Supplemental Digital Content is available in the text
Keywords: alcoholism, clinical and radiological features, Marchiafava–Bignami disease, MRI, prognosis
Abstract
Marchiafava–Bignami disease (MBD) is a rare neurological disease usually associated with chronic alcoholism and characterized by demyelination and necrosis. Our aims were to describe the clinicoradiological features and identify factors that may affect the prognosis of patients with MBD.
We examined clinical manifestations, laboratory results, and neuroradiological features of 9 patients with MBD. The patients were classified into 2 subgroups (favorable and poor outcome subgroups) based on the Modified Oxford Handicap Scale (MOSH). In addition, we compared the clinical and neuroimaging features between the 2 subgroups.
Nine adult male patients (age of onset range 37–62 years, with a mean age of 47.00 ± 14.50 years) were included in this study. According to MOSH, 4 patients were placed in the poor outcome subgroup (MOHS ≥ 3), 5 patients were placed in the favorable outcome subgroup (MOHS ≤ 2). Relatively high score of MAST-C (≥6) (P = .008), extracallosal lesions (P = .048), GCS (P = .026), cerebral lobe impairment (P = .048) was significantly more common in the poor outcome subgroup.
Clinical manifestations of MBD are variable and lack specificity. Early diagnosis by relatively specific performance of bisymmetric lesions in corpus callosum of diffusion-weighted imaging (DWI) may affect the prognosis. The prognosis of patients with severe disturbance of consciousness, heavy alcohol consumption, extracallosal lesions, cerebral lobe impairment is probably unfavorable.
1. Introduction
Marchiafava–Bignami disease (MBD) is a toxic, demyelinating, and necrotic central nervous system disorder associated with chronic alcoholism that occasionally occurs in the patients who are not alcoholics but chronically malnourished.[1,2] Deficiency of group B vitamins might be the etiology of MBD; it is reported that many patients recover after treatment with B vitamins; however, others do not recover.[3]
Increasing evidence shows that lesions in MBD are not confined to the corpus callosum, but also appear in other regions of brain, such as subcortical regions, cerebral lobes, hemispheric white matter, and basal ganglia.[4] When other regions of the brain are involved, MBD manifests with severe neurological dysfunction and has a poor prognosis.[5] The presentations of MBD are nonspecific.[6] In the acute phase, the patients always have several neurological symptoms, such as confusion, seizure, dysarthria, limb hypertonic, and delirium/coma. The typical MRI features of MBD are symmetric lesions of the corpus callosum, which usually are restricted to the genu, body, or splenium. The impaired area has edematous changes with or without demyelination, which appears as a high signal lesion on T2-weighted imaging (T2WI)/FLAIR and diffusion-weighted imaging (DWI). As the acute stage passes, edematous changes gradually subside and the high signal changes to a normal signal. If the impairment progresses to permanent myelin impairment and necrosis, the MRI of the affected region shows atrophy and cystic transformation.[7] With early diagnosis and effective treatment, the patients can recover and serial MRI can demonstrate complete disappearance of the lesions of the corpus callosum. In contrast, cases with lesions in other regions of the brain, especially cortical lesions, have unfavorable outcomes.[8]
During the previous 4 years, we have collected 9 patients with MBD. To describe the clinicoradiological features and identify factors that may affect prognosis, we retrospectively examined clinical manifestations, laboratory findings, and neuroradiological features of 9 patients with MBD. Our aim is to summarize the clinical and radiology features of MBD in our hospital, and to assess the effect of different factors for prognosis, we hope our study could provide a reference for clinical diagnosis and treatment.
2. Materials and methods
2.1. Patient selection
We examined clinical manifestations, laboratory results, and neuroradiological features of 9 patients with MBD who presented with neurological symptoms, such as seizure, cognitive impairment, limb hypertonic, delirium/coma, and dysarthria. All patients were hospitalized between January 2010 and January 2014. Two board-certified neurologists examined each patient to provide a definite diagnosis of MBD (Table 1).
Table 1.
Clinical manifestations, time course, and therapy of 9 patients with MBD.
