Abstract
Plants, bacteria and some fungi are known to produce indole-3-acetic acid (IAA) by employing various pathways. Among these pathways, the indole-3-pyruvic acid (IPA) pathway is the best studied in green plants and plant-associated beneficial microbes. While IAA production circuitry in plants has been studied for decades, little is known regarding the IAA biosynthesis pathway in fungal species. Here, we present the first data for IAA-producing genes and the associated biosynthesis pathway in a non-pathogenic fungus, Neurospora crassa. For this purpose, we used a computational approach to determine the genes and outlined the IAA production circuitry in N. crassa. We then validated these data with experimental evidence. Here, we describe the homologous genes that are present in the IPA pathway of IAA production in N. crassa. High-performance liquid chromatography and thin-layer chromatography unambiguously identified IAA, indole-3-lactic acid (ILA) and tryptophol (TOL) from cultures supplemented with tryptophan. Deletion of the gene (cfp) that encodes the enzyme indole-3-pyruvate decarboxylase, which converts IPA to indole-3-acetaldehyde (IAAld), results in an accumulation of higher levels of ILA in the N. crassa culture medium. A double knock-out strain (Δcbs-3;Δahd-2) for the enzyme IAAld dehydrogenase, which converts IAAld to IAA, shows a many fold decrease in IAA production compared with the wild type strain. The Δcbs-3;Δahd-2 strain also displays slower conidiation and produces many fewer conidiospores than the wild type strain.
Introduction
Indole-3-acetic acid (IAA) is considered the main auxin in plants and a major regulator of plant growth and development. Although the physiological role of auxins in plants is well understood and several IAA biosynthesis pathways have been proposed, only a limited number of biosynthesis genes have been characterized to date despite decades of research [1, 2]. Tryptophan is considered the major source of IAA synthesis in plants, but in some cases, other indole derivatives such as indole-3-acetamide and indole-3-ethanol (TOL) are also employed [3–5]. IAA can either be produced from the precursor molecule tryptophan through several pathways involving different intermediates, or via a tryptophan-independent pathway [6]. Tryptophan-dependent pathways include the (i) indole-3-pyruvic acid (IPA), (ii) YUC flavin monooxygenase, (iii) indole-3-acetaldoxime (IAOx), (iv) indole-3-acetamide (IAM) and (v) tryptamine (TAM) pathways [1, 7, 8]. The most studied is the IPA pathway, which involves three steps. It is initiated by the oxidative transamination of tryptophan to IPA, proceeds via decarboxylation of IPA to indole-3-acetaldehyde (IAAld) by indole-3-pyruvate decarboxylase, and ends by converting IAAld to IAA as the final product via the enzymatic reaction of IAAld dehydrogenase. In the YUC flavin monooxygenase pathway, IPA is directly converted to IAA by the enzymatic action of the flavin monooxygenase-like proteins (YUC family), which has been proposed to be ‘the main auxin biosynthesis pathway in Arabidopsis’ [7]. IAAld serves as a key intermediate for two additional IAA biosynthesis pathways and can be generated directly from tryptophan or via the intermediate compound tryptamine [9].
Microorganisms, including bacteria and fungi, also produce IAA [10, 11]. Unlike the situation in plants, the physiological role of microbial auxins is quite unclear. A number of biosynthetic genes involved in microbial IAA production have been identified or discussed previously [12–14]. In pathogenic bacteria, the IAM pathway is considered the major route for IAA production, yet IAA synthesis via the IPA pathway has been described in a broad range of plant growth-promoting bacterial species [13, 15, 16]. These two pathways use different routes for IAA production via different intermediates. IAA production in different fungi has been documented and shown to be dependent on tryptophan [10, 11, 17, 18]. The IPA pathway-mediated IAA biosynthesis route has been conclusively demonstrated only in the smut fungus Ustilago maydis [15, 17], and genes have been identified for the same pathway in the ectomycorrhizal fungus Tricholoma vaccinum [19]. U. maydis and T. vaccinum are the only fungal species known to date from which IAA metabolic genes from the IPA pathway have been isolated and functionally characterized. In addition, the root endophyte Piriformospora indica has been shown to produce IAA and indole-3-lactic acid (ILA) through IPA as an intermediate compound. Elimination of the tryptophan aminotransferase gene from P. indica results in a significant reduction in IAA and ILA production [20]. A tryptophan-independent IAA biosynthetic pathway has not been described in most fungi and is known only from yeast species [21, 22]. In Fusarium, however, instead of IPA, the IAM pathway is utilized or IAA biosynthesis [14].
In recent literature, the IAA-producing genes and biosynthetic circuitry have been demonstrated exclusively in phytopathogenic or other plant-associated fungi. However, IAA, a basic signaling molecule, has been shown to play a role in interspecies communication and signal transduction [23–25]. This knowledge led us to explore the presence of IAA biosynthetic genes and a functional IAA biosynthesis pathway in the non-pathogenic fungus Neurospora crassa. IAA production in N. crassa has been shown and requires an external supply of tryptophan [18, 26]. We studied IAA biosynthetic genes present in the IPA, YUC, IAM and TAM pathways. Here, we identify, for the first time, N. crassa genes that contribute functionally to tryptophan-dependent IAA biosynthesis. These data provide initial insight in auxin production in a non-pathogenic filamentous fungus.
Results
A few reports have described auxin production in the filamentous fungus N. crassa [18, 26–29]. However, none of these studies has investigated the genes required for IAA production. Our study aimed to comprehensively analyze the potential of N. crassa to produce auxin and to identify the pathway(s) and gene functionality using both bioinformatics and experimental analyses.
The amino acid sequences of four key enzymes from different fungal species, i.e., tryptophan aminotransferase, pyruvate decarboxylase, aldehyde dehydrogenase, and flavin-monooxygenase, were selected for sequence and structural analyses. Alignments of these enzyme sequences demonstrated that all the key amino acid residues that are important for binding to the respective ligands are conserved in the fungal enzyme sequences. In many cases, specific groups of proteins were identified and categorized by their ligand binding motifs, which are known as signature motifs for that particular group of proteins. We implemented a motif searching approach to identify specific ligand binding motifs and categorize the studied enzymes accordingly. Three copies of IAAld dehydrogenase (cbs-3, ahd-3 and ahd-2) from N. crassa were identified and found in six other species. A single copy of each tryptophan aminotransferase (aro-8), pyruvate decarboxylase (cfp) and flavin-monooxygenase (mox-2) from N. crassa was identified and also identified in eight other species. Sequence-based functional conservation analysis suggested that these genes have been conserved throughout fungal evolution (S1–S4 Figs).
The N. crassa genes that were identified and used for bioinformatics as well as for experimental studies were aro-8 (NCU09116), cfp (NCU02193), cbs-3 (NCU03415), ahd-3 (NCU09648), ahd-2 (NCU00378) and mox-2 (NCU03755). Site-specific binding of enzymes to their respective cofactors is essential to determine their active states. The ligand binding sites for the group of homologous enzymes analyzed in this study have been published previously [30–33]. Genes were named according to the N. crassa gene nomenclature and used throughout this study. For clarity, a conversion table is presented that includes the N. crassa genes and their corresponding general auxin gene nomenclature for the respective enzymes (details in S1 Table and the Materials and Methods section).
Key amino acid residues that are important for enzymatic function are conserved
Although the alignments were carried out using multiple species, the focal species of our investigations was N. crassa because of the availability of thousands of knock-out mutants [34]. Aminotransferase enzymes are homologous to pyridoxal-5'-phosphate (PLP)-dependent enzymes, which share a common ancient evolutionary origin. They catalyze the deamination and racemization reactions of amino acids in different species lineages [35]. The amino acid residues that are known to bind to PLP are conserved in the tryptophan aminotransferase homolog in N. crassa. The data indicate that residues Y180, N302, D330, Y333, K381, and R388, present in the N. crassa homolog of tryptophan aminotransferase are involved in ligand binding (Fig 1). Pyruvate carboxylase catalyzes the carboxylation reaction of pyruvate to oxaloacetate. Thiamine pyrophosphate (TPP) acts as a ligand in the carboxylation reaction [36]. Residues G36, E59, T81, V84, G396, W419, G420, I422, V449, G450, D451, and G452 in the pyruvate decarboxylase homolog from N. crassa appeared to be significantly conserved when aligned with known ligand binding residues, and these residues were also highly conserved in other species (S5 Fig). NAD serves as a cofactor for aldehyde dehydrogenase enzymes. Compared with the known template, IAAld dehydrogenase from N. crassa was observed to contain the conserved amino acid residues that are important for NAD binding within the enzymatic catalytic site. The data suggest that the conserved residues from N. crassa aldehyde dehydrogenase involved in ligand binding were N167, T242, G243, E266, L267, G268, C300, E397, F399, and F463. These residues are also conserved in other species (S6 Fig). Flavin-monooxygenase or YUC family proteins are known to have an FAD binding signature motif known as a -GXGXXG- motif [30] (“x” denotes any amino acid). The data suggest that the MOX-2, the YUC homolog from N. crassa has a nucleotide binding motif consisting of G17, A18, G19, P20, A21, and G22 (S7 Fig).
Fig 1. Multiple sequence alignment of tryptophan aminotransferase homologs from different fungal species.
Residues with 100% identity are shown in yellow font with a black background, residues with 75% identity are shown in black font with a red background, and residues with 50% identity are shown in black font with a green background. PLP binding residues are marked with a pink star at the top. Sequences are presented in a discontinuous fashion. Blue discontinuous symbols are shown when there is a discontinuity of the sequence.
