Abstract
Standard treatment of primary and metastatic brain tumors includes high dose megavoltage radiation to the cranial vault. About half of patients survive >6 months, many attain long term control or cure, but 50-90% of survivors overall exhibit disabling cognitive dysfunction. The radiation cognitive syndrome is poorly understood and there is no effective prevention or long-term treatment. Attention has primarily focused on mechanisms of disability appearing at six months to one year after radiotherapy. However, a range of studies have revealed that CNS alterations and dysfunction develop much earlier than 6 months following radiation exposure. This has prompted the recent hypothesis that relatively subtle early forms of radiation induced CNS damage may drive chronic pathophysiology leading to permanent cognitive decline. Within this perspective, the present review presents evidence of acute CNS irradiation triggered inflammation, and injury to neuronal lineages, accessory cells and their progenitors, and loss of supporting structure integrity. Moreover, injury related processes set in motion soon after intracranial irradiation may interact and synergize to alter the neuronal and supporting cell progenitor signaling environment in stem cell niches in the brain, and specifically in the hippocampus, a structure critical to memory and cognition. Changed niche conditions may cause a sustained decline in neurons and progressive deterioration of cognition. The concluding discussion addresses, (1) what further data is needed, and (2) potential treatment interventions, identified via recent findings on acute CNS radiation injury, that may reverse degenerative processes before they can cause permanent cognitive disability.
I. Introduction
Every year many hundreds of thousands of patients worldwide undergo radiotherapy for primary brain tumors and for brain metastases originating from extracranial tumors.1-5 Radiation is an indispensable treatment mainstay for the majority of these brain tumors.6-9 Brain radiotherapy is subdivided into whole brain radiotherapy (WBRT) in which the entire brain and brainstem are irradiated, and partial brain radiotherapy (PBRT) which includes treatment of the tumor or tumor bed and surrounding margin, and some healthy brain tissue subject to incidental irradiation 4,10,11 Stereotactic radiosurgery (SRS) relies on precise 3D imaging and localization to deliver ablative doses of radiation to the tumor, and can significantly reduce exposure of healthy brain tissue.12 These modes of brain radiotherapy fulfill any of a range of clinical objectives including; (1) long term tumor control or cure as mono or combined therapy, (2) salvage treatment for slowing tumor growth or palliation, and (3) prophylaxis to kill metastatic cells that would otherwise become established in the brain.6,13-15
Presently about 100,000 brain tumor patients per year in the U.S. receiving brain irradiation survive >6 months, and 50-90% of these individuals exhibit cognitive dysfunction which is often progressive and disabling.3,16-18 Affected cognitive domains include learning, memory, processing speed, attention, and executive function.8,19 Patient quality of life (QOL) is now accorded great importance in clinical oncology, and radiotherapy-induced cognitive decline erodes patients' perception of QOL as revealed by a questionnaire from the cognitive section of the European Organization for Research and Treatment of Cancer (EORTC).20-22,18,22 The World Health Organization (who) describes health as a “state of complete physical, mental, and social well-being and not merely the absence of disease or infirmity” (http://www.who.int/about/definition/en/print.html). The Response Assessment in Neuro-Oncology (RANO) working group recommended that neurocognitive outcome be considered as one of the primary endpoints in brain tumor clinical trials.23 Despite the importance and clear concern about radiation induced cognitive decline, the pathophysiology driving the progression of this syndrome remains poorly understood, and there are no effective preventative measures or long-term treatments.24
The genesis of radiation induced cognitive decline is complex with multiple interacting and synergistic mechanisms.25-27 Historically, the primary focus has been on markers of damage and cognitive decline appearing over 6 months to 1 year or more after irradiation.5 For example, white matter deterioration has been presumed to be a major factor underlying progressive cognitive decline that is generally apparent a year or so after brain irradiation.18 Questions inevitably arose because of reports describing the absence of demonstrable white matter changes despite the presence of cognitive impairment.18,28,29 More sensitive imaging modalities such as diffusion tensor imaging (DTI) along with new lines of inquiry and enhanced experimental techniques in animal and cell based systems have been able to reveal subtle evidence of damage to white matter, the cortex, and a range of neuroanatomical domains at various levels of resolution much sooner than 6 months.5,18 In many studies CNS changes were observed within hours, days, or weeks of irradiation. For the present review the term early refers to acute events developing at 4 weeks (1 month) or less after radiation exposure.27,30,31
We and others postulate that previously undetected and comparatively subtle early manifestations of irradiation damage to the central nervous system (CNS) may synergize over time to form long-term macro and microstructural abnormalities, resulting in permanent cognitive disability.5,18,25 Early changes below the gross anatomical level, including a decline in oligodendrocytes, microvascular damage, subtle loss of white matter integrity imaged with DTI, neuroinflammation, and disturbances of neuronal micromorphophysiology, may interact and progressively alter neuronal stem cell niches to impede neuronal function, viability, and progenitor cell differentiation.5,18,30 This scenario may result in long-term dysfunction and depletion of neurons and contribute to cognitive decline.4,5,32,33
This review begins by delineating the rationale for CNS radiotherapy and identifying the impacted cognitive domains. This is followed by a description of the time course of cognitive deterioration, and the evidence acquired from preclinical models supporting the concept that early radiation induced subtle changes in the brain are precursors to long-term, permanent CNS dysfunction (Figure 1). Evidence gathered via the longitudinal imaging of brain injury in human radiotherapy patients is then addressed in the context of (1) early and long-term CNS deterioration and cognitive disability, and (2) major hypotheses of radiation induced cognitive decline. The concluding discussion transitions to future directions and to potentially relevant opportunities for early therapeutic interventions, based on early CNS damage that may be exploited to impede progressive cognitive deterioration.3-5,18,30,33,34
Figure 1. Evidence for early radiation injury to the CNS.
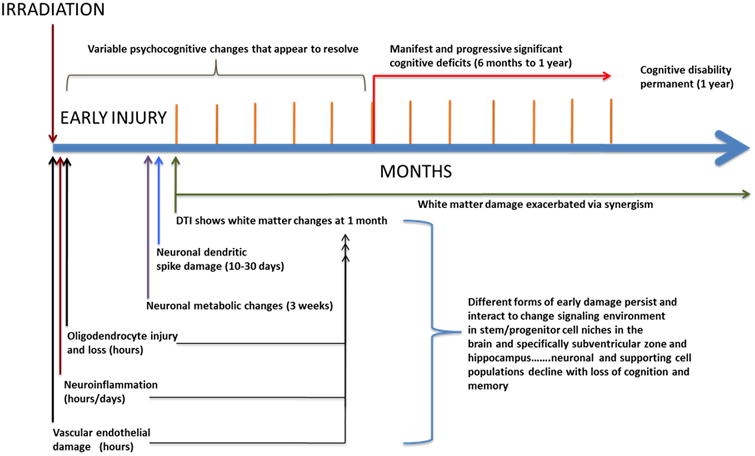
A proposed time course and scheme by which early damage becomes chronic and the interactions leading to permanent cognitive disability.
II. Intracranial Radiotherapy--- Effective but with Serious Risks to Cognition
The initial barrier to success in treating brain tumors is resistance and local treatment failure allowing tumor recurrence and expansion.35,36 Multiple biological processes contribute to treatment failure in both primary and metastatic brain tumors, but it is generally held that the key obstacle is the inability of drugs to attain effective intra-tumoral concentrations.36,37 In contrast radiation uniformly penetrates both the brain and tumor parenchyma, engages macroscopic and microscopic disease, overcomes resistant cells, and thus is an essential therapeutic modality.6,8
For example in adult medulloblastoma patient cohorts with nondisseminated disease, craniospinal irradiation and chemotherapy have achieved a 5 year survival of 70%.38
Despite its undisputed therapeutic importance radiation often unavoidably damages the brain and affects cognition.27 The neurocognitive domains thought to be most affected by radiation are verbal and non-verbal memory, executive function, sustained attention, and information processing speed.8,39 The hippocampus is instrumental in learning and memory, while the prefrontal cortex (PFC) plays a key role in executive functioning and data shows it is affected by radiation.27,40-42 The underlying brain networks involved in sustained attention and processing speed are thought to be more distributed in nature, with most evidence suggesting that they rely on a complex frontal-subcortical network that involves both cortical and white matter structures.43,44 Radiation injury may affect some or all of these pathways, is multifactorial and complex, and is characterized by vascular abnormalities, inflammation, gliosis, demyelination, and often at high doses, white matter necrosis.3,45
III. The Time Course of Neurocognitive Dysfunctionin Radiotherapy Patients
Wu and colleagues have noted that attention has largely focused on potential mechanisms of cognitive deterioration appearing at six months to one year following brain irradiation, rather than early abnormalities and how they may evolve over time.5,46 The cognitive decline developing after 6 months is held to be progressive and irreversible and is thus of the greatest concern (Figure 2).18,6,27 In the adult patient population, which is the focus of the present review, radiation induced neurocognitive decline follows a biphasic pattern beginning with a transient cognitive decline at approximately 4 months posttreatment, followed by an improvement, and then a progressive, irreversible deterioration in cognitive functioning at 12 months or later after irradiation.6 However, it is important to note that tumor progression during this time period may adversely affect cognition, confounding imaging and the measurement of radiation-induced cognitive decline in many studies. Radiation necrosis occurs in a minority of patients and its probability is low at doses below 50 Gy given in 2 Gy fractions.6,47 However, the incidence increases substantially with escalating radiation dose, fraction size, and the use of chemotherapy.47,48 The most frequent neurotoxic effect of cranial irradiation at any patient age is not focal necrosis but diffuse cerebral injury.49 Importantly, radiation-induced cognitive decline occurs at doses much lower than those that can cause radionecrosis.50
Figure 2.
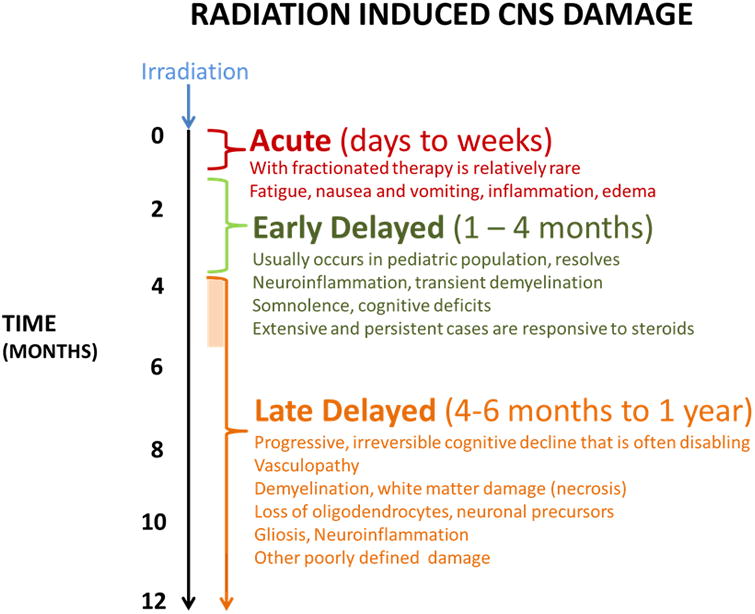
Manifestations and time course of radiation induced CNS injury and cognitive decline.
