]
- 2. Lim J, Kwok K. The effects of varying break length on attention and time on task. Hum Factors. 2016; 58(3): 472–481. [DOI] [PubMed] [Google Scholar]
- 3. Hancock PA, Hart SG. Defeating terrorism: what can human factors/ergonomics offer?Ergon Des. 2002; 10(1): 6–16. [Google Scholar]
- 4. McFadden SM, Vimalachandran A, Blackmore E. Factors affecting performance on a target monitoring task employing an automatic tracker. Ergonomics. 2004; 47(3): 257–280. [DOI] [PubMed] [Google Scholar]
- 5. Caldwell JA. Fatigue in aviation. Travel Med Infect Dis. 2005; 3(2): 85–96. [DOI] [PubMed] [Google Scholar]
- 6. Basner M, Rubinstein J, Fomberstein KM et al. . Effects of night work, sleep loss and time on task on simulated threat detection performance. Sleep. 2008; 31(9): 1251–1259. [PMC free article] [PubMed] [Google Scholar]
- 7. Satterfield BC, Van Dongen HPA. Occupational fatigue, underlying sleep and circadian mechanisms, and approaches to fatigue risk management. Fatigue. 2013; 246(3): 118–136. [Google Scholar]
- 8. Verster JC, Roth T. Vigilance decrement during the on-the-road driving tests: the importance of time-on-task in psychopharmacological research. Accid Anal Prev. 2013; 58: 244–248. [DOI] [PubMed] [Google Scholar]
- 9. Van Dongen HPA, Balkin TJ, Hursh SR. Performance deficits during sleep loss and their operational consequences. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practices of Sleep Medicine. 6th ed Philadelphia, PA: Elsevier B.V; 2016: 682–688. [Google Scholar]
- 10. Ackerman PL, ed. Cognitive Fatigue: Multidisciplinary Perspective on Current Research and Future Applications. Washington, DC: American Psychological Association; 2011. [Google Scholar]
- 11. Bills AG. Blocking: a new principle of mental fatigue. Am J Psychol. 1931; 43(2): 230–245. [Google Scholar]
- 12. Kribbs NB, Dinges DF.. Vigilance decrement and sleepiness; In: Ogilvie RD, Harsh JR, eds. Sleep Onset: Normal and Abnormal Processes. Washington, DC: American Psychological Association; 1994: 113–125. [Google Scholar]
- 13. Doran SM, Van Dongen HPA, Dinges DF. Sustained attention performance during sleep deprivation: evidence of state instability. Arch Ital Biol. 2001; 139(3): 253–267. [PubMed] [Google Scholar]
- 14. Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008; 1129: 305–322. [DOI] [PubMed] [Google Scholar]
- 15. Van Dongen HPA, Belenky G, Krueger JM. Investigating the temporal dynamics and underlying mechanisms of cognitive fatigue. In: Ackerman PL, ed. Cognitive Fatigue: Mulitidisciplinary Perspectives on Current Research and Future Applications. Washington, DC: American Psychological Association; 2011: 127–147. [Google Scholar]
- 16. Honn KA, Riedy SM, Grant DA. Validation of a portable, touch-screen psychomotor vigilance test. Aerosp Med Hum Perform. 2015; 86(5): 428–434. [DOI] [PubMed] [Google Scholar]
- 17. Gunzelmann G, Moore LR, Gluck KA, Van Dongen HPA, Dinges DF. Fatigue in sustained attention: generalizing mechanisms for time awake to time one task. In: Ackerman PL, ed. Cognitive Fatigue: Mulitidisciplinary Perspectives on Current Research and Future Applications. Washington, DC: American Psychological Association; 2011: 83–101. [Google Scholar]
- 18. Veksler BZ, Gunzelmann G. Functional equivalence of sleep loss and time on task effects in sustained attention. Cognitive Sci. 2017. [Published online ahead of print March 22, 2017]. doi:10.1111/cogs.12489. [DOI] [PubMed] [Google Scholar]
- 19. Van Dongen HPA, Belenky G, Krueger JM. A local, bottom-up perspective on sleep deprivation and neurobehavioral performance. Curr Top Med Chem. 2011; 11(19): 2414–2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krueger JM, Rector DM, Roy S, Van Dongen HPA, Belenky G, Panksepp J. Sleep as a fundamental property of neuronal assemblies. Nat Rev Neurosci. 2008; 9(12): 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van Dongen HPA, Rector DM, Belenky G, Krueger JM. Sleep, a localized phenomenon of the brain. In: Kryger MH, ed. Atlas of Clinical Sleep Medicine. Philadelphia, PA: Elsevier Saunders; 2010: 27–29. [Google Scholar]