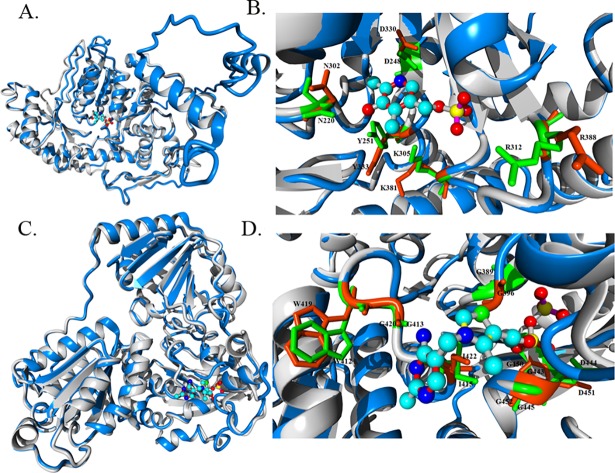
Together with the sequence analysis, we performed a structural analysis of candidate enzymes to provide evidence for their involvement in IAA biosynthesis. In this context, sequence and structural analysis data were supportive and complemented each other [37]. All key amino acid residues from each enzyme present in the auxin biosynthesis pathway were sequentially conserved in the focal species N. crassa as well as in other fungal species. Evidence for structural conservation of the enzymatic catalytic sites of the analyzed proteins is also provided. The results of the structural analysis are presented in two ways. First, the overall structure of the predicted protein model is aligned with a known enzyme with a known function (Fig 2A and 2B). Then, an in-depth analysis of each amino acid residue present in the enzymatic catalytic site and responsible for ligand binding is provided (Fig 2B and 2D). The same analysis was performed for ARO-8 and CFP (Fig 2), IAAld dehydrogenase and MOX-2 (S8 Fig). We checked for the putative ligand binding site in the predicted structures using COFACTOR [38]. COFACTOR in silico analysis makes predictions about the ligand binding sites and plausible ligands that can bind to the given protein structure.
Fig 2. Structural alignment and characterization of ligand binding sites of predicted N. crassa enzymes.
Predicted structures and known templates are shown in blue and gray, respectively. Residues present in predicted enzymes and templates are indicated in orange and green, respectively. All structures are rendered as a ribbon. Key amino acid residues involved in ligand binding are rendered in a stick model. Residues in close vicinity are only highlighted. Ligands are presented in a ball and stick model. (A) The overall structural alignment of predicted ARO-8 structure with a known enzyme structure (4JE5). PLP is bound inside the enzymatic catalytic site. (B) Structural insight into the ligand binding site of both the predicted and 4JE5 known structure. (C) Structural alignment of the predicted structure of the pyruvate decarboxylase homolog from N. crassa with a known pyruvate decarboxylase structure (2VJY). TPP is bound inside the enzymatic catalytic site. (D) Characterization of the ligand binding site of the predicted enzyme using the 2VJY structure as a template.
COFACTOR analysis of the putative N. crassa aromatic aminotransferase (ARO-8) suggests that it has a strong binding match (BS-score 1.20) for PLP (a BS-score >1 signifies strong binding). PLP serves as a coenzyme in transamination, decarboxylation, and deamination reactions of amino acids. The aromatic aminotransferase Aro8 enzyme in a complex with PLP from Saccharomyces cerevisiae [39] was used as a template (PDB ID: 4JE5) for the structural alignment. A structural alignment between the predicted ARO-8 from N. crassa and the template structure showed that despite the presence of sequence dissimilarities, protein folding was very similar in both protein structures (Fig 2A). Analysis of the PLP binding site revealed key amino acid residues that were positionally conserved in the predicted structure and mimicked a conformation similar to the template structure, which facilitated proper ligand binding (Fig 2B). Analysis of the ligand binding of the predicted structure of pyruvate decarboxylase from N. crassa indicated that thiamine pyrophosphate (TPP) had a greater potential to serve as a ligand (BS-score 1.46). Along with many other enzymatic reactions, TPP is known as a coenzyme for pyruvate decarboxylase. To explore the ligand binding site and compare the overall topology, pyruvate decarboxylase from Enterobacter cloacae [40] was used as a structural template (PDB ID: 1OVM). Structural alignment of pyruvate decarboxylase from N. crassa with the above-mentioned template (Fig 2C) indicated that the TPP binding site was similar and that all the key amino acid residues were positionally conserved in the enzymatic active site (Fig 2D). Aldehyde dehydrogenase (IAD) is grouped within a wide class of enzymes, namely, the dehydrogenases. We observed a good amount of sequence conservation among three of the IAD enzymes from N. crassa (CBS-3, AHD-3 and AHD-2) itself, and therefore only the CBS-3 structure is shown herein. The NAD binding pocket was also found in the predicted CBS-3 homolog of N. crassa and was compared with the known NAD binding enzyme [41] (PDB ID: 1NZX) (S8 Fig). MOX-2, which is also known as flavin-containing monooxygenase (FMO), is known to be functional when bound to the NADPH-FAD complex. The nucleotide binding sequence GAGPSG from S. pombe stabilizes the binding of FAD [30]. The present results showed that the putative FMO from N. crassa folded in such a fashion that the GXGXXG motifs (‘X’ denoting any amino acid) of both the template (PDB ID: 1VQW) and predicted structure were properly aligned (S8 Fig).
IAA production in N. crassa is tryptophan dependent
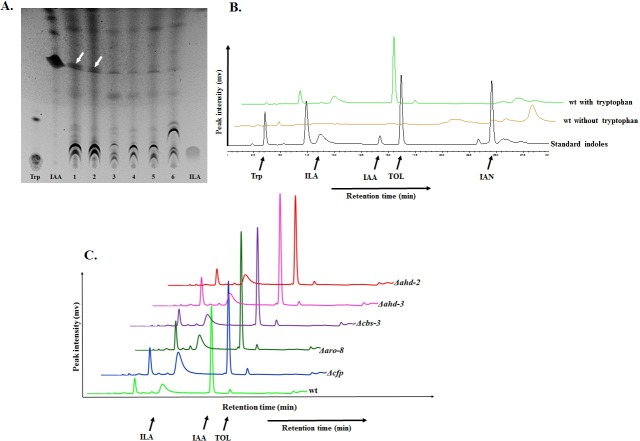
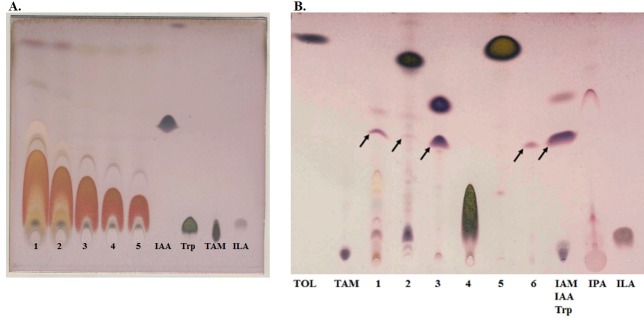
It is known that external supplementation with tryptophan leads to IAA biosynthesis in N. crassa [26, 42]. In this study, for the first time we have analyzed the production of a wide range of indolic compounds including IAA, ILA and TOL by N. crassa employing different chromatographic techniques. N. crassa strains grown without supplementation of external tryptophan show no traces of IAA in the sample-extracts (S9 Fig). While similar data have been reported earlier [18], those data were based on the Avena curvature test. Tryptophan feeding tests were performed with various concentration of tryptophan to identify the threshold concentration of tryptophan in IAA production by N. crassa. Cultures without any supplementation were used as control. Compounds were extracted from the culture and loaded onto a silica plate along with standard indolic compounds for TLC assay. We found that N. crassa can produce IAA with externally supplemented tryptophan in as low concentration as 1 μM (Fig 3A). Lower concentrations of 100, 50 and 25 nM tryptophan produced no detectable IAA. Total indole including IAA production by the wild type N. crassa strain increases with increasing concentration of tryptophan supplementation (S10 Fig).
Fig 3. IAA and related indolic compound production by N. crassa upon tryptophan supplementation.
N. crassa produces IAA and other related indolic compounds when supplemented with tryptophan. (A) TLC analysis to find the lowest concentration of tryptophan that can be used by N. crassa to produce IAA. White arrows in lane 1 and 2 indicate traces of IAA. Different tryptophan concentrations were used in lane 1 (5 μM), 2 (1 μM), 3 (100 nM), 4 (50 nM), 5 (25 nM), and 6 (without tryptophan). (B) HPLC analysis of the metabolites produced by wild type N. crassa with and without tryptophan supplementation for 72 hours. (C) Metabolite profiling of wild type as well as single knock-out N. crassa strains using HPLC. Single knock-outs for the genes specifically present in the IPA pathway of IAA biosynthesis were used. The following standards were used: tryptophan (Trp), indole-3-lactic acid (ILA), indole-3-acetic acid (IAA), tryptophol (TOL) and indole-3-acetonitrile (IAN).
For HPLC analysis, a fixed tryptophan concentration level of 400 mg/L (1.96 mM) was used. The 72-hour-old wild type fungal cultures supplemented with and without tryptophan were analyzed by HPLC. We found that cultures without tryptophan supplementation did not produce any of the indoles, while HPLC peaked for different indolic compounds over time from the tryptophan supplemented culture, which were identified based on the corresponding standards that were run along with the samples (Fig 3B). We found that among all the indolic compounds that were analyzed, IAA was the most abundant compound. Apart from IAA, indole-3-lactic acid and tryptophol were also identified under the same cultural and experimental conditions.
Towards pathway elucidation in N. crassa
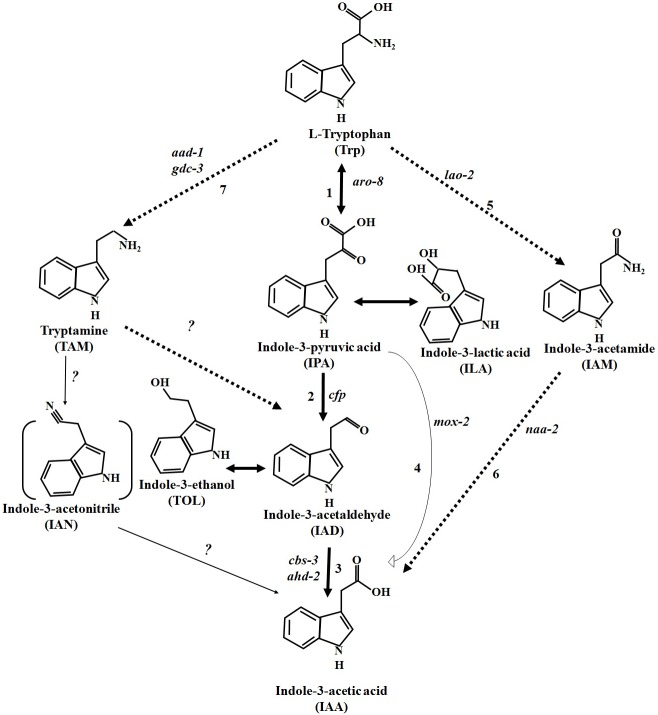
In bacteria and plants, many IAA biosynthetic genes have been identified, and as a result, many pathways have also been proposed. In this study, we performed, for the first time, a comprehensive analysis of N. crassa encompassing different IAA biosynthesis pathways that were previously proposed in microorganisms and plants. We identified IAA biosynthetic genes from different pathways in N. crassa and placed them in the IAA producing network for a better understanding of the IAA-producing circuitry (Fig 4). We took advantage of this proposed IAA-producing pathway for the systemic analysis of IAA producing gene(s).