IV. Early Radiation Induced CNS Abnormalities in Preclinical Animal Models
Perhaps somewhat surprisingly, early CNS functional changes developing in animal models after irradiation were noted decades ago. Gangloff reported that altered hippocampal spike activity persisting for 7 days appeared in the cat and rabbit following 4 Gy of X-rays.51 Bassant and Court found immediate alterations in the firing patterns of rabbit hippocampal neurons with 4 Gy of gamma radiation.52 Pellmar and Lepinski found evidence that 5 Gy to the Guinea pig resulted in a decrease of synaptic efficacy soon after irradiation, with apparent recovery by 5 days.53 These results suggest that the hippocampus expresses radiation induced damage early, and an important conceptual question that has not been addressed until quite recently is whether early changes are somehow related to later dysfunction, progressive deterioration, and permanent cognitive deficits.
Early Radiation Injury to Brain Blood Vessels
Early vascular disruption in preclinical models such as the rabbit ear, evidenced by edema developing within hours of a single radiation dose, followed by vascular dysfunction months later, was first reported in the 1950s.54,55 Since then a considerable body of accumulated evidence has shown that radiation early destabilizes the plasma membrane of a range of cell types including the vascular endothelium.3,56 Cranial irradiation has reduced endothelial cell density in rat brains within a day of 5-200 Gy, and this dose-independent loss persisted for one month after exposure, followed by a slow dose-independent decrease in cell number for 6 months, and thereafter the cell population remained depleted.31 Microvascular sequelae of endothelial degeneration seen in humans may occur months to years after the initial radiation induced damage, and include telangiectasias, microvascular dilatation, and thickening and hyalinization of the vessel wall.57 Such changes can trigger ischemic strokes, brain microbleeds, and occlusion of small vessels, leading to local necrosis and extravasation with subsequent demyelinization.58 Intracerebral cavernous malformations which are susceptible to bleeding have been observed in human patients with a median latency of 3 to 6 years following CNS irradiation.45,59,60 Disturbances of cerebral blood flow have been associated with cognitive debility, and the extent of hypoperfusion has correlated with the depth of cognitive deterioration.61,62 Vascular damage and associated sequelae leading to poor perfusion of brain tissue can secondarily cause local demyelination and focal necrosis which degrade cognition.47 Vascular endothelial and glial cell loss has been presumed to cause white matter damage and subsequent cognitive impairment.63 White matter networks play an essential role in supporting cortical function and cognitive dysfunction has been linked to damage within these networks (Figures 3 & 4).27,64 However, radiation induced vasculopathy alone may not entirely account for the progressive cognitive decline that occurs after WBRT. Greene-Schloesser et al note that in the rat, drugs such as the PPARγ agonist, pioglitazone, and the ACE inhibitor Ramipril do not effect improvement in the microvasculature after it has been damaged and depleted after irradiation, but both agents have been reported to prevent radiation induced cognitive decline.18,65,66
Figure 3. Primary radiation induced CNS abnormalities.
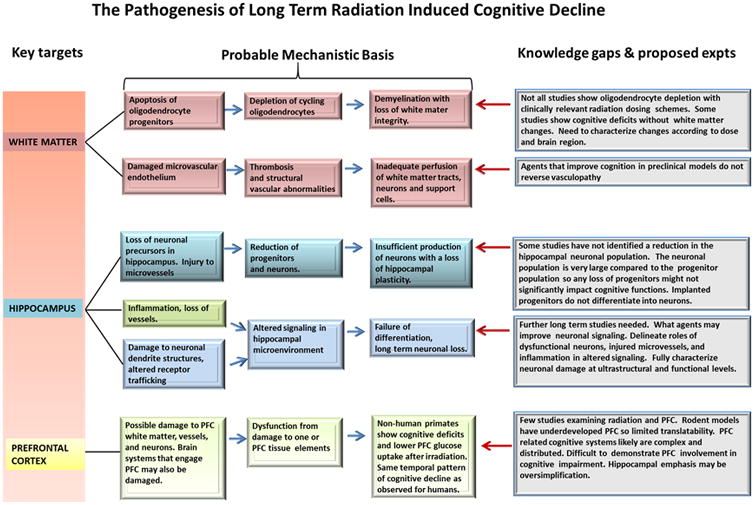
Detailed scheme shows major proposed mechanisms contributing to radiation induced cognitive decline.
Figure 4. The presumed origins of white matter damage.
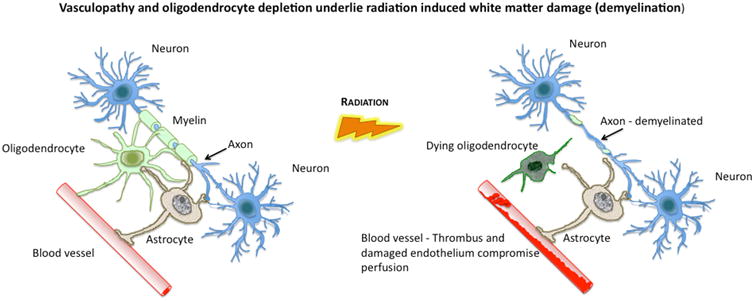
White matter provides essential connectivity for cortical function. Oligodendrocytes establish and maintain myelin around white matter axons (left panel) and their destruction (right panel) results in a loss of myelin integrity. Damage to feeding microvessels (right panel) also compromises white matter but also has adverse effects on the perfusion of other key CNS elements such as astrocytes which provide metabolic and functional support to neurons.
Astrocytes – Early Radiation Effects
Glial cells comprise a major proportion of the cellular population of the brain, they control a range of essential functions, and have long been implicated as a causative factor in radiation induced cognitive decline.67 How glial cells are affected by radiation and how they contribute to radiation induced cognitive decline remains unclear. Astrocytes are glial cells that have a major role in the mature CNS, supporting neurons, maintaining homeostasis, and modulating neurotransmitter dynamics.68 Following intracranial irradiation astrocytes proliferate and form scar tissue, referred to as reactive gliosis.69 Hwang et al reported that a single dose of 15 Gy radiation to the rat brain increased immunostaining of GFAP in astrocytes at 6 hours with increased staining at 24 hours, which the authors interpreted as the onset of gliosis.70,71 Identifying features of reactive gliosis are hypertrophy of astrocytic processes, upregulation of intermediate filaments, and increased glial fibrillary acidic protein (GFAP) expression.70,72 Their experiments suggested that PGE2 released from irradiated microglia is a trigger of radiation-induced gliosis.70 How astrocytic activation and dysfunction may affect white matter, may be affected by neuroinflammation, and in general contribute to cognitive decline requires further investigation. It has been reported that irradiation of microglia-astrocyte co-cultures and astrocyte monocultures results in the expression of inflammatory cytokines.73 Some evidence indicates that treatment with Ang 1-7 peptide may prevent radiation-induced MAP kinase activation and inflammation, which could in theory reduce radiation-induced cognitive impairment.73
Oligodendrocytes – Early Radiation Effects
Oligodendrocytes are glial cells thought to be the most radiation sensitive cell type in the CNS, they produce and maintain myelin sheaths along neuronal axons. Early developing global reductions in their numbers has been observed in rodent brains within a few hours of intracranial irradiation.74,75 The majority of cycling cells in the adult brain (>75%) are oligodendroglial precursors and loss of these cells is presumed to diminish the replacement of oligodendrocytes.76 Following cranial radiation in animals manifestations of white matter injury such as focal demyelinization and necrosis have been observed.77 Panagiotakis et al using rats head irradiated with 25 Gy of 250 Kv X-rays observed a reduction in oligodendroglial lineage cells and ultrastructural evidence of myelin fragmentation, but did not find evidence of vascular damage.76
The Role of Cranial Radiation Dosing Regimenin Glial Loss and White Matter Damage
Comparatively recent reports suggest that the nature of the brain irradiation dosing scheme appears to significantly influence specific pathologies in the human and animal brain (see Table 1).9,28,78 In rats following a high single intracranial dose (25 Gy) of 250 kV X-rays, Panagiotakis et al assessed oligodendrocyte lineage cell populations over 15 months.76 Markers for mature (MBP) and developing (NG2, O4, PDGFRA) oligodendrocytes revealed that cell numbers at all differentiation stages showed an initial early drop followed by a variable pattern for several months, and finally an apparently permanent decline at about 12 to15 months after irradiation.76 Moreover, at the 15 month time point a diffuse pattern of myelin loss was observed throughout the corpus callosum as well as the fimbriae, the external capsule and the deep white matter.76 In contrast, fractionated intracranial irradiation was investigated by Shi et al and delivered as 9 exposures to 5 Gy over 4.5 weeks for a total of 45 Gy in middle aged (12 month) Fischer 344× Brown Norway rats, a regimen that closely emulated human radiotherapy treatment schedules.29 At 12 months post-irradiation white matter gross morphology and structural integrity were normal (Table 1).29 Importantly, despite normal appearing CNS anatomy in the Shi et al study, a fractionated 45 Gy intracranial radiation dose in this F344 rat model has caused hippocampal and non-hippocampal dependent cognitive deficits, along with glutamate receptor abnormalities.79 These results align with other studies in preclinical models and with human subjects which have both uncovered moderate cognitive deficits in the absence of evident structural changes on neuroimaging.57 Chen et al exposed rat pup subventricular zone progenitor cells in vitro to 8 Gy of γ-rays and concluded that neither the percentage of stem cells nor their proliferation was affected, although progression through the cell cycle was markedly delayed, and, interestingly, the proliferation of a glial-restricted precursor was increased.80
Table 1. Evidence for White Matter Damage After Brain Irradiation.
| Species | Subject Stage (Adult/Pediatric/Elderly) | Radiation Dose (Gy) WBRT/ PBRT | Fraction (Gy) | Assay | White Matter Changes | Time (mos) | Reference |
|---|---|---|---|---|---|---|---|
| Human | Adult | 50 – 81 | 1.8 or 2 | DTI | Yes | 1.5 | Nagesh et al, 2008148 |
| Human | Adult | 50 - 60 | 1.8 or 2 | Histology | Yes | - | Burger et al, 2006170 |
| Human | Adult | 35 | 2.5 or 3 | MRI | Yes | 11-51 | Fuji et al, 2006171 |
| Human | Adult | 60 tumor only | 2 | DTI | No | 6 | Prust et al, 2015131 |
| Human | Adult | 59 - 60 | 1.8 or 2 | DTI | Yes** | 12 | Connor et al, 201627 |
| Human | Adult | 45-59.4 | 1.8 | DTI | Yes** | 36-108 | Ravn et al, 2013146 |
| Human | Pediatric | 36 WB; 54 local | Typically 1.8 | DTI | Yes | 30 | Mabbott et al, 2006172 |
| Human | Elderly >60 years | 50 | 1.8 or 2 | MRI | Prevalent | 38 | Tsuruda et al, 198777 |
| Mouse | Adult | 30 | 30 | Histology | Yes | 8 | Chiang et al, 1993173 |
| Rat | Adult | 17.5 | 17.5* | Histology | Yes | 10 | Calvo, et al, 198863,173 |
| Rat | Adult | 45 | 5* | Histology | No | 12 | Shi et al, 200929 |
| Monkey | Adult (Rhesus) | 40 | 2* | Histology | No | 6, 12 | Nakagaki et al, 1976188 |
| Monkey | Adult (Rhesus) | 60 | 2 | Histology | Yes | 6, 12 | Nakagaki et al, 1976188 |
| Monkey | Adult (Rhesus) | 80 | 2 | Histology | Yes | 6, 12 | Nakagaki et al, 1976188 |
WBRT = whole brain irradiation
PBRT = partial brain irradiation
DTI = diffusion tensor imaging
MRI = magnetic resonance imaging
Note apparent effect of dose rate; according to histology low dose rate avoided white matter abnormalities, despite cognitive deficits in this model and with this dosing schedule.