- 22. Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. Neuroimage. 2010; 49(4): 3426–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Asplund CL, Chee MWL. Time-on-task and sleep deprivation effects are evidenced in overlapping brain areas. Neuroimage. 2013; 82: 326–335. [DOI] [PubMed] [Google Scholar]
- 24. Vandenbergh DJ, Persico AM, Hawkins AL et al. . Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992; 14(4): 1104–1106. [DOI] [PubMed] [Google Scholar]
- 25. Lim J, Ebstein R, Tse CY et al. . Dopaminergic polymorphisms associated with time-on-task declines and fatigue in the Psychomotor Vigilance Test. PLoS One. 2012; 7(3): e33767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Holst SC, Müller T, Valomon A, Seebauer B, Berger W, Landolt HP. Functional polymorphisms in dopaminergic genes modulate neurobehavioral and neurophysiological consequences of sleep deprivation. Sci Rep. 2017; 7: 45982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holst SC, Bersagliere A, Bachmann V, Berger W, Achermann P, Landolt HP. Dopaminergic role in regulating neurophysiological markers of sleep homeostasis in humans. J Neurosci. 2014; 34(2): 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001; 21(5): 1787–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wesensten NJ, Belenky G, Thorne DR, Kautz MA, Balkin TJ. Modafinil vs. caffeine: effects on fatigue during sleep deprivation. Aviat Space Environ Med. 2004; 75(6): 520–525. [PubMed] [Google Scholar]
- 30. Lachman HM, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM. Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996; 6(3): 243–250. [DOI] [PubMed] [Google Scholar]
- 31. Goel N, Banks S, Lin L, Mignot E, Dinges DF. Catechol-O-methyltransferase Val158Met polymorphism associates with individual differences in sleep physiologic responses to chronic sleep loss. PLoS One. 2011; 6(12): e29283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bodenmann S, Xu S, Luhmann UF et al. . Pharmacogenetics of modafinil after sleep loss: catechol-O-methyltransferase genotype modulates waking functions but not recovery sleep. Clin Pharmacol Ther. 2009; 85(3): 296–304. [DOI] [PubMed] [Google Scholar]
- 33. Wilson AG, di Giovine FS, Blakemore AI, Duff GW. Single base polymorphism in the human tumour necrosis factor alpha (TNF alpha) gene detectable by NcoI restriction of PCR product. Hum Mol Genet. 1992; 1(5): 353. [DOI] [PubMed] [Google Scholar]
- 34. Satterfield BC, Wisor JP, Field SA, Schmidt MA, Van Dongen HPA. TNFα G308A polymorphism is associated with resilience to sleep deprivation-induced psychomotor vigilance performance impairment in healthy young adults. Brain Behav Immun. 2015; 47: 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Churchill L, Rector DM, Yasuda K et al. . Tumor necrosis factor alpha: activity dependent expression and promotion of cortical column sleep in rats. Neuroscience. 2008; 156(1): 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Grant DA, Honn KA, Layton ME, Riedy SM, Van Dongen HPA. 3-minute smartphone-based and tablet-based psychomotor vigilance tests for the assessment of reduced alertness due to sleep deprivation. Behav Res Methods. 2017; 49(3): 1020–1029. [DOI] [PubMed] [Google Scholar]
- 37. Chavali VP, Riedy SM, Van Dongen HPA. Signal-to-noise ratio in PVT performance as a cognitive measure of the effect of sleep deprivation on the fidelity of information processing. Sleep. 2017; 40(3): zsx016. doi:10.1093/sleep/zsx016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whitney P, Hinson JM, Jackson ML, Van Dongen HPA. Feedback blunting: total sleep deprivation impairs decision making that requires updating based on feedback. Sleep. 2015; 38(5): 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Felten A, Montag C, Markett S, Walter NT, Reuter M. Genetically determined dopamine availability predicts disposition for depression. Brain Behav. 2011; 1(2): 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ozen S, Alikasifoglu M, Bakkaloglu A et al. . Tumour necrosis factor alpha G→A -238 and G→A -308 polymorphisms in juvenile idiopathic arthritis. Rheumatology (Oxford). 2002; 41(2): 223–227. [DOI] [PubMed] [Google Scholar]