Fig 4. Proposed tryptophan-dependent IAA biosynthetic pathway in N. crassa.
The pathway with solid bold arrows was functionally characterized by the identification of novel genes, and intermediate compounds were also identified and estimated. Pathways with dashed bold arrows were proposed accordingly based on the feeding test with IAM and TAM. Solid thin arrows denote pathways proposed in green plants but not known in fungi including N. crassa. Unidentified homologues of the genes in N. crassa for the respective biosynthetic steps are indicated by question marks. IAN, with bracketed lines, has not been identified in the N. crassa culture. All other indoles without any brackets (except IAM and TAM) were identified from the culture upon tryptophan feeding. Indole-3-pyruvic acid (IPA) and indole-3-acetaldehyde (IAAld) are very unstable compounds and spontaneously convert into indole-3-lactic acid (ILA) and indole-3-ethanol (TOL), respectively. ILA and TOL can be readily identified in culture as a proxy for IPA and IAAld, respectively. Numbers in the figure reflect different enzymes: 1—tryptophan aminotransferase, 2—pyruvate decarboxylase, 3—aldehyde dehydrogenase, 4—flavin monooxygenase, 5—L-amino acid oxidase, 6—N-acylethanolamine amidohydrolase, 7 –aromatic-L-amino acid decarboxylase and glutamate decarboxylase.
The cfp gene is one of the key regulators of the IPA pathway in N. crassa
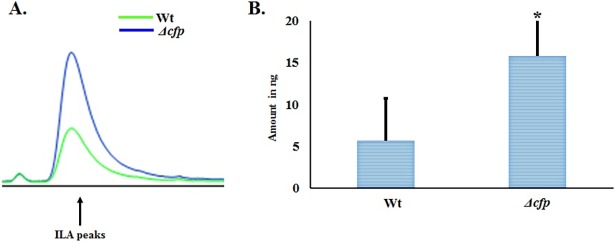
We found that, irrespective of their genetic background, all the strains produced IAA and related indolic compounds, but few knock-out strains produced indoles in higher or lower abundance (Fig 3C). HPLC analysis of 72-hour fungal cultures supplemented with 400 mg/L (1.96 mM) tryptophan revealed that the Δcfp knock-out strain produced a maximum amount of indole-3-lactic acid (Fig 5A). We estimated that the amount of ILA was 64.4% higher in Δcfp (15.78 ng) compared with wild type (5.62 ng) (Fig 5B). In the pathway analysis of knock-out strains, the most pronounced effect on IAA production was found in the Δcbs-3;Δahd-2 double knock-out strain (Fig 6). HPLC peaks for wild type, Δcbs-3, Δahd-2 and Δcbs-3;Δahd-2 are shown in different colors, as mentioned in the figure (Fig 6A). Based on the HPLC peak areas and those of the standards, we estimated that the amount of IAA in the Δcbs-3;Δahd-2 double knock-out strain (3.52 ng) was 44% lower than in the wild type strain (6.28 ng) (Fig 6B) under the same cultural and extraction conditions. The other genes that were studied in the IAA biosynthesis pathways were mox-2, lao-2 (tryptophan-2-monooxygenase), naa-2 (indole-3-acetamide hydrolase) aad-1 (aromatic–L-amino acid decarboxylase) and gdc-3 (glutamate decarboxylase). Neither the single knock-outs nor any of the double or triple knock-out strains had any effect on the level of IAA production (S12 Fig).
Fig 5. Effect of the Δcfp strain on ILA production in N. crassa.
(A) HPLC peaks (peak intensity is given in mv as in Fig 3) of indole-3-lactic acid (ILA) from the wild type and Δcfp strain are shown in different colors. (B) Amount of ILA produced in 72-hour cultures of wild type and Δcfp knock-out strains supplemented with tryptophan. * p < 0.05.
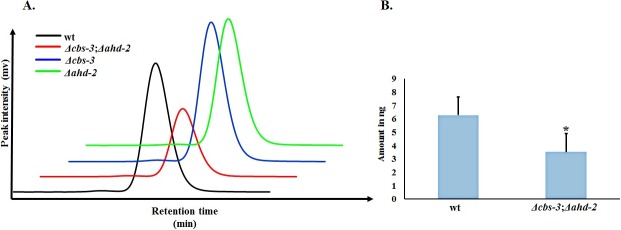
Fig 6. Reduction in IAA production by the synergistic effect of the Δcbs-3;Δahd-2 double knock-out in N. crassa.
Individual knock-out strains for the aldehyde dehydrogenase genes cbs-3 and ahd-2 did not have any significant effect on IAA production, but the synergistic effect of the Δcbs-3;Δahd-2 double knock-out resulted in a strong reduction of IAA production. (A) HPLC peaks of IAA for wild type along with other knock-out strains are shown in different colors as mentioned in the figure. IAA production was decreased in the Δcbs-3;Δahd-2 double knock-out strain compared with the wild-type strain. (B) Comparison of the amount of IAA produced by wild type and Δcbs-3;Δahd-2 strains in 72 hours. * p < 0.05.
Fewer conidiospores in the Δcbs-3;Δahd-2 double knock-out strain compared to wild type
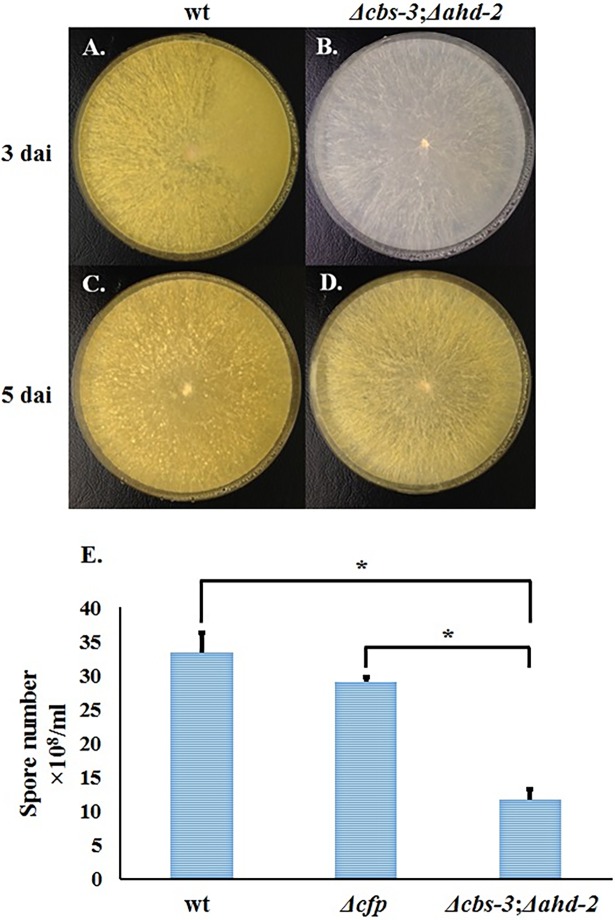
We observed the lowest amount of IAA production in the Δcbs-3;Δahd-2 double-knock-out strain. We also checked its ability to produce conidia by culturing wild type and knock-out strains on an agar plate containing Vogel’s Minimal Medium (VMM). The double knock-out strain (Fig 7B and 7D) for the genes Δcbs-3 and Δahd-2 showed much less conidiation than their wild type counterpart (Fig 7A and 7C), even after five days of inoculation. After culturing on solid medium for 10–15 days, the total number of conidiospores produced by the Δcbs-3;Δahd-2 strain was less than that of the wild type strain (Fig 7E).
Fig 7. Conidiospore production by the Δcbs-3;Δahd-2 double mutant.
(A) and (C) are wild type strains, and (B) and (D) are Δcbs-3;Δahd-2 double knock-out strains after growth for three and five days, respectively. (E) A numerical comparison of conidia production by the Δcbs-3;Δahd-2 double knock-out strain with wild-type and the Δcfp single knock-out strain. dai: days after inoculation. * p < 0.05.
Indole production in N. crassa is time-dependent and affected by knock-out of the IAA-producing genes
IAA production in fungi is time-dependent, and various indolic compounds start to accumulate in the culture medium over time [20, 42]. In this study, we demonstrated how IAA and related indole production changes over time in different N. crassa strains with different mutations of the IAA-producing genes (S13 Fig). Our results suggested that the production of IAA and other related indolic compounds by the wild type strain in culture medium supplemented with tryptophan began after ~12 hours of inoculation and declined over time (S13A Fig). The Δcfp strain showed a similar indole production pattern as the wild type strain, with notably higher ILA production throughout the experimental time frame (96 hours) (S13B Fig). The Δcbs-3;Δahd-2 double knock-out strain started to produce IAA later than the wild type strain (S13C Fig). None of the 72-hour-old fungal cultures showed any trace of tryptophan in HPLC and TLC analysis (Fig 3B and S13A Fig), but the Δcbs-3;Δahd-2 double knock-out strain showed an accumulation of tryptophan up to 48 hours after inoculation compared with the wildtype strain (S13C Fig and S13D Fig). These results suggest that the Δcbs-3;Δahd-2 strain had not only a reduced level of IAA production but also a lower efficiency in tryptophan utilization.