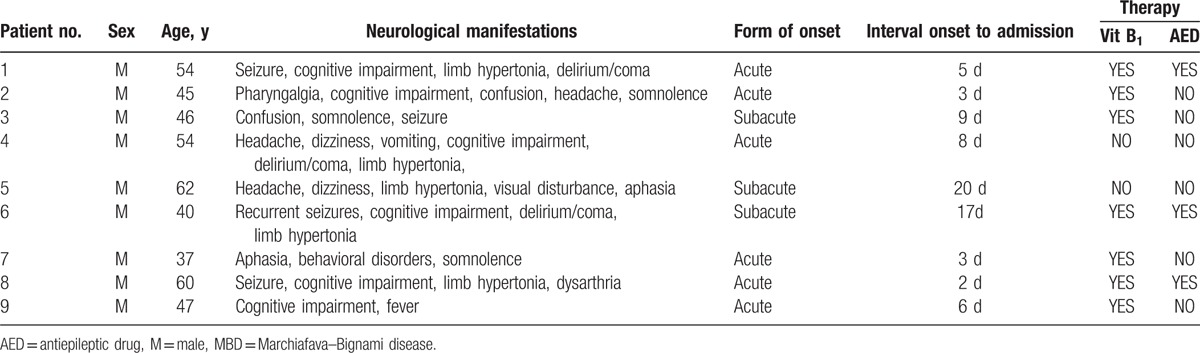
Nine adult male patients with MBD were identified. The age of onset ranged from 37 to 62 years; mean age was 47.00 ± 14.50 years. Three suspected cases were excluded because these individuals were nonalcoholics and had diabetes for several years.
2.2. MRI examinations
All 9 patients underwent their initial MRI from 0 to 3 days after hospitalization. MRI was performed using 3.0 T MR equipment with the following parameters: T1-weighted imaging (T1WI): TR/TE of 530 millisecond/7.2 millisecond; T2WI: TR/TE of 5200 millisecond/120 millisecond, slice thickness 4 mm, interslice gap of 2 mm, and matrix size was 512 × 512; DWI: TR/TE of 2500 millisecond/67 millisecond, field of view was 230 mm, matrix size was 144 × 144, b values were from 0 to 1000 second/mm2, slice thickness was 6.0 mm, and interslice gap was 1.0 mm; fast fluid attenuated inversion recovery (FLAIR) imaging: TR/TE was 7000 millisecond/100 millisecond, field of view was 230 mm, TI was 2500 millisecond, and matrix size was 512 × 512.
MRI findings, such as shape of splenial lesion, the regions of the CC impairment, other involved regions of the CC, DWI, and T2WI/FLAIR findings, enhancement effect, and reversal of lesion during follow-up; description of chronic alcoholism; and laboratory results, were comprehensively analyzed.
2.3. Evaluation of neurological and cognitive function
Neurological function was evaluated by the Modified Oxford Handicap Scale (MOHS). The MOHS is similar to the modified Rankin scale and has values from 0 to 6:0 represents no symptoms or completely independent functioning and 6 indicates the patient has died. MOHS scores of 0–2 indicate a good functional outcome. The MOHS was determined 30 days after admission; the patients were categorized into 2 subgroups that corresponded to the severity of the disability. A favorable outcome was defined as “no or mild disability” (MOHS ≤ 2), and a poor outcome was defined as a “moderate or severe disability” (MOHS ≥ 3).
Cognitive function was evaluated using the Abbreviated Mental Test (AMT).[6] The AMT uses a 10-item scale: age, time of day, year, place, recognition of people, date of birth, national day, president, counting backward from 20 to 1, and recall of an address. Each scale is measures with a score and the maximum score is 10. The Glasgow Coma Scale (GCS) was used to assess the severity of impaired consciousness.
2.4. Evaluation of consumption of alcohol
The consumption of alcohol was evaluated using Michigan Alcoholism Screening Test (MAST-C).[9] MAST was first introduced in 1971 and was developed based on the items used in alcoholism surveys by previous investigators. The MAST-C has 24 items and requires 15 to 30 minutes to complete by a physician. The questions contain interpersonal relationships, work performance, health status, legal issues, and family problems. A total score of 6 or more indicates hazardous drinking or alcohol dependence.
Additionally, clinical manifestations and laboratory findings were compared with the MRI findings of these patients.
2.5. Statistical analysis
We compared clinical manifestations, laboratory results, and neuroradiological features between 2 subgroups using Fisher exact test for categorical variables and the Mann–Whitney U test for continuous variables. All statistical analysis was performed with the Statistical Package for Social Sciences (SPSS) version 17.0 program, and a value of P < .05 was considered statistically significant.
