Abstract
Climate changes, including chronic changes in precipitation amounts, will influence plant physiology and growth. However, such precipitation effects on switchgrass, a major bioenergy crop, have not been well investigated. We conducted a two-year precipitation simulation experiment using large pots (95 L) in an environmentally controlled greenhouse in Nashville, TN. Five precipitation treatments (ambient precipitation, and -50%, -33%, +33%, and +50% of ambient) were applied in a randomized complete block design with lowland "Alamo" switchgrass plants one year after they were established from tillers. The growing season progression of leaf physiology, tiller number, height, and aboveground biomass were determined each growing season. Precipitation treatments significantly affected leaf physiology, growth, and aboveground biomass. The photosynthetic rates in the wet (+50% and +33%) treatments were significantly enhanced by 15.9% and 8.1%, respectively, than the ambient treatment. Both leaf biomass and plant height were largely increased, resulting in dramatically increases in aboveground biomass by 56.5% and 49.6% in the +50% and +33% treatments, respectively. Compared to the ambient treatment, the drought (-33% and -50%) treatments did not influence leaf physiology, but the -50% treatment significantly reduced leaf biomass by 37.8%, plant height by 16.3%, and aboveground biomass by 38.9%. This study demonstrated that while switchgrass in general is a drought tolerant grass, severe drought significantly reduces Alamo’s growth and biomass, and that high precipitation stimulates its photosynthesis and growth.
Introduction
Due to fossil fuel combustion and land-use change, global climate change has been accelerating over the past decades [1]. Global land surface temperature is expected to increase 1.1–6.4°C by the end of the century. The increase in temperature affects the hydrological cycle, and causes more extreme precipitation events [2]. For example, Easterling et al. [3] reported that intensity of precipitation during the growing seasons could increase, resulting in more droughts and flooding in the United States (US). Intense droughts and excessive flooding in California are projected to increase by at least 50% by the end of the 21st century [4]. Changes in precipitation intensities will alter soil water availability and influence ecosystem productivity and biomass [5–11].
To reduce both fossil fuel dependence and greenhouse gas emissions, bioenergy/biofuel crops are promoted as part of the solutions [12–14]. The U.S. Energy Independence and Security Act (EISA) of 2007 mandates at least 36 million gallons of biofuel production a year to displace gasoline by 2022. The U.S. Department of Energy estimated that 907 million Mg of biomass are needed annually to replace 30% of the 2004 petroleum consumption in the US by 2030 [15]. Since cellulosic biofuel crops often grow in less productive soils, and require few inputs of water, fertilizer, and pesticides, the demand of cellulosic biofuels such as switchgrass is increasing [16–19].
Switchgrass (Panicum virgatum L.) is a perennial C4 grass widely distributed from southern Canada to the US and Mexico. It is one of the most dominant grass species within the tallgrass prairie ecosystem [20,21]. The characteristics of switchgrass that make it more attractable than other grass species include the production of high biomass, high nutrient use efficiencies, tolerant of a broad range of environmental conditions, and ability to sequester atmospheric carbon in the soil [18,22,23]. For example, Schmer et al. [23] reported that the annual switchgrass biomass averaged 5.2–11.1 Mg ha-1. Biomass production of switchgrass may be influenced by many factors, such as soil nutrients, varieties, and climatic factors like precipitation [24–27].
Research on switchgrass has been conducted over the past decades, particularly on variety comparisons, field management schemes, nutrient limitation responses, and life cycle assessments [21,28]. Physiological studies have been mostly limited to the study of differences among switchgrass cultivars and different agricultural practices [25, 29–32]. Different switchgrass varieties show different leaf photosynthetic rates, ranging from 25.4 to 35.4 μmol CO2 m-2s-1 [33]. Compared to other crop types, the effects of climate change, such as water stresses on switchgrass, have not been well investigated [21,34,35]. Some studies compared differences in annual precipitation and temperature and found that inter-annual precipitation influences soil water availability and then the physiology of switchgrass [21,29]. The soil water stress significantly reduced switchgrass aboveground biomass, establishment rates of stands, and physiological responses [36–39]. But few experimental studies have been conducted to investigate the responses of switchgrass physiology and growth to climate changes [21,35,39].
Precipitation is a very important factor influencing ecosystem productivity and biomass [5,6,32,34,40–42]. This study was designed to determine the effects of sustained precipitation changes on leaf physiology, growth, and biomass of switchgrass. Specifically, we tested 1) whether there were significant effects of the precipitation treatments on switchgrass physiology, growth, and biomass? and 2) how were switchgrass biomass related to plant physiological and environmental factors?
Materials and methods
Experimental facility and design
The experiment was conducted in an environmentally controlled greenhouse at Tennessee State University Agricultural Research and Demonstration Center (Latitude 36.12'N, Longitude 86.89'W, Elevation 127.6 m) in Nashville, Tennessee [43]. Roof panels on the greenhouse opened automatically during clear days and closed during rain. Wall panels also opened automatically to further regulate the temperature, and closed when the temperature was below 20°C. The temperature in the greenhouse was controlled by a Wadsworth Step Up Control system (Arvada, CO). Panels were controlled by a Micro Grow Commercial Greenhouse Control (Temecula, CA) with inputs from rain and wind speed sensors. Temperature varied during the day and night. Light in the greenhouse averaged about 80% of full sunlight.
Switchgrass was grown in large pots (95 L, 50 cm Diameter and 50 cm Height) placed on the greenhouse floor. There were holes in the bottom of the pots to allow free draining. Pots were filled with top soil from an Armour silt loam soil, with pH = 6.2 and low in phosphorus and potassium. No fertilizer was applied during this study. Seeds of “Alamo” switchgrass were planted in a field plot in April 2011, and two-year old switchgrass plants with two to three tillers were transplanted in the large pots in May, 2013. Five plants were planted in each pot with one in the center. Plants were harvested three times during each growing season, at the end of April, July, and October each year. Thus, the whole growing season was separated into three harvest periods (February-April; May-July; August-October).
Five simulated precipitation treatments were applied during the 2014 and 2015 growing seasons in a completely randomized block design with five blocks. Precipitation treatments were defined relative to an ambient precipitation treatment, which applied the annual amount and monthly distribution of rainfall from 1969, which typified the amount and seasonality of precipitation over the past 100 years (1903–2012) for Nashville, TN. Two drought treatments (-33% and -50% of ambient precipitation), and two wet treatments (+33%, and +50% of ambient precipitation) were also used. For the ambient precipitation, monthly precipitation varied from 6.12 cm in May to 15.57 cm in October with a mean monthly precipitation of 9.80 cm (Annual precipitation amount was 1176 cm; S1 Fig). Precipitation treatment applications were automated using a watering timer controller (RSC600i, Raindrip, Inc., Woodland Hills, CA). In 2013, the ambient level treatment was applied to all pots to minimize water stress during establishment. The precipitation treatments began on February 01, 2014. In 2014, pots were watered every three days, three times each day at midnight, in the early morning and later afternoon. Application amounts were adjusted monthly to match monthly variation in precipitation. In 2015, water was added twice each day at midnight and in the early morning, with the same total monthly precipitation amount. In June and July of 2015, we had two incidents where several pots received natural precipitation because the rain sensor failure prevented closing of the roof vents. To compensate for this additional irrigation water, we reduced irrigation in affected pots.
Measurements
Soil temperature and moisture sensors were buried at 20 cm depth in each pot to continuously monitor soil temperature and moisture using the Watermark Monitor 900M (Irrometer Inc., Riverside, CA). The data were recorded every hour. The soil moisture sensor measures soil matric potential in centibar (cb), which is equal to kilopascal (kPa), over a range of 0 to -239 cb. The larger cb number means the higher soil water content [44].