Feeding test performed with other indolic compounds and the amino acid tyrosine
In an earlier study, it was mentioned that tyrosine may act as a precursor for IAA production in fungi [18], while some other studies utilized tryptamine. However, none of the tested fungi showed IAA production when supplemented with tryptamine [18, 20]. We performed feeding experiments with tyrosine together with indolic compounds such as tryptamine, indole-3-acetamide (IAM), indole-3-lactic acid, tryptophol (TOL) and indole-3-acetic acid (IAA) to assess their ability to produce IAA. Our results showed that the wild type strain could not produce IAA even when supplemented with tyrosine at a relatively high concentration of 5 mM. (Fig 8A). The effect of tyrosine was also checked by feeding the wild type N. crassa strain with different tyrosine concentrations and performing subsequent metabolite analysis on TLC plates. However, tyrosine-mediated IAA biosynthesis was not found in the same experimental setup with any of the concentrations used. In contrast, both tryptamine and IAM supplementation led to IAA production, although at different concentrations: 1 mM IAM was sufficient to induce IAA production (S14 Fig), but tryptamine had to be supplemented at 2.5 mM (Fig 8B).
Fig 8. Indole production by the wt N. crassa strain supplemented with tyrosine and other indoles.
Indole production by wt N. crassa was checked by supplementation with different concentrations of tyrosine. (A) lanes 1–5 tyrosine in 5 mM, 2.5 mM, 1.25 mM, 625 μM and 312.5 μM. (B) TLC analysis to determine the ability to produce indoles, including IAA, by various indolic compounds. Black arrows indicate traces of IAA. Indolic compounds that were used as supplements are as follows: lane 1—mM tryptophan, lane 2–2.5 mM tryptamine, 3–1 mM indole-3-acetamide, 4–1 mM indole-3-lactic acid, 5–1 mM tryptophol, 6–10 μM IAA. Following standards were used: tryptophol (TOL), tryptamine (TAM), indole-3-acetamide (IAM), indole-3-acetic acid (IAA), tryptophan (Trp), indole-3-pyruvic acid (IPA) and indole-3-lactic acid (ILA).
The tryptophan supplemented culture produced a maximum number of indolic compounds compared to other supplements tested in this study (Fig 8B and S14 Fig). We also observed that indolic compounds, including IAA from the tryptophan-supplemented culture, gradually declined over time (S13A Fig and S14 Fig), but the opposite was observed for the IAM-supplemented culture (S14 Fig), in which the IAA level tended to increase. These results clearly suggest that not only tryptophan but also tryptamine and indole-3-acetamide (IAM), the main precursor molecule for bacterial IAA biosynthesis [13, 16], can be utilized as a precursor molecule for IAA biosynthesis in N. crassa.
Discussion
Microorganisms, including fungi and bacteria, are capable of producing indole-3-acetic acid [1, 5, 10, 14, 17, 18, 20, 26, 43]. While many fungi have been shown to produce IAA using various IAA biosynthetic routes [5, 14, 15, 20], a limited number of IAA biosynthetic genes have been characterized in detail despite many efforts [1, 44]. IAA synthesis via the IPA pathway has been described in a broad range of plant growth-promoting bacterial species [13, 16] and in a few fungal species [15, 19, 20]. However, the presence of IAA in N. crassa has been documented in only a few studies [26, 42], and neither the biosynthetic genes nor the biosynthesis pathway have been explored. Here, we showed for the first time, that genes in the IPA pathway of IAA production are present and functionally active in N. crassa. Although a homologue of the gene encoding indole-3-pyruvate decarboxylase (cfp) has not been found in green plants [1], our results clearly demonstrated that cfp is present in a wide range of fungi. Furthermore, it appears to be fully functional in N. crassa. TLC and HPLC analyses of N. crassa wild type and knock-out strains for the IPA pathway used in this study revealed that N. crassa produces IAA via the IPA route. Additionally, we report that deletion of the IAA-producing genes in the IPA pathway of N. crassa results in altered production of IAA as well as other related indolic compounds. Tryptophan is needed as an external supplement for IAA production in N. crassa [26, 42]. We also assessed the potential for tyrosine to produce IAA because tyrosine has been mentioned as a potential precursor candidate leading to IAA in fungi [18]. However, our result confirmed that N. crassa cannot use tyrosine as a precursor molecule to produce IAA (Fig 8A).
Identification and functional characterization of indole-3-pyruvate decarboxylase in N. crassa
By employing homology searching, we identified a pyruvate decarboxylase gene in N. crassa (NCU02193), which is named as “cellular filamentous protein” (cfp) in FGSC as well as in “FungiDB”. Herein we report that cfp from N. crassa contains binding sites for thiamine pyrophosphate (TPP or ThPP), which acts as a ligand for decarboxylase enzymes (S5 Fig, Fig 2C and 2D). In the proposed IPA pathway from various studies [1, 32], it has been shown that indole-3-pyruvic (IPA) acid is an unstable compound and that a dynamic conversion occurs between IPA and indole-3-lactic acid (ILA). Our HPLC analysis showed that a Δcfp N. crassa strain produced more ILA as compared with the wild type strain (Fig 5). Hence, our results are consistent with the observed inability of fungi to convert IPA into indole-3-acetaldehyde (IAAld) in the absence of the cfp gene, leading to increased IPA levels and hence further accumulation of ILA. These results unambiguously confirm that cfp is present and functionally contributes its enzymatic role in the IPA pathway mediated IAA biosynthesis in N. crassa.
Indole-3-acetaldehyde dehydrogenase genes are direct contributors to IAA production in N. crassa
The functional identification and synergistic effects of two IAAld dehydrogenase genes in the pathogenic fungus U. maydis have been previously studied [17]. In our study, we reported the presence of more than one copy of the genes encoding IAAld dehydrogenase in N. crassa (cbs-3, ahd-2 and ahd-3) and their functional contribution towards IAA biosynthesis. Individual knock-outs of any of these genes did not have any significant effect on IAA production (Fig 6). A significant decrease in IAA formation was only observed in the Δcbs-3;Δahd-2 double knock-out N. crassa strain. These findings align perfectly with the same scenario in a previous study conducted in U. maydis [15]. A single copy IAAld dehydrogenase (iad) mutant did not cause any reduction in IAA formation in U. maydis, while an iad1iad2 double mutant strain exhibited a many fold reduction in IAA formation [15, 17]. Taken together, these results support the presence of multiple highly interconnected and well regulated aldehyde dehydrogenase genes acting in the IAA biosynthesis pathway of N. crassa. The synergistic effect reflected in the Δcbs-3;Δahd-2 double knock-out implies that IAA production in N. crassa via the IPA pathway proceeds through the production of indole-3-acetaldehyde (IAAld) and that the last step of this pathway is under tight regulation by multiple genes. Here, we report, for the first time, a novel aldehyde dehydrogenase gene (ahd-2) and its contribution towards IAA biosynthesis in N. crassa.
IAA biosynthesis in N. crassa is activated and regulated spatiotemporally
In the present study, we examined IAA production by N. crassa with different supplements at different time points. Our results demonstrated that tryptophan is the only amino acid among tryptophan, tyrosine and histidine that can be used as a precursor for IAA biosynthesis in N. crassa (histidine supplementation in S15 Fig). The presence of multiple IAA biosynthesis pathways in microorganisms has been previously reported. For example, Erwinia herbicola pv. gypsophilae has both IPA and IAM pathways [45], wherein the IPA pathway is involved in epiphytic fitness while the IAM pathway is responsible for pathogenicity. Our study showed that tryptophan-supplemented cultures produce many indolic compounds other than IAM over time. In contrast, IAM supplementation resulted only in IAA production as the major indolic compound, along with very few other unidentified compounds. The IAM pathway has been shown to be present in the filamentous fungi Fusarium [14] and in Colletotrichum gloeosporioides f. sp. Aeschynomene [5]. In our study, we did not detect any trace of IAM produced by N. crassa strain(s) by TLC and HPLC analyses. However, we found that IAM supplementation in the N. crassa culture medium also led to IAA production. These results clearly suggest that tryptophan is used as a primary source for IAA production in N. crassa and that IAA synthesis proceeds through the IPA pathway to produce intermediate indolic compounds such as ILA and TOL, which are side products of the IPA pathway. The IAM pathway in N. crassa is activated only if IAM is supplemented in the medium; in its absence, no trace of IAM was detected in the metabolite analysis, which further suggests that the IAM pathway in N. crassa is a reserved and very specific secondary pathway for IAA production.
Tryptamine (TAM) supplementation produced a minute amount of IAA production when supplemented at a relatively higher concentration (Fig 8B), suggesting that although tryptamine supplementation leads to IAA production, the TAM pathway itself is inefficient and requires an additional secondary IAA production pathway in N. crassa. We also used single, double and triple knock-out strains encompassing other IAA biosynthetic routes. We studied IAA production using knock-out strains for IAA-biosynthetic genes from the IPA, YUC, IAM and TAM pathways. Our findings revealed that only those genes present in the IPA pathway were active and appeared to be produce IAA most efficiently in N. crassa.
The Δcbs-3;Δahd-2 double knock-out strain produces fewer conidiospores, which is a significant finding because it suggests a biological function of auxin in the fungus. We conclude that aberrations in IAA-producing genes have a direct effect on conidiation as well as overall fungal growth.
Materials and methods
Identification of IAA-producing genes in N. crassa
Homologous protein sequences of tryptophan aminotransferase (ARO-8), indole-3-pyruvate decarboxylase (CFP), indole-3-acetaldehyde (IAAld) dehydrogenases and flavin monooxygenase (MOX-2) from different fungal species were obtained by BLAST [46] analysis and used in both bioinformatics as well as in experimental studies. First, Neurospora crassa protein sequences for the enzymes mentioned above were determined using the Broad Institute database (http://www.broadinstitute.org) and FungiDB (http://fungidb.org/fungidb/). Then, homologous sequences in other species were checked and retrieved by bidirectional BLAST using the Broad Institute database, FungiDB and the National Centre for Biotechnology Information (NCBI) [47, 48]. Only full-length sequences of the above-mentioned enzymes from different fungal species were considered for further analysis. Sequences were aligned using MUltiple Sequence Comparison by Log-Expectation (MUSCLE) [49, 50], and the alignments were visualized, edited, and presented using GeneDoc [51]. In a similar way, homologous sequences of tryptophan-2-monooxygenase (LAO-2), indole-3-acetamide hydrolase (NAA-2), aromatic-L-amino acid decarboxylase (aad-1) and glutamate decarboxylase (gdc-3) were obtained only for N. crassa and were used only for experimentation along with other genes. Throughout this study, we used the names of the genes and their corresponding enzymes per N. crassa conventions. However, we also found that some genes were annotated differently in different databases. For this reason, a description of the gene identity with its annotations is presented in S1 Table, showing annotations for both N. crassa as well as the general nomenclature for enzymes.
