Abstract
Laccase-mediator systems (LMS) have been widely studied for their capacity to oxidize the nonphenolic subunits of lignin (70–90% of the polymer). The phenolic subunits (10–30% of the polymer), which can also be oxidized without mediators, have received considerably less attention. Consequently, it remains unclear to what extent the presence of a mediator influences the reactions of the phenolic subunits of lignin. To get more insight in this, UHPLC-MS was used to study the reactions of a phenolic lignin dimer (GBG), initiated by a laccase from Trametes versicolor, alone or in combination with the mediators HBT and ABTS. The role of HBT was negligible, as its oxidation by laccase occurred slowly in comparison to that of GBG. Laccase and laccase/HBT oxidized GBG at a comparable rate, resulting in extensive polymerization of GBG. In contrast, laccase/ABTS converted GBG at a higher rate, as GBG was oxidized both directly by laccase but also by ABTS radical cations, which were rapidly formed by laccase. The laccase/ABTS system resulted in Cα oxidation of GBG and coupling of ABTS to GBG, rather than polymerization of GBG. Based on these results, we propose reaction pathways of phenolic lignin model compounds with laccase/HBT and laccase/ABTS.
Keywords: Oxidation, Radical coupling, Trametes versicolor, ABTS, Hydroxybenzotriazole
Short abstract
This paper contributes to a more fundamental understanding of lignin modification by laccase-mediator systems.
Introduction
Lignin is one of the most abundant polymers in nature as a part of plant cell walls and is currently considered as a sustainable precursor for chemicals or materials.1−4 It is built up from sinapyl alcohol, coniferyl alcohol, and p-coumaroyl alcohol (S, G, and H units, respectively), which couple via radical polymerization to form a variety of C–C and C–O interunit linkages. The β-O-4 linkage is the most abundant one, accounting for approximately 45–94% of the total interunit linkages, in lignins mildly isolated from plant materials.3 Lignin contains phenolic and nonphenolic subunits, which account for 10–30 and 70–90% of the polymer, respectively.5
Although the valorization of lignin is still underexploited,3 three main routes are considered relevant. The first is via lignin polymerization, which may result in improved binders and adhesives.6 Second, lignin depolymerization may lead to high-value low-molecular-weight aromatics.1,2 A third option is the functionalization of lignin via grafting of specific molecules onto lignin.7 A green alternative for lignin valorization is via biocatalysis and, in particular, laccase is known as one of the key activities toward lignin. Various lignin modifications are reported for laccase, such as polymerization,4,8−10 depolymerization,11−13 Cα oxidation,14 and demethylation.15 It is poorly understood, however, how to direct lignin modification by laccase toward one of these modifications. Thus, for an effective use of laccase in lignin valorization, it is essential to have a better understanding on how to control lignin modification by laccase.
Laccases (E.C. 1.10.3.2) are oxidases that couple the reduction of molecular oxygen to the one-electron oxidation of a wide variety of aromatic substrates. The most powerful laccases are produced by white-rot fungi and can have redox potentials up to 800 mV vs NHE.3 With respect to laccase activity toward lignin, it is important to distinguish the phenolic and nonphenolic subunits in lignin. The phenolic subunits can directly be oxidized by laccase, as the redox potentials of such subunits are sufficiently low. In contrast, the nonphenolic parts have redox potentials up to 1500 mV vs NHE and are, therefore, recalcitrant to oxidation by laccase alone.16 Nevertheless, when laccase is combined with a so-called mediator, oxidation of nonphenolic lignin structures is possible as well.17 In such a laccase/mediator system (LMS), the mediator is first oxidized by the laccase, after which it can oxidize nonphenolic substrates via different mechanisms, such as electron transfer (ET) or radical hydrogen atom transfer (HAT).18 As a LMS is required for the oxidation of the nonphenolic subunits in lignin and the phenolic subunits can be oxidized by laccase alone, research on lignin modification by LMS has mainly focused on the reactivity of nonphenolic lignin subunits,14,17−21 and several reaction pathways have been described for such incubations.22−24 Nevertheless, as lignin also contains a considerable amount of phenolic subunits, it is important to understand how these subunits react when incubated with LMS.
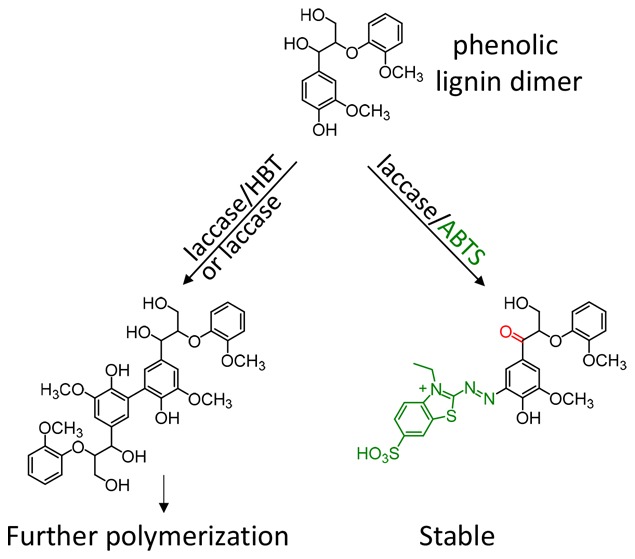
Research on lignin conversion by laccase and LMS is often performed using lignin model compounds. Phenolic lignin model compounds have been used but most often in the absence of mediators.25−27 A few studies have been published on the reactions of phenolic lignin model compounds in LMS incubations, and mainly polymerization was shown to occur. Conclusions about the degree of polymerization reached were obtained, but no insights about the structure of the (initially) formed reaction products could be provided.28,29 Consequently, it remains unclear how the phenolic subunits of lignin are modified by LMS and to what extent mediators play a role in such modifications.
In the current research, we used the phenolic β-O-4 linked lignin model compound, 1-(4-hydroxy-3-methoxyphenyl)-2-(2-methoxyphenoxy)propane-1,3-diol, (guaiacylglycerol-β-guaiacyl ether, GBG), to mimic the phenolic subunits in lignin. We employed RP-UHPLC-PDA-ESI-MSn, to study detailed reaction pathways of GBG initiated by laccase (from Trametes versicolor) with or without a mediator. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and 1-hydroxybenzotriazole (HBT) were used as mediators, as these are the most commonly used mediators in literature. MALDI-TOF-MS was used to investigate the formation of larger reaction products.
Materials and Methods
Materials
Guaiacylglycerol-β-guaiacyl ether (GBG; Figure 1) was obtained from TCI chemicals (Toyo, Japan), and 2,5-dihydroxybenzoic acid (DHB) was obtained from Bruker Daltonics (Bremen, Germany). Laccase from Trametes versicolor and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO, U.S.A.). The laccase was partially purified, after which the activity was determined spectrophotometrically by oxidation of ABTS17 (1 U = 1 μmol ABTS oxidized per minute; see the Supporting Information, S2 for details). Water was prepared using a Milli-Q water purification system (Merck Millipore, Billerica, MA).