Very low total doses elicited white matter DTI changes.
Early Post-Irradiation Neuroinflammation, Vascular Damage, and the Hippocampus
Adult neurogenesis mainly occurs in two brain regions, (1) the hippocampal dentate gyrus and subgranular zone (SGZ) and (2) the subventricular zone (SVZ).81,82 Radiation is now known to suppress the proliferation of hippocampal SGZ progenitor cells and their differentiation into neurons.83-86 Murine hippocampal cells undergo reduced neurogenesis after 5 Gy or 10 Gy of intracranial X-irradiation, and the mice exhibit reduced cognitive abilities in maze tests.87,88 Radiation induced hippocampal impairment is currently thought to derive from damage to differentiated neural cells, altered neurogenesis,89 and a resultant loss of hippocampal plasticity.83,84,90 In line with this theory, Acharya et al. reported that human neural stem cell transplantation attenuated radiation-induced cognitive dysfunction in head irradiated mice.91,92 The hippocampus, entorhinal cortex, perirhinal cortex, and parahippocampal cortex are particularly sensitive to vascular injury, and radiation has been hypothesized to cause vascular rarefaction in the hippocampus and cognitive dysfunction that is reversible with hypoxia.62,93
Astrocytes and microglia respond to brain irradiation by secreting factors that induce widespread neuroinflammation that can impact cell function and differentiation in the CNS.25,94 Proinflammatory cytokines such as interleukin-1β, tumor necrosis factor-α, interleukin-6 and interleukin-18 have been measured in specific regions of the brain following radiation exposure.95 Inflammatory markers such as GFAP, intercellular adhesion molecule-1 and NF-κb have also been identified in irradiated brain tissue.96 COX-2 pathways have been implicated in neuroinflammation following intracranial irradiation and the expression of prostaglandin E2 has been suggested as a cause of radiation gliosis.70,97 Monje et al examined neuropathological markers of neurogenesis and inflammation in the human hippocampus after intracranial radiation treatment for early myelogenous leukemia and MB and found evidence of inflammation with virtually complete inhibition of neurogenesis.98 Inflammation in the hippocampal microenvironment causes microvascular damage which also results in the release of signaling molecules, thereby changing the progenitor cell microenvironment which suppresses differentiation to the neuronal phenoptype.94,98-101 A trophic relationship exists between microvessels and neural progenitors, and the levels of VEGF in the hippocampus have been shown to affect hippocampal angiogenesis and neurogenesis, and to modulate hippocampal plasticity of mature neurons.102,103 The above findings suggest potential therapeutic strategies such as the use of anti-inflammatory agents and implantation of neural stem cells.98,94 Jenrow et al reported that pharmacologically based reduction of neuroinflammation during a 9 month period following a single intracranial 10 Gy dose of gamma radiation to adult male Fisher F344 rats resulted in a higher proportion of hippocampal progenitors adopting a neural instead of a glial fate compared to untreated controls.10 The authors suggested that this outcome was due to an improved neurogenic signaling microenvironment. Importantly, the comparatively long time span of this study points to the possibility that neuroinflammation may be chronic after irradiation, and exerts protracted effects on the neuronal population, glial cells, and white matter, thus driving progressive cognitive deterioration.10,27
V. Early Radiation Damage to the Neuronal Lineage and Progressive Cognitive Dysfunction
Classically the brain has been regarded as a radio resistant organ, and neurons as essentially inert to radiation.5,18,104 However, some writers drawing on both old and new observations posit that differentiated neurons are not inert to radiation as previously thought, and suggest that neuronal dysfunction and not neuronal loss is the driver of radiation induced cognitive debility.5,90 In accordance with this view, Lee and colleagues found reduced hippocampal neurogenesis following irradiation of young adult male rats despite pharmacologic blockade of Angiotensin II mediated inflammation, yet, importantly, these animals did not exhibit radiation induced cognitive impairment.66 Perhaps this was because the neuronal precursor population is small compared to the mature neuronal compartment and hence its disruption may be insufficient to cause identifiable hippocampal cognitive deficits.34
Shi and coworkers demonstrated cognitive impairment in rats after fractionated WBRT, yet quantitative analysis of hippocampal neuron number and myelin integrity in the same model indicated neither hippocampal neuron loss nor changes in myelin integrity.2,17,29,79 Why this study differs from other reports is not clear, possibilities include the rodent model and radiation dosing scheme. Another possibility is that microanatomical and functional deficits at the neuronal level occur soon after irradiation, without an immediate loss of neuronal density, apparent white matter damage, or detectable loss of gray matter volume.
Early Neuronal Microanatomical Abnormalities After Irradiation
Robbins et al (2011) performed a head radiation study using healthy adult male rhesus monkeys, without chemotherapy or surgery.105 These authors reported that 40 Gy in 5 Gy fractions were associated with impaired Delayed-Match-to-Sample (DMS) cognitive performance and reduced prefrontal cortical glucose uptake.105 The temporal pattern of cognitive decline in the monkeys over the course of 11 months closely mirrored the apparent cognitive course of human intracranial radiotherapy patients.105 In the context of the present review, a key question is whether the cognitive decline observed for rhesus monkeys was preceded by early CNS damage. Possible microanatomical correlates of early radiation induced neuronal dysfunction include changes in neuronal dendritic spine density and morphology. Dendritic spines are sites of synaptic contact and their morphophysiology is thought to provide the basis of specific components of cognition such as long-term potentiation (LTP) and plasticity.106-108
Dendritic spines are actin-rich protrusions of neuronal dendrites and are the site of excitatory synaptic transmission.109 They are highly dynamic structures populated by NMDA-type glutamate receptors which are activated by strong synaptic input. 109 Activation triggers calcium flux into the spines and cytoskeletal rearrangement that generates larger spines with stronger synapses. Such changes in synaptic strength are held to comprise the primary basis of learning and memory.109 Frankfurt and Luine related histology and behavioral tests in rats and identified a clear relationship between dendritic spine density in the hippocampus and memory.107 In the PFC early stress in humans and animals and mental illness in human patients can cause atrophy of dendrites and spines, and this is associated with working memory impairment.110 Dendritic spines in the pyramidal neurons of the PFC and the hippocampus, brain structures which orchestrate executive function and memory, respectively, change with aging and this correlates with behavioral decline.111 Brizee reported changes in dendritic morphology following prenatal irradiation of squirrel monkeys with 2 Gy of X-rays.112 Parihar and Limoli and Parihar found changes in dendritic morphology and plasticity in hippocampal slices from mice following doses of 0.1 and 1 Gy of protons or 1 and 10 Gy of X-rays at early time points, 10 and 30 days post-irradiation.30,34 Significant reductions in the number of dendritic branches, branch points, and dendritic length were observed at 30 days indicating a reduction in dendritic complexity.30,34
Early Alterations in the Neuronal Signaling and the Hippocampal Progenitor Cell Microenvironment After Intracranial Irradiation
The hippocampus depends on the differentiation of thousands of neurons each day to subserve cognition and memory.113 These neurons are generated from progenitors in the subgranular zone of the dentate gyrus (DG) which secrete VEGF.114,115 Immature neurons are mainly post-mitotic and migrate into the DG granule cell layer (GCL) where they differentiate into mature neurons.116 Mature glutamatergic neurons in the GCL of the DG form spines in the molecular layer of the hippocampus where they receive input from the entorhinal cortex (EC).116 Ambient GABA (γ-aminobutyric acid) is instrumental to the proper maturation of neurons117 This process of neurogenesis is supported by an exquisite niche comprised of glial cells, neurons, astrocytes which secrete Wnt, oligodendrocytes, extracellular matrix and remodeling microvasculature.32,114,118 Damage to any component of this niche may be expected to dysregulate neurogenesis. Implantation of neuronal precursors has been found to mitigate radiation induced cognitive decline, although Monje and colleagues (2002) found that transplants of non-irradiated neural precursor cells did not differentiate into neurons in the irradiated hippocampus.89,91,119 The authors suggested the failure to differentiate was caused by, (1) an altered microenvironment due to disruption of the microvascular angiogenesis associated with adult neurogenesis, and (2) a marked increase in the number of activated microglia causing inflammation within the neurogenic zone.89,93 In a recent study however, Bostrom reported that a high single dose of 8 Gy to the brain of young male C57BL/6 mice caused over one year, progressive depletion of neurogenesis without disruption of the neurovascular niche.120
Several authors have speculated that early dysfunction of surviving neuronal cells alters the signaling microenvironment thus influencing progenitor cell differentiation and cognitive capacity over the long-term.5,121,122 For example Wu et al reported that the ex vivo irradiated rat hippocampal dentate gyrus exhibited early decreases in tyrosine phosphorylation and loss of excitatory N-methyl-D-aspartate receptors (NMDARs) from the cell surface.5 At the same time the surface expression of gamma-aminobutyric acid receptors (GABARs) was increased.5 Moore and coworkers (2015) observed subtle changes related to both hippocampal and cortical signaling (2014), discovering that the Homer1a receptor binding protein in young adult male Fischer 344 × Brown Norway rats at 48 h after 40 Gy of fractionated WBRT was elevated in the hippocampus and decreased in the cortex.4 Homer1a engages only family 1 metabotropic glutamate receptors (mGluR) and inhibits their binding to the synapse.123 At 2 months following irradiation homer1a expression was reduced in both the cortex and hippocampus, and correlated with downregulation of hippocampal glutamate receptor 1 and protein kinase Cγ, and cortical up-regulation of glutamate receptor 1 and protein kinase Cγ.4 Overexpression of Homer1a in the hippocampus is known to abolish maintenance of CA3-CA1 long-term potentiation (LTP), synaptic plasticity and to impair working memory.124-126 These are functional hippocampal effects that are duplicated by radiation exposure.46,127 Over the long term, months to years, dysregulated signaling may cause hippocampal precursor cells to differentiate into glia rather than neurons, resulting in a loss of neurons and the plasticity required for learning, memory, and other aspects of cognition.102,128-130 Importantly, other radiation induced effects such as inflammation may interact with signaling changes within the neurogenic SGZ niche to worsen dysregulation of progenitor cells in the hippocampus and elsewhere in the brain.