For the structural analysis, we implemented two independent methods to establish the consistency and reliability of each method, as well as the structure. We used the Ab initio method from the I-TASSER (http://zhanglab.ccmb.med.umich.edu/I-TASSER/) online-server [52] and the "SWISS-MODEL Repository portal" (http://www.expasy.org/) for homology modeling [53]. For the Ab initio method, corresponding enzyme sequences from N. crassa were submitted to the I-TASSER server, and the best model was chosen based on the C-scores. To choose a proper homologous structural template, individual enzymes were analyzed using BLAST against the PDB database (www.rcsb.org) prior to homology modeling. Suitable models were chosen for homology modeling based on the BLAST scores. We then predicted and cross-checked the cofactor binding properties in our predicted models by assessing the "structure-based function prediction" using the COFACTOR online server (http://zhanglab.ccmb.med.umich.edu/COFACTOR/) [38] for ARO-8, CFP, CBS-3 and MOX-2. COFACTOR makes predictions about the ligands and their binding sites on the structures. The reliability of ligand binding is defined by the BS-score. A score >1 reflects significant ligand binding in the structure. Ligand-binding sites present on both N. crassa enzymes and template structures (those used for homology modeling) were identified by alignments with known proteins. Finally, I-TASSER-predicted enzyme structures were aligned with template structures from the PDB. This structural alignment helps to obtain information about key residues inside enzymatic catalytic sites.
Structure optimization
All the predicted structures generated by I-TASSER or SWISS-MODEL were evaluated in the PDBsum database using PROCHECK analysis [52]. As two independent approaches (Ab initio and homology modeling) were used for structure prediction, we further checked the consistency and reliability of the models by structural alignment of the same enzyme predicted by different methods.
Molecular visualization
All the molecular visualization and editing was performed using PyMol (The PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, LLC.) and YASARA (http://www.yasara.org/). Images were processed using POV-Ray (http://www.povray.org/).
Fungal strains and culture media
All the fungal strains, including wild type and single knock-out strains (S1 Table), were purchased from the fungal genetics stock center (FGSC) (http://www.fgsc.net/). All the strains are viable without any special supplementation. Single knock-out strains carry a hph (hygromycin B phosphotransferase) cassette in place of the ORF of the targeted genes [34]. Unless otherwise mentioned, the strains are always maintained and/or cultured on Vogel’s minimal medium (VMM) containing 2 ml of 50× Vogel’s salts (150 g Na3 citrate, 250 g of KH2PO4, 100 g of NH4NO3, 10 g of MgSO4, 5 g of CaCl2, 5 ml of trace element solution, 25 mg of biotin for a total volume of 1000 ml), and 2 g of sucrose, (2 g agar for solid media) for a total volume of 100 ml. Per the requirements, strains with two different mating types were crossed on Westergaard crossing medium containing 25 ml 4× Westergaard solution (4 g of KNO3, 4 g of KH2PO4, 4.1 g of MgSO4, 0.8 g of CaCl2, 0.4 g of NaCl, 400 μl of trace element solution, 10 mg of biotin for a total volume of 1000 ml), 2 g of sucrose, adjusted to pH 6.5, and 2 g of agar for a total volume of 100 ml. After successful crossing, ascospores were collected and spread on sorbose-glucose-fructose medium (20× SGF) (20 g of sorbose, 0.5 g of glucose, 0.5 g of fructose for a total volume of 100 ml) plates (for each 100 ml of total medium, 2 ml of 50× Vogel’s salts and 2 g of agarose were made up to 95 ml and autoclaved at 121°C for 20 minutes followed by the addition of 5 ml of 20× SGF). For DNA isolation, fungi were grown in liquid VMM medium, and for indole quantification and testing, liquid VMM medium was supplemented either with L-tryptophan (Sigma-Aldrich) or other supplementation as needed. All liquid cultures for indolic compound testing were performed in 50-ml flasks and incubated at 28°C on a rotary shaker.
Setting for crosses, isolation of ascospores and propagation
Crosses were set up on plates containing Westergaard crossing medium [54]. The strains were allowed to grow at 25°C. After ~14 days of setting up the cross, the ascospores were collected. The ascospores were activated at 60°C for 45 minutes [55] and spread on SGF-agar plates. Next, one to two isolated colonies were transferred to VMM slant tubes and maintained at 25°C for a few days until they grew well and produced conidiospores.
Estimation of the conidial number from different strains
Strains were inoculated in a 150–200 ml Erlenmeyer flask with 50 ml of VMM-agar medium and maintained for 10–21 days at 25°C in a fungal growth chamber. Next, 40 ml of 1 M sorbitol + 0.1 ml of Tween® 20 solution was poured carefully into the flask, which was then sealed with Parafilm and shaken well to remove all the spores from the solution. The mixture of spores, sorbitol and Tween® was then poured onto gauze and passed through a funnel for filtering, collected in 50-ml tubes and centrifuged at 4,000 rpm for 5 minutes. The spores were re-suspended and centrifuged with 50 ml of 1 M sorbitol only. This step was performed twice. Finally, the conidia were collected in a 1.5-ml tube with 500 μl of 1 M sorbitol, diluted at a ratio of 1:100 and counted using a hemocytometer. The formula applied for counting conidia is as follows: n (number of spores in large squares) / 4 = K ×108 spores/ml. All counting was performed in triplicate and repeated several times.
DNA isolation, primer designing and confirmation of the knockout strains by PCR
DNA isolation for screening purposes in search of positive knock-out strains was performed using the Quick-DNA™ Fungal/Bacterial Miniprep Kit (Zymo Research, USA), and the DNA concentration was measured in NanoDrop 2000 Spectrophotometer (Thermo Scientific, PEQLAB). As all single knock-out strains had a hph-cassette in the position of ORF of the targeted gene, all the subsequent knock-out strains (double, triple, etc.) also had the hph-cassette in the targeted positions. A successful hph-cassette exchange with the targeted gene was confirmed in two steps: first, by checking for the middle portion of the ORF by designing primers specific for the ORF of the targeted gene, and second, by designing primers such that the forward primer is always in the 5’ UTR of the targeted gene and the reverse primer was in the hph-cassette. Therefore, the reverse primer (5’ ATCCACTTAACGTTACTGAAATCTCCAAC 3’) was always same for all knock-out strains. A detailed list of primers is provided in S2 Table in the supplementary information. Each double or triple knock-out strain was first checked on an agarose gel after PCR, and then the DNA band from the 5’ UTR along with hph-cassette of the respective gene was sequenced (Sanger sequencing).
Extraction of indolic compounds and analysis
Estimation of IAA and total indoles
IAA and other indole derivatives were estimated by the Salkowski method [56, 57]. Samples were filtered using cheesecloth, and the supernatant was collected. One milliliter of supernatant was mixed with 1 ml of Salkowski reagent (1.015 g of FeCl3. 6H2O, 150 ml of H2SO4, 250 ml of ddH2O) in a light-protected tube. The pH was adjusted to 2.8 with 25% HCl. The tubes were incubated for 30 minutes at 30°C in the dark for color development, and then the absorbance at OD540 was determined. The regression line and correlation coefficient (r2 >0.998) were determined using commercially available IAA (Sigma-Aldrich) standards prepared from serial dilutions of 100 mM stock solutions. All experiments were conducted with three replicates and were repeated at least three times.
Feeding test for IAA production with indole derivatives and other supplements
For the feeding tests, conidiospores were germinated in 50 ml of VMM medium with various supplements. Fungal indole production was checked by tryptophan supplementation at a final concentration of 1.96 mM unless otherwise mentioned. The IAA final concentration was set to 10 μM in the culture medium because this concentration has been reported to be the optimized one for maximum conidial germination [27]. Tryptamine (TAM), indole-3-acetamide (IAM) and tyrosine were used at different concentrations according to the needs of the experiments. A fixed concentration of indole-3-lactic acid (ILA) and tryptophol (TOL) was used in a final volume 2.5 mM each. The culture was induced in the dark at 28°C by shaking at 150 rpm. Negative controls were mock-inoculated. To assess the ability to produce IAA by the mentioned indoles, a feeding experiment was performed coupled to a time course assay. In the time course assay, wild type and different knock-out N. crassa strains were supplemented with different indolic compounds, and IAA production was checked every 12 or 24 hours for a total length of 96 or 72 hours, respectively, according to the experimental requirements.
TLC analysis
Fungal cultures were filtered through cheesecloth and collected in a tube. The pH was adjusted to 2.8 and mixed with equal volume of ethyl acetate (1:1 v/v). The aliquots were extracted by shaking at 200 rpm for 3 hours in the dark. After phase separation, 1.5 ml of the ethyl acetate fraction was removed to a light protected tube, and ethyl acetate was evaporated in a UNIVAPO 100H (UNIEQUIP, Planegg, Germany) rotating vacuum. The solid residue was dissolved in 60 μl of ethyl acetate and loaded onto silica gel 60 F254 plates (Merck, Darmstadt, Germany). The TLC plates were run for 1–1.5 hours with a buffer of chloroform: methanol: water (84: 14: 1), and color was developed by spraying a mixture of van Urk and Salkowski reagents at a ratio of 1: 3 [57] and subsequent incubation at 90°C for 10 min. The indole derivatives were identified by the retention factor (Rf) and colors of the bands by loading 2 μl of 20 mM standard compounds on the plate.