2.6. Standard protocol approvals, registrations, and patient consents
This study was approved by the Ethics Committee of Shengjing Hospital, China Medical University. All participants provided written informed consent before inclusion in the study.
3. Results
3.1. Clinical features and patient outcomes
The details of clinical manifestations, clinical course, and therapy of 9 patients with MBD are displayed in Table 1. Nine adult male patients with MBD were identified. The age of onset ranged from 37 to 62 years; mean age was 47.00 ± 14.50 years. Three suspected cases were excluded because these individuals were nonalcoholics and had diabetes for several years.
The etiology of MBD is considered related to chronic alcoholism. Among the 9 patients, the mean history of drinking ethanol was 18.78 years (range 4–30 years). Four (44.4%) patients scored over 6 of MAST-C. The form of onset was acute in 6 (66.7%) patients. The mean time interval from onset to admission was 8.11 days (range, 2–20 days). Four (44.4%) patients had seizures (cases 1, 3, 6, and 8) accompanying cognitive impairment and limb hypertonic. Other symptoms such as delirium/coma, pharyngalgia, confusion, headache, somnolence, dizziness, vomit, aphasia, behavioral disorders, and dysarthria were also found. The mean GCS was 10.00 ± 5.00 (range 6–10). The mean AMT for all 9 patients was 6.00 ± 4.00 (range 3–9).
3.2. Laboratory examination findings
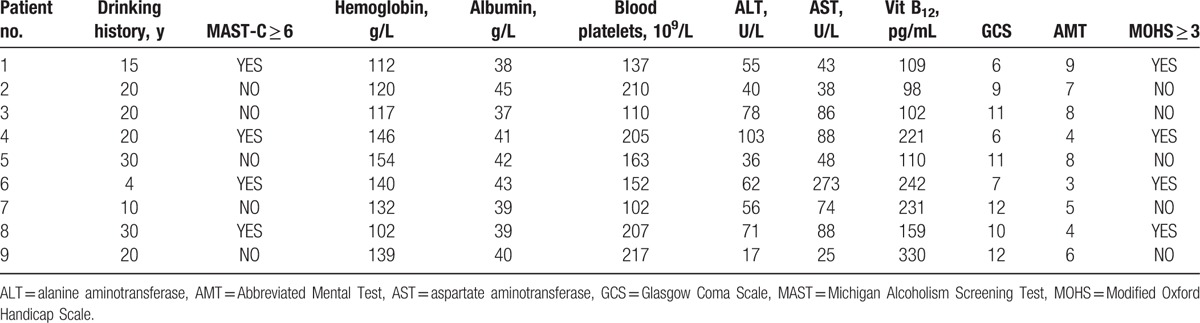
The laboratory findings are summarized in Table 2. The mean hemoglobin was 132.00 ± 28.50 g/L (range, 102–154). The mean albumin was 40.00 ± 4.00 g/L (range, 37–45). The mean blood platelet count was 163.00 ± 85.00 × 109/L (range, 102–217). The mean alanine aminotransferase (ALT) was 56.00 ± 36.50 U/L (range, 17–103). The mean aspartate aminotransferase (AST) was 74.00 ± 47.50 U/L (range, 25–27). The mean B12 was 159.00 ± 131.00 pg/mL (range, 98–330) (Table 2).
Table 2.
Laboratory findings of 9 patients with MBD.