VI. Neuroimaging Evidence for Radiation Induced CNS Changes in the Context of Time Course and Prevalent Biological Hypotheses of Potential Mechanisms
Structural Changes in White and Gray Matter Brain Territories
Presently imaging is the only way by which to chronicle CNS damage in a radiotherapy patient over time. White matter plays a key role in linking cortical territories in cognition while gray matter (cortex) especially in the frontal, temporal and hippocampal regions plays a vital role in memory and cognition. Detection and interpretation of white matter damage is challenging with standard MR imaging approaches. The extent of detectable white matter damage may depend on age (Table 1, Figure 5).18,77,105,113 One study of glioblastoma patients found that standard chemoradiation led to progressive volume loss of the non-tumor bearing brain hemisphere, with DTI changes in the subventricular (progenitor cell) zone.131 Karunamuni et al used cortical volumetry of high resolution MRI and showed dose-dependent cortical atrophy in high grade glioma patients one year after PBRT (Figure 6).132 This study also showed a greater degree of dose-dependent cortical atrophy in the limbic and temporal lobes. These authors went on to study the selective vulnerability of cerebral cortex, showing that regions critical for higher-order cognition (entorhinal cortex and inferior parietal cortex) are the most sensitive to radiation-related atrophy.133 The same authors in another study modeled radiation-induced cortical atrophy, showing feasibility of sparing eloquent cortex with advanced planning techniques.134
Figure 5. The severity of radiation induced white matter damage increases with age.
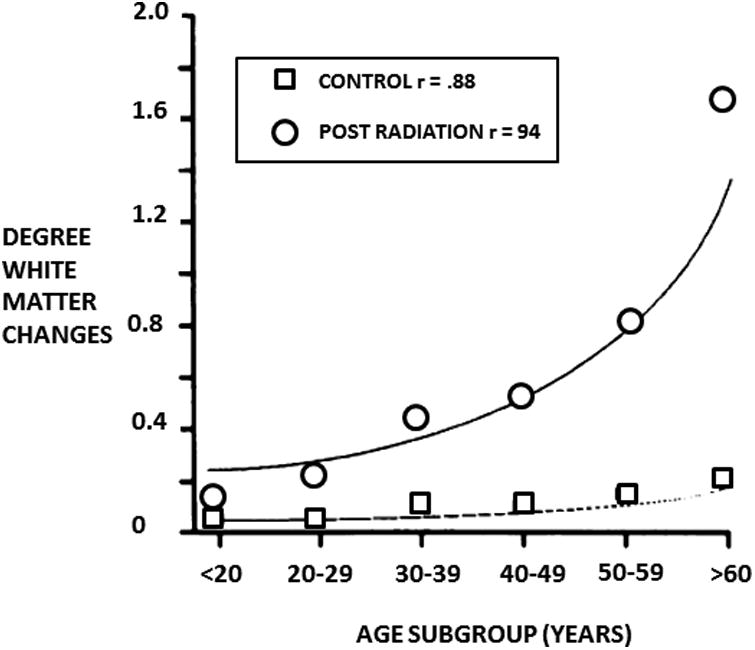
Graph of the average degree of supratentorial white matter changes versus age. Therapeutic and controls groups are included, the curves were fitted and correlation calculated using second-order polynomial regression. Taken from Tsuruda et al (AJR 149:165-171, 1987)77. Used with permission.
Figure 6. Cortical thinning according to dose at one year post-irradiation.
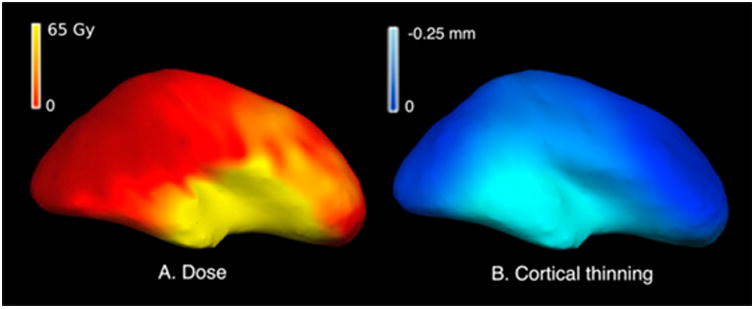
Cortical surface representation of (A) radiation dose in Gy and (B) cortical thinning at 1 year observed in an example patient. Regions receiving a higher dose show a greater degree of cortical thinning at 1 year post radiation therapy.132
Imaging Changes in the Hippocampus
Neuroimaging evidence of radiation-induced hippocampal changes are mixed. A study in head and neck cancer patients with low-dose incidental RT to the hippocampus found no volume changes, and the study cited above in glioblastoma patients found no hippocampal volume changes 6 months after start of chemoradiation.131,135 However one recent study found progressive hippocampal volume loss after chemo-radiation in malignant glioma patients.136 Seibert and colleagues analyzed hippocampal volume changes after PBRT in patients with primary brain tumors, and found significant radiation dose-dependent atrophy with a 6% volume loss in high-dose regions after one year, Figure 7.137 Studies correlating imaging biomarkers with neurocognitive effects in brain tumor patients are underway. A pilot functional imaging study using FDG PET showed that decreases in CNS metabolism after radiotherapy correlated with neurocognitive decline for problem solving and cognitive flexibility.61 No early changes seem have been reported in neuroimaging studies, but changes in neuronal structure and function may occur early along with vascular damage and inflammation, eventually leading to volume changes that are detectable on imaging.
Figure 7. Radiation dose-dependent hippocampal atrophy.
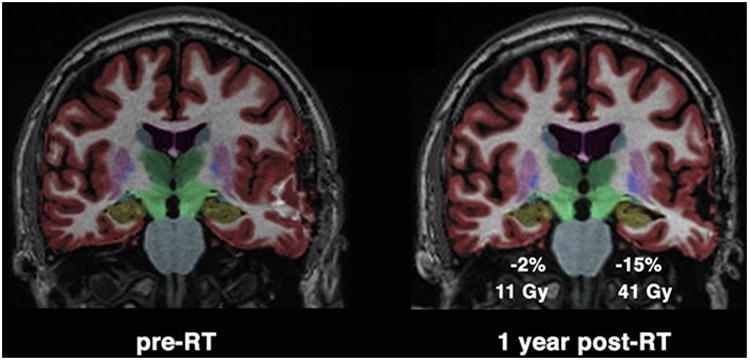
Coronal projection of brain MRI from a glioma patient pre-radiotherapy (pre-RT) on left, and one year post-radiotherapy (post-RT) on right. Color overlay shows automated segmentation of hippocampus in yellow. Greater percent decrease in hippocampal volume was seen in the hippocampus with higher mean dose of 41 Gy. 137
Early Neuronal Abnormalities Detected by MR Spectroscopy
Neuronal damage in patients may be signaled early after irradiation by changes in brain chemical constituents.138 N-acetylaspartate (NAA) is a neuronal marker, and Walecki et al reported a decline in the Cr/NAA ratio in brain tumor patients one month after WBRT with 60 Gy given in fractions of 1.8-2 Gy.138 The authors attributed this effect to neuronal dysfunction rather than neuronal loss.138 Sundgren et al also found early NAA decreases that persisted, being evident at 3 weeks, 1 month and 6 months after 50.4-59.4 Gy of fractionated PBRT.139 These radiotherapy patients had low grade glioma (LGG)or benign brain tumors, and, importantly, NAA changes developed in brain regions that appeared normal on MRI imaging.139 Movsas et al in a small study examined NAA levels in lung cancer patients receiving either palliative or prophylactic fractionated 30-37.5 Gy WBRT, and found a statistically valid decrease in NAA, with no significant difference between prophylactic and palliative groups.140 The timeframes generally described by these studies align with the timeframe for radiation induced changes in neuronal architecture reported by Parihar et al.30,34
Early White Matter Changes After Irradiation Revealed by Diffusion Imaging (DI)
Novel, high resolution imaging techniques can be used to study radiation-induced brain injury with precision and high spatial resolution. Diffusion-weighted imaging (DWI) is more a sensitive MR based technique by which microstructural changes in white matter integrity may be detectable141,142. DWI measures and models the diffusion of water at the cellular level143 and diffusion-tensor imaging (DTI) models the motion of water as an ellipse, with derived metrics allowing the study of white matter integrity.144 Several studies have used DTI to study the effects of radiation on normal appearing brain white matter, specifically as biomarkers of demyelination and axonal dysfunction shown in preclinical models.27,131,142,145,146 While one study suggested that DTI was only sensitive to radiation changes above a threshold dose of 45-50 Gy,147 others have shown changes in DTI metrics with doses as low as 5-15 Gy and dose dependent DTI changes.27,146,148 Importantly, Connor et al found CNS white matter changes on DTI appearing as early as one month after irradiation of glioma patients.27
Diffusion Imaging and Synergism Between Different Forms of CNS Radiation Damage
Connor and colleagues utilized advanced diffusion imaging with different diffusion weightings, showing dose and time dependent white matter changes which favor a primarily extra-axonal component of white matter injury.27 This suggests early changes in the white matter microenvironment like neuroinflammation may contribute to white matter/axonal injury. Another recent study found that diffusion changes in individual white matter bundles were associated with maximum dose to those tracts, implying serial radiation injury.149 There also appears to be regional susceptibility of white matter damage. A study of medulloblastoma survivors found greater diffusion changes in the frontal versus parietal lobes.150 Another study found prominent diffusion changes in the fornix and cingula after WBRT.151 Several DTI studies have correlated white matter diffusion metrics with neurocognitive changes, for example Chapman and colleagues found that diffusion changes in the parahippocampal cingulum were associated with late decline in verbal recall after radiotherapy.152 Khong correlated changes in diffusion parameters after WBRT in childhood cancer survivors with intelligence quotient scores.153
VII. Conclusions
Given the fact that the CNS contains many structures potentially sensitive to radiation, it is probable that the irreversible cognitive deterioration that so often follows intracranial radiotherapy is multifactorial in its origins.27 The present review posits the concept that early brain tissue abnormalities, which have in fact recently been identified, may lead to chronic interactive pathologies that progress to permanent cognitive deterioration. For example early inflammation affects the hippocampal microenvironment and may exacerbate dysregulated neurogenesis because of neuronal damage and altered signaling in the progenitor cell microenvironment. Widespread inflammation may affect white matter integrity and oligodendrocyte progenitors elsewhere in the brain.154 In addition white matter comprises part of progenitor cell niches in the brain and thus may adversely affect progenitors and repair mechanisms.155 A key need at the present juncture is a framework of how differential brain compartment susceptibilities are distributed across patient populations and in distinct preclinical models. This needs to be accompanied by an experimentally validated definition of the nature and extent of early CNS damage according to specific radiation dose ranges and fractionation schedules, and how such early damage evolves and synergizes over the long term to create permanent cognitive disability.26