Maximum leaf photosynthetic rate, stomatal conductance, and transpiration were measured five times during each harvest period using a Li-6400XT Portable Photosynthesis System (Li-Cor, Inc., Lincoln, NE). The fully expanded young leaves of four or five selected tillers in each pot were measured between 10:00am and 3:00pm. Leaf chamber photosynthetic photon flux density was set at 2000 μmol m-2s-1. Reference CO2 was set at ambient CO2 concentration in the greenhouse at the measurement (~400 ppm). Temperature was not controlled during the measurements. Measurements were conducted biweekly. Leaves were randomly selected each time for measurements. Instantaneous water use efficiency (WUEi) was calculated as a ratio of leaf photosynthesis and transpiration. Leaf temperature was measured at the same time.
The maximum height, average height, and number of tillers in each pot were measured at the end of each harvest period. Maximum height was the measured of the tallest tiller and the average tiller height was measured by averaging five tillers in each pot. Biomass was measured every period after harvesting the aboveground tillers in the pot, dried at 75°C for more than 24 hr to constant mass, and weighed. All plants in the pots were harvested each time. Due to relatively small areas in pots, we did not harvest and measure belowground biomass. Aboveground biomass was reported on a dry basis. Leaf and stem biomass were separately measured and leaf:stem ratio was calculated. Biomass-based WUE (WUEb) was calculated as a ratio of aboveground biomass and total water amount applied during each harvest period.
Statistical analysis
Data analysis was performed using SAS software 9.3 (SAS Inc. Cary, NC) [45]. The effects of precipitation treatment, year, harvest period, and block on soil moisture, soil temperature, leaf physiology, plant height, number of tillers, and aboveground biomass were analyzed using repeated measure analysis of variance (ANOVA). When a significant effect at α = 0.05 level was detected, least significant difference (LSD) was used for multiple comparisons. Regression analysis was conducted to develop the relationships among photosynthesis, transpiration, WUE, number of tillers, tiller height, aboveground biomass, soil temperature, and soil moisture. Bivariate regression was first used to detect the relationships between two variables; then stepwise multiple regression was applied to derive the optimal regression models for physiology, growth, and biomass of switchgrass under all precipitation treatments.
Results
Switchgrass physiological variables and growth before the precipitation treatments
In 2013 before the precipitation treatments were applied, there were no significant effects in leaf photosynthesis, stomatal conductance, and tiller growth among treatment plots (S1 Table). Only blocks showed significant effects. We set blocks that paralleled to the greenhouse side wall. The significant block effects indicated that the potential effects of the environmental differences due to pot settings could be partitioned by the block arrangements. Further analyses focused only on the data collected after the precipitation treatments.
Seasonal variations of soil temperature and moisture among precipitation treatments
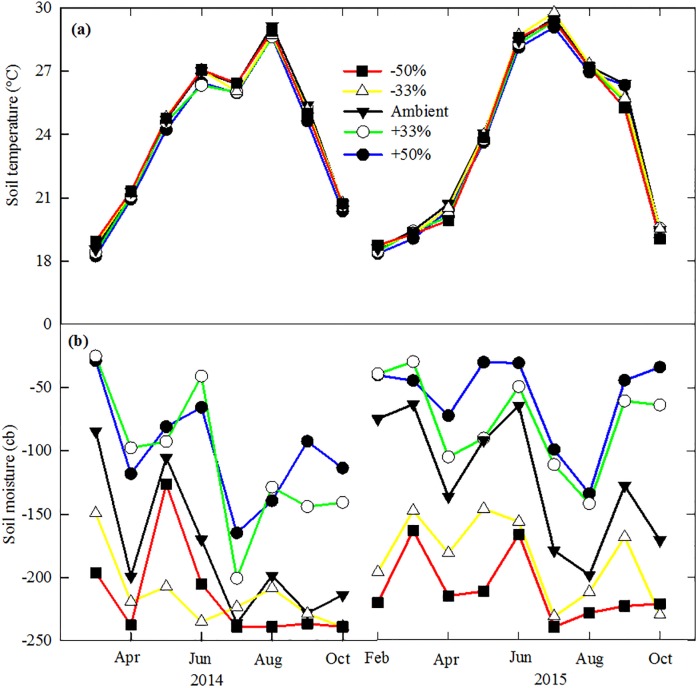
The ANOVA test showed that there were significant differences in soil moisture among the precipitation treatments, years and blocks, but no significant difference in soil temperature among the precipitation treatments (Table 1). Soil moisture decreased with the decrease in the amount of water applied. The soil moisture in the +50% treatment was the highest and the -50% precipitation treatments had the lowest soil moisture (Table 2). No difference in soil moisture between the -33% and -50% treatments was detected. Mean soil temperature was about 23°C for all treatments (Table 2).
Table 1. Significance of the effects of precipitation treatments, year, their interaction, and block on soil moisture and temperature using ANOVA.
Numbers are F values. Stars indicate the level of significance (* = p<0.05, ** = p<0.01).
| Source | Soil Moisture (cb) | Soil Temperature (°C) |
|---|---|---|
| Block | 5.27** | 23.55** |
| Precipitation | 128.19** | 1.33 |
| Year | 51.74** | 5.12* |
| Precipitation*Year | 1.57 | 0.65 |
Table 2. Multiple comparisons of soil moisture and soil temperature under five precipitation treatments.
Same letters indicate no significant difference in a column.
| Precipitation Treatment | Soil Moisture (cb) | Soil Temperature (°C) |
|---|---|---|
| +50% | -81.03±5.26a | 23.72±0.30a |
| +33% | -92.90±5.39b | 23.78±0.28a |
| Ambient | -149.98±5.78b | 24.09±0.29a |
| -33% | -200.12±4.00c | 23.95±0.29a |
| -50% | -206.73±4.04c | 23.84±0.29a |
Soil temperature showed strong seasonal variations among all treatments in both growing seasons (Fig 1a). The mean monthly soil temperature ranged from 18.6°C to 29.4°C during the growing seasons, with the highest temperature appeared in July or August. Soil moisture also varied seasonally following the precipitation pattern (Fig 1b; S1 Fig), and in the ambient precipitation treatment ranged from -63 to -236 cb during the growing seasons. Soil moistures in the -33% and -50% treatments were below -145 cb, and were mostly above -145 cb in the +33% and +50% treatments.
Fig 1. Monthly mean soil temperature and soil moisture in each precipitation treatment from February to October in 2014 and 2015.
Seasonal variations of leaf photosynthesis and transpiration of different precipitation treatments
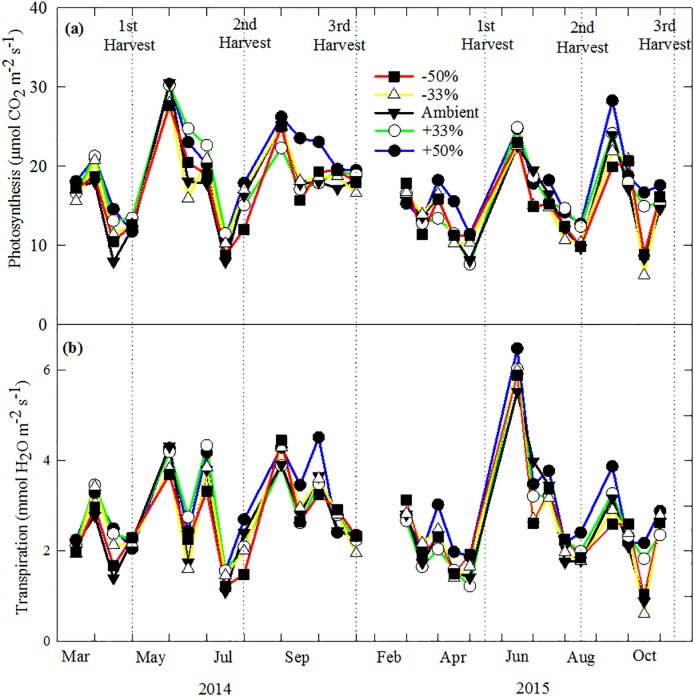
The seasonal patterns in leaf photosynthesis and transpiration rates were similar for all treatments. The highest rates occurred in new tillers following each harvest, and rates declined until the next harvest (Fig 2). At each measurement time, the +50% and +30% treatments tended to have high values in leaf photosynthesis and transpiration rates than other treatments. Compared to the ambient precipitation treatment, the +50% treatment enhanced the photosynthesis mostly by 21.3% and transpiration by 22.0%.