Figure 1.
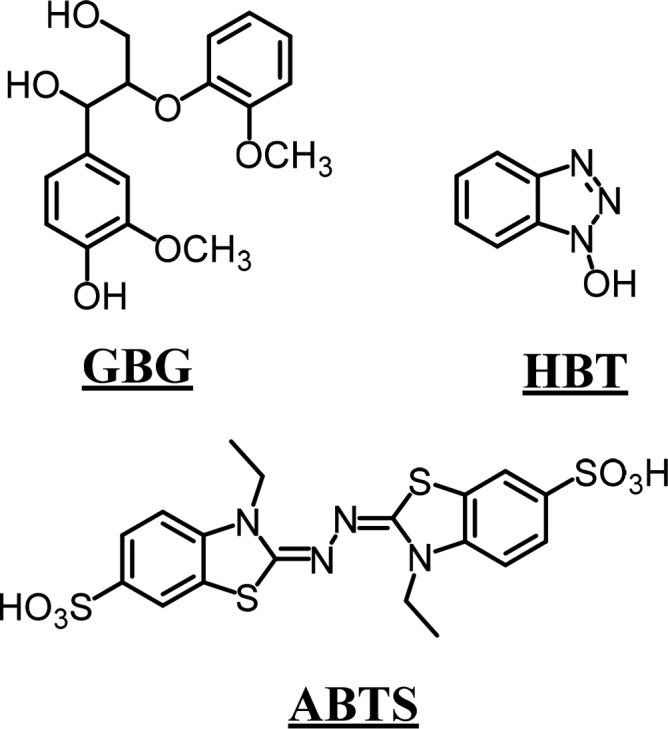
Molecular structures of guaiacylglycerol-β-guaiacyl ether (GBG), 1-hydroxybenzotriazole (HBT), and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS).
Incubation of GBG with Laccase and Laccase/Mediator Systems
GBG was dissolved at 0.5 mM in sodium phosphate buffer (50 mM, pH 4) with or without an equimolar concentration of ABTS or HBT. Laccase was added to reach a final substrate and mediator concentration of 0.4 mM and a laccase activity of 0.1 U mL–1. The mixtures were incubated at 40 °C and 400 rpm in a thermomixer (Eppendorf, Hamburg, Germany). After 1, 2, 5, 10, 15, 20, 30, and 60 min, and after 24 h, 50 μL of the sample was transferred to another tube, and 5 μL sodium azide (20 mM) was added to stop the reaction.30 As no azide-related products were detected and reaction products were stable in the presence of an excess of azide, the addition of azide was considered not to influence the reaction product profile (results not shown). The resulting samples were diluted 10 times in Milli-Q water and were centrifuged (10 000g, 5 min, 20 °C) prior to analysis by RP-UHPLC-PDA-MSn. A detailed description of the RP-UHPLC-PDA-MSn analysis can be found in S3 and S4 of the Supporting Information.
Incubation of GBG with ABTS Radical Cations
GBG was dissolved at 0.5 mM in sodium phosphate buffer (50 mM, pH 4). ABTS (2 mM) was incubated with 0.20 U mL–1 laccase (2 min, 40 °C) and directly centrifuged over an Amicon Ultra-4 centrifugal filter (Merck Millipore) with a normalized molecular weight limit of 10 kDa. The dark green filtrate was incubated with the GBG solution at a ratio of 1:4 (2 min, 40 °C). As a control, the same incubation was done using ABTS that was not treated with laccase.
Sample Preparation for Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry (MALDI-TOF)
The samples were prepared as described in the previous section, with the adaptation that the reaction was stopped after 7, 15, and 60 min. Samples were diluted 2× in methanol, and a saturated solution of DHB was used as matrix. In a second experiment, similar incubations with laccase and laccase/HBT were stopped after 2, 5, 10, 15, and 20 min. For details about MALDI-TOF settings see the Supporting Information, S6.
Results and Discussion
Reaction Products of GBG Incubated with Laccase
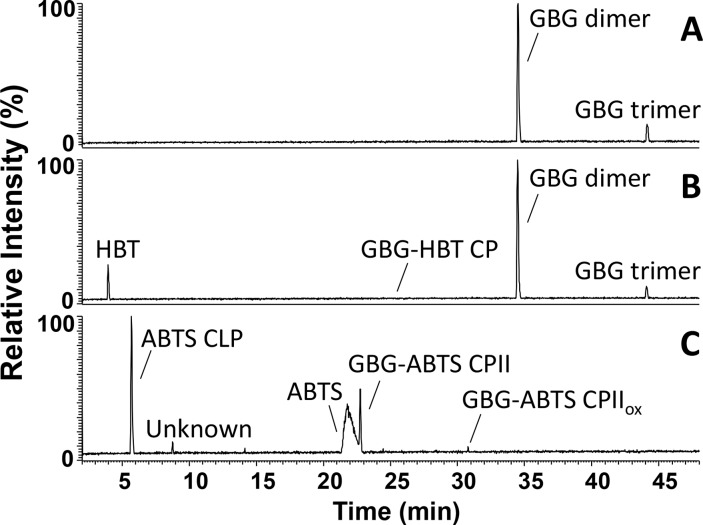
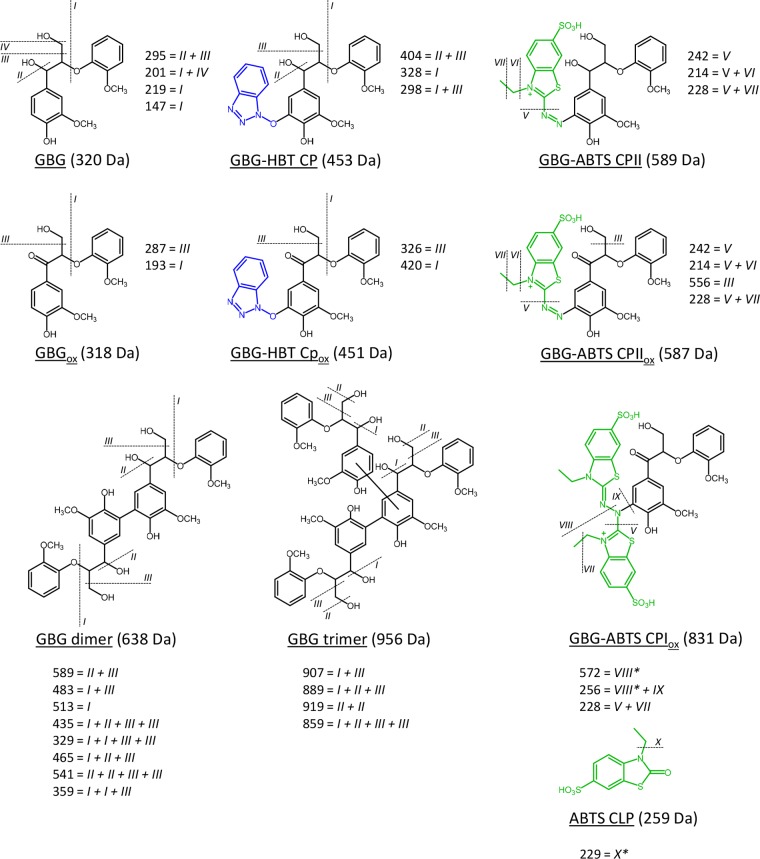
GBG (Figure 1) was, first, incubated with laccase without addition of mediators. From the UHPLC-MS chromatograms, two main reaction products were observed upon incubation of GBG, both having their largest intensities during the first 20 min (Figure 2, Table 1, and Figure S1). In addition, remaining GBG was detected (only trace amounts after 2 min). Using accurate mass determination the reaction products were found to correspond with molecular formulas C34H38H12 and C51H56O18 (Table 1). Based on their molecular formulas and the fact that the fragmentation patterns showed high similarities with GBG (Table 1 and Figure 3), these products were annotated as a dimer of GBG (molecular weight (Mw) = 2 × GBG–2H) and a trimer of GBG (Mw = 3 × GBG–4H; Table 1), formed via radical coupling. Recently, oligomerization of GBG was reported by others, and it was shown that coupling between two GBG radicals occurs via C–C bond formation.31 The same was suggested for the subsequent formation of a trimer, although no experimental evidence was given. Therefore, we have specified the coupling position in the dimer but not of the second coupling in the trimer in Figure 3. In addition to the dimer and trimer, only traces of other reaction products were observed (Table S1). After 24 h, precipitation was observed and no peaks were detected in the chromatograms, suggesting ongoing polymerization of the initially formed dimer and trimer. Thus, the activity of laccase alone was found to result in polymerization of GBG. This conclusion is in line with results on laccase activity toward phenolic model compounds reported by others.28,31
Figure 2.