The lack of an overview and mechanistic understanding of radiation induced cognitive decline has forced clinical investigators to base the selection of candidate therapeutics on extrapolations from other CNS disorders, with the unsurprising result that patient responses have been mixed and short term in nature. Methylphenidate, which in the past has yielded some beneficial results, and memantine, which is in clinical testing, are both well tolerated and may be helpful in select radiotherapy patients.156,157-159 A current clinical trial (NRG CC-001) is examining memantine and WBRT with and without hippocampal sparing. Acetylcholinesterase inhibitors, e.g., donepezil, are being pursued as they have produced some positive results in Alzheimers and other dementias.18,160,161 On the other hand anti-VEGF agents in brain tumor patients have elicited serious concerns since in clinical testing the small molecule VEGF-A inhibitor bevacizumab appears to have worsened cognitive decline by possibly further inhibiting hippocampal plasticity.162
New strategies may evolve from recent demonstrations that neuronal architecture may be damaged by radiation. Protein Kinase C (PKC) over expression reduces neuronal dendritic spine density in the hippocampus and PFC.163,164 Hence PKC inhibitors such as chelerythrine, and staurosporine analogs such as midostaurin which has been approved for use in patients, may help protect against deleterious changes in dendritic architecture.164,165 Benzothiazole amphiphiles promote the formation of dendritic spines in primary hippocampal neurons and may help overcome the effects of radiation.166 In addition recent data suggests that stem and progenitor cells are likely key targets in radiation brain injury and cognitive decline, stem cell niches may be compromised by inflammation, vascular damage, and extracellular matrix destruction. Stem cell niches are distributed throughout the brain, so one approach may be to ‘re-engineer’ CNS stem cell niches soon after irradiation.167 Aggressive and early use of anti-inflammatory agents may prove beneficial, along with direct injection or targeted administration of nanoparticles bearing biomimetic extracellular scaffolds which have shown benefits in animal models of inflammatory CNS injury, gels, bioactive signaling molecules for stem and microvessel endothelial cells, and stem or progenitor cells.168 Moreover, small molecules such as fluoxetine, which is a selective serotonin reuptake inhibitor, have promoted the maturation of neurons and enhanced neurogenesis in animal models.169
Future studies may offer translational promise by focusing on newly emerging data showing early post-irradiation changes that have the potential to impact neuronal support elements and the signaling environment in neuronal progenitor cell niches within the brain. In this regard progress may be expected based on, (1) the careful consideration of radiation dosing regimens, (2) more sensitive brain imaging methods for longitudinal biomarker18 and anatomically oriented studies, especially in the context of CNS changes developing before 6 months after irradiation, and not all patients may be equally vulnerable (3) subtle morphologic and functional changes on the neuronal level,18 (4) the role of inflammation, and (5) the long-term signaling microenvironment in terms of neuronal progenitor cell differentiation. In aggregate these elements may facilitate a deeper appreciation of evolving radiation induced brain abnormalities at multiple levels. This would certainly establish a more concrete foundation for rationally based studies aimed towards the identification of early preventative measures and therapies to impede degenerative processes before cognitive decline can become permanent.
Key Points.
Intracranial radiotherapy leads to permanent and significant cognitive disability in 50-90% of patients.
The pathophysiology remains poorly understood and there are no effective preventative measures or long-term treatments.
Historically, the primary focus has been on markers of damage and cognitive decline appearing 6 months to 1 year or more after irradiation.
More sensitive imaging and new lines of inquiry have revealed subtle evidence of central nervous system (CNS) damage much sooner than 6 months after radiation.
These early forms of CNS damage may persist and synergize over time to form long-term and irreversible deficits in neuronal and supporting cell lineages vital to cognition.
Consideration of early forms of radiation induced CNS damage may help identify early treatments to reverse degenerative processes before they can cause permanent disability
Review Criteria.
The database searched was primarily PUBMED together with extensive google inquiries based on search terms including; cognitive decline/deterioration and radiation, intra/cranial radiation/ radiotherapy, progenitor cells, glioma, medulloblastoma, radiotherapy brain tumors, radiation vascular, glial cells and radiation, hippocampus physiology and anatomy, hippocampus and radiation, inflammation and radiation, neurons and radiation, among others too numerous to list. Primarily peer reviewed research papers and recent reviews were examined. Publications listed in review articles were acquired and considered, and frequently their bibliographies were also pursued. Publications older than 1990 were generally not included, the majority of papers are 2-3 years old, and the emphasis was on publications less than 10 years old. However, some older papers showing early radiation effects in the brain and vasculature were included, as these had been ignored for many years, but are now important landmarks and relevant to recent thinking that acute effects do occur and that these may evolve and conspire to cause permanent cognitive disability after radiotherapy.
Biographies
Milan Makale, PhD, Assistant Professor, Radiation Medicine and Applied Sciences, UC San Diego Moores Cancer Center, Dr. Milan Makale is an assistant professor at the UC San Diego Moores Cancer Center. Dr. Makale's background is in radiation biology and bioengineering, and his research interests are focused on improving the efficacy and tolerability of radiation therapy for cancer. Dr. Makale is developing tumor selective small molecule and nanoparticle based radiation sensitizers for solid tumor therapy. He is also pursuing improved understanding and characterization of normal tissue injury that is associated with radiotherapy, and is exploring nanoparticle and stem cell niche engineering based approaches to promote tissue recovery.
Carrie McDonald, PhD, ABPP-CN, Associate Professor of Psychiatry, Center for Multimodal Imaging and Genetics (CMIG) University of California, San Diego, Dr. Carrie McDonald is an Associate Professor of Psychiatry at UC San Diego and a board-certified neuropsychologist with expertise in the presurgical evaluation of patients with epilepsy and primary brain tumors. Her research focuses on multimodal imaging of patients with epilepsy for predicting cognitive and seizures outcomes following neurosurgical interventions, as well as understanding the effects of radiation therapy on cognitive decline in patients with brain tumors. She directs the Brain Tumor Neuropsychological Assessment Clinic at UC San Diego and is an active member of the Center for Multimodal Imaging and Genetics.
Jona Hattangadi-Gluth, MD, Assistant Professor of Radiation Medicine and Applied Sciences, Associate Director, Imaging Research, Center for Translational Radiation Medicine and Imaging, Dr. Jona Hattangadi-Gluth is a board-certified radiation oncologist and Chief of the Central Nervous System Tumor Service in Radiation Medicine at UC San Diego. She is a national expert in the treatment of patients with brain tumors, serving on the National Comprehensive Cancer Network panel for the treatment of brain tumors. Her research focuses on the use of advanced MRI techniques in radiation planning and measuring response to radiation in brain tissue. She is the principal investigator on several investigator-initiated clinical trials including a longitudinal study of advanced diffusion imaging and serial neurocognitive testing to measure radiation-induced injury in brain tumor patients.
Santosh Kesari, MD, PhD, Chair, Department of Translational Neuro-oncology and Neurotherapeutics, John Wayne, Cancer Institute. Director of Neuro-oncology, Providence Saint John’s Health Center, Dr. Santosh Kesari is a board-certified neurologist and neuro-oncologist and is currently Chair, Department of Translational Neuro-oncology and Neurotherapeutics, John Wayne Cancer Institute. He is also Director of Neuro-oncology, Providence Saint John's Health Center and Member, Los Angeles Biomedical Research Institute. Dr. Kesari's research focuses on improving our understanding of the molecular and biological basis of brain tumors. A physician/scientist, Kesari leverages his background in normal tissue and tumor biology, and radiation therapy to, (1) better understand the natural history of brain tumors and identify potential therapeutics, and (2) develop strategies for addressing the recovery of normal tissues after chemoradiotherapy.