Fig 2. Monthly mean photosynthesis and transpiration in each precipitation treatment from February to October in 2014 and 2015.
Overall effects of precipitation treatment, harvest period, year, and their interactions on switchgrass physiology, growth, and biomass
Results of ANOVA showed that precipitation treatments, harvest periods, and growing season had significant effects on most of plant physiological variables, plant growth, stem biomass, and aboveground biomass (Table 3). Leaf biomass and leaf:stem ratio were significantly influenced by precipitation treatments, harvest period, but did not change between years. WUEi (a measure of carbon fixed relative to water transpired) did not change among the precipitation treatments, and number of tillers, and height were not different between the two years. The interactive effects of precipitation treatment and harvest period were significant for photosynthesis, stomatal conductance, number of tillers, height, leaf biomass, stem biomass, and aboveground biomass. Significant interactive effects between precipitation treatments and growing season year were found for stomatal conductance.
Table 3. Significance of the effects of treatment, harvest period, their interactions, and block on leaf physiology, growth, and biomass using ANOVA in two years.
| Source | Pn | gs | E | WUEi | WUEb | Ntiller | Hmax | Hmean | Babove | BLeaf | BStem | LS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Block | 1.12 | 2.51* | 4.21** | 6.97** | 10.92** | 1.67 | 22.39** | 36.75** | 12.48** | 7.78** | 11.60** | 2.91* |
| Precipitation | 16.31** | 5.32** | 8.63** | 0.81 | 2.83* | 4.02** | 67.57** | 65.06** | 36.61** | 21.58** | 32.87** | 2.47* |
| Year | 138.67** | 67.42** | 10.71** | 42.30** | 19** | 24.56** | 1.04 | 3.10 | 7.41** | 0.06 | 8.32** | 2.11 |
| Harvest | 94.32** | 93.14** | 89.34** | 15.29** | 117.14** | 33.04** | 284.52** | 235.58** | 152.01** | 65.02** | 190.86** | 34.57** |
| Precipitation x Harvest | 2.14* | 2.81** | 0.89 | 0.37 | 1.59 | 3.23* | 26.13** | 18.94** | 3.99** | 2.68** | 4.13* | 1.53 |
| Precipitation x Year | 0.85 | 2.58* | 1.02 | 0.70 | 0.67 | 0.38 | 0.56 | 0.37 | 0.17 | 0.45 | 0.08 | 0.34 |
Pn: Leaf maximum photosynthesis (μmol CO2 m-2s-1); gs: Stomatal conductance (mol H2O m-2s-1); E: Transpiration (mmol H2O m-2s-1); WUEi: Instantaneous water use efficiency (μmol mmol-1); WUEb: Biomass-based water use efficiency (g L-1); Ntiller: Number of tillers; Hmax: Maximum plant height (cm); Hmean: Mean plant height (cm); Babove: Aboveground biomass (g pot-1); Bleaf: Leaf biomass (g pot-1); Bstem: Stem biomass (g pot-1); LS: Leaf and stem biomass ratio. Numbers are F values. Stars indicate the level of significance (* = p<0.05, ** = p<0.01).
Effects of precipitation treatment on switchgrass physiology, growth, and biomass
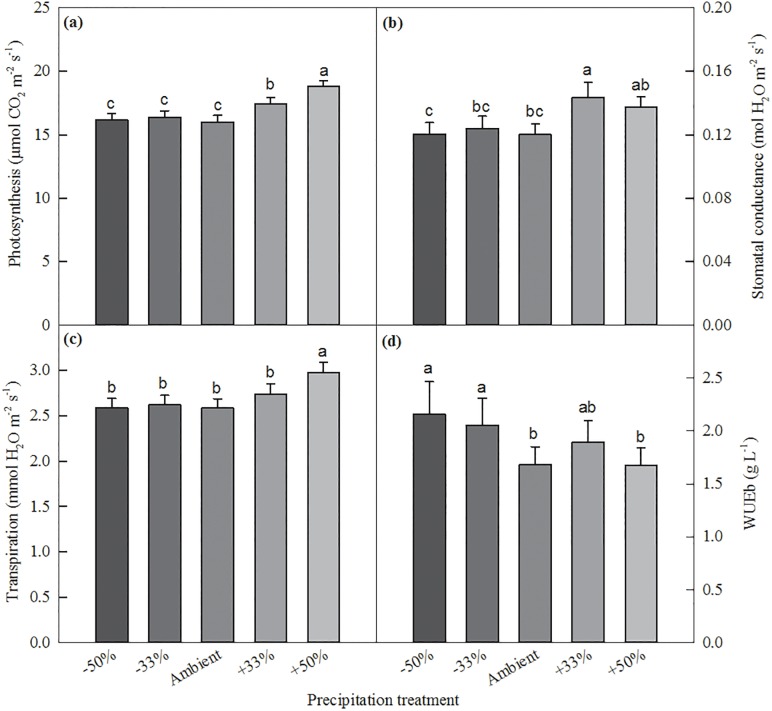
The precipitation treatment significantly influenced most of the physiological variables measured (Table 3). Among all treatments, the +50% treatment had the highest photosynthesis rate (18.74 μmol CO2 m-2s-1) (Fig 3a), 15.9% higher than the ambient treatment. The +33% treatment had lower photosynthesis than the +50% treatment, but was 8.1% higher than the ambient treatment. No difference in photosynthesis was found between the ambient precipitation and two drought treatments. For stomatal conductance, the +33% treatment had significant higher value than the -50 treatment, but there was no difference among other three treatments (Fig 3b). Similar response pattern of transpiration was found as photosynthesis (Fig 3c). As a result, WUEi wasn’t influenced by the precipitation treatment. WUEb was slightly higher in the drought treatments than the ambient precipitation (Fig 3d). The wet treatments did not influence WUEb.
Fig 3. Multiple comparisons of leaf photosynthesis, stomatal conductance, transpiration, and water use efficiency among different precipitation treatments.
Differences of variables among treatments labeled with the same letter are not significant at α = 0.05 level.