RP-UHPLC-MS chromatograms (negative mode) of GBG incubated for 5 min with laccase (A), laccase/HBT (B), and laccase/ABTS (C). Chromatograms of other time points can be found in the Supporting Information.
Table 1. Compounds Detected with UHPLC-PDA-ESI-ITMS and UHPLC-PDA-ESI-FTMS after Incubation of GBG with Laccase from T. versicolor in the Presence or Absence of ABTS and HBTa.
| RT (min) | tentative annotation | molecular formula | ionization | observed/calculated mass | mass error (ppm) | MS2 fragments | λmax(nm) |
|---|---|---|---|---|---|---|---|
| Reaction Products of GBG Incubated with Laccase Alone | |||||||
| 20.8 | GBG | C17H20O6 | [M+Na]+ | 320.12556/320.12599 | –1.34 | 295 (55), 302 (16), 201 (8), 147 (6), 219 (5), 176 (4) | 278 |
| 34.8 | GBG dimer | C34H38O12 | [M–H]− | 638.23720/638.23633 | 1.36 | 589 (100), 483 (62), 513 (28), 435 (26), 329 (20), 465 (12), 541 (12), 359 (8) | 278 |
| 44.7 | GBG trimer | C51H56O18 | [M–H]− | 956.34775/956.34666 | 1.13 | 907 (100), 889 (60), 919 (30), 859 (20) | N.D. |
| Reaction Products of GBG Incubated with Laccase/HBT | |||||||
| 3.9 | HBT | C6H5N3O | [M–H]− | 135.04329/135.04326 | 0.23 | 106 (100), 78 (4) | 306, 276, 269 |
| [M+H]+ | 91 (63), 80 (40), 53 (18), 107 (8) | ||||||
| 7.2 | BT | C6H5N3 | [M+H]+ | 119.04832/119.04835 | –0.23 | N.D. | 260, 280 |
| 20.9 | GBG | C17H20O6 | [M+Na]+ | 320.12556/320.12599 | –1.34 | 295, 302, 201, 219, 147, 176 | 278 |
| 25.1 | GBG-HBT CP | C23H23N3O7 | [M–H]− | 453.15375/453.15360 | 0.33 | 404 (100), 328 (38), 298 (30) | 330 |
| 31.9 | GBG-HBT CPox | C23H21N3O7 | [M–H]− | 451.13838/451.13795 | 0.95 | 420 (100), 310 (27), 326 (15) | N.D. |
| 34.4 | GBG dimer | C34H38O12 | [M–H]− | 638.23720/638.23633 | 1.36 | 589 (100), 483 (62), 513 (28), 435 (26), 329 (20), 465 (12), 541 (12), 359 (8) | 278 |
| 44.7 | GBG trimer | C51H56O18 | [M–H]− | 956.34775/956.34666 | 1.13 | 907 (100), 889 (60), 919 (30), 859 (20) | N.D. |
| Reaction Products of GBG Incubated with Laccase/ABTS | |||||||
| 5.6 | ABTS CLP | C9H9NO4S2 | [M–H]− | 258.99757/258.99730 | 1.05 | 229 (37) | 256, 292, 285 |
| 8.7 | unknown | unknown | [M–H]− | 329.99756 | 302 (100), 238 (29), 222 (9) | 278 | |
| 17.0 | unknown | C25H25N4O8S4 | [M–2H]− | 637.05667/637.05553 | 1.79 | 363 (100), 378 (95), 228 (34), 605 (31), 405 (26), 257 (25), 335 (18) | 305 |
| 21.4 | ABTS | C18H18N4O6S4 | [M–2H]2– | 514* | N.D. | 342 | |
| 22.7 | GBG-ABTS CPII | C26H27N3O9S2 | [M–H]− | 589.11946/589.118876 | 0.99 | 242 (100), 214 (61), 228 (10) | 550 |
| 24.4 | GBG-ABTS CPIox | C35H35N4O12S4 | [M–3H]2– | 831.11369/831.11289 | 0.96 | 572 (100), 228 (43), 256 (42) | 305, 278 |
| [M–2H]− | N.D. | ||||||
| 28.3 | GBGox | C17H18O6 | [M–H]− | 318.11017/318.11034 | –0.52 | 287 (100), 193 (71) | 280, 311 |
| [M+H]+ | 167 (100), 149 (70), 271 (68), 151 (59), 195 (44), 269 (32), 177 (26), 289 (22) | ||||||
| [M+Na]+ | 311 (100), 218 (16), 146 (11), 187 (6) | ||||||
| 30.7 | GBG-ABTS CPIIox | C26H25N3O9S2 | [M–H]− | 587.10366/587.10322 | 0.76 | 242, 214, 556, 228 | 585, 448, 328 |
MS2 fragments and λmax were determined using UHPLC-PDA-ESI-ITMS. All other values were obtained using UHPLC-PDA-ESI-FTMS. Relative intensities of MS2 fragments are shown between brackets.* For ABTS, only data from UHPLC-PDA-ESI-ITMS were used, as FTMS only showed in-source fragmentation. N.D. = not detected.