3.3. MRI findings
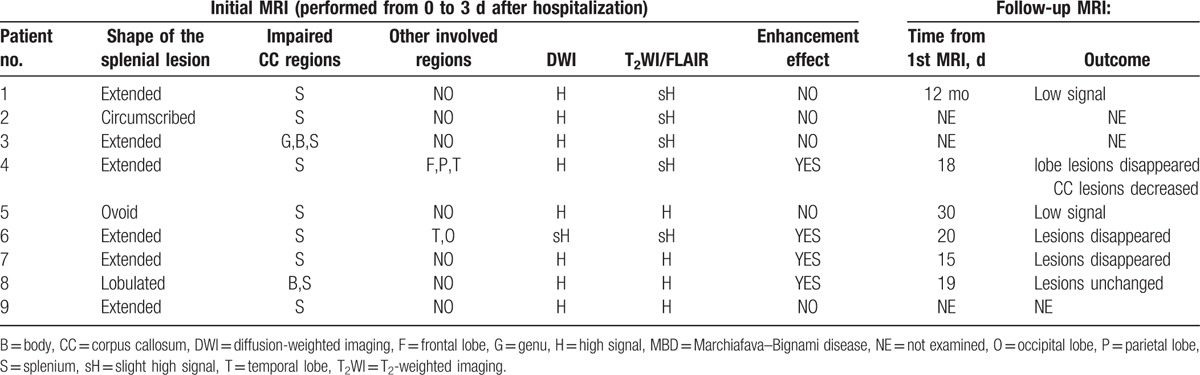
MRI findings of the 9 patients with MBD are displayed in Table 3. All 9 patients were performed MRI from 0 to 3 days after hospitalization. Splenial lesion were most often extended in shape (cases 1, 3, 4, 6, 7, 9) but also were circumscribed shape (case 2), ovoid shape (case 5), and lobulated in shape (case 8). The impaired callosal regions included only the splenium (cases 1–9), the genu, body and splenium (case 3), and the body and splenium (case 8). Other involved parts in CC were frontal lobe, parietal lobe, and temporal lobe (case 4). Temporal lobe and occipital lobe were involved (case 6). The involved region showed high signal intensity on FLAIR/T2-weighted sequences and DWI, with (cases 4, 6, 7, 8) or without (cases 1, 2, 3, 5, 9) enhancement effect. The follow-up MRI was performed 15 days to 12 months (case 2, 3, 9 were not examined). Reversal of lesion in follow-up MRI was low signals (cases 1, 5), Lobe lesions disappeared and CC lesions decreased (case 4), lesions disappeared (case 6, 7), or unchanged (case 8) (Table 3).
Table 3.
MRI findings of 9 patients with MBD.
3.4. Analysis of 2 subgroups and prognostic factors
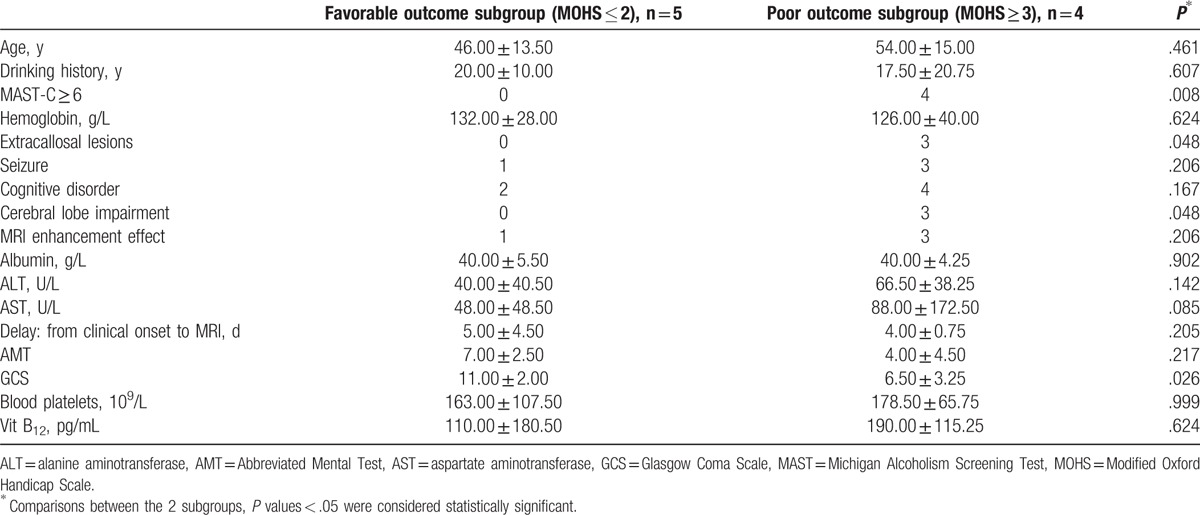
Table 4 displays the comparisons between the 2 MOHS-based subgroups on demographic, clinical, laboratory, and radiological characteristics. Four patients were classified as belonging to the poor outcome subgroup, and the other 5 patients were classified to the favorable outcome subgroup. The GCS value confirmed the apparent difference between the 2 subgroups (P = .026). The etiology of MBD is considered related to chronic alcoholism; the relatively high score of MAST-C (≥6), differed significantly between the subgroups (P = .008). The poor outcome subgroup more frequently had extracallosal lesions (P = .048). Similarly, cerebral lobe impairment was more frequent in the poor outcome group (P = .048), which indicates these factors mentioned above may affect the prognosis of MBD. However, neither the frequency of a cognitive disorder (P = .167), nor vitamin B12 level (P = .624) differed between the subgroups (Table 4).