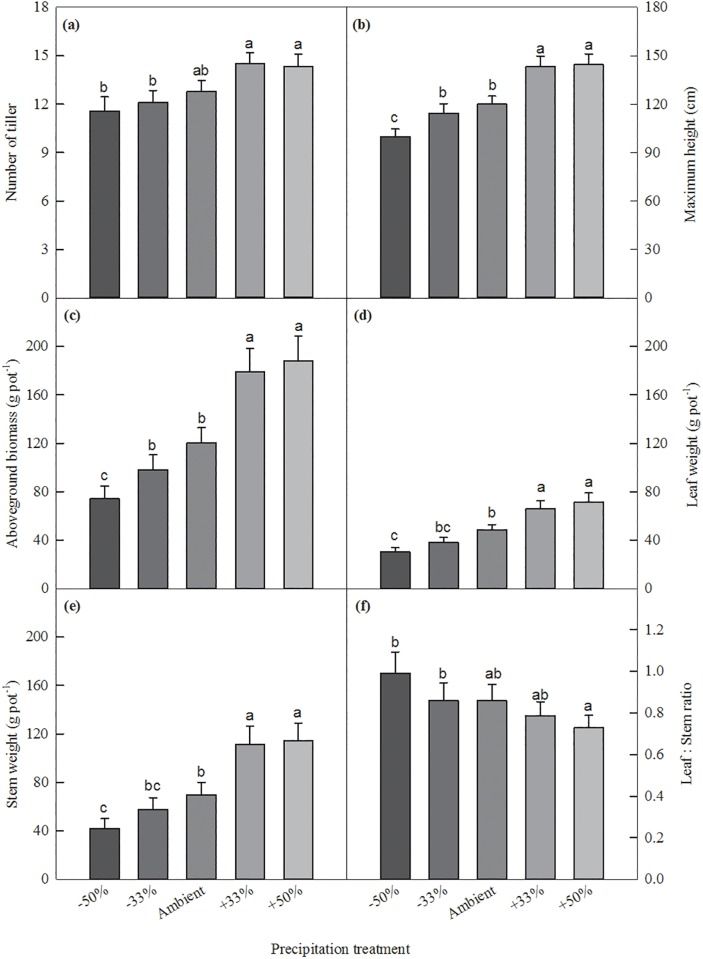
Precipitation significantly influenced plant growth and biomass (Table 3). Compared to the ambient precipitation, the wet (+30% and +33%) treatments did not influence the number of tillers produced, but the two drought (-50% and -33%) treatments produced significantly lower number of tillers (Fig 4a). The plants in the wet treatments grew significantly taller (144.2 cm, 20.2%) than the ambient precipitation, and the -50% treatment significantly reduced plant height (100.7 cm, 16.3%). Aboveground biomass was increased by 56.7% to 185.8 g pot-1 in the +50% treatment, by 49.6% in the +33% treatment, and reduced by 38.9% to 72.5 g pot-1 in the -50% treatment, compared to the ambient precipitation (118.6 g pot-1; Fig 4c). Both leaf and stem biomass were increased in the wet treatments, and the severe drought -50% treatment significantly reduced the leaf and stem biomass (Fig 4d and 4e). Leaf biomass and stem biomass were increased by 46.6% and 63.8% in the +50% treatment, and reduced by 37.8% and 39.9% in the -50% treatment, respectively. Leaf:stem ratio in the drought treatments was significantly higher than the +50 treatment (Fig 4f). But no difference in leaf:stem ratio was found between the ambient precipitation with either the drought or wet treatment.
Fig 4. Multiple comparisons of number of tillers, maximum height, and biomass of leaf, stem and total plant, and leaf:stem ratio among different precipitation treatments.
Differences of variables among treatments labeled with the same letter are not significant at α = 0.05 level.
Effects of harvest period on switchgrass physiology, plant growth, and biomass
Significant differences for leaf physiology, growth, and biomass were observed among the three harvest periods (Table 4). The maximum leaf photosynthesis, stomatal conductance and WUEi were higher in the 3rd harvest period (August-November), but height of plants were significantly higher in the 1st (February-April) and 2nd (May-July) harvests. The highest leaf transpiration and WUEb appeared during the 2nd harvest period. Leaf biomass, stem biomass, and aboveground biomass were higher in the 2nd harvest period, and much lower in the 3rd harvest. Leaf:stem ratio was lower in the 2nd harvest period than other two periods.
Table 4. Multiple comparisons of leaf physiology, growth, and biomass of switchgrass among three different harvest periods.
| Harvest Period | Pn | gs | E | WUEi | WUEb | Ntiller | Hmax | Hmean | Babove | BLeaf | BStem | LS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feb-Apr | 14.61c | 0.093c | 2.27c | 6.67b | 1.91b | 9.53b | 140.0a | 85.58a | 125.30b | 59.30b | 66.94b | 0.97a |
| May-Jul | 17.78b | 0.132b | 3.03a | 6.59b | 2.98a | 14.49a | 144.4a | 84.52a | 213.98a | 70.31a | 146.45a | 0.54b |
| Aug-Oct | 18.95a | 0.165a | 2.83b | 7.25a | 0.78c | 15.00a | 90.2b | 49.27b | 56.71c | 24.66c | 26.41c | 1.03a |
Pn: Leaf maximum photosynthesis (μmol CO2 m-2s-1); gs: Stomatal conductance (mol H2O m-2s-1); E: Transpiration (mmol H2O m-2s-1); WUEi: Instantaneous water use efficiency (μmol mmol-1); WUEb: Biomass-based water use efficiency (g L-1); Ntiller: Number of tillers; Hmax: Maximum plant height (cm); Hmean: Mean plant height (cm); Babove: Aboveground biomass (g pot-1); Bleaf: Leaf biomass (g pot-1); Bstem: Stem biomass (g pot-1); LS: Leaf and stem biomass ratio. Means followed by the same letter in a column are not significantly different at the α = 0.05 level.
Variations in switchgrass physiology, plant growth and biomass between the two years
All variables measured showed significant differences between two growing seasons. Leaf photosynthesis, transpiration, WUEi and WUEb were higher in 2014 than in 2015 (Table 5). Plant heights and leaf:stem ratio were similar in two years. The aboveground biomass, leaf and stem biomass in 2014 were also significantly higher than these in 2015.
Table 5. Multiple comparisons of leaf physiology, growth and biomass of switchgrass.
| Year | Pn | gs | E | WUEi | WUEb | Ntiller | Hmax | Hmean | Babove | BLeaf | Bstem | LS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 18.42a | 0.144a | 2.80a | 7.08a | 2.15a | 11.52a | 125.42a | 74.10a | 142.05a | 54.50a | 86.77a | 0.81a |
| 2015 | 15.57b | 0.112b | 2.64b | 6.49b | 1.64b | 14.57b | 124.16a | 71.98a | 121.95b | 49.90b | 72.64b | 0.87a |
Pn: Leaf maximum photosynthesis (μmol CO2 m-2s-1); gs: Stomatal conductance (mol H2O m-2s-1); E: Transpiration (mmol H2O m-2s-1); WUEi: Instantaneous water use efficiency (μmol mmol-1); WUEb: Biomass-based water use efficiency (g L-1); Ntiller: Number of tillers; Hmax: Maximum plant height (cm); Hmean: Mean plant height (cm); Babove: Aboveground biomass (g pot-1); Bleaf: Leaf biomass (g pot-1); Bstem: Stem biomass (g pot-1); LS: Leaf and stem biomass ratio. Means followed by the same letter in a column are not significantly different at the α = 0.05 level.