References
- 1.Chi A, Komaki R. Treatment of brain metastasis from lung cancer. Cancers (Basel) 2010;2:2100–2137. doi: 10.3390/cancers2042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi L, et al. Aging masks detection of radiation-induced brain injury. Brain Res. 2011;1385:307–316. doi: 10.1016/j.brainres.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greene-Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res. 2013;19:2294–2300. doi: 10.1158/1078-0432.CCR-11-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore ED, Kooshki M, Wheeler KT, Metheny-Barlow LJ, Robbins ME. Differential expression of Homer1a in the hippocampus and cortex likely plays a role in radiation-induced brain injury. Radiat Res. 2014;181:21–32. doi: 10.1667/RR13475.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu PH, et al. Radiation induces acute alterations in neuronal function. PLoS One. 2012;7:e37677. doi: 10.1371/journal.pone.0037677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McTyre E, Scott J, Chinnaiyan P. Whole brain radiotherapy for brain metastasis. Surg Neurol Int. 2013;4:S236–244. doi: 10.4103/2152-7806.111301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owonikoko TK, et al. Current approaches to the treatment of metastatic brain tumours. Nat Rev Clin Oncol. 2014;11:203–222. doi: 10.1038/nrclinonc.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDuff SG, et al. Neurocognitive assessment following whole brain radiation therapy and radiosurgery for patients with cerebral metastases. J Neurol Neurosurg Psychiatry. 2013;84:1384–1391. doi: 10.1136/jnnp-2013-305166. [DOI] [PubMed] [Google Scholar]
- 9.Marsh JC, Gielda BT, Herskovic AM, Abrams RA. Cognitive Sparing during the Administration of Whole Brain Radiotherapy and Prophylactic Cranial Irradiation: Current Concepts and Approaches. J Oncol. 2010;2010:198208. doi: 10.1155/2010/198208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jenrow KA, et al. Selective inhibition of microglia-mediated neuroinflammation mitigates radiation-induced cognitive impairment. Radiat Res. 2013;179:549–556. doi: 10.1667/RR3026.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippitz B, et al. Stereotactic radiosurgery in the treatment of brain metastases: the current evidence. Cancer Treat Rev. 2014;40:48–59. doi: 10.1016/j.ctrv.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Phillips MH, Stelzer KJ, Griffin TW, Mayberg MR, Winn HR. Stereotactic radiosurgery: a review and comparison of methods. J Clin Oncol. 1994;12:1085–1099. doi: 10.1200/JCO.1994.12.5.1085. [DOI] [PubMed] [Google Scholar]
- 13.Bilimagga RS, et al. Role of palliative radiotherapy in brain metastases. Indian J Palliat Care. 2009;15:71–75. doi: 10.4103/0973-1075.53588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadasadawala T, Gupta S, Bagul V, Patil N. Brain metastases from breast cancer: management approach. J Cancer Res Ther. 2007;3:157–165. doi: 10.4103/0973-1482.37409. [DOI] [PubMed] [Google Scholar]
- 15.Packer RJ, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children's Cancer Group Study. J Clin Oncol. 1999;17:2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 16.Davis CM, et al. Effects of X-ray radiation on complex visual discrimination learning and social recognition memory in rats. PLoS One. 2014;9:e104393. doi: 10.1371/journal.pone.0104393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi L, Molina DP, Robbins ME, Wheeler KT, Brunso-Bechtold JK. Hippocampal neuron number is unchanged 1 year after fractionated whole-brain irradiation at middle age. Int J Radiat Oncol Biol Phys. 2008;71:526–532. doi: 10.1016/j.ijrobp.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greene-Schloesser D, et al. Radiation-induced brain injury: A review. Front Oncol. 2012;2:73. doi: 10.3389/fonc.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer SL, Reddick WE, Gajjar A. Understanding the cognitive impact on children who are treated for medulloblastoma. J Pediatr Psychol. 2007;32:1040–1049. doi: 10.1093/jpepsy/jsl056. [DOI] [PubMed] [Google Scholar]
- 20.Outcomes of cancer treatment for technology assessment and cancer treatment guidelines. American Society of Clinical Oncology. J Clin Oncol. 1996;14:671–679. doi: 10.1200/JCO.1996.14.2.671. [DOI] [PubMed] [Google Scholar]
- 21.Gustafsson M, Edvardsson T, Ahlstrom G. The relationship between function, quality of life and coping in patients with low-grade gliomas. Support Care Cancer. 2006;14:1205–1212. doi: 10.1007/s00520-006-0080-3. [DOI] [PubMed] [Google Scholar]
- 22.Frost MH, Sloan JA. Quality of life measurements: a soft outcome--or is it? Am J Manag Care. 2002;8:S574–579. [PubMed] [Google Scholar]
- 23.Lin NU, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol. 2013;14:e407–416. doi: 10.1016/S1470-2045(13)70308-5. [DOI] [PubMed] [Google Scholar]
- 24.Lee YW, Cho HJ, Lee WH, Sonntag WE. Whole brain radiation-induced cognitive impairment: pathophysiological mechanisms and therapeutic targets. Biomol Ther (Seoul) 2012;20:357–370. doi: 10.4062/biomolther.2012.20.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attia A, Page BR, Lesser GJ, Chan M. Treatment of radiation-induced cognitive decline. Curr Treat Options Oncol. 2014;15:539–550. doi: 10.1007/s11864-014-0307-3. [DOI] [PubMed] [Google Scholar]
- 26.Rooney JW, Laack NN. Pharmacological interventions to treat or prevent neurocognitive decline after brain radiation. CNS Oncol. 2013;2:531–541. doi: 10.2217/cns.13.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connor M, et al. Dose-dependent white matter damage after brain radiotherapy. Radiother Oncol. 2016 doi: 10.1016/j.radonc.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong CL, Gyato K, Awadalla AW, Lustig R, Tochner ZA. A critical review of the clinical effects of therapeutic irradiation damage to the brain: the roots of controversy. Neuropsychol Rev. 2004;14:65–86. doi: 10.1023/b:nerv.0000026649.68781.8e. [DOI] [PubMed] [Google Scholar]
- 29.Shi L, et al. Maintenance of white matter integrity in a rat model of radiation-induced cognitive impairment. J Neurol Sci. 2009;285:178–184. doi: 10.1016/j.jns.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parihar VK, et al. Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Struct Funct. 2015;220:1161–1171. doi: 10.1007/s00429-014-0709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ljubimova NV, Levitman MK, Plotnikova ED, Eidus L. Endothelial cell population dynamics in rat brain after local irradiation. Br J Radiol. 1991;64:934–940. doi: 10.1259/0007-1285-64-766-934. [DOI] [PubMed] [Google Scholar]
- 32.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Peiffer AM, et al. Neuroanatomical target theory as a predictive model for radiation-induced cognitive decline. Neurology. 2013;80:747–753. doi: 10.1212/WNL.0b013e318283bb0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parihar VK, Limoli CL. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci U S A. 2013;110:12822–12827. doi: 10.1073/pnas.1307301110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal S, Manchanda P, Vogelbaum MA, Ohlfest JR, Elmquist WF. Function of the blood-brain barrier and restriction of drug delivery to invasive glioma cells: findings in an orthotopic rat xenograft model of glioma. Drug Metab Dispos. 2013;41:33–39. doi: 10.1124/dmd.112.048322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Furnari FB, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 37.Puhalla S, et al. Unsanctifying the sanctuary: challenges and opportunities with brain metastases. Neuro Oncol. 2015;17:639–651. doi: 10.1093/neuonc/nov023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Packer RJ, Zhou T, Holmes E, Vezina G, Gajjar A. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children's Oncology Group trial A9961. Neuro Oncol. 2013;15:97–103. doi: 10.1093/neuonc/nos267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saury JM, Emanuelson I. Cognitive consequences of the treatment of medulloblastoma among children. Pediatr Neurol. 2011;44:21–30. doi: 10.1016/j.pediatrneurol.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann M. The human frontal lobes and frontal network systems: an evolutionary, clinical, and treatment perspective. ISRN Neurol. 2013;2013:892459. doi: 10.1155/2013/892459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parihar VK, et al. What happens to your brain on the way to Mars. Sci Adv. 2015;1 doi: 10.1126/sciadv.1400256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuster JM. Frontal lobe and cognitive development. J Neurocytol. 2002;31:373–385. doi: 10.1023/a:1024190429920. [DOI] [PubMed] [Google Scholar]
- 44.Braun U, et al. Dynamic reconfiguration of frontal brain networks during executive cognition in humans. Proc Natl Acad Sci U S A. 2015;112:11678–11683. doi: 10.1073/pnas.1422487112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy ES, et al. Review of cranial radiotherapy-induced vasculopathy. J Neurooncol. 2015;122:421–429. doi: 10.1007/s11060-015-1732-2. [DOI] [PubMed] [Google Scholar]
- 46.Pellmar TC, Schauer DA, Zeman GH. Time- and dose-dependent changes in neuronal activity produced by X radiation in brain slices. Radiat Res. 1990;122:209–214. [PubMed] [Google Scholar]
- 47.Ruben JD, et al. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65:499–508. doi: 10.1016/j.ijrobp.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, et al. Radiation induced temporal lobe necrosis in patients with nasopharyngeal carcinoma: a review of new avenues in its management. Radiat Oncol. 2011;6:128. doi: 10.1186/1748-717X-6-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dropcho EJ. Neurotoxicity of radiation therapy. Neurol Clin. 2010;28:217–234. doi: 10.1016/j.ncl.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 50.Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215–1228. doi: 10.1016/0360-3016(80)90175-3. [DOI] [PubMed] [Google Scholar]
- 51.Gangloff H, Haley TJ. Effects of X-irradiation on spontaneous and evoked brain electrical activity in cats. Radiat Res. 1960;12:694–704. [PubMed] [Google Scholar]
- 52.Bassant MH, Court L. Effects of whole-body gamma irradiation on the activity of rabbit hippocampal neurons. Radiat Res. 1978;75:593–606. [PubMed] [Google Scholar]
- 53.Pellmar TC, Lepinski DL. Gamma radiation (5-10 Gy) impairs neuronal function in the guinea pig hippocampus. Radiat Res. 1993;136:255–261. [PubMed] [Google Scholar]
- 54.Gerstner HB, Brooks PM, Smith SA. Effect of x-radiation on the flow of perfusion fluid through the isolated rabbit's ear. Am J Physiol. 1955;182:459–461. doi: 10.1152/ajplegacy.1955.182.3.459. [DOI] [PubMed] [Google Scholar]
- 55.Krueger H, Wagelie EG, Bogart R. Radiation and responses of rabbit ear artery to xylene, alcohol, and epinephrine. Radiat Res. 1967;30:420–430. [PubMed] [Google Scholar]
- 56.Li YQ, Chen P, Haimovitz-Friedman A, Reilly RM, Wong CS. Endothelial apoptosis initiates acute blood-brain barrier disruption after ionizing radiation. Cancer Res. 2003;63:5950–5956. [PubMed] [Google Scholar]
- 57.Brown WR, Thore CR, Moody DM, Robbins ME, Wheeler KT. Vascular damage after fractionated whole-brain irradiation in rats. Radiat Res. 2005;164:662–668. doi: 10.1667/rr3453.1. [DOI] [PubMed] [Google Scholar]
- 58.Finet P, Rooijakkers H, Godfraind C, Raftopoulos C. Delayed compressive angiomatous degeneration in a case of mesial temporal lobe epilepsy treated by gamma knife radiosurgery: case report. Neurosurgery. 2010;67:218–220. doi: 10.1227/01.NEU.0000370011.36820.ED. discussion 220. [DOI] [PubMed] [Google Scholar]
- 59.Desai SS, Paulino AC, Mai WY, Teh BS. Radiation-induced moyamoya syndrome. Int J Radiat Oncol Biol Phys. 2006;65:1222–1227. doi: 10.1016/j.ijrobp.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 60.Ullrich NJ, et al. Moyamoya following cranial irradiation for primary brain tumors in children. Neurology. 2007;68:932–938. doi: 10.1212/01.wnl.0000257095.33125.48. [DOI] [PubMed] [Google Scholar]