HPLC analysis
HPLC analysis was performed in a 10 A Model HPLC (Shimadzu, Japan) equipped with a NUCLEODUR® 100–5 C18 reverse column (250 mm length × 4 mm internal diameter), RF-10A XL fluorescence detector at a 280-nm wavelength. Following extraction with ethyl acetate, the dry pallet was dissolved in 60 μl of buffer containing 25% methanol and 1% acetic acid, pH 4.5. The same buffer was used to dilute the sample further at a ratio of 99:1 (buffer: sample). Indoles were separated on a 20–100% (v/v) MeOH- water gradient as solvent B and 2.5% acetic acid pH 3.8 set with 4 M KOH as solvent A over 45 minutes with a flow rate of 1 ml/min. Under these conditions, all the standard indolic compounds were detected within the first 30 minutes. IAA was detected and confirmed by the retention time and co-migration (spiking with an authentic standard). Including IAA, all other indolic compounds were detected, and integration of the different peaks was conducted according to the standards. Excluding tryptophan, other indolic compound standard stocks were prepared in methanol. The standard tryptophan stock was prepared in HPLC grade water. In each HPLC run, a standard mixture of different indoles was used at a 2 mM concentration each, diluted in sample-dissolving buffer. Standard curves for different indolic compounds were generated using three different concentration (5 μM, 2 μM and 1 μM) with four different injection volumes (2 μl, 6 μl, 10 μl and 15 μl) of each concentration. Thus, for each standard 12 (twelve), data points were obtained for the calibration curves. Calibration curves were plotted as the injected analyte mass vs peak area. The curves were then fitted, and a linear regression equation as well as coefficient (r2) were calculated. All the experiments were performed at least with three replicates and repeated several times.
Supporting information
Residues with 100% identity are shown in yellow font on a black background, residues with 75% identity are shown in black font on a red background, and residues with 50% identity are shown in black font on a green background. PLP binding residues are denoted by a pink star on top.
(PDF)
The black background with yellow font, red background with black font and green background with black fonts represent 100%, 75% and 50% identity of the residues, respectively. TPP binding residues are denoted by pink stars on top of the alignment.
(PDF)
Amino acid residues with black background yellow font represent 100% sequence identity; 75% identity is shown in black font on a red background, and 50% sequence identity is shown in black font on a green background. NAD serves as cofactor for the enzyme aldehyde dehydrogenase. NAD binding residues are denoted by a pink star the top of the residues.
(PDF)
MOX-2 or flavin monooxygenase is known to bind FAD through its nucleotide binding motif (GXGXXG). The GXGXXG motif is indicated by a bar, and conserved residues are highlighted with pink stars. “X” represents any amino acid. Residues in yellow font on a black background are 100% identical, and residues in black font on a red background are 75% identical. (the MOX-2 homolog in U. mydis was not found using the same BLAST search with the same template used for the other homologs.)
(PDF)
A black background with yellow font, red background with black font and green background with black font represents 100%, 75% and 50% identity of the residues, respectively. TPP binding residues are denoted by pink stars. A blue discontinuous symbol shows a discontinuity in sequence.
(TIF)
Amino acid residues in yellow font on a black background represent 100% sequence identity, in black font on a red background represent 75% identity and in black font on a green background represent 50% sequence identity. NAD serves as cofactor for the enzyme aldehyde dehydrogenase. NAD binding residues are indicated by a pink star on top of the residues.
(TIF)
MOX-2 or flavin monooxygenase is known to bind to FAD through its nucleotide binding motif (GXGXXG). GXGXXG motif is shown as a bar, and conserved residues are highlighted by pink stars. Residues in yellow font on a black background are 100% identical and residues, and residues in black font on a red background are 75% identical.
(TIF)
Predicted structures and known templates are shown in blue and gray, respectively. Residues present on predicted enzymes and templates are marked with orange and green, respectively. All structures are rendered as a ribbon. Key amino acid residues involved in ligand binding are rendered as a stick model. Residues those are at close vicinity are only highlighted. Ligands are shown as a ball and stick model. (a) Overall structural alignment of the predicted CBS-3 structure with the known enzyme structure (1NZX). NAD is bound inside enzymatic catalytic site. (b) Structural insight of the ligand binding site of both the predicted and 1NZX known structure. (c) Structural alignment of the predicted structure of flavin monooxygenase homolog from N. crassa with the known flavin monooxygenase structure (1VQW). (d) Characterization of the ligand binding site of the predicted enzyme using the 1VQW structure as a template.
(TIF)
Lower panel of the figure with numbers denotes the standard peaks of the various indolic compounds. Except indole-3-lactic acid (ILA) produced by Δcfp strain, no other indolic compounds comparable to the standard peaks were found within the time window set for the analysis. Genes that were analyzed from different pathways are as follows: aro-8aro-8aro-8, cfp, cbs-3, mox-2, lao-2 (tryptophan-2-monooxygenase) and naa-2 (indole-3-acetamide hydrolase). lao-2 and naa-2 are involved in IAM pathway of IAA biosynthesis. Standard peaks are: lane 1—tryptophan, lane 2—indole-3-lactic acid, lane 3—tryptamine, lane 4—indole-3-acetamide, lane 5—indole-3-acetic acid, lane 6—tryptophol, lane 7 -indole-3-acetonitrile.
(TIF)
Different tryptophan concentrations ranging from 2 mM to 12.5 μM were used.
(PDF)
Two samples, namely, S1 and S8, were confirmed. S1 is a double knock-out strain for genes lao-2 and naa-2, and S8 is a double knock-out strain for genes lao-2 and aro-8. Lane 1 and 2 were loaded with the PCR products of 5’ UTR amplifications of lao-2 and naa-2 ORFs, respectively, along with the hph cassette. Lane 3 and 4 were loaded with the PCR products of mid-part of lao-2 and naa-2 gene ORFs respectively. Lane 5 and 6 were loaded with the PCR products of 5’ UTR amplifications of lao-2 and aro-8 ORFs, respectively, along with the hph cassette. Lane 7 and 8 were loaded with the PCR products of mid-part of lao-2 and aro-8 ORFs respectively. Lane 9, 10 and 11 were loaded with the PCR products of mid-part of lao-2, naa-2 and aro-8 ORFs, respectively, from wild type strain (wt). M—DNA marker.
(TIF)
Graphs with different colors represent different knock-out strains. A specific graph for a particular knock-out strain is mentioned in the figure with color bars. Standard peaks are ILA: indole-3-lactic acid, IAA: indole-3-acetic acid and TOL: tryptophol.
(TIF)
Changes in IAA and indolic compounds metabolism over time in wild type and mutant N. crassa supplemented with 1.96 mM tryptophan. (A) Wild type N. crassa strain was grown with tryptophan supplementation, and samples were collected in every 12 hours for a total of 96 hours. The following standards were used: tryptophol (TOL), tryptamine (TAM), indole-3-acetamide (IAM), indole-3-acetic acid (IAA), tryptophan (Trp), indole-3-pyruvic acid (IPA) and indole-3-lactic acid (ILA). (B) Δcfp knock-out strain was grown with tryptophan supplementation. At every 12-hour interval, samples were collected. Black arrow marks indicate ILA spots. The following standards were used: tryptophol (TOL), indole-3-acetamide (IAM), indole-3-acetic acid (IAA), tryptophan (Trp), indole-3-pyruvic acid (IPA) and indole-3-lactic acid (ILA). (C) The Δcbs-3Δahd-2 double knock-out strain was grown with tryptophan supplementation. At every 12-hour interval, samples were collected and analyzed. Black arrow marks indicate tryptophan spots. The following standards were used: tryptophol (TOL), indole-3-acetamide (IAM), indole-3-acetic acid (IAA), tryptophan (Trp), indole-3-pyruvic acid (IPA), and indole-3-lactic acid (ILA). (D) HPLC analysis of 48 hours old wild type and Δcbs-3;Δahd-2 double knock-out N. crassa culture supplemented with tryptophan. After 48 hours of inoculation Δcbs-3;Δahd-2 double knock-out strain shows a peak for tryptophan while corresponding peak from wild type strain has not been observed.
(TIF)
Different indolic compounds were supplemented to wild type N. crassa culture media for checking their role in IAA production over time. tryptophan (Trp), tryptamine (TAM), indole-3-acetamide (IAM) and indole-3-acetic acid (IAA) were used for the assay. Samples were collected in 24-hour intervals. The numbers below the lanes represent the time in hours. Following standards were used: tryptamine (TAM), indole-3-acetamide (IAM), indole-3-acetic acid (IAA), tryptophan (Trp), and indole-3-pyruvic acid (IPA).
(TIF)
Fungal culture was supplemented with 2 mM histidine. Lane 1: Fungal culture was supplemented with 1.96 mM of tryptophan. Lane 2: Fungal culture was supplemented with 1 mM of tryptophan. Lane 3: Fungal culture was supplemented with 2 mM of histidine. IAA: indole-3-acetic acid standard.
(TIF)
(PDF)
(PDF)
Acknowledgments
We would like to thank Prof. Wolfgang Bilger from the department of Eco-physiology of Plants, Christian-Albrechts-University for providing the HPLC facility and Jens Hermann for necessary technical support. We acknowledge the Fungal Genetics Stock Center (FGSC) for providing the N. crassa strains. Dr. Krisztina Kolláth-Leiß is acknowledged for discussion and scientific comments.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work. However PS received a stipend from the Max-Planck-International-Research-School Evolutionary Biology.