Relationships among aboveground biomass, leaf physiological variables, soil temperature, and soil moisture of different precipitation treatments
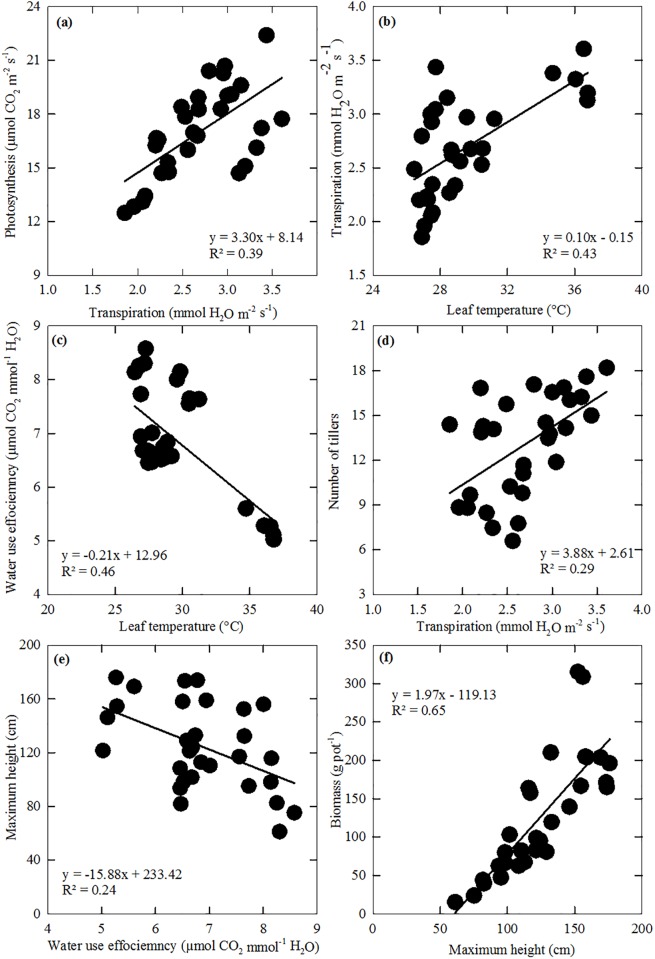
Bivariate regression results showed that leaf photosynthesis was significantly correlated with transpiration (Fig 5a), similar to previous studies [21,46,47]. Transpiration increased with leaf temperature (Fig 5b) and WUEi was negatively correlated to transpiration (Fig 5c). Tiller number was positively influenced by transpiration (Fig 5d), but height was negatively related to WUEi (Fig 5e). A strong positive relationship was found between biomass and plant height (Fig 5f).
Fig 5. Relationships among leaf photosynthesis, transpiration, water use efficiency (WUEi), number of tillers, plant height, biomass, leaf temperature, soil temperature, and soil moisture under all precipitation treatments.
Sample size n = 30. All models are significant at α = 0.05 level.
Multiple regression analysis showed that leaf photosynthesis was correlated to transpiration, positively related to soil moisture, but negatively related to leaf temperature (Table 6). Transpiration was correlated to photosynthesis, and positively related to leaf temperature. WUEi was negatively related to leaf temperature and positively related to soil temperature. The number of tillers increased with WUEi and soil moisture. Plant height was positively related to soil moisture, but negatively to number of tillers and WUEi. Aboveground biomass was positively influenced by WUEi, plant height, and soil temperature, but negatively influenced by leaf temperature.
Table 6. Relationships of leaf physiological variables, growth, and biomass with soil temperature, soil moisture, and other variables.
| Response variable | Model | R2 |
|---|---|---|
| Photosynthesis | Pn = 17.60+4.98E-0.72Tl+0.29Ts | 0.81* |
| Transpiration | E = -2.36+0.12Pn+0.10Tl | 0.86** |
| WUEi | WUEi = 12.61–0.28Tl+0.11Ts | 0.54** |
| Number of tillers | Ntiller = 4.53+4.21E+0.018M | 0.42** |
| Maximum Height | Hmax = 374.6+0.30M -23.59WUEi-3.20Ntiller | 0.59** |
| Aboveground Biomass | Babove = -563.20+0.18M+24.93WUEi+10.43Ts+2.33Hmax | 0.95** |
Pn: Leaf maximum photosynthesis (μmol CO2 m-2s-1); E: transpiration (mmol H2O m-2s-1); WUEi: water use efficiency (μmol mmol-1); Ntiller: number of tillers; Hmax: maximum plant height (cm); Babove: Aboveground biomass (g pot-1); Tl: leaf temperature (°C); Ts: soil temperature (°C); M: soil moisture (cb); R2: coefficient of determination. Stars indicate the level of significance (* = p<0.05, ** = p<0.01).
Discussion
The primary objective of this study was to understand the physiological variables and growth responses of switchgrass to precipitation changes. We found that increased precipitations significantly enhanced plant aboveground biomass by stimulating leaf photosynthesis, but mostly, by increasing leaf biomass, stem biomass and height, compared to the ambient precipitation. The significantly reduced aboveground biomass in the severe drought (-50%) treatment was caused by the reduced growth of leaf and stem, not by leaf photosynthesis changes. Among all precipitation treatments, leaf photosynthesis and transpiration were significantly correlated and influenced by temperature. Plant growth and biomass were influenced by soil moisture and water use efficiency. This study provided direct evidence that sustained precipitation changes could have significant impacts on switchgrass physiology and growth, and severe drought would significantly reduce switchgrass growth and biomass. It is worth noting that biomass yield of switchgrass Alamo could be negatively affected by water logging due to heavy precipitation in poorly drained fields [48,49].
In this study, we found that drought treatments reduced plant growth and biomass compared to the ambient precipitation treatment, but did not change much of leaf photosynthesis and transpiration rates, particularly under the -33% treatment (Fig 3). The reduced growth and biomass of switchgrass under the drought treatments have been reported in previous studies. For example, Sanderson & Reed [29] and Hartman et al. [21] found that switchgrass tiller height, tiller number, and aboveground biomass were reduced by water deficits. Barney et al. [50] also reported that drought treatments reduce tiller number, leaf area, and biomass production by up to 80%. Wang et al. [51] found that leaf photosynthesis was not influenced by drought treatment when leaf water potential was larger than -1 Mpa, but decreased with decreasing leaf water potential when it was lower than -1 Mpa. In this study, we did not find significant differences in tiller number between the drought and ambient precipitation treatments, but leaf biomass, stem biomass, and height of switchgrass were significantly reduced in the severe drought treatment.
Lower levels of precipitation amounts and soil moisture contents often limit the stomatal conductance leading to the photosynthesis rate decreases, and finally decrease plant height and biomass [30]. However, we did not find any significant differences in leaf photosynthesis, transpiration and WUEi between the drought treatments and the ambient precipitation (Fig 3). This seemed to be contradictory to some previous studies, as leaf photosynthetic rate is often reduced under the drought treatments. For example, Barney et al. [50] showed a 50% reduction in switchgrass leaf photosynthetic rate when the soil water potential was below -1.5 MPa. Knapp [52] reported that under severe water stress, switchgrass photosynthesis decreases dramatically. The different responses in this study could be related to our growing conditions and harvests. Temperature in the greenhouse was higher than the outside field and plants had extended growing seasons. During the whole growing seasons, plants were harvested three times. The differences in leaf photosynthesis among precipitation treatments tended to be smaller when plants were very young or before harvests. In addition, biomass may have a poor relationship with photosynthetic rate (carbon gain) in drought treatments [21,53]. A recent study of switchgrass genotypes growing under different temperatures showed that variations in leaf-level photosynthesis may not scale up to final biomass yield, as leaf area, leaf architecture, and canopy development could contribute to final biomass yield [54].
The increased precipitation treatments increased photosynthesis, transpiration, maximum tiller height and biomass, compared to the ambient precipitation treatment (Fig 3), similar to some precious studies [55,56]. Plants in the wet treatments had higher photosynthetic rates, grew taller, and produced more biomass compared to those in the ambient precipitation treatment (Table 2). Hartman et al. [21] found that increased precipitation increased leaf photosynthesis and stomatal conductance in early growing seasons, but not in the middle or late seasons. Increased precipitation also stimulated switchgrass growth by producing more tillers and biomass. Abdulahi et al. [8] demonstrated that more frequent irrigation could mostly increase switchgrass biomass. A meta-synthesis of switchgrass yield showed that the annual yield of switchgrass increased with annual precipitation amount [57]. The large increases in aboveground biomass under the wet treatments in this study were mostly caused by the enhanced leaf growth and stem development, with small increases in tiller number. Both plant height, biomass and number of tillers increased with soil moisture, but biomass was more closely related to plant height than number of tillers among all precipitation treatments, indicating that precipitation stimulates more individual tiller growth to access light more than vegetation spread.