- 61.Hahn CA, et al. Dose-dependent effects of radiation therapy on cerebral blood flow, metabolism, and neurocognitive dysfunction. Int J Radiat Oncol Biol Phys. 2009;73:1082–1087. doi: 10.1016/j.ijrobp.2008.05.061. [DOI] [PubMed] [Google Scholar]
- 62.Abayomi OK. Pathogenesis of cognitive decline following therapeutic irradiation for head and neck tumors. Acta Oncol. 2002;41:346–351. doi: 10.1080/028418602760169389. [DOI] [PubMed] [Google Scholar]
- 63.Calvo W, Hopewell JW, Reinhold HS, Yeung TK. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br J Radiol. 1988;61:1043–1052. doi: 10.1259/0007-1285-61-731-1043. [DOI] [PubMed] [Google Scholar]
- 64.Filley CM. White matter dementia. Ther Adv Neurol Disord. 2012;5:267–277. doi: 10.1177/1756285612454323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao W, et al. Administration of the peroxisomal proliferator-activated receptor gamma agonist pioglitazone during fractionated brain irradiation prevents radiation-induced cognitive impairment. Int J Radiat Oncol Biol Phys. 2007;67:6–9. doi: 10.1016/j.ijrobp.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 66.Lee TC, et al. Chronic administration of the angiotensin-converting enzyme inhibitor, ramipril, prevents fractionated whole-brain irradiation-induced perirhinal cortex-dependent cognitive impairment. Radiat Res. 2012;178:46–56. doi: 10.1667/rr2731.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hansson E. Astroglia from defined brain regions as studied with primary cultures. Prog Neurobiol. 1988;30:369–397. doi: 10.1016/0301-0082(88)90008-1. [DOI] [PubMed] [Google Scholar]
- 68.Pal B. Astrocytic Actions on Extrasynaptic Neuronal Currents. Front Cell Neurosci. 2015;9:474. doi: 10.3389/fncel.2015.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Doyle DM, Einhorn LH. Delayed effects of whole brain radiotherapy in germ cell tumor patients with central nervous system metastases. Int J Radiat Oncol Biol Phys. 2008;70:1361–1364. doi: 10.1016/j.ijrobp.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 70.Hwang SY, et al. Ionizing radiation induces astrocyte gliosis through microglia activation. Neurobiol Dis. 2006;21:457–467. doi: 10.1016/j.nbd.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 71.Chiang CS, McBride WH, Withers HR. Radiation-induced astrocytic and microglial responses in mouse brain. Radiother Oncol. 1993;29:60–68. doi: 10.1016/0167-8140(93)90174-7. [DOI] [PubMed] [Google Scholar]
- 72.Ho G, Zhang C, Zhuo L. Non-invasive fluorescent imaging of gliosis in transgenic mice for profiling developmental neurotoxicity. Toxicol Appl Pharmacol. 2007;221:76–85. doi: 10.1016/j.taap.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 73.Moore ED, Kooshki M, Metheny-Barlow LJ, Gallagher PE, Robbins ME. Angiotensin-(1-7) prevents radiation-induced inflammation in rat primary astrocytes through regulation of MAP kinase signaling. Free Radic Biol Med. 2013;65:1060–1068. doi: 10.1016/j.freeradbiomed.2013.08.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li YQ, Jay V, Wong CS. Oligodendrocytes in iation-induced inflammation in rat primaryergo radiation-induced apoptosis. Cancer Res. 1996;56:5417–5422. [PubMed] [Google Scholar]
- 75.Kurita H, et al. Radiation-induced apoptosis of oligodendrocytes in the adult rat brain. Neurol Res. 2001;23:869–874. doi: 10.1179/016164101101199324. [DOI] [PubMed] [Google Scholar]
- 76.Panagiotakos G, et al. Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS One. 2007;2:e588. doi: 10.1371/journal.pone.0000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuruda JS, et al. Radiation effects on cerebral white matter: MR evaluation. AJR Am J Roentgenol. 1987;149:165–171. doi: 10.2214/ajr.149.1.165. [DOI] [PubMed] [Google Scholar]
- 78.Padovani L, Andre N, Constine LS, Muracciole X. Neurocognitive function after radiotherapy for paediatric brain tumours. Nat Rev Neurol. 2012;8:578–588. doi: 10.1038/nrneurol.2012.182. [DOI] [PubMed] [Google Scholar]
- 79.Shi L, et al. Spatial learning and memory deficits after whole-brain irradiation are associated with changes in NMDA receptor subunits in the hippocampus. Radiat Res. 2006;166:892–899. doi: 10.1667/RR0588.1. [DOI] [PubMed] [Google Scholar]
- 80.Chen H, et al. Ionizing Radiation Perturbs Cell Cycle Progression of Neural Precursors in the Subventricular Zone Without Affecting Their Long-Term Self-Renewal. ASN Neuro. 2015;7 doi: 10.1177/1759091415578026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hellstrom NA, Bjork-Eriksson T, Blomgren K, Kuhn HG. Differential recovery of neural stem cells in the subventricular zone and dentate gyrus after ionizing radiation. Stem Cells. 2009;27:634–641. doi: 10.1634/stemcells.2008-0732. [DOI] [PubMed] [Google Scholar]
- 82.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mizumatsu S, et al. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003;63:4021–4027. [PubMed] [Google Scholar]
- 84.Kalm M, Karlsson N, Nilsson MK, Blomgren K. Loss of hippocampal neurogenesis, increased novelty-induced activity, decreased home cage activity, and impaired reversal learning one year after irradiation of the young mouse brain. Exp Neurol. 2013;247:402–409. doi: 10.1016/j.expneurol.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 85.Manda K, Ueno M, Anzai K. Cranial irradiation-induced inhibition of neurogenesis in hippocampal dentate gyrus of adult mice: attenuation by melatonin pretreatment. J Pineal Res. 2009;46:71–78. doi: 10.1111/j.1600-079X.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- 86.Tada E, Parent JM, Lowenstein DH, Fike JR. X-irradiation causes a prolonged reduction in cell proliferation in the dentate gyrus of adult rats. Neuroscience. 2000;99:33–41. doi: 10.1016/s0306-4522(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 87.Rola R, et al. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Raber J, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- 89.Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002;8:955–962. doi: 10.1038/nm749. [DOI] [PubMed] [Google Scholar]
- 90.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res. 2000;153:357–370. doi: 10.1667/0033-7587(2000)153[0357:trotcn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 91.Acharya MM, et al. Human neural stem cell transplantation ameliorates radiation-induced cognitive dysfunction. Cancer Res. 2011;71:4834–4845. doi: 10.1158/0008-5472.CAN-11-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Acharya MM, Roa DE, Bosch O, Lan ML, Limoli CL. Stem cell transplantation strategies for the restoration of cognitive dysfunction caused by cranial radiotherapy. J Vis Exp. 2011 doi: 10.3791/3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Warrington JP, Csiszar A, Mitschelen M, Lee YW, Sonntag WE. Whole brain radiation-induced impairments in learning and memory are time-sensitive and reversible by systemic hypoxia. PLoS One. 2012;7:e30444. doi: 10.1371/journal.pone.0030444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 95.Lee WH, Sonntag WE, Mitschelen M, Yan H, Lee YW. Irradiation induces regionally specific alterations in pro-inflammatory environments in rat brain. Int J Radiat Biol. 2010;86:132–144. doi: 10.3109/09553000903419346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ballesteros-Zebadua P, Chavarria A, Celis MA, Paz C, Franco-Perez J. Radiation-induced neuroinflammation and radiation somnolence syndrome. CNS Neurol Disord Drug Targets. 2012;11:937–949. doi: 10.2174/1871527311201070937. [DOI] [PubMed] [Google Scholar]
- 97.Kyrkanides S, et al. Cyclooxygenase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Brain Res Mol Brain Res. 2002;104:159–169. doi: 10.1016/s0169-328x(02)00353-4. [DOI] [PubMed] [Google Scholar]
- 98.Monje ML, et al. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Ann Neurol. 2007;62:515–520. doi: 10.1002/ana.21214. [DOI] [PubMed] [Google Scholar]
- 99.Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ingraham JP, Forbes ME, Riddle DR, Sonntag WE. Aging reduces hypoxia-induced microvascular growth in the rodent hippocampus. J Gerontol A Biol Sci Med Sci. 2008;63:12–20. doi: 10.1093/gerona/63.1.12. [DOI] [PubMed] [Google Scholar]
- 101.Park JA, Choi KS, Kim SY, Kim KW. Coordinated interaction of the vascular and nervous systems: from molecule- to cell-based approaches. Biochem Biophys Res Commun. 2003;311:247–253. doi: 10.1016/j.bbrc.2003.09.129. [DOI] [PubMed] [Google Scholar]
- 102.Licht T, et al. Reversible modulations of neuronal plasticity by VEGF. Proc Natl Acad Sci U S A. 2011;108:5081–5086. doi: 10.1073/pnas.1007640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kang SG, et al. Isolation and perivascular localization of mesenchymal stem cells from mouse brain. Neurosurgery. 2010;67:711–720. doi: 10.1227/01.NEU.0000377859.06219.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim JH, Jenrow KA, Brown SL. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat Oncol J. 2014;32:103–115. doi: 10.3857/roj.2014.32.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robbins ME, Bourland JD, Cline JM, Wheeler KT, Deadwyler SA. A model for assessing cognitive impairment after fractionated whole-brain irradiation in nonhuman primates. Radiat Res. 2011;175:519–525. doi: 10.1667/RR2497.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gonzalez Burgos I, Nikonenko I, Korz V. Dendritic spine plasticity and cognition. Neural Plast. 2012;2012:875156. doi: 10.1155/2012/875156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Frankfurt M, Luine V. The evolving role of dendritic spines and memory: Interaction(s) with estradiol. Horm Behav. 2015;74:28–36. doi: 10.1016/j.yhbeh.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yuste R, Bonhoeffer T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu Rev Neurosci. 2001;24:1071–1089. doi: 10.1146/annurev.neuro.24.1.1071. [DOI] [PubMed] [Google Scholar]
- 109.Schwechter B, Tolias KF. Cytoskeletal mechanisms for synaptic potentiation. Commun Integr Biol. 2013;6:e27343. doi: 10.4161/cib.27343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hains AB, Yabe Y, Arnsten AF. Chronic Stimulation of Alpha-2A-Adrenoceptors With Guanfacine Protects Rodent Prefrontal Cortex Dendritic Spines and Cognition From the Effects of Chronic Stress. Neurobiol Stress. 2015;2:1–9. doi: 10.1016/j.ynstr.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pereira AC, et al. Glutamatergic regulation prevents hippocampal-dependent age-related cognitive decline through dendritic spine clustering. Proc Natl Acad Sci U S A. 2014;111:18733–18738. doi: 10.1073/pnas.1421285111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brizzee KR, Ordy JM, Kaack MB, Beavers T. Effect of prenatal ionizing radiation on the visual cortex and hippocampus of newborn squirrel monkeys. J Neuropathol Exp Neurol. 1980;39:523–540. doi: 10.1097/00005072-198009000-00002. [DOI] [PubMed] [Google Scholar]
- 113.Shors TJ, Anderson ML, Curlik DM, 2nd, Nokia MS. Use it or lose it: how neurogenesis keeps the brain fit for learning. Behav Brain Res. 2012;227:450–458. doi: 10.1016/j.bbr.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kirby ED, Kuwahara AA, Messer RL, Wyss-Coray T. Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc Natl Acad Sci U S A. 2015;112:4128–4133. doi: 10.1073/pnas.1422448112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eriksson PS, et al. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 116.Seib DR, Martin-Villalba A. Neurogenesis in the Normal Ageing Hippocampus: A Mini-Review. Gerontology. 2015;61:327–335. doi: 10.1159/000368575. [DOI] [PubMed] [Google Scholar]