- 41. Valomon A, Holst SC, Bachmann V et al. . Genetic polymorphisms of DAT1 and COMT differentially associate with actigraphy-derived sleep-wake cycles in young adults. Chronobiol Int. 2014; 31(5): 705–714. [DOI] [PubMed] [Google Scholar]
- 42. Almpanidou P, Hadjigeorgiou G, Gourgoulianis K, Papadimitriou A. Association of tumor necrosis factor-α gene polymorphism (-308) and obstructive sleep apnea-hypopnea syndrome. Hippokratia. 2012; 16(3): 217–220. [PMC free article] [PubMed] [Google Scholar]
- 43. Ratcliff R, Van Dongen HPA. Diffusion model for one-choice reaction-time tasks and the cognitive effects of sleep deprivation. Proc Natl Acad Sci USA. 2011; 108(27): 11285–11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lucci G, Berchicci M, Perri RL, Spinelli D, Di Russo F. Effect of target probability on pre-stimulus brain activity. Neuroscience. 2016; 322: 121–128. [DOI] [PubMed] [Google Scholar]
- 45. Beste C, Güntürkün O, Baune BT, Domschke K, Falkenstein M, Konrad C. Double dissociated effects of the functional TNF-α -308G/A polymorphism on processes of cognitive control. Neuropsychologia. 2011; 49(2): 196–202. [DOI] [PubMed] [Google Scholar]
- 46. Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-O-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006; 60(2): 141–151. [DOI] [PubMed] [Google Scholar]
- 47. Braver TS. The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci. 2012; 16(2): 106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Volkow ND, Tomasi D, Wang GJ et al. . Evidence that sleep deprivation downregulates dopamine D2R in ventral striatum in the human brain. J Neurosci. 2012; 32(19): 6711–6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tomasi D, Wang GJ, Volkow ND. Association between striatal dopamine D2/D3 receptors and brain activation during visual attention: effects of sleep deprivation. Transl Psychiatry. 2016; 6(5): e828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ettinger U, Merten N, Kambeitz J. Meta-analysis of the association of the SLC6A3 3’-UTR VNTR with cognition. Neurosci Biobehav Rev. 2016; 60: 72–81. [DOI] [PubMed] [Google Scholar]
- 51. Faraone S, Spencer T, Madras B, Zhang-James Y, Biederman J. Functional effects of dopamine transporter gene genotypes on in vivo dopamine transporter functioning: A meta-analysis. Mol Psychiatry. 2014; 19(8): 880–889. [DOI] [PubMed] [Google Scholar]
- 52. Volkow ND, Wang G-J, Fowler JS et al. . Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001; 21(2): 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Greer SM, Goldstein AN, Knutson B, Walker MP. A genetic polymorphism of the human dopamine transporter determines the impact of sleep deprivation on brain responses to rewards and punishments. J Cogn Neurosci. 2016; 28(6): 803–810. [DOI] [PubMed] [Google Scholar]
- 54. Hillion J, Canals M, Torvinen M et al. . Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J Biol Chem. 2002; 20(17): 18091–18097. [DOI] [PubMed] [Google Scholar]
- 55. Basheer R, Strecker RE, Thakkar MM, McCarley RW. Adenosine and sleep-wake regulation. Prog Neurobiol. 2004; 73(6): 379–396. [DOI] [PubMed] [Google Scholar]
- 56. Bodenmann S, Hohoff C, Freitag C et al. . Polymorphisms of ADORA2A modulate psychomotor vigilance and the effects of caffeine on neurobehavioural performance and sleep EEG after sleep deprivation. Br J Pharmacol. 2012; 165(6): 1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bishop DV. Genes, cognition, and communication: insights from neurodevelopmental disorders. Ann N Y Acad Sci. 2009; 1156: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.