WUE measures carbon/biomass produced relative to water consumed [47,58]. WUEi was not influenced by the different precipitation treatments. This is a little surprising, as we expected that WUEi could be enhanced by precipitation increases. We did find that leaf photosynthetic rates were significantly higher under the +33% and +50% treatments, but water uses (transpirations) were also enhanced, as a results, WUEi did not change. Barney et al. [50] reported a similar result. WUEi was 6 μmol mmol-1 following a moisture stress period (20% deficit). This value was similar to our results and within the normal range (5.8–6.8 μmol mmol-1, [50]). But Hartman et al. [21] reported a higher WUEi in the decreased precipitation treatment compared to the increased precipitation treatment, and WUEi decreased over the growing season with a range of 3.16 to 4.71 μmol mmol-1 [59]. They found that switchgrass lowered transpiration rates and stomatal conductance under moisture deficit conditions. In this study, no significant difference in transpiration was found between the drought and the ambient precipitation treatments over the two growing seasons. We found slightly higher biomass-based WUEb under the drought treatments, as less water was applied though the biomass was lower in the drought treatments. It is worth noting that higher WUE is a good property for plant species/varieties, but treatments that increasing WUE may not always contribute to more plant growth or higher biomass, as observed in the drought treatments of this study.
Leaf physiology, plant growth and biomass of switchgrass differed significantly during the three harvest periods and interactively with precipitation treatments. The difference in leaf photosynthetic rate among harvest periods could be related to the changes in leaf temperature. Temperature was lower during the 1st harvest and higher during the 3rd harvest, and resulted in a lower photosynthetic rate during the 1st harvest and a higher photosynthesis during the 3rd harvest period. Within each harvest period, leaf photosynthesis and stomatal conductance declined over time (Fig 2). Similar results were reported by Hartman & Nippert [59] who reported that maximum leaf photosynthesis decreased from 30 to 10 μmol m-2 s-1 over the course of the growing season. Gao et al. [30] also reported that leaf photosynthesis of switchgrass in arid environments decreased from 17 μmol m-2 s-1 in May to 8 μmol m-2 s-1 in September. The declines of photosynthesis could be related to leaf development and soil moisture conditions [60] (Fig 1). Soil moisture often strongly influences the xylem pressure potential to cause the performance change of stomatal and leaf photosynthesis [29]. The last harvest had significantly lower aboveground biomass and plant height, compared to previous harvests, this could be due to higher air temperature and lower soil moisture contents. Lower biomass in late harvest of switchgrass has also been reported in field studies [12,61]. Leaf:stem ratio also varied among three harvests. Similar changes were found at development stages or under different environmental factors [62–64]. For example, Somleva et al. [62] showed that leaf:stem ratio of switchgrass dropped from 0.9–1.1 in vegetative tillers to 0.5–0.7 in tillers at a reproductive stage. In a field study, Tian et al. [63] found that leaf:stem ratio of Alamo decreased from ~1.0 in early vegetative stage to 0.20–0.25 at harvest in October, lower than our results. Changes of leaf:stem ratio at different harvests were mainly due to non-synchronized growths of plant organs, but might also be related to growing temperature [64].
Significant differences in leaf physiology and aboveground biomass were found between the two growing seasons, with the values in 2015 lower than those in 2014. The reasons for this could be due to the difference in temperature and nutrient deficiencies in the soil [65,66]. Air temperature in the greenhouse was set within a temperature range and controlled by the Wadsworth Step Up control system that automatically opened and closed the roofs and window panels. It seems that soil temperature in the greenhouse during the 2015 season was slightly higher than in 2014, particularly from June to August (Fig 1a). High summer temperature might reduce biomass production. In addition, no fertilizer was applied during the 3 years of the experiment. Nutrient limitation might have contributed to the decrease in biomass production in 2015. Further studies are needed to test the interaction of nutrient and precipitation change and whether nutrient is still sufficient for switchgrass growth [29, 67].
Conclusions
To conclude, we found significant effects of precipitation changes on leaf physiology and plant growth and biomass of switchgrass. Under the reduced precipitation treatments, leaf maximum photosynthetic rate and transpiration were not significantly influenced. But the severe drought treatment significantly reduced leaf biomass, stem biomass, and plant height, suggesting that lower water availability had more influences on leaf and stem development than physiology, and caused the reduced aboveground biomass. To develop high producing switchgrass cultivars for drier environments, attention should be paid to the traits related to leaf initiation and development. While switchgrass can tolerate drought conditions, precipitation increases could significantly enhance leaf photosynthesis, transpiration, and more on leaf and stem growth, and increase aboveground biomass. Thus, adequate irrigation under the drought condition could improve switchgrass growth and biomass. To verify whether the results from this mesocosm study could be applied in the field condition, more field experiments with multiple levels of precipitation treatment with switchgrass need to be conducted.
Supporting information
(DOCX)
Numbers are F values. Stars indicate the level of significance (* = p<0.05, ** = p<0.01).
(DOCX)
Acknowledgments
The authors thank Eddie Williams for his assistance in soil preparation, and Xiaotao Ding and Dr. Jianping Wu for their help with field measurements.
Data Availability
All relevant data are available from figshare at https://doi.org/10.6084/m9.figshare.5740266.v1.