- 117.Christian KM, Song H, Ming GL. Functions and dysfunctions of adult hippocampal neurogenesis. Annu Rev Neurosci. 2014;37:243–262. doi: 10.1146/annurev-neuro-071013-014134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lie DC, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 119.Acharya MM, Christie LA, Hazel TG, Johe KK, Limoli CL. Transplantation of human fetal-derived neural stem cells improves cognitive function following cranial irradiation. Cell Transplant. 2014;23:1255–1266. doi: 10.3727/096368913X670200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bostrom M, Kalm M, Karlsson N, Hellstrom Erkenstam N, Blomgren K. Irradiation to the young mouse brain caused long-term, progressive depletion of neurogenesis but did not disrupt the neurovascular niche. J Cereb Blood Flow Metab. 2013;33:935–943. doi: 10.1038/jcbfm.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–2431. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- 122.Wong CS, Van der Kogel AJ. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv. 2004;4:273–284. doi: 10.1124/mi.4.5.7. [DOI] [PubMed] [Google Scholar]
- 123.Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Roloff AM, Anderson GR, Martemyanov KA, Thayer SA. Homer 1a gates the induction mechanism for endocannabinoid-mediated synaptic plasticity. J Neurosci. 2010;30:3072–3081. doi: 10.1523/JNEUROSCI.4603-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Celikel T, et al. Select overexpression of homer1a in dorsal hippocampus impairs spatial working memory. Front Neurosci. 2007;1:97–110. doi: 10.3389/neuro.01.1.1.007.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tappe-Theodor A, Fu Y, Kuner R, Neugebauer V. Homer1a signaling in the amygdala counteracts pain-related synaptic plasticity, mGluR1 function and pain behaviors. Mol Pain. 2011;7:38. doi: 10.1186/1744-8069-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou FW, Roper SN. Impaired hippocampal memory function and synaptic plasticity in experimental cortical dysplasia. Epilepsia. 2012;53:850–859. doi: 10.1111/j.1528-1167.2012.03431.x. [DOI] [PubMed] [Google Scholar]
- 128.Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- 129.Deisseroth K, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 130.Ge S, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Prust MJ, et al. Standard chemoradiation for glioblastoma results in progressive brain volume loss. Neurology. 2015;85:683–691. doi: 10.1212/WNL.0000000000001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Karunamuni R, et al. Dose-Dependent Cortical Thinning After Partial Brain Irradiation in High-Grade Glioma. Int J Radiat Oncol Biol Phys. 2016;94:297–304. doi: 10.1016/j.ijrobp.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Seibert, et al. Selective cortical vulnerability. IJROBP. 2016 abstract. [Google Scholar]
- 134.Karunamuni RA, et al. Radiation sparing of cerebral cortex in brain tumor patients using quantitative neuroimaging. Radiother Oncol. 2016 doi: 10.1016/j.radonc.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Olsson E, et al. Hippocampal volumes in patients exposed to low-dose radiation to the basal brain. A case-control study in long-term survivors from cancer in the head and neck region. Radiat Oncol. 2012;7:202. doi: 10.1186/1748-717X-7-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nolen SC, et al. The effects of sequential treatments on hippocampal volumes in malignant glioma patients. J Neurooncol. 2016;129:433–441. doi: 10.1007/s11060-016-2188-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Seibert, et al. Radiation dose-dependent hippocampal atrophy…. IJROBP. 2016 doi: 10.1016/j.ijrobp.2016.10.035. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Walecki J, et al. Role of short TE 1H-MR spectroscopy in monitoring of post-operation irradiated patients. Eur J Radiol. 1999;30:154–161. doi: 10.1016/s0720-048x(99)00053-4. [DOI] [PubMed] [Google Scholar]
- 139.Sundgren PC, et al. Metabolic alterations: a biomarker for radiation-induced normal brain injury-an MR spectroscopy study. J Magn Reson Imaging. 2009;29:291–297. doi: 10.1002/jmri.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Movsas B, et al. Quantifying radiation therapy-induced brain injury with whole-brain proton MR spectroscopy: initial observations. Radiology. 2001;221:327–331. doi: 10.1148/radiol.2212001648. [DOI] [PubMed] [Google Scholar]
- 141.Robbins ME, et al. Imaging radiation-induced normal tissue injury. Radiat Res. 2012;177:449–466. doi: 10.1667/rr2530.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang S, et al. Radiation induced brain injury: assessment of white matter tracts in a pre-clinical animal model using diffusion tensor MR imaging. J Neurooncol. 2013;112:9–15. doi: 10.1007/s11060-012-1031-0. [DOI] [PubMed] [Google Scholar]
- 143.White NS, et al. Diffusion-weighted imaging in cancer: physical foundations and applications of restriction spectrum imaging. Cancer Res. 2014;74:4638–4652. doi: 10.1158/0008-5472.CAN-13-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chan KC, et al. MRI of late microstructural and metabolic alterations in radiation-induced brain injuries. J Magn Reson Imaging. 2009;29:1013–1020. doi: 10.1002/jmri.21736. [DOI] [PubMed] [Google Scholar]
- 146.Ravn S, Holmberg M, Sorensen P, Frokjaer JB, Carl J. Differences in supratentorial white matter diffusion after radiotherapy--new biomarker of normal brain tissue damage? Acta Oncol. 2013;52:1314–1319. doi: 10.3109/0284186X.2013.812797. [DOI] [PubMed] [Google Scholar]
- 147.Haris M, et al. Serial diffusion tensor imaging to characterize radiation-induced changes in normal-appearing white matter following radiotherapy in patients with adult low-grade gliomas. Radiat Med. 2008;26:140–150. doi: 10.1007/s11604-007-0209-4. [DOI] [PubMed] [Google Scholar]
- 148.Nagesh V, et al. Radiation-induced changes in normal-appearing white matter in patients with cerebral tumors: a diffusion tensor imaging study. Int J Radiat Oncol Biol Phys. 2008;70:1002–1010. doi: 10.1016/j.ijrobp.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhu T, et al. Effect of the Maximum Dose on White Matter Fiber Bundles Using Longitudinal Diffusion Tensor Imaging. Int J Radiat Oncol Biol Phys. 2016;96:696–705. doi: 10.1016/j.ijrobp.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Qiu D, Kwong DL, Chan GC, Leung LH, Khong PL. Diffusion tensor magnetic resonance imaging finding of discrepant fractional anisotropy between the frontal and parietal lobes after whole-brain irradiation in childhood medulloblastoma survivors: reflection of regional white matter radiosensitivity? Int J Radiat Oncol Biol Phys. 2007;69:846–851. doi: 10.1016/j.ijrobp.2007.04.041. [DOI] [PubMed] [Google Scholar]
- 151.Chapman CH, et al. Regional variation in brain white matter diffusion index changes following chemoradiotherapy: a prospective study using tract-based spatial statistics. PLoS One. 2013;8:e57768. doi: 10.1371/journal.pone.0057768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chapman CH, et al. Diffusion tensor imaging of normal-appearing white matter as biomarker for radiation-induced late delayed cognitive decline. Int J Radiat Oncol Biol Phys. 2012;82:2033–2040. doi: 10.1016/j.ijrobp.2011.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Khong PL, et al. White matter anisotropy in post-treatment childhood cancer survivors: preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006;24:884–890. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- 154.Pleasure D, Soulika A, Singh SK, Gallo V, Bannerman P. Inflammation in white matter: clinical and pathophysiological aspects. Ment Retard Dev Disabil Res Rev. 2006;12:141–146. doi: 10.1002/mrdd.20100. [DOI] [PubMed] [Google Scholar]
- 155.Nunes MC, et al. Identification and isolation of multipotential neural progenitor cells from the subcortical white matter of the adult human brain. Nat Med. 2003;9:439–447. doi: 10.1038/nm837. [DOI] [PubMed] [Google Scholar]
- 156.Meyers CA, Weitzner MA, Valentine AD, Levin VA. Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. J Clin Oncol. 1998;16:2522–2527. doi: 10.1200/JCO.1998.16.7.2522. [DOI] [PubMed] [Google Scholar]
- 157.Brown PD, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15:1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McGough JJ, et al. Once-daily OROS methylphenidate is safe and well tolerated in adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2006;16:351–356. doi: 10.1089/cap.2006.16.351. [DOI] [PubMed] [Google Scholar]
- 159.Parsons CG, Danysz W, Quack G. Memantine is a clinically well tolerated N-methyl-D-aspartate (NMDA) receptor antagonist--a review of preclinical data. Neuropharmacology. 1999;38:735–767. doi: 10.1016/s0028-3908(99)00019-2. [DOI] [PubMed] [Google Scholar]
- 160.Howard R, et al. Donepezil and memantine for moderate-to-severe Alzheimer's disease. N Engl J Med. 2012;366:893–903. doi: 10.1056/NEJMoa1106668. [DOI] [PubMed] [Google Scholar]
- 161.Kleinberg L. Neurocognitive challenges in brain tumor survivors: is there anything we can do? J Clin Oncol. 2015;33:1633–1636. doi: 10.1200/JCO.2014.60.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fathpour P, et al. Bevacizumab treatment for human glioblastoma. Can it induce cognitive impairment? Neuro Oncol. 2014;16:754–756. doi: 10.1093/neuonc/nou013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Calabrese B, Halpain S. Essential role for the PKC target MARCKS in maintaining dendritic spine morphology. Neuron. 2005;48:77–90. doi: 10.1016/j.neuron.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 164.Hains AB, et al. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proc Natl Acad Sci U S A. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Starr P. Midostaurin the First Targeted Therapy to Improve Survival in AML: Potentially Practice-Changing. Am Health Drug Benefits. 2016;9:1–21. [PMC free article] [PubMed] [Google Scholar]
- 166.Cifelli JL, Dozier L, Chung TS, Patrick GN, Yang J. Benzothiazole Amphiphiles Promote the Formation of Dendritic Spines in Primary Hippocampal Neurons. J Biol Chem. 2016;291:11981–11992. doi: 10.1074/jbc.M115.701482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Williams CA, Lavik EB. Engineering the CNS stem cell microenvironment. Regen Med. 2009;4:865–877. doi: 10.2217/rme.09.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tysseling-Mattiace VM, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28:3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang HD, Dunnavant FD, Jarman T, Deutch AY. Effects of antipsychotic drugs on neurogenesis in the forebrain of the adult rat. Neuropsychopharmacology. 2004;29:1230–1238. doi: 10.1038/sj.npp.1300449. [DOI] [PubMed] [Google Scholar]
- 170.Burger PC, Mahley MS, Jr, Dudka L, Vogel FS. The morphologic effects of radiation administered therapeutically for intracranial gliomas: a postmortem study of 25 cases. Cancer. 1979;44:1256–1272. doi: 10.1002/1097-0142(197910)44:4<1256::aid-cncr2820440415>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 171.Fujii O, et al. White matter changes on magnetic resonance imaging following whole-brain radiotherapy for brain metastases. Radiat Med. 2006;24:345–350. doi: 10.1007/s11604-006-0039-9. [DOI] [PubMed] [Google Scholar]
- 172.Mabbott DJ, Noseworthy MD, Bouffet E, Rockel C, Laughlin S. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: correlation with IQ. Neuro Oncol. 2006;8:244–252. doi: 10.1215/15228517-2006-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chiang CS, McBride WH, Withers HR. Myelin-associated changes in mouse brain following irradiation. Radiother Oncol. 1993;27:229–236. doi: 10.1016/0167-8140(93)90079-n. [DOI] [PubMed] [Google Scholar]
- 174.Nakagaki H, Brunhart G, Kemper TL, Caveness WF. Monkey brain damage from radiation in the therapeutic range. J Neurosurg. 1976;44:3–11. doi: 10.3171/jns.1976.44.1.0003. [DOI] [PubMed] [Google Scholar]