Figure 3.
Proposed structures of reaction products of GBG after incubation with laccase and LMS. The dotted lines represent the proposed fragmentation pattern, resulting in the MS2 fragments reported in Table 1. The fragmentation pattern of GBG and GBG-ABTS CPIox originate from the parent ions [M+Na]+ and [M–3H]2–, respectively. All other patterns originate from the parent ion [M–H]−. In the coupling products and oligomers, the positions of coupling could not be identified based on MS2 spectra and are therefore based on the literature.31,34,35 The position of the second interunit bond in the GBG trimer is not specified, as there is no experimental evidence for either C–C or C–O linkage. Fragmentations with an * (in GBG-ABTS CPIox and ABTS CLP) are suggested to be radical fragmentations.
Reaction Products of GBG Incubated with a Laccase/HBT System
When GBG was incubated with laccase in combination with HBT, the reaction products formed were very comparable to those formed in the presence of laccase alone (Figure 2, Table 1, and Figure S1). The same dimer and trimer were identified based on retention time, accurate mass, and fragmentation pattern (Figure 2 and Table 1). Also the amounts were comparable, based on UV280 peak area. In addition to the dimer and trimer, a small peak (hardly visible) corresponding to the molecular formula C23H23N3O7 was observed. This, in combination with a fragmentation pattern similar to that of GBG (Table 1 and Figure 3), suggested the formation of a GBG-HBT radical coupling product (GBG-HBT CP, Figure 3). After prolonged incubation times (1 h) C23H21N3O7 was detected, which, most likely, corresponded to further oxidation of GBG-HBT CP to GBG-HBT CPox (Figure 3). It was recently shown for the first time that N–OH type mediators, such as HBT, couple to lignin upon addition of laccase.7 Our results confirm that such coupling can occur at the phenolic moieties of lignin but also indicate that the extent of coupling between GBG and HBT is small as compared to GBG oligomerization. The proposed structures of all coupling products are depicted in Figure 3, together with their proposed MS2 fragmentation pathways. After 24 h, no GBG-related peaks were detected anymore and precipitation was observed. Approximately 45% of the original HBT was still present, as determined from UV280 peak areas (data not shown). Part of it was converted to benzotriazole (BT), which is inactive as a mediator.32 This conversion of HBT to BT has been described by others32,33 and also occurred in a control reaction with only HBT and laccase without GBG present (data not shown). Most likely, BT is formed upon a reaction of HBT radicals with aromatic amino acids of the enzyme.33
Reaction Products of GBG Incubated with a Laccase/ABTS System
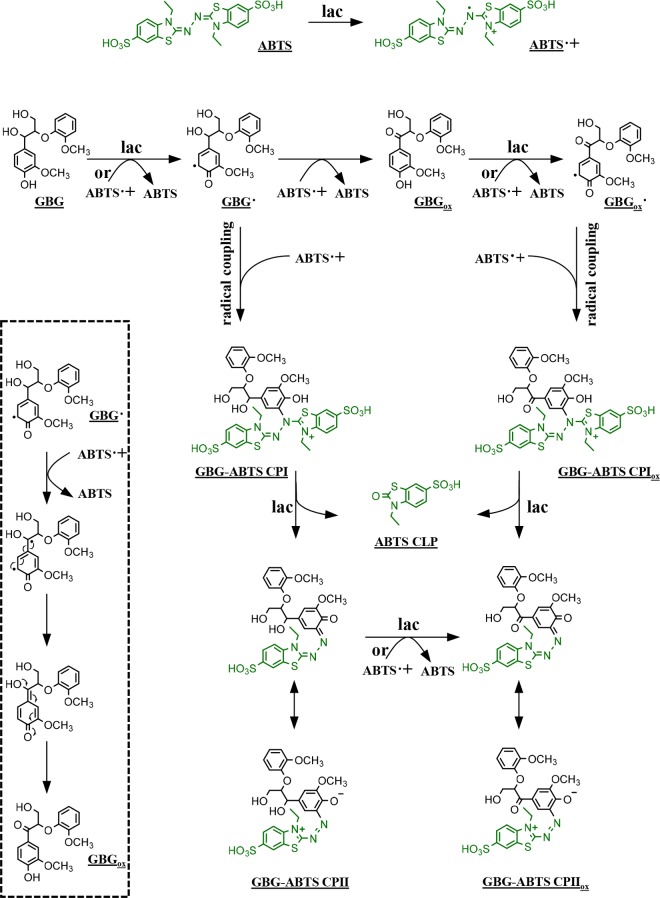
When GBG was incubated with laccase and ABTS, a larger variety of reaction products was detected compared to the incubations described above (Figure 2, Table 1, and Figure S1). Molecules with molecular formulas C17H18O6 and C35H35N4O12S4 were detected, especially after short reaction times (1–15 min). These were annotated as Cα oxidized GBG and a radical coupling product between oxidized GBG and ABTS (GBGox and GBG-ABTS CPIox in Table 1 and Figure 3), respectively. A relatively small peak corresponding to a coupling of nonoxidized GBG and ABTS (GBG-ABTS CPI) has been observed as well but inconsistently. Likely, the latter coupling product is prone to react further. The structure and fragmentation pattern of GBG-ABTS CPI can be found in Figure S4. In addition, reaction products with molecular formulas C26H27N3O9S2, C26H25N3O9S2 were found. Based on these formulas and their fragmentation patterns, these products were annotated as GBG-ABTS CPII and GBG-ABTS CPIIox, respectively (Table 1 and Figure 3). Similar coupling products, between a phenolic compound and part of the ABTS molecule, have been reported for hydroxybenzoic acids34 and catechin,35 using NMR and mass spectrometry, respectively. To our knowledge, this is the first time that such coupling products are shown for lignin model compounds. The main MS2 fragments (m/z 242 and 214 in negative mode) match with the fragments reported for the catechin-ABTS products (m/z 244 and 216 in positive mode).35 As the adducts identified with NMR spectroscopy29 resulted from C–N coupling (rather than O–N coupling), the adducts in Figure 3 are also shown with a C–N bond between the GBG and ABTS moiety. In addition to these coupling products, a major peak was detected corresponding to C9H9NO4S2. As this molecule was hardly formed in a control reaction with only ABTS and laccase (data not shown), it was considered to be an ABTS cleavage product (ABTS CLP) formed upon coupling of GBG and ABTS. Lastly, a reaction product was detected with molecular formula C25H25N4O8S4. This could correspond to a radical coupling product of guaiacol and ABTS. It should be mentioned that for the formation of such a product cleavage of GBG to form guaiacol is required, either before or during coupling with ABTS. Besides the detection of C25H25N4O8S4, no indications were found for cleavage of GBG. In contrast to the incubation with laccase alone and laccase with HBT, no GBG was detected at all incubation times, suggesting a fast conversion of GBG into reaction products. The dimer and trimer found for incubations with laccase alone and laccase/HBT were absent at all incubation times when ABTS was used. After 24 h, approximately 29% of the initial amount ABTS was still present (data not shown). Whereas others reported polymerization as the main result of laccase/ABTS activity toward phenolic substrates,28,29 our results suggest that GBG preferably couples to ABTS, rather than to another GBG molecule.