Funding Statement
This work was supported by the USDA- Capacity Building Grant (2013-38821-21390) and Evans-Allen grant, and National Science Foundation (1504886, 1623085). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, et al. (2013) Carbon and other biogeochemical cycles. In: Stocker TF, Qin D, Plattner GK (Eds.), Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, pp. 465–570
- 2.Huntington TG (2006) Evidence for intensification of the global water cycle: review and synthesis. J. Hydrol. 319 (1):83–95 [Google Scholar]
- 3.Easterling DR, Meehl GA, Parmesan C, Changnon SA, Karl TR, Mearns LO (2002) Climate extremes: observations, modeling, and impacts. Science 289 (5487):2068–2074 [DOI] [PubMed] [Google Scholar]
- 4.Yoon JH, Wang SY, Gillies RR, Kravitz B, Hipps L, Rasch PJ (2015) Increasing water cycle extremes in California and relation to ENSO cycle under global warming. Nat. Commun. 6:8657 doi: 10.1038/ncomms9657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fay PA, Carlisle JD, Knapp AK, Blair JM, Collins SL (2003) Productivity responses to altered rainfall patterns in a C4 dominated grassland. Oecologia 137(2):245–251 doi: 10.1007/s00442-003-1331-3 [DOI] [PubMed] [Google Scholar]
- 6.Hui D, Jackson RB (2006) Geographic and interannual variability in biomass partitioning in grassland ecosystems: a synthesis of field data. New Phytol. 169(1):85–93 doi: 10.1111/j.1469-8137.2005.01569.x [DOI] [PubMed] [Google Scholar]
- 7.Wu Z, Dijkstra P, Koch GW, Penuelas J, Hungate BA (2011) Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob. Change Biol. 17(2):927–942 [Google Scholar]
- 8.Abdulahi AA, Aliero BL, Aliero AA, Zuru AA (2013) Effects of irrigation regime, organic and inorganic mineral source on growth and yield components of switchgrass (Panicum virgatum L.) in upland and lowland conditions in Sokoto, Nigeria. Pak. J. Biol. Sci. 16(2):51–58 [DOI] [PubMed] [Google Scholar]
- 9.Frank D, Reichstein M, Bahn M, Thonicke K, Frank D, Mahecha MD, et al. (2015) Effects of climate extremes on the terrestrial carbon cycle: concepts, processes and potential future impacts. Glob. Chang. Biol. 21(8):2861–2880 doi: 10.1111/gcb.12916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu X, Sherry RA, Niu S, Li D, Luo Y (2013) Net primary productivity and rain-use efficiency as affected by warming, altered precipitation, and clipping in a mixed-grass prairie. Glob. Change Biol. 19(9):2753–2764 [DOI] [PubMed] [Google Scholar]
- 11.Knapp AK, Hoover DL, Wilcox KR, Avolio ML, Koerner SE, La Pierre KJ, et al. (2015) Characterizing differences in precipitation regimes of extreme wet and dry years: implications for climate change experiments. Glob. Chang. Biol. 21(7):2624–2633 [DOI] [PubMed] [Google Scholar]
- 12.Vogel KP, Brejda JJ, Walters DT, Buxton DR (2002) Switchgrass biomass production in the Midwest USA. Agron. J. 94(3):413–420 [Google Scholar]
- 13.Long SP, Spence AK (2013) Toward cool C4 crops. Annu. Rev. Plant Biol. 64:701–722 doi: 10.1146/annurev-arplant-050312-120033 [DOI] [PubMed] [Google Scholar]
- 14.DeLucia EH, Gomez-Casanovas N, Greenberg JA, Hudiburg TW, Kantola IB, Long SP, et al. (2014) The theoretical limit to plant productivity, Environ. Sci. Technol. 48(16):9471–9477 doi: 10.1021/es502348e [DOI] [PubMed] [Google Scholar]
- 15.Perlack RD, Wright LL, Turhollow AF, Graham RL, Stokes BJ, Erbach DC (2005) Biomass as Feedstock for a Bioenergy and Bioproducts In: Industry: The Technical Feasibility of a Billion-Ton Annual Supply. Washington DC: U.S. Department of Agriculture, U.S. Department of Energy. [Google Scholar]
- 16.McLaughlin SB, Kszos LA (2005) Development of switchgrass (Panicum virgatum) as a bioenergy feedstock in the United States. Biomass Bioenergy 28(6):515–535 [Google Scholar]
- 17.Sanderson MA, Adler PR, Boateng AA, Casler MD, Sarah G (2006) Switchgrass as a biofuel feedstock in the USA. Can. J. Plant Sci. 86(5):1315–1325 [Google Scholar]
- 18.Gelfand I, Sahajpal R, Zhang X, Izaurralde RC, Gross KL, Robertson GP (2013) Sustainable bioenergy production from marginal lands in the US Midwest. Nature 493 (7433):514–517 doi: 10.1038/nature11811 [DOI] [PubMed] [Google Scholar]
- 19.Pedroso GM, van Kessel C, Six J, Putnam DH, Linquist BA (2014) Productivity, 15N dynamics and water use efficiency in low- and high-input switchgrass systems. GCB Bioenergy 6(6):704–716 [Google Scholar]
- 20.Weaver JE, Fitzpatrick TJ (1932) Ecology and relative importance of the dominants of tall-grass prairie. Bot. Gaz. 93(2):113–150 [Google Scholar]
- 21.Hartman JC, Nippert JB, Springer CJ (2012) Ecotypic responses of switchgrass to altered precipitation. Funct. Plant Biol. 39(2):126–136 [DOI] [PubMed] [Google Scholar]
- 22.Fike JH, Parrish DJ, Wolf DD, Balasko JA, Green J, Rasnake M, et al. (2006) Switchgrass production for the upper southeastern USA: influence of cultivar and cutting frequency on biomass yields. Biomass Bioenergy 30(3):207–213 [Google Scholar]
- 23.Schmer MR, Vogel KP, Mitchell RB, Perrin RK (2008) Net energy of cellulosic ethanol from switchgrass. Proc. Natl. Acad. Sci. U.S.A. 105(2):464–469 doi: 10.1073/pnas.0704767105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dukes JS, Chiariello NR, Cleland EE, Moore LA, Shaw MR, Thayer S, et al. (2005) Responses of grassland production to single and multiple global environmental changes. PLoS Biol. 3(10):1829–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parrish D, Fike JH (2005) The biology and agronomy of switchgrass for biofuels. Crit. Rev. Plant Sci. 24(5–6):423–459 [Google Scholar]
- 26.Wilson DM, Dalluge DL, Rover M, Heaton EA, Brown RC (2013) Crop management impacts biofuel quality: influence of switchgrass harvest time on yield, nitrogen and ash of fast pyrolysis products. Bioenergy Res. 6(1):103–113 [Google Scholar]
- 27.Haankuku C, Epplin FM (2015) Biomass yield enhancement required for a replacement switchgrass variety. Agron. J. 107(1):287–295 [Google Scholar]
- 28.Oliver RJ, Finch JW, Taylor G (2009) Second generation bioenergy crops and climate change: a review of the effects of elevated atmospheric CO2 and drought on water use and the implications for yield. GCB Bioenergy 1(2):97–114 [Google Scholar]
- 29.Sanderson MA, Reed RL (2000) Switchgrass growth and development: water, nitrogen, and plant density effects. J. Range Manage. 53(2):221–227 [Google Scholar]
- 30.Albaugh JM, Albaugh TJ, Heiderman RR, Leggett Z, Stape JL, King K, et al. (2014) Evaluating changes in switchgrass physiology, biomass, and light-use efficiency under artificial shade to estimate yields if intercropped with Pinus taeda L. Agroforestry syst. 88(3):489–503 [Google Scholar]
- 31.Gao ZJ, Xu BC, Wang J, Huo LJ, Li S (2015) Diurnal and seasonal variations in photosynthetic characteristics of switchgrass in semiarid region on the Loess Plateau of China. Photosynthetica 53(4):489–498 [Google Scholar]
- 32.Haworth M, Cosentino SL, Marino G, Brunetti C, Scordia D, Testa G, et al. (2016) Physiological responses of Arundo donax ecotypes to drought: a common garden study. GCB Bioenergy doi: 10.1111/gcbb.12348 [Google Scholar]
- 33.Sanderson MA, Reed RL, McLaughlin SB, Wullschleger SD, Conger BV, Parrish DJ, et al. (1996) Switchgrass as a sustainable bioenergy crop. Bioresour. Technol. 56(1):83–93 [Google Scholar]
- 34.Hui D, Tian H, Luo Y (2012) Impacts of climatic changes on biogeochemical cycling in terrestrial ecosystems, In: Chen W, Seiner J, Suzuki T, Lackner M (Eds.), Handbook of Climate Change Mitigation, Springer, New York: pp. 436–470 [Google Scholar]