Table 4.
Comparison of demographic characteristics, clinical manifestations, laboratory findings, and radiological features of the 2 subgroups.
4. Discussion
4.1. Clinical course and manifestations
In MBD, symptom onset can be acute, subacute, or chronic. The onset in the 9 patients we studied was either acute or subacute. The acute form of MBD includes severe disturbance of consciousness, seizures, and limb hypertonia. The subacute form includes confusion, dysarthria, behavioral abnormalities, somnolence, and visual disturbance.[10] The clinical manifestations of MBD are not specific to this disease; mild clinical signs such as headache, dizziness, emotional and psychotic symptoms, depression, apathy, and hemiparesis are regarded as initial manifestations. However, if diagnosis and treatment are not timely, MBD may progress to coma or even death. According to our study and other case reports, patients with severe disturbance of consciousness and neurocognitive deficits appear to have a poor prognosis, and lesions may not disappear.[11,12] Acute MBD needs to be distinguished from Wernicke encephalopathy, another alcohol-related disease that presents with ataxia, ophthalmoplegia, nystagmus, and confusion. The chronic form of MBD, which presents with dementia, needs to be differentiated from Alzheimer disease.[1,2]
4.2. MRI features
MBD is rare and does not have a typical presentation, so it is difficult to diagnose or differentiate from other diseases at an early stage. However, due to advances in MRI, early diagnosis of MBD has become possible. MRI detects lesions of high signal intensity in T2WI/FLAIR and DWI, especially in the corpus callosum, which indicate the presence of cytotoxic edema in MBD.[13,14] Lesions are also found in other regions of the brain, such as cerebral lobes, hemispheric white matter, and basal ganglia; such extracallosal lesions are found primarily in patients with a poor prognosis and severely impaired cognitive.[15–19] MRI plays a dominant role in the early diagnosis of MBD, facilitates early invention of acute MBD, and also predicts its prognosis.[20] (Supplemental Figure 1).
4.3. Pathophysiological mechanism of MBD
The pathophysiological mechanism of MBD is currently unclear; several case reports discuss possible mechanisms of MBD, but all the discussions remain uncertain and inconclusive. Possible mechanisms include cytotoxic edema, the breakdown of the blood–brain barrier, demyelination, and necrosis. The splenium has more myelin than any other part of the callosum. So there is a plausible explanation why intramyelinic edema may be a possible mechanism for lesions in callosum. However, the cortical lesions cannot be explained by this hypothesis.[3] Cortical lesions are considered to indicate Morel's laminar sclerosis, which has previously been reported in postmortem examinations.[17] Cytotoxic edema is proposed as the possible underlying mechanism in the early stage when hyperintense lesions are seen on DWI, while demyelination and necrosis may play a role in later stages.[21] However, neither of these mechanisms could explain why the corpus callosum is vulnerable in MBD. (Supplemental Figure 2).