Reaction Pathways of Laccase and Laccase/HBT with GBG
To get more insight into the course of the reactions occurring during incubation of GBG with laccase and the laccase/mediator systems, product formation was monitored at nine different time points within the first hour. Hereto, peak areas from extracted ion chromatograms of the reaction products (Table 1) relative to the time point with the highest area were plotted as a function of incubation time (Figure 4). For clarity, only GBG-related reaction products are shown.
Figure 4.
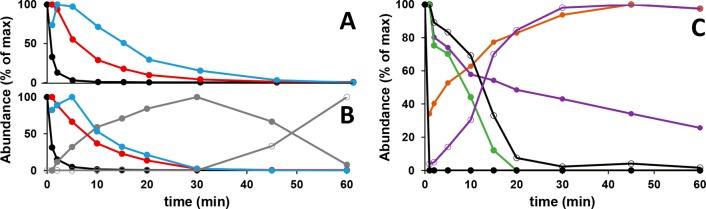
Normalized MS peak areas in time of reaction products formed upon incubation of GBG with laccase alone (A), laccase/HBT (B), and laccase/ABTS (C). Areas were obtained from extracted ion chromatograms: GBG (m/z 343) (black dot), GBG dimer (m/z 637) (red dot), GBG trimer (m/z 955) (blue dot), GBG-HBT CP (m/z 452) (grey dot), GBG-HBT CPox (m/z 450) (open dot, grey line), GBGox (m/z 319 and 341) (open dot, black line), GBG-ABTS CPIox (m/z 414) (green dot), GBG-ABTS CPII (m/z 588) (purple dot), GBG-ABTS CPIIox (m/z 586) (open dot, purple line) and ABTS CLP (m/z 258) (orange dot). For the abundance (Y axis), the time point with the largest peak area was set to 100%. For the other time points, the abundance was calculated as the peak area relative to the area of this largest peak. Amounts withdrawn per time point and injected to UHPLC-MS analysis were the same for all time points.
Figure 4A,B shows that, under the conditions used, a fast conversion of GBG to oligomeric products occurred. Already in the first minute, 67 and 69% of the GBG was converted by laccase and laccase/HBT, respectively. For both laccase and laccase/HBT it was shown that the dimer and the trimer gradually decreased in abundance and disappeared within 1 h. The decrease of the dimer in the first 20 min occurred together with an increase in higher oligomers, indicating further polymerization (Figure S2). In the incubation with laccase/HBT, the coupling product GBG-HBT CP increased during the first 30 min, and then decreased again. The decrease of GBG-HBT CP coincided with an increase in its oxidized equivalent GBG-HBT CPox, confirming that GBG-HBT CP is oxidized further to GBG-HBT CPox. For such a conversion from alcohol to ketone at Cα, a benzylic radical should be formed. This may have occurred via radical hydrogen abstraction by a HBT radical. As a phenolic lignin structure itself could also act as HAT-type mediator,36 radical hydrogen abstraction may also have occurred by a phenoxyl radical of a GBG-related structure. If the latter indeed occurred, the oxidant probably was an oligomeric GBG radical, as all GBG was converted before conversion of GBG-HBT CP to GBG-HBT CPox took place (Figure 4).
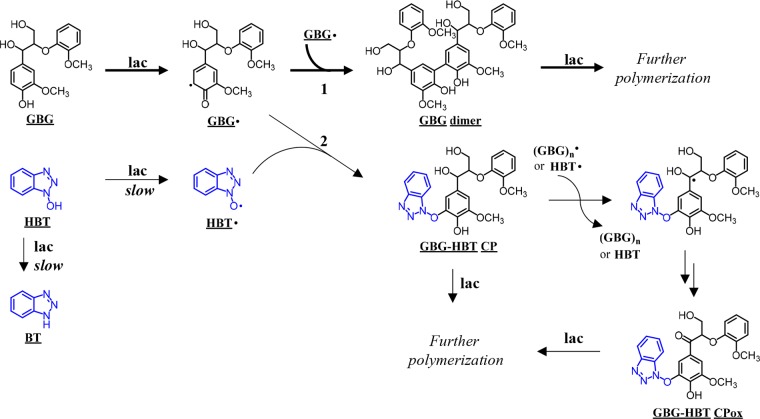
These findings indicate that upon incubation with both laccase and laccase/HBT, extensive polymerization of GBG takes place. Radical coupling between GBG and HBT occurred but only to a small extent. The coupling product was further oxidized and is probably prone to further polymerization. The rate of conversion of GBG and its oligomers does not seem to be influenced by the presence of HBT, suggesting that the role of HBT is negligible when laccase/HBT is used on phenolic substrates. A schematic overview of the proposed reactions is depicted in Figure 5.
Figure 5.
Overview of proposed reactions occurring after incubation of GBG with laccase alone or a laccase/HBT system. The thick arrows represent the major reaction pathway. The reactions represented by thin arrows do occur, but slower and/or to a smaller extent. In the case of laccase alone, all GBG reacts via route 1. In the case of a laccase/HBT system, GBG reacts via route 1 and 2, but route 1 is favored due to the slow oxidation of HBT. Oxidation of GBG-HBT CP is proposed to occur via radical hydrogen abstraction by either a HBT radical (HBT•) or an oligomeric GBG radical ((GBG)n•). The C–C coupling in the dimer is based on Ramalingam et al.31 For clarity, reductions of O2 to H2O by laccase are not shown in the figure.
Reaction Pathways of Laccase/ABTS with GBG
Similar to the incubations with laccase and laccase/HBT, the reaction of GBG with laccase/ABTS was monitored over time. Figure 4C shows that all GBG was converted within the first minute of incubation. Based on this and on the annotations discussed above, it can be stated that the laccase/ABTS system differs from laccase and laccase/HBT in two ways: (i) GBG is converted faster (higher rate) and (ii) GBG is converted into different reaction products (other pathways).
A possible explanation for the higher conversion rate might be the rapid formation of ABTS radical cations (ABTS•+), which might undergo a redox reaction with GBG. ABTS•+ has been shown to induce oxidative polymerization of creosol, a phenolic monomeric lignin model compound29 and to undergo a redox reaction with vanillyl alcohol.37 To check whether a similar interaction between GBG and ABTS•+ occurred, GBG was incubated with laccase-free ABTS•+. GBG was indeed converted upon addition of ABTS•+, resulting in several reaction products: GBGox, GBG dimer, oxidized GBG dimer, and GBG-ABTS CPI (Figure S3). The annotation of the latter two products can be found in Table S2 and Figure S4. As no GBG conversion was observed in a control reaction with neutral ABTS (Figure S3B), it was concluded that the higher GBG conversion rate by the laccase/ABTS system is caused by the fact that GBG is oxidized via two mechanisms: directly by laccase and via a redox reaction with ABTS•+. It cannot be excluded that also the dication ABTS++ (formed via disproportionation of ABTS•+) is involved in the oxidation of GBG. Nevertheless, as it has been shown with cyclic voltammetry that vanillyl alcohol can rapidly be oxidized by ABTS•+,37 we assume that the same applies to GBG.