References
- 1.Mano Y, Nemoto K. The pathway of auxin biosynthesis in plants. J Expe Bot. 2012;63(8): 2853–72. doi: 10.1093/jxb/ers091 . [DOI] [PubMed] [Google Scholar]
- 2.Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Ann Botany. 2005;95(5):707–35. doi: 10.1093/aob/mci083 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Normanly J. Approaching cellular and molecular resolution of auxin biosynthesis and metabolism. Cold Spring Harbor Perspect Biol. 2010;2(1):a001594 doi: 10.1101/cshperspect.a001594 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandberg G. Biosynthesis and metabolism of indole-3-ethanol and indole-3-acetic acid by Pinus sylvestris L. needles. Planta. 1984;161(5):398–403. doi: 10.1007/BF00394569 . [DOI] [PubMed] [Google Scholar]
- 5.Robinson M, Riov J, Sharon A. Indole-3-acetic acid biosynthesis in Colletotrichum gloeosporioides f. sp. aeschynomene. Appl Environ Microbiol. 1998;64(12):5030–2. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sitbon F, Astot C, Edlund A, Crozier A, Sandberg G. The relative importance of tryptophan-dependent and tryptophan-independent biosynthesis of indole-3-acetic acid in tobacco during vegetative growth. Planta. 2000;211(5):715–21. doi: 10.1007/s004250000338 . [DOI] [PubMed] [Google Scholar]
- 7.Mashiguchi K, Tanaka K, Sakai T, Sugawara S, Kawaide H, Natsume M, et al. The main auxin biosynthesis pathway in Arabidopsis. Proc Natl Acad Sci U S A. 2011;108(45):18512–7. doi: 10.1073/pnas.1108434108 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao Y. Auxin biosynthesis and its role in plant development. Ann Rev Plant Biol. 2010;61:49–64. doi: 10.1146/annurev-arplant-042809-112308 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szkop M, Bielawski W. A simple method for simultaneous RP-HPLC determination of indolic compounds related to bacterial biosynthesis of indole-3-acetic acid. Antonie van Leeuwenhoek. 2013;103(3):683–91. doi: 10.1007/s10482-012-9838-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsavkelova EA, Klimova SY, Cherdyntseva TA, Netrusov AI. Microbial producers of plant growth Stimulators and their practical use: A review. Prikl Biokhim Mikrobiol. 2006;42(2):117–26. . [PubMed] [Google Scholar]
- 11.Tudzynski B, Sharon A. Biosynthesis, biological role and application of fungal phytohormones In: Osiewacz HD, editor. The Mycota, Industrial Applications. Berlin: Springer-Verlag; 2002. p. 183–211. [Google Scholar]
- 12.Costacurta A, Vanderleyden J. Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol. 1995;21(1):1–18. doi: 10.3109/10408419509113531 . [DOI] [PubMed] [Google Scholar]
- 13.Patten CL, Glick BR. Bacterial biosynthesis of indole-3-acetic acid. Can J Microbiol. 1996;42(3):207–20. . [DOI] [PubMed] [Google Scholar]
- 14.Tsavkelova E, Oeser B, Oren-Young L, Israeli M, Sasson Y, Tudzynski B, et al. Identification and functional characterization of indole-3-acetamide-mediated IAA biosynthesis in plant-associated Fusarium species. Fungal genet Biol. 2012;49(1):48–57. doi: 10.1016/j.fgb.2011.10.005 . [DOI] [PubMed] [Google Scholar]
- 15.Basse CW, Lottspeich F, Steglich W, Kahmann R. Two potential indole-3-acetaldehyde dehydrogenases in the phytopathogenic fungus Ustilago maydis. Eur J Biochem. 1996;242(3):648–56. . [DOI] [PubMed] [Google Scholar]
- 16.Spaepen S, Vanderleyden J, Remans R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev. 2007;31(4):425–48. doi: 10.1111/j.1574-6976.2007.00072.x . [DOI] [PubMed] [Google Scholar]
- 17.Reineke G, Heinze B, Schirawski J, Buettner H, Kahmann R, Basse CW. Indole-3-acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumour formation. Mol Plant Pathol. 2008;9(3):339–55. doi: 10.1111/j.1364-3703.2008.00470.x . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gruen HE. Auxins and fungi. Annu Rev Plant Physiol. 1959;10:405–40. [Google Scholar]
- 19.Krause K, Henke C, Asiimwe T, Ulbricht A, Klemmer S, Schachtschabel D, et al. Biosynthesis and Secretion of Indole-3-Acetic Acid and Its Morphological Effects on Tricholoma vaccinum-Spruce Ectomycorrhiza. Appl Environ Microbiol. 2015;81(20):7003–11. doi: 10.1128/AEM.01991-15 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hilbert M, Voll LM, Ding Y, Hofmann J, Sharma M, Zuccaro A. Indole derivative production by the root endophyte Piriformospora indica is not required for growth promotion but for biotrophic colonization of barley roots. New Phytol. 2012;196(2):520–34. doi: 10.1111/j.1469-8137.2012.04275.x . [DOI] [PubMed] [Google Scholar]
- 21.Rao RP, Hunter A, Kashpur O, Normanly J. Aberrant synthesis of indole-3-acetic acid in Saccharomyces cerevisiae triggers morphogenic transition, a virulence trait of pathogenic fungi. Genetics. 2010;185(1):211–20. doi: 10.1534/genetics.109.112854 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun PF, Fang WT, Shin LY, Wei JY, Fu SF, Chou JY. Indole-3-acetic acid-producing yeasts in the phyllosphere of the carnivorous plant Drosera indica L. PloS One. 2014;9(12):e114196 doi: 10.1371/journal.pone.0114196 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooke TJ, Poli D, Sztein AE, Cohen JD. Evolutionary patterns in auxin action. Plant Mol Biol. 2002;49(3–4):319–38. . [PubMed] [Google Scholar]
- 24.Sztein AE, Cohen JD, Cooke TJ. Evolutionary patterns in the auxin metabolism of green plants. Int J Plant Sci. 2000;161(6):849–59. [Google Scholar]
- 25.Fu SF, Wei JY, Chen HW, Liu YY, Lu HY, Chou JY. Indole-3-acetic acid: A widespread physiological code in interactions of fungi with other organisms. Plant Signal Behav. 2015;10(8):e1048052 doi: 10.1080/15592324.2015.1048052 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kollath-Leiss K, Bonniger C, Sardar P, Kempken F. BEM46 Shows Eisosomal Localization and Association with Tryptophan-Derived Auxin Pathway in Neurospora crassa. Eukaryot Cell. 2014;13(8): 1051–1063. doi: 10.1128/EC.00061-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura T, Kawanabe Y, Takiyama E, Takahashi N, Murayama T. Effects of auxin and gibberellin on conidial germination in Neurospora crassa. Plant Cell Physiol. 1978;19(4):705–9. [Google Scholar]
- 28.Nakamura T MC, Ozaki Y, Saotome M, Murayama T. Effects of auxin and gibberellin on conidial germination and elongation of young hyphae in a cyclic 3′:5′ adenosine monophosphate-dependent protein kinase mutant of Neurospora crassa. Plant Growth Regul. 1988;7(3):201–7. [Google Scholar]
- 29.Tomita KM T. Nakamura T. Effects of Auxin and Gibberellin on Elongation of Young Hyphae in Neurospora crassa. Plant Cell Physiol. 1984;25(2):355–8. [Google Scholar]
- 30.Eswaramoorthy S, Bonanno JB, Burley SK, Swaminathan S. Mechanism of action of a flavin-containing monooxygenase. Proc Natl Acad Sci U S A. 2006;103(26):9832–7. doi: 10.1073/pnas.0602398103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zuther K, Mayser P, Hettwer U, Wu W, Spiteller P, Kindler BL, et al. The tryptophan aminotransferase Tam1 catalyses the single biosynthetic step for tryptophan-dependent pigment synthesis in Ustilago maydis. Mol Microbiol. 2008;68(1):152–72. doi: 10.1111/j.1365-2958.2008.06144.x . [DOI] [PubMed] [Google Scholar]
- 32.Phi QT, Park YM, Ryu CM, Park SH, Ghim SY. Functional identification and expression of indole-3-pyruvate decarboxylase from Paenibacillus polymyxa E681. J Microbiol Biotechnol. 2008;18(7):1235–44. . [PubMed] [Google Scholar]
- 33.Liu ZJ, Sun YJ, Rose J, Chung YJ, Hsiao CD, Chang WR, et al. The first structure of an aldehyde dehydrogenase reveals novel interactions between NAD and the Rossmann fold. Nat Struct Biol. 1997;4(4):317–26.. [DOI] [PubMed] [Google Scholar]
- 34.Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, et al. A high-throughput gene knockout procedure for Neurospora reveals functions for multiple transcription factors. Proc Natl Acad Sci U S A. 2006;103(27):10352–7. doi: 10.1073/pnas.0601456103 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Facchini PJ, Huber-Allanach KL, Tari LW. Plant aromatic L-amino acid decarboxylases: evolution, biochemistry, regulation, and metabolic engineering applications. Phytochemistry. 2000;54(2):121–38. . [DOI] [PubMed] [Google Scholar]
- 36.Pietrzak I. [Vitamin disturbances in chronic renal insufficiency. I. Water soluble vitamins]. Przegl Lek. 1995;52(10):522–5. . [PubMed] [Google Scholar]
- 37.Epstein CJ. Relation of protein evolution to tertiary structure. Nature. 1964;203:1350–2. . [DOI] [PubMed] [Google Scholar]
- 38.Roy A, Yang J, Zhang Y. COFACTOR: an accurate comparative algorithm for structure-based protein function annotation. Nucleic Acids Res. 2012;40:W471–7. doi: 10.1093/nar/gks372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bulfer S, Brunzelle J, Trievel R. Crystal structure of Saccharomyces cerevisiae Aro8, a putative α-aminoadipate aminotransferase. Protein Sci. 2013;22(10):1417–24. doi: 10.1002/pro.2315 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schutz A, Sandalova T, Ricagno S, Hubner G, Konig S, Schneider G. Crystal structure of thiamindiphosphate-dependent indolepyruvate decarboxylase from Enterobacter cloacae, an enzyme involved in the biosynthesis of the plant hormone indole-3-acetic acid. Eur J Biochem. 2003;270(10):2312–21. . [DOI] [PubMed] [Google Scholar]
- 41.Perez-Miller SJ, Hurley TD. Coenzyme isomerization is integral to catalysis in aldehyde dehydrogenase. Biochemistry. 2003;42(23):7100–9. doi: 10.1021/bi034182w . [DOI] [PubMed] [Google Scholar]
- 42.Tomita K, Kitsuwa T, Murayama T, Nakamura T. Identification of indol-3-acetic acid in Neurospora crassa. Agric Biol Chem. 1987;51:2633–4. [Google Scholar]
- 43.Gunasekaran M, Weber DJ. Auxin production of three phytopathogenic fungi. Mycologia. 1972;64(5):1180–3. . [PubMed] [Google Scholar]
- 44.Hütter R, Niederberger P, DeMoss JA. Tryptophan biosynthetic genes in eukaryotic microorganisms. Annu Rev Microbiol. 1986;40:55–77. doi: 10.1146/annurev.mi.40.100186.000415 . [DOI] [PubMed] [Google Scholar]