4.4. Prognostic factors
The results of this study indicate that heavy alcohol consumption (MAST-C ≥ 6), extracallosal lesions, cerebral lobe impairment, and patients with severe disturbances of consciousness estimated by GCS may have a poor prognosis (MOHS ≥ 3). MBD is a disease related to chronic alcohol consumption, but also occurs in nonalcoholics. Chronic malnutrition might be another etiology of MBD because some patients completely recover after treatment with B vitamin compounds.[22] In MBD, extracallosal lesions are primarily discovered in cortical areas, which indicate Morel's laminar sclerosis. Such lesions are also observed in Wernicke's encephalopathy, heavy alcohol consumption, and chronic malnutrition. Autopsy reports of alcoholic patients with MBD found that the third layer of the cortex was primarily affected.[23] Some researchers have proposed that the cortex and the corpus callosum are the most vulnerable regions of acute MBD. The cortical lesions might not be caused by heavy alcohol consumption but by deficiency of thiamine.[24] Lesions in other brain regions are primarily found in patients with a poor prognosis and severe cognitive impairment. In contrast, patients with circumscribed lesions in the corpus callosum who receive an early diagnosis and appropriate treatment have a favorable prognosis.[25] In addition, MRI, particularly DWI, can be a prognosis indicator of clinical recovery and neuroradiological changes.[26]
4.5. Differential diagnosis
The differential diagnosis for MBD consists of other chronic, alcohol-related diseases. MBD needs to be distinguished from Wernicke's encephalopathy, an alcohol-related disease that may occur together with MBD that presents with ataxia, ophthalmoplegia, nystagmus, and confusion. Wernicke encephalopathy can be diagnosed by involvement of medial thalamic nuclei, hypothalamus, mamillary bodies, and periaqueductal grey matter. Pontine and extrapontine myelinolysis can also be differentiated by the involvement of the central pons, basal ganglia, thalami, lateral geniculate body, cerebellum, and cerebral cortex.[1,2] Other demyelinating diseases represented by multiple sclerosis also need to identify with MBD. Compared to other diseases, the characteristic MRI presentation of MBD is bisymmetric lesions of the corpus callosum; MBD lesions could also impair the cortex, subcortical area, and the white matter.[11]
4.6. Study limitations and strength
Although these results described the clinicoradiological features of MBD in adults using rigorous, systematic methods, the present study still has some limitations. First, our study is a single center, and there may be selective bias. Second, another major limitation is the relatively small number of patients with MBD we studied; this may affect the subgroups we classified and explain the limited spectrum of we observed. For example, the onset of all 9 patients was either acute or subacute; we did not observe patients with chronic onset. However, we regard our sample as representative. Adding more patients with MBD and a longer follow-up intervals might resolve this major limitation. Third, the present study chose the MOHS as the standard for prognosis, which was confined to detection methods and consistency of follow-up. Whether different lengths of follow-up affect the prognosis of MBD remains unknown. In addition, the patients we studied were all chronic alcoholics, we did not compare other possible etiologies that could lead to MBD. The major strengths of the present study are that it provided reliable, consistently collected data from patients with MBD and compared 2 subgroups using a systematic method, which supplement the deficiency of current studies.
5. Conclusion
In summary, the results of the present study indicated that in MBD patients with an acute to subacute onset, those who have high alcohol consumption (MAST-C ≥ 6), extracallosal lesions, cerebral lobe impairment, and severe disturbances of consciousness estimated by GCS have a poor prognosis (MOHS ≥ 3). MRI can reveal possible pathophysiological mechanism of MBD at an early stage, facilitate early diagnosis and intervention, and evaluate the prognosis.
Supplementary Material
Footnotes
Abbreviations: DWI = diffusion-weighted imaging, MBD = Marchiafava–Bignami disease, T1WI = T1-weighted imaging, T2WI = T2-weighted imaging.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Arbelaez A, Pajon A, Castillo M. Acute Marchiafava-Bignami disease: MR findings in two patients. AJNR Am J Neuroradiol 2003;24:1955–7. [PMC free article] [PubMed] [Google Scholar]