Figure S3 also shows that Cα oxidized GBG is formed upon addition of ABTS•+. The fact that Cα oxidized products were not observed in incubations with laccase alone implies that Cα oxidation is caused by ABTS•+ rather than laccase. Figure 4C shows that the concentrations of GBGox and GBG-ABTS CPIox are highest after 1 min of incubation and that their concentration decreased relatively fast during the first 20 min. GBG-ABTS CPII was also highest after 1 min, but its decrease was slower. In contrast, the concentrations of GBG-ABTS CPIIox and ABTS CLP gradually increased during the first hour of incubation, suggesting that they were formed upon conversion of the other reaction products. After 24 h, most reaction products from Figure 4C had disappeared, except for ABTS CLP and GBG-ABTS CPIIox. The areas of these peaks had even increased with 33 and 23%, respectively (data not shown). These results indicate that GBG formed coupling products with ABTS in the presence of laccase, after which a part of the ABTS moiety was cleaved off. The ultimate product formed after coupling of ABTS and GBG was GBG-ABTS CPIIox. To our knowledge, coupling of ABTS to lignin and lignin model compounds has been suggested by others,8,28 but the structure of such lignin-ABTS coupling products has not been elaborated so far.
Based on the above, an overview of the course of the reactions leading to the formation of GBG-ABTS CPIIox was constructed (Figure 6). We suggest that, after oxidation, GBG• undergoes two follow-up reactions: (i) radical coupling with ABTS•+ to form an intermediate product GBG-ABTS CPI or (ii) a second oxidation (by ABTS•+) to form GBGox. In the latter case, GBGox can be further oxidized (by laccase or ABTS•+) to GBGox•, which then also couples to ABTS•+ to form GBG-ABTS CPIox. GBG-ABTS CPI and GBG-ABTS CPIox are subsequently converted to GBG-ABTS CPII and GBG-ABTS CPIIox, upon which ABTS CLP was split off as a byproduct. The exact mechanism behind this is unknown, but it is suggested to involve an oxidation step, as the intermediates (GBG-ABTS CPI and GBG-ABTS CPIox) did not react further after inactivation of the laccase (Figure 4). Eventually, the formed GBG-ABTS CPII can be oxidized to the ultimate reaction product GBG-ABTS CPIIox.
Figure 6.
Overview of proposed reactions occurring after incubation of GBG with a laccase/ABTS system. GBG can be oxidized directly by laccase or indirectly via ABTS•+, which is formed by laccase. The GBG radicals may react further to GBGox or may undergo radical coupling with ABTS•+. After coupling of GBG and ABTS, further rearrangements take place in which part of the ABTS molecule is cleaved from the reaction product. The inset shows the proposed mechanism of GBGox formation from GBG•. For clarity, reductions of O2 to H2O by laccase are not shown in the figure.
Formation of Larger Oligomeric Reaction Products
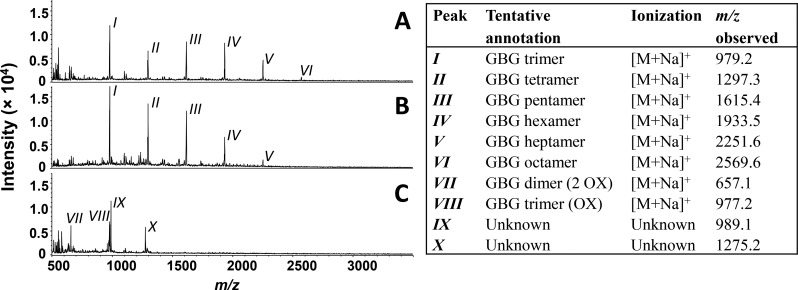
To investigate whether larger reactions products were formed upon incubation with laccase or LMS, MALDI-TOF spectra were recorded. After 1 h of incubation with laccase and laccase/HBT, GBG oligomers up to DP 8 and DP 7 were detected, respectively (Figure 7). As precipitation of reaction products occurred in both incubations and the mixture still contained small amounts of insoluble material after dilution in methanol, it cannot be excluded that insoluble oligomers of higher DP were formed. Besides the GBG oligomers, no other peaks were detected, suggesting that incubation of GBG with laccase or laccase/HBT (almost) solely resulted in oligomerization of GBG. In contrast, a completely soluble reaction mixture was obtained after incubation with laccase/ABTS, in which all of the GBG oligomers were absent. Instead, reaction products with m/z 657, 977, 989, and 1275 were detected. Assuming that these m/z values, like all GBG oligomers, originate from Na+ adducts, it seems plausible that m/z 657 and 977 correspond to a GBG dimer with two oxidized Cα groups (Mw = 2 × GBG-6H) and a GBG trimer with one oxidized Cα group (Mw = 3 × GBG-6H), respectively. Although these products were not detected using UHPLC-MS, a GBG dimer and oxidized dimer were detected upon incubation with ABTS•+ (Figure S3). It can therefore not be excluded that GBG is converted into (oxidized) oligomers in the laccase/ABTS system. Another indication for this is that all GBG had reacted during the first minute, but approximately 45% of the ABTS was still present after 1 h. Since an equimolar ratio of GBG and ABTS was used, it is impossible that all GBG coupled to ABTS and that GBG-ABTS CPIIox is the only reaction product. Furthermore, polymerization of a phenolic model compound with laccase and ABTS has been shown before, using SEC28 and MALDI-TOF.29
Figure 7.
MALDI-TOF spectra (left) of GBG after 60 min incubation with laccase alone (A), laccase/HBT (B), and laccase/ABTS (C) measured in positive mode and corresponding tentative peak annotations (right). OX = Cα hydroxyl oxidized to ketone.