- 35.O'Keefe K, Tomeo N, Nippert JB, Springer CJ (2013) Population origin and genome size do not impact Panicum virgatum (switchgrass) responses to variable precipitation. Ecosphere 4(3):1–19 [Google Scholar]
- 36.Muir JP, Sanderson MA, Ocumpaugh WR, Jones RM, Reed RL (2001) Biomass production of ‘Alamo’ switchgrass in response to nitrogen, phosphorus, and row spacing. Agron J. 93(4):896–901 [Google Scholar]
- 37.Heaton E, Voigt T, Long SP (2004) A quantitative review comparing the yields of two candidate C4 perennial biomass crops in relation to nitrogen, temperature and water. Biomass Bioenergy 27(1):21–30 [Google Scholar]
- 38.Berdahl JD, Frank AB, Krupinsky JM, Carr PM, Hanson JD, Johnson HA (2005) Biomass yield, phenology, and survival of diverse switchgrass cultivars and experimental strains in western North Dakota. Agron. J. 97(2):549–555 [Google Scholar]
- 39.Deng Q, Aras S, Yu CL, Dzantor EK, Fay PA, Luo Y, et al. (2017) Effects of precipitation changes on aboveground net primary production and soil respiration in a switchgrass field. Agriculture, Ecosystems, and Environment 248:29–37 [Google Scholar]
- 40.Fay PA, Kaufman DM, Nippert JB, Carlisle JD, Harper CW (2008) Changes in grassland ecosystem function due to extreme rainfall events: implications for responses to climate change. Glob. Chang. Biol. 14(7):1600–1608 [Google Scholar]
- 41.Deng Q, Hui D, Zhang D, Zhou G, Liu J, Liu S, et al. (2012) Effects of precipitation increase on soil respiration: a three-year field experiment in subtropical forests in China, PLoS One 7 (7). doi: 10.1371/journal.pone.0041493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Wang Q, Liu Y, Cui J, Ma X, Gu M, et al. (2015) Coupling effects of water availability and pH on switchgrass and the optimization of these variables for switchgrass productivity determined by response surface methodology. Biomass Bioenergy 83:393–402 [Google Scholar]
- 43.Yu C-L, Hui D, Deng Q, Dzantor EK, Fay PA, Shen W, et al. (2017) Responses of switchgrass soil respiration and its components to precipitation gradient in a mesocosm study. Plant and Soil (in press). doi: 10.1007/s11104-017-3370-2 [Google Scholar]
- 44.Irmak S, Haman DZ (2001) Performance of the WaterMark Granular matrix sensor in sandy soils. Appl. Eng. Agric. 17(6):787 [Google Scholar]
- 45.Hui D, Jiang C (1996) Practical SAS Usage, Beijing, China. [Google Scholar]
- 46.Collatz GJ, Ribas-Carbo M, Berry JA (1992) Coupled photosynthesis-stomatal conductance model for leaves of C4 plants. Functional Plant Biol. 19(5):519–538 [Google Scholar]
- 47.Hui D, Luo Y, Johnson DW, Cheng W, Coleman JS, Sims DA (2001) Canopy radiation and water use efficiency as affected by elevated CO2. Glob. Chang. Biol. 7(1):75–91 [Google Scholar]
- 48.Tian S, Youssef MA, Chescheir GM, Skaggs RW, Cacho J, Nettles J (2016) Development and preliminary evaluation of an integrated field scale model for perennial bioenergy grass ecosystems in lowland areas. Environmental Modelling and Software 84:226–239 [Google Scholar]
- 49.Mooney DF, Roberts RK, English BC, Tyler DD, Larson JA (2009) Yield and breakeven price of 'Alamo' switchgrass for biofuels in Tennessee. Agron. J. 101:1234–1242 [Google Scholar]
- 50.Barney JN, Mann JJ, Kyser GB, Blumwald E, van Deynze A, DiTomaso JM (2009) Tolerance of switchgrass to extreme soil moisture stress: ecological implications. Plant Sci. 177(6):724–732 [Google Scholar]
- 51.Wang B, Seiler J, Mei C (2016) A microbial endophyte enhanced growth of switchgrass two drought cycles improving leaf level physiology and leaf development. Environmental and Experimental Botany 122:100–108 [Google Scholar]
- 52.Knapp AK (1984) Water relations and growth of three grasses during wet and drought years in a tallgrass prairie. Oecologia 65(1):35–43 doi: 10.1007/BF00384460 [DOI] [PubMed] [Google Scholar]
- 53.Nippert JB, Fay PA, Knapp AK (2007) Photosynthetic traits in C3 and C4 grassland species in mesocosm and field environments. Environ. Exp. Bot. 60(3):412–420 [Google Scholar]
- 54.Cordero A, Osborne BA (2016) Variation in leaf-level photosynthesis among switchgrass genotypes exposed to low temperatures does not scale with final biomass yield, GCB Bioenergy doi: 10.1111/gcbb.12349 [Google Scholar]
- 55.Heckathorn SA, DeLucia EH (1994) Drought-induced nitrogen retranslocation in perennial C4 grasses of tallgrass prairie. Ecology 75(7):1877–1886 [Google Scholar]
- 56.Loik ME (2006) Sensitivity of water relations and photosynthesis to summer precipitation pulses for Artemisia tridentata and Purshia tridentate. Plant Ecol. 191(1):95–108 [Google Scholar]
- 57.Wang DAN, Lebauer DS, Dietze MC (2010) A quantitative review comparing the yield of switchgrass in monocultures and mixtures in relation to climate and management factors. GCB Bioenergy 2:16–25 [Google Scholar]
- 58.Medrano H, Tomás M, Martorell S, Flexas J, Hernández E, Rosselló J, et al. (2015) From leaf to whole-plant water use efficiency (WUE) in complex canopies: limitations of leaf WUE as a selection target. Crop J. 3(3):220–228 [Google Scholar]
- 59.Hartman JC, Nippert JB (2013) Physiological and growth responses of switchgrass (Panicum virgatum L.) in native stands under passive air temperature manipulation. GCB Bioenergy 5(6):683–692 [Google Scholar]
- 60.Wang B, Seiler J, Mei C (2015) Burkholderia phytofirmans stain PsJN advanced development and altered leaf physiology of switchgrass. Biomass and Bioenergy 83:493–500 [Google Scholar]
- 61.Lemus R, Parrish DJ, Wolf DD (2014) Switchgrass cultivar/ecotype selection and management for biofuels in the upper Southeast USA. Scientific World J. doi: 10.1155/2014/937594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Somleva MN, Snell KD, Beauliu JJ, Peoples OP, Garrion BR, Patterson NA (2008) Production of polyhydroxybutyrate in switchgrass, a value-added co-product in an important lignocellulosic biomass crop. Plant Biotechnology Journal 6:663–678 doi: 10.1111/j.1467-7652.2008.00350.x [DOI] [PubMed] [Google Scholar]
- 63.Tian S, Cacho JF, Youssef MA, Chescheir GM, Nettles JE (2015) Switchgrass growth and morphological changes under established pine-grass agroforestry systems in the lower coastal plain of North Carolina, United States. Biomass and Bioenergy 83:233–244 [Google Scholar]
- 64.Kandel TP, Wu Y, Kakani VG (2013) Growth and yield responses of switchgrass ecotypes to temperature. American Journal of Plant Sciences 4:1173–1180 [Google Scholar]
- 65.Zhu Y, Fan X, Hou X, Wu J, Wang T (2014) Effect of different levels of nitrogen deficiency on switchgrass seedling growth. Crop J. 2(4):223–234 [Google Scholar]
- 66.Zhou X, Fei S, Sherry R, Luo Y (2012) Root biomass dynamics under experimental warming and doubled precipitation in a tallgrass prairie. Ecosystems 15:542–554 [Google Scholar]
- 67.Luo Y, Gerten D, Le Maire G, Parton WJ, Weng E, Zhou X, et al. (2008) Modelled interactive effects of precipitation, temperature, and CO2 on ecosystem carbon and water dynamics in different climatic zones. Glob. Chang. Biol. 14(9):1986–1999 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Numbers are F values. Stars indicate the level of significance (* = p<0.05, ** = p<0.01).
(DOCX)
Data Availability Statement
All relevant data are available from figshare at https://doi.org/10.6084/m9.figshare.5740266.v1.