- [2].Kakkar C, Prakashini K, Polnaya A. Acute Marchiafava-Bignami disease: clinical and serial MRI correlation. BMJ Case Rep 2014;2014:502–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Carrilho PE, Santos MB, Piasecki L, et al. Marchiafava-Bignami disease: a rare entity with a poor outcome. Rev Bras Ter Intensiva 2013;25:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee SH, Kim SS, Kim SH, et al. Acute Marchiafava-Bignami disease with selective involvement of the precentral cortex and splenium: a serial magnetic resonance imaging study. Neurologist 2011;17:213–7. [DOI] [PubMed] [Google Scholar]
- [5].Namekawa M, Nakamura Y, Nakano I. Cortical involvement in Marchiafava-Bignami disease can be a predictor of a poor prognosis: a case report and review of the literature. Intern Med 2013;52:811–3. [DOI] [PubMed] [Google Scholar]
- [6].Pan CW, Wang X, Ma Q, et al. Cognitive dysfunction and health-related quality of life among older Chinese. Sci Rep 2015;5:17301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tung CS, Wu SL, Tsou JC, et al. Marchiafava-Bignami disease with widespread lesions and complete recovery. AJNR Am J Neuroradiol 2010;31:1506–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bano S, Mehra S, Yadav SN, et al. Marchiafava-Bignami disease: role of neuroimaging in the diagnosis and management of acute disease. Neurol India 2009;57:649–52. [DOI] [PubMed] [Google Scholar]
- [9].Hsueh YJ, Chu H, Huang CC, et al. Psychometric properties of the Chinese version of the Michigan Alcoholism Screening Test (MAST-C) for patients with alcoholism. Perspect Psychiatr Care 2014;50:83–92. [DOI] [PubMed] [Google Scholar]
- [10].Kumar KS, Challam R, Singh WJ. Marchiafava–Bignami disease: a case report. J Clin Diagn Res 2014;8:RD01–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bilgic B, Bilgin AA, Buluth H, et al. A Marchiafava–Bignami patient with mild symptoms and the role of diffusion-weighted magnetic resonance imaging in the diagnosis. Arch Neuropsychiatry 2011;48:277–80. [Google Scholar]
- [12].Salazar G, Fragoso M, Espanol G, et al. Primary degeneration of the corpus callosum (Marchiafava-Bignami disease): 2 unusual clinical presentations. Neurologia 2013;28:587–9. [DOI] [PubMed] [Google Scholar]
- [13].Yoshizaki T, Hashimoto T, Fujimoto K, et al. Evolution of callosal and cortical lesions on MRI in Marchiafava-Bignami disease. Case Rep Neurol 2010;2:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hlaihel C, Gonnaud PM, Champin S, et al. Diffusion-weighted magnetic resonance imaging in Marchiafava-Bignami disease: follow-up studies. Neuroradiology 2005;47:520–4. [DOI] [PubMed] [Google Scholar]
- [15].Tuntiyatorn L, Laothamatas J. Acute Marchiafava-Bignami disease with callosal, cortical, and white matter involvement. Emerg Radiol 2008;15:137–40. [DOI] [PubMed] [Google Scholar]
- [16].Menegon P, Sibon I, Pachai C, et al. Marchiafava-Bignami disease: diffusion-weighted MRI in corpus callosum and cortical lesions. Neurology 2005;65:475–7. [DOI] [PubMed] [Google Scholar]
- [17].Kawarabuki K, Sakakibara T, Hirai M, et al. Marchiafava-Bignami disease: magnetic resonance imaging findings in corpus callosum and subcortical white matter. Eur J Radiol 2003;48:175–7. [DOI] [PubMed] [Google Scholar]
- [18].Ihn YK, Hwang SS, Park YH. Acute Marchiafava-Bignami disease: diffusion-weighted MRI in cortical and callosal involvement. Yonsei Med J 2007;48:321–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Johkura K, Naito M, Naka T. Cortical involvement in Marchiafava-Bignami disease. AJNR Am J Neuroradiol 2005;26:670–3. [PMC free article] [PubMed] [Google Scholar]
- [20].Paidipati Gopalkishna Murthy K. Magnetic resonance imaging in Marchiafava-Bignami syndrome: a cornerstone in diagnosis and prognosis. Case Rep Radiol 2014;2014:609708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Staszewski J, Macek K, Stepien A. Reversible demyelinisation of corpus callosum in the course of Marchiafava-Bignami disease. Neurol Neurochir Pol 2006;40:156–61. [PubMed] [Google Scholar]
- [22].Celik Y, Temizoz O, Genchellac H, et al. A non-alcoholic patient with acute Marchiafava-Bignami disease associated with gynecologic malignancy: paraneoplastic Marchiafava-Bignami disease? Clin Neurol Neurosurg 2007;109:505–8. [DOI] [PubMed] [Google Scholar]
- [23].Bellido S, Navas I, Aranda MA, et al. Unusual MRI findings in a case of Marchiafava Bignami disease. Neurology 2012;78:1537. [DOI] [PubMed] [Google Scholar]
- [24].Gimeno MJ, Lasierra R, Pina JI. Marchiafava-Bignami disease. Four case reports. Rev Neurol 2002;35:596–8. [PubMed] [Google Scholar]
- [25].Helenius J, Tatlisumak T, Soinne L, et al. Marchiafava-Bignami disease: two cases with favourable outcome. Eur J Neurol 2001;8:269–72. [DOI] [PubMed] [Google Scholar]
- [26].Oster J, Doherty C, Grant PE, et al. Diffusion-weighted imaging abnormalities in the splenium after seizures. Epilepsia 2003;44:852–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