Role of the Mediator in LMS Incubations of GBG
Lignin contains 70–90% nonphenolic and 10–30% phenolic subunits.5 As the phenolic lignin structures can be oxidized by laccase alone, the question remains whether and how the mediator plays a role in the reaction rate and pathway when phenolic structures are incubated with LMS. Based on the results discussed above, it can be concluded that the role of HBT is very limited, in contrast to ABTS, which strongly influenced both rate and pathway. Whereas the efficiency of a mediator toward nonphenolic lignin structures has been reported to depend on its stability as a radical and to its potency to abstract a radical from a nonphenolic substrate38 (via electron transfer or radical hydrogen atom transfer), we hypothesized that the role of the mediator toward phenolic substrates is dominated by the rate of its oxidation. The large difference in redox potentials, i.e., 1.08 V vs NHE for HBT and 0.69 V vs NHE for ABTS/ABTS•+,38 suggests that the oxidation of HBT by laccase occurs much slower than that of ABTS to ABTS•+. This was confirmed by oxygen consumption measurements of both mediators and GBG with laccase (Figure S5). Oxygen consumption of ABTS is 1.9 times faster than that of GBG, whereas no substantial oxygen consumption of HBT with laccase was detected within the time frame used. So, although oxidized HBT may probably undergo a (redox) reaction with GBG, the conversion of GBG by laccase alone is already complete before a substantial amount of HBT is oxidized. Consequently, the role of HBT is negligible in this system. In contrast, the fast conversion of ABTS to ABTS•+ allows ABTS•+ to compete with GBG radicals in coupling reactions and to induce Cα oxidations before GBG radicals start to polymerize. These results indicate that the influence of the mediator on the conversion of a phenolic lignin structure is highly dependent on its oxidation rate by laccase. It should be noted that the formation of substrate-mediator adducts may also be influenced by the bulkiness and presence of reactive groups on the mediator, but the comparison between HBT and ABTS does not give any insights in this.
Conclusions
The mediators HBT and ABTS strongly differed regarding their influence on GBG conversion by laccase. The influence of HBT was negligible, but ABTS strongly increased the conversion rate of GBG and altered the followed reaction pathways. Whereas laccase and laccase/HBT incubations resulted in extensive polymerization of GBG, incubation with the laccase/ABTS system mainly resulted in Cα oxidation and coupling between GBG and ABTS. This difference in influence between HBT and ABTS can be explained by the much higher oxidation rate of ABTS by laccase, as compared to that of HBT. Extrapolating to polymeric lignin, our results suggest that the use laccase/ABTS will most likely result in a large degree of ABTS grafting on the phenolic lignin subunits, whereas such grafting reactions are less likely to occur with laccase/HBT. As the GBG-ABTS adducts were found to be relatively stable, this suggests that, once ABTS has been grafted on phenolic lignin subunits, further polymerization by laccase is blocked. These insights can be helpful for choosing the optimal mediator in a LMS, in which the mediator should not only be selected based on its efficiency in oxidation of nonphenolic lignin subunits but also on its reactivity with the phenolic subunits.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acssuschemeng.7b03451.
Detailed experimental information (laccase purification, LC-MS and MALDI-TOF-MS), LC-MS data of minor reaction products, LC-MS chromatograms of all time points, MALDI-TOF spectra (2–20 min), LC-MS results of GBG oxidation byABTS•+, and O2 consumption measurements. (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Yao B.; Ji Y. Lignin biodegradation with laccase-mediator systems. Front. Energy Res. 2014, 2, 12. 10.3389/fenrg.2014.00012. [DOI] [Google Scholar]
- Bugg T. D.; Rahmanpour R. Enzymatic conversion of lignin into renewable chemicals. Curr. Opin. Chem. Biol. 2015, 29, 10–17. 10.1016/j.cbpa.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Munk L.; Sitarz A. K.; Kalyani D. C.; Mikkelsen J. D.; Meyer A. S. Can laccases catalyze bond cleavage in lignin?. Biotechnol. Adv. 2015, 33 (1), 13–24. 10.1016/j.biotechadv.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Areskogh D.; Li J.; Gellerstedt G. R.; Henriksson G. Investigation of the molecular weight increase of commercial lignosulfonates by laccase catalysis. Biomacromolecules 2010, 11 (4), 904–910. 10.1021/bm901258v. [DOI] [PubMed] [Google Scholar]
- Lundquist K.; Parkås J. Different types of phenolic units in lignins. BioResources 2011, 6 (2), 920–926. [Google Scholar]
- Huber D.; Ortner A.; Daxbacher A.; Nyanhongo G. S.; Bauer W.; Guebitz G. M. Influence of oxygen and mediators on laccase-catalyzed polymerization of lignosulfonate. ACS Sustainable Chem. Eng. 2016, 4 (10), 5303–5310. 10.1021/acssuschemeng.6b00692. [DOI] [Google Scholar]
- Munk L.; Punt A.; Kabel M.; Meyer A. S. Laccase catalyzed grafting of–N–OH type mediators to lignin via radical–radical coupling. RSC Adv. 2017, 7 (6), 3358–3368. 10.1039/C6RA26106J. [DOI] [Google Scholar]
- Bourbonnais R.; Paice M.; Reid I.; Lanthier P.; Yaguchi M. Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2, 2′-azinobis (3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl. Environ. Microbiol. 1995, 61 (5), 1876–1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moya R.; Saastamoinen P.; Hernández M.; Suurnäkki A.; Arias E.; Mattinen M.-L. Reactivity of bacterial and fungal laccases with lignin under alkaline conditions. Bioresour. Technol. 2011, 102 (21), 10006–10012. 10.1016/j.biortech.2011.08.046. [DOI] [PubMed] [Google Scholar]
- Prasetyo E. N.; Kudanga T.; Østergaard L.; Rencoret J.; Gutiérrez A.; José C.; Santos J. I.; Nieto L.; Jiménez-Barbero J.; Martínez A. T. Polymerization of lignosulfonates by the laccase-HBT (1-hydroxybenzotriazole) system improves dispersibility. Bioresour. Technol. 2010, 101 (14), 5054–5062. 10.1016/j.biortech.2010.01.048. [DOI] [PubMed] [Google Scholar]
- Fernaud J. H.; Carnicero A.; Perestelo F.; Cutuli M. H.; Arias E.; Falcón M. Upgrading of an industrial lignin by using laccase produced by Fusarium proliferatum and different laccase-mediator systems. Enzyme Microb. Technol. 2006, 38 (1-2), 40–48. 10.1016/j.enzmictec.2005.01.043. [DOI] [Google Scholar]