- 45.Manulis S, Haviv-Chesner A, Brandl MT, Lindow SE, Barash I. Differential involvement of indole-3-acetic acid biosynthetic pathways in pathogenicity and epiphytic fitness of Erwinia herbicola pv. gypsophilae. Mol Plant Microbe Interact. 1998;11(7):634–42. doi: 10.1094/MPMI.1998.11.7.634 . [DOI] [PubMed] [Google Scholar]
- 46.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215(3):403–10. doi: 10.1016/S0022-2836(05)80360-2 . [DOI] [PubMed] [Google Scholar]
- 47.Wheeler DL, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, et al. Database resources of the National center for biotechnology information. Nucleic Acids Res. 2006;34:D173–80. doi: 10.1093/nar/gkj158 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wheeler DL, Barrett T, Benson DA, Bryant SH, Canese K, Chetvernin V, et al. Database resources of the National center for biotechnology information. Nucleic Acids Res. 2007;35:D5–12. doi: 10.1093/nar/gkl1031 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC bioinformatics. 2004;5:113 doi: 10.1186/1471-2105-5-113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–7. doi: 10.1093/nar/gkh340 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nicholas K, Nicholas H, Deerfield D. GeneDoc: analysis and visualization of genetic variation. Embnew news. 1997;4(14). [Google Scholar]
- 52.Zhang Y. I-TASSER server for protein 3D structure prediction. BMC bioinformatics. 2008;9:40 doi: 10.1186/1471-2105-9-40 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18(15):2714–23. doi: 10.1002/elps.1150181505 . [DOI] [PubMed] [Google Scholar]
- 54.Westergaard M, Mitchell HK. Neurospora-V—a Synthetic Medium Favoring Sexual Reproduction. Am J Bot. 1947;34(10):573–7. [Google Scholar]
- 55.Lindegren CC. The Genetics of Neurospora. I. The Inheritance of Response to Heat- Treatment. B Torrey Bot Club. 1932;59:85–102. [Google Scholar]
- 56.Glickmann E, Dessaux Y. A critical examination of the specificity of the salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61(2):793–6. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ehmann A. The van urk-Salkowski reagent—a sensitive and specific chromogenic reagent for silica gel thin-layer chromatographic detection and identification of indole derivatives. J Chromatogr. 1977;132(2):267–76. . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Residues with 100% identity are shown in yellow font on a black background, residues with 75% identity are shown in black font on a red background, and residues with 50% identity are shown in black font on a green background. PLP binding residues are denoted by a pink star on top.
(PDF)
The black background with yellow font, red background with black font and green background with black fonts represent 100%, 75% and 50% identity of the residues, respectively. TPP binding residues are denoted by pink stars on top of the alignment.
(PDF)
Amino acid residues with black background yellow font represent 100% sequence identity; 75% identity is shown in black font on a red background, and 50% sequence identity is shown in black font on a green background. NAD serves as cofactor for the enzyme aldehyde dehydrogenase. NAD binding residues are denoted by a pink star the top of the residues.
(PDF)
MOX-2 or flavin monooxygenase is known to bind FAD through its nucleotide binding motif (GXGXXG). The GXGXXG motif is indicated by a bar, and conserved residues are highlighted with pink stars. “X” represents any amino acid. Residues in yellow font on a black background are 100% identical, and residues in black font on a red background are 75% identical. (the MOX-2 homolog in U. mydis was not found using the same BLAST search with the same template used for the other homologs.)
(PDF)
A black background with yellow font, red background with black font and green background with black font represents 100%, 75% and 50% identity of the residues, respectively. TPP binding residues are denoted by pink stars. A blue discontinuous symbol shows a discontinuity in sequence.
(TIF)
Amino acid residues in yellow font on a black background represent 100% sequence identity, in black font on a red background represent 75% identity and in black font on a green background represent 50% sequence identity. NAD serves as cofactor for the enzyme aldehyde dehydrogenase. NAD binding residues are indicated by a pink star on top of the residues.
(TIF)
MOX-2 or flavin monooxygenase is known to bind to FAD through its nucleotide binding motif (GXGXXG). GXGXXG motif is shown as a bar, and conserved residues are highlighted by pink stars. Residues in yellow font on a black background are 100% identical and residues, and residues in black font on a red background are 75% identical.
(TIF)
Predicted structures and known templates are shown in blue and gray, respectively. Residues present on predicted enzymes and templates are marked with orange and green, respectively. All structures are rendered as a ribbon. Key amino acid residues involved in ligand binding are rendered as a stick model. Residues those are at close vicinity are only highlighted. Ligands are shown as a ball and stick model. (a) Overall structural alignment of the predicted CBS-3 structure with the known enzyme structure (1NZX). NAD is bound inside enzymatic catalytic site. (b) Structural insight of the ligand binding site of both the predicted and 1NZX known structure. (c) Structural alignment of the predicted structure of flavin monooxygenase homolog from N. crassa with the known flavin monooxygenase structure (1VQW). (d) Characterization of the ligand binding site of the predicted enzyme using the 1VQW structure as a template.
(TIF)
Lower panel of the figure with numbers denotes the standard peaks of the various indolic compounds. Except indole-3-lactic acid (ILA) produced by Δcfp strain, no other indolic compounds comparable to the standard peaks were found within the time window set for the analysis. Genes that were analyzed from different pathways are as follows: aro-8aro-8aro-8, cfp, cbs-3, mox-2, lao-2 (tryptophan-2-monooxygenase) and naa-2 (indole-3-acetamide hydrolase). lao-2 and naa-2 are involved in IAM pathway of IAA biosynthesis. Standard peaks are: lane 1—tryptophan, lane 2—indole-3-lactic acid, lane 3—tryptamine, lane 4—indole-3-acetamide, lane 5—indole-3-acetic acid, lane 6—tryptophol, lane 7 -indole-3-acetonitrile.
(TIF)
Different tryptophan concentrations ranging from 2 mM to 12.5 μM were used.
(PDF)
Two samples, namely, S1 and S8, were confirmed. S1 is a double knock-out strain for genes lao-2 and naa-2, and S8 is a double knock-out strain for genes lao-2 and aro-8. Lane 1 and 2 were loaded with the PCR products of 5’ UTR amplifications of lao-2 and naa-2 ORFs, respectively, along with the hph cassette. Lane 3 and 4 were loaded with the PCR products of mid-part of lao-2 and naa-2 gene ORFs respectively. Lane 5 and 6 were loaded with the PCR products of 5’ UTR amplifications of lao-2 and aro-8 ORFs, respectively, along with the hph cassette. Lane 7 and 8 were loaded with the PCR products of mid-part of lao-2 and aro-8 ORFs respectively. Lane 9, 10 and 11 were loaded with the PCR products of mid-part of lao-2, naa-2 and aro-8 ORFs, respectively, from wild type strain (wt). M—DNA marker.
(TIF)
Graphs with different colors represent different knock-out strains. A specific graph for a particular knock-out strain is mentioned in the figure with color bars. Standard peaks are ILA: indole-3-lactic acid, IAA: indole-3-acetic acid and TOL: tryptophol.
(TIF)
Changes in IAA and indolic compounds metabolism over time in wild type and mutant N. crassa supplemented with 1.96 mM tryptophan. (A) Wild type N. crassa strain was grown with tryptophan supplementation, and samples were collected in every 12 hours for a total of 96 hours. The following standards were used: tryptophol (TOL), tryptamine (TAM), indole-3-acetamide (IAM), indole-3-acetic acid (IAA), tryptophan (Trp), indole-3-pyruvic acid (IPA) and indole-3-lactic acid (ILA). (B) Δcfp knock-out strain was grown with tryptophan supplementation. At every 12-hour interval, samples were collected. Black arrow marks indicate ILA spots. The following standards were used: tryptophol (TOL), indole-3-acetamide (IAM), indole-3-acetic acid (IAA), tryptophan (Trp), indole-3-pyruvic acid (IPA) and indole-3-lactic acid (ILA). (C) The Δcbs-3Δahd-2 double knock-out strain was grown with tryptophan supplementation. At every 12-hour interval, samples were collected and analyzed. Black arrow marks indicate tryptophan spots. The following standards were used: tryptophol (TOL), indole-3-acetamide (IAM), indole-3-acetic acid (IAA), tryptophan (Trp), indole-3-pyruvic acid (IPA), and indole-3-lactic acid (ILA). (D) HPLC analysis of 48 hours old wild type and Δcbs-3;Δahd-2 double knock-out N. crassa culture supplemented with tryptophan. After 48 hours of inoculation Δcbs-3;Δahd-2 double knock-out strain shows a peak for tryptophan while corresponding peak from wild type strain has not been observed.
(TIF)
Different indolic compounds were supplemented to wild type N. crassa culture media for checking their role in IAA production over time. tryptophan (Trp), tryptamine (TAM), indole-3-acetamide (IAM) and indole-3-acetic acid (IAA) were used for the assay. Samples were collected in 24-hour intervals. The numbers below the lanes represent the time in hours. Following standards were used: tryptamine (TAM), indole-3-acetamide (IAM), indole-3-acetic acid (IAA), tryptophan (Trp), and indole-3-pyruvic acid (IPA).
(TIF)
Fungal culture was supplemented with 2 mM histidine. Lane 1: Fungal culture was supplemented with 1.96 mM of tryptophan. Lane 2: Fungal culture was supplemented with 1 mM of tryptophan. Lane 3: Fungal culture was supplemented with 2 mM of histidine. IAA: indole-3-acetic acid standard.
(TIF)
(PDF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.