- Shleev S.; Persson P.; Shumakovich G.; Mazhugo Y.; Yaropolov A.; Ruzgas T.; Gorton L. Interaction of fungal laccases and laccase-mediator systems with lignin. Enzyme Microb. Technol. 2006, 39 (4), 841–847. 10.1016/j.enzmictec.2006.01.010. [DOI] [Google Scholar]
- Xia Z.; Yoshida T.; Funaoka M. Enzymatic synthesis of polyphenols from highly phenolic lignin-based polymers (lignophenols). Biotechnol. Lett. 2003, 25 (1), 9–12. 10.1023/A:1021705426181. [DOI] [PubMed] [Google Scholar]
- Bourbonnais R.; Paice M.; Freiermuth B.; Bodie E.; Borneman S. Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl. Environ. Microbiol. 1997, 63 (12), 4627–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourbonnais R.; Paice M. G. Demethylation and delignification of kraft pulp by Trametes versicolor laccase in the presence of 2, 2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate). Appl. Microbiol. Biotechnol. 1992, 36 (6), 823–827. 10.1007/BF00172202. [DOI] [Google Scholar]
- Couto S. R.; Herrera J. L. T. Industrial and biotechnological applications of laccases: a review. Biotechnol. Adv. 2006, 24 (5), 500–513. 10.1016/j.biotechadv.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Bourbonnais R.; Paice M. G. Oxidation of non-phenolic substrates: an expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267 (1), 99–102. 10.1016/0014-5793(90)80298-W. [DOI] [PubMed] [Google Scholar]
- Baiocco P.; Barreca A. M.; Fabbrini M.; Galli C.; Gentili P. Promoting laccase activity towards non-phenolic substrates: a mechanistic investigation with some laccase–mediator systems. Org. Biomol. Chem. 2003, 1 (1), 191–197. 10.1039/B208951C. [DOI] [PubMed] [Google Scholar]
- Muheim A.; Fiechter A.; Harvey P. J.; Schoemaker H. E. On the mechanism of oxidation of non-phenolic lignin model compounds by the laccase-ABTS couple. Holzforschung 1992, 46 (2), 121–126. 10.1515/hfsg.1992.46.2.121. [DOI] [Google Scholar]
- Eggert C.; Temp U.; Dean J. F.; Eriksson K.-E. L. A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett. 1996, 391 (1), 144–148. 10.1016/0014-5793(96)00719-3. [DOI] [PubMed] [Google Scholar]
- Astolfi P.; Brandi P.; Galli C.; Gentili P.; Gerini M. F.; Greci L.; Lanzalunga O. New mediators for the enzyme laccase: mechanistic features and selectivity in the oxidation of non-phenolic substrates. New J. Chem. 2005, 29 (10), 1308–1317. 10.1039/b507657a. [DOI] [Google Scholar]
- Kawai S.; Nakagawa M.; Ohashi H. Aromatic ring cleavage of a non-phenolic β-O-4 lignin model dimer by laccase of Trametes versicolor in the presence of 1-hydroxybenzotriazole. FEBS Lett. 1999, 446 (2–3), 355–358. 10.1016/S0014-5793(99)00247-1. [DOI] [PubMed] [Google Scholar]
- Kawai S.; Asukai M.; Ohya N.; Okita K.; Ito T.; Ohashi H. Degradation of a non-phenolic β-O-4 substructure and of polymeric lignin model compounds by laccase of Coriolus versicolor in the presence of 1-hydroxybenzotriazole. FEMS Microbiol. Lett. 1999, 170 (1), 51–57. 10.1111/j.1574-6968.1999.tb13354.x. [DOI] [Google Scholar]
- Christopher L. P.; Yao B.; Ji Y. Lignin biodegradation with laccase-mediator systems. Front. Energy Res. 2014, 2, 12. 10.3389/fenrg.2014.00012. [DOI] [Google Scholar]
- Kawai S.; Umezawa T.; Shimada M.; Higuchi T. Aromatic ring cleavage of 4, 6-di (tert-butyl) guaiacol, a phenolic lignin model compound, by laccase of Coriolus versicolor. FEBS Lett. 1988, 236 (2), 309–311. 10.1016/0014-5793(88)80043-7. [DOI] [PubMed] [Google Scholar]
- Kawai S.; Umezawa T.; Higuchi T. Degradation mechanisms of phenolic β-1 lignin substructure model compounds by laccase of Coriolus versicolor. Arch. Biochem. Biophys. 1988, 262 (1), 99–110. 10.1016/0003-9861(88)90172-5. [DOI] [PubMed] [Google Scholar]
- Areskogh D.; Li J.; Nousiainen P.; Gellerstedt G.; Sipilä J.; Henriksson G. Oxidative polymerisation of models for phenolic lignin end-groups by laccase. Holzforschung 2010, 64 (1), 21–34. 10.1515/hf.2010.001. [DOI] [Google Scholar]
- Rittstieg K.; Suurnäkki A.; Suortti T.; Kruus K.; Guebitz G. M.; Buchert J. Polymerization of guaiacol and a phenolic β-O-4-substructure by Trametes hirsuta laccase in the presence of ABTS. Biotechnol. Prog. 2003, 19 (5), 1505–1509. 10.1021/bp034054z. [DOI] [PubMed] [Google Scholar]
- Potthast A.; Rosenau T.; Koch H.; Fischer K. The reaction of phenolic model compounds in the laccase-mediator system (LMS) investigations by matrix assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS). Holzforschung 1999, 53 (2), 175–180. 10.1515/HF.1999.029. [DOI] [Google Scholar]
- Johannes C.; Majcherczyk A. Laccase activity tests and laccase inhibitors. J. Biotechnol. 2000, 78 (2), 193–199. 10.1016/S0168-1656(00)00208-X. [DOI] [PubMed] [Google Scholar]
- Ramalingam B.; Sana B.; Seayad J.; Ghadessy F.; Sullivan M. Towards understanding of laccase-catalysed oxidative oligomerisation of dimeric lignin model compounds. RSC Adv. 2017, 7 (20), 11951–11958. 10.1039/C6RA26975C. [DOI] [Google Scholar]
- Li K.; Helm R. F.; Eriksson K. E. L. Mechanistic studies of the oxidation of a non-phenolic lignin model compound by the laccase/1-hydroxybenzotriazole redox system. Biotechnol. Appl. Biochem. 1998, 27 (3), 239–243. [Google Scholar]
- Sealey J.; Ragauskas A. J. Investigation of laccase/N-hydroxybenzotriazole delignification of kraft pulp. J. Wood Chem. Technol. 1998, 18 (4), 403–416. 10.1080/02773819809349588. [DOI] [Google Scholar]
- Matsumura E.; Yamamoto E.; Numata A.; Kawano T.; Shin T.; Murao S. Structures of the laccase-catalyzed oxidation products of hydroxy-benzoic acids in the presence of ABTS [2, 2′-Azino-di-(3-ethylbenzothiazoline-6-sulfonic Acid)]. Agric. Biol. Chem. 1986, 50 (5), 1355–1357. 10.1080/00021369.1986.10867576. [DOI] [Google Scholar]
- Osman A.; Wong K.; Fernyhough A. ABTS radical-driven oxidation of polyphenols: Isolation and structural elucidation of covalent adducts. Biochem. Biophys. Res. Commun. 2006, 346 (1), 321–329. 10.1016/j.bbrc.2006.05.118. [DOI] [PubMed] [Google Scholar]
- Cañas A. I.; Camarero S. Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28 (6), 694–705. 10.1016/j.biotechadv.2010.05.002. [DOI] [PubMed] [Google Scholar]
- Bourbonnais R.; Leech D.; Paice M. G. Electrochemical analysis of the interactions of laccase mediators with lignin model compounds. Biochim. Biophys. Acta, Gen. Subj. 1998, 1379 (3), 381–390. 10.1016/S0304-4165(97)00117-7. [DOI] [PubMed] [Google Scholar]
- Fabbrini M.; Galli C.; Gentili P. Comparing the catalytic efficiency of some mediators of laccase. J. Mol. Catal. B: Enzym. 2002, 16 (5), 231–240. 10.1016/S1381-1177(01)00067-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.