Supplemental Digital Content is available in the text
Keywords: antiretroviral therapy, cost–benefit analysis, HIV, young people
Abstract
Objectives:
To analyze the cost effectiveness of short-cycle therapy (SCT), where patients take antiretroviral (ARV) drugs 5 consecutive days a week and have 2 days off, as an alternative to continuous ARV therapy for young people infected with human immunodeficiency virus (HIV) and taking efavirenz-based first-line ARV drugs.
Methods:
We conduct a hierarchical cost-effectiveness analysis based on data on clinical outcomes and resource use from the BREATHER trial. BREATHER is a randomized trial investigating the effectiveness of SCT and continuous therapy in 199 participants aged 8 to 24 years and taking efavirenz-based first-line ARV drugs in 11 countries worldwide. Alongside nationally representative unit costs/prices, these data were used to estimate costs and quality adjusted life years (QALYs). An incremental cost-effectiveness comparison was performed using a multilevel bivariate regression approach for total costs and QALYs. Further analyses explored cost-effectiveness in low- and middle-income countries with access to low-cost generic ARV drugs and high-income countries purchasing branded ARV drugs, respectively.
Results:
At 48 weeks, SCT offered significant total cost savings over continuous therapy of US dollar (USD) 41 per patient in countries using generic drugs and USD 4346 per patient in countries using branded ARV drugs, while accruing nonsignificant total health benefits of 0.008 and 0.009 QALYs, respectively. Cost-effectiveness estimates were similar across settings with access to generic ARV drugs but showed significant variation among high-income countries where branded ARV drugs are purchased.
Conclusion:
SCT is a cost-effective treatment alternative to continuous therapy for young people infected with HIV in countries where viral load monitoring is available.
1. Introduction
Thirty years after acquired immunodeficiency syndrome (AIDS) was first recognized, considerable progress has been made in combating the epidemic. The public health landscape was transformed with the emergence of effective human immunodeficiency virus-1 (HIV-1) therapies and the subsequent global expansion of access to these treatments.[1,2] Evidence has shown that people who have access to antiretroviral (ARV) drugs early in the course of infection may live a near-normal lifespan.[3]
Despite these achievements, challenges still exist for vulnerable groups, such as young people who are more likely to drop out of care and have lower viral suppression and adherence rates than adults.[4–7] The 2016 World Health Organization HIV Treatment Guidelines called for adolescent friendly treatment guidelines, yet the evidence on approaches to achieve this remains limited.[8,9]
One option that offers promise is short-cycle therapy (SCT), in which patients have weekends off from taking long-acting ARV drugs. This was shown to be virologically noninferior to continuous treatment in the BREATHER trial, which assessed young people, as well as among adults in small adult trials.[10–13] Findings from a qualitative study using a subsample of BREATHER showed that participants described a positive SCT experience and a preference to SCT over continuous therapy.[14]
As yet no information exists to guide policymakers about the value for money of SCT compared with continuous therapy for HIV-positive young people. This study investigates the cost-effectiveness of SCT in the 11 countries that took part in the BREATHER trial and explores if the economic results could be applicable to other settings.
2. Methods
The cost-effectiveness analysis compared SCT and continuous therapy using individual patient-level data from BREATHER on resource use and quality adjusted life years (QALYs) over a 48-week time horizon. Participants were aged 8 to 24 years, and must have been stable on first-line efavirenz with 2 nucleoside reverse transcriptase inhibitors with HIV-1 ribonucleic acid viral load <50 copies/mL for 12 months or longer. The trial protocol was approved by the ethics committees in participating centers in Europe, Africa, and the United States. Parents or guardians and older participants provided written consent; young children gave assent appropriate for age and knowledge of HIV status, as per guidelines for each participating country. The trial is described in detail elsewhere.[10] Participants were randomized 2 to 4 weeks after screening and then assessed clinically, including viral load and T lymphocytes measurements, at weeks 4 and 12, and then every 12 weeks for a total of 48 weeks’ follow-up.
Due to heterogeneity in ART prices across countries, the trial sample was divided into 2 groups: countries that access generic drugs through the Global Fund for AIDS, tuberculosis, and malaria procurement systems (“generic”: Thailand, Uganda, and Ukraine); those who pay for brand name ARV drugs (“branded”: Argentina, Belgium, Denmark, Germany, Ireland, Spain, the United Kingdom, and the United States).
Resource use data is taken from the BREATHER trial from case report forms using a healthcare provider perspective, which includes only direct medical costs. Unit prices for the generic medications were extracted from the Global Fund and Médecins Sans Frontières.[15,16] For the high-income countries, the ARV drug costs were obtained from local sources.[17–24] The costs of inpatient care were obtained from the WHO-CHOICE dataset, while test costs were obtained from other studies.[25–30] Monetary values are presented in 2015 US dollar (USD) .
Quality of life (QoL) was measured in the trial using the Pediatric Quality of Life (PedsQL) tool at randomization (0 weeks), 24 and 48 weeks. Although children-specific and widely validated, PedsQL is a nonpreference-based measure so cannot directly be used to calculate QALYs.[31,32] To obtain QALYs, the PedsQL responses were mapped onto the EQ-5D health status descriptive tool using results from a previous exercise conducted in the United Kingdom (UK).[33]
A bivariate model specification was used to model costs and health outcomes simultaneously.[34] The use of a multilevel specification was assessed as the trial data were hierarchical with patients nested into sites nested into countries (presenting a 3-level structure). Due to the objective of generating evidence for any country considering SCT, the appropriate hierarchical estimation method was the random-effects specification rather than a fixed-effects approach.[35] Potential patient-level covariates were identified from the information collected in BREATHER.
Results are reported as incremental net monetary benefit (difference in outcomes multiplied by the cost-effectiveness threshold, less difference in costs). Two types of results were obtained: “pooled” (i.e., branded and generic groups) and country-specific (analyses by country). Country-specific values were estimated through empirical Bayes predictions (shrinkage estimators) using the random-coefficients specification.[35] The cost-effectiveness thresholds were drawn from Woods et al.[36] Positive incremental NMB indicates an intervention is cost-effective.
Values were missing for ART doses (12.1%), ART intake frequency (number of pills taken/day, 16.2%)1, and PedsQL measure for weeks 0, 24, and 48 at 19.6%, 17.6%, and 16.6%, respectively. Unit costs of laboratory tests were missing for 4 countries: Denmark, Spain, Germany, and Belgium.
As doses and intake frequency were similar between patients within the same cluster, ART cost data were imputed at the resource use level, using the country-specific mode. Where the cost of laboratory tests was not available, the highest unit cost in the generic/branded drug group to which the country belongs was used.
On the health benefit side of the trial, a descriptive analysis of missing data was performed in order to select the best method for handling the missing values (see Supplementary Material: eMethods for further details). According to this analysis, the data were nonmonotone missing at random with multiple follow-ups. Therefore, the best technique for imputing missing values is multiple imputation (MI).[37] To consider the hierarchical structure of the data, a 2-level structure in the imputation process was made using the software Realcom. The missing utility values were predicted in terms of gender, age group, and total cost at 6 months. The MI process was validated by comparing the distributions of the observed with the imputed data sets.
3. Results
3.1. Quality of life and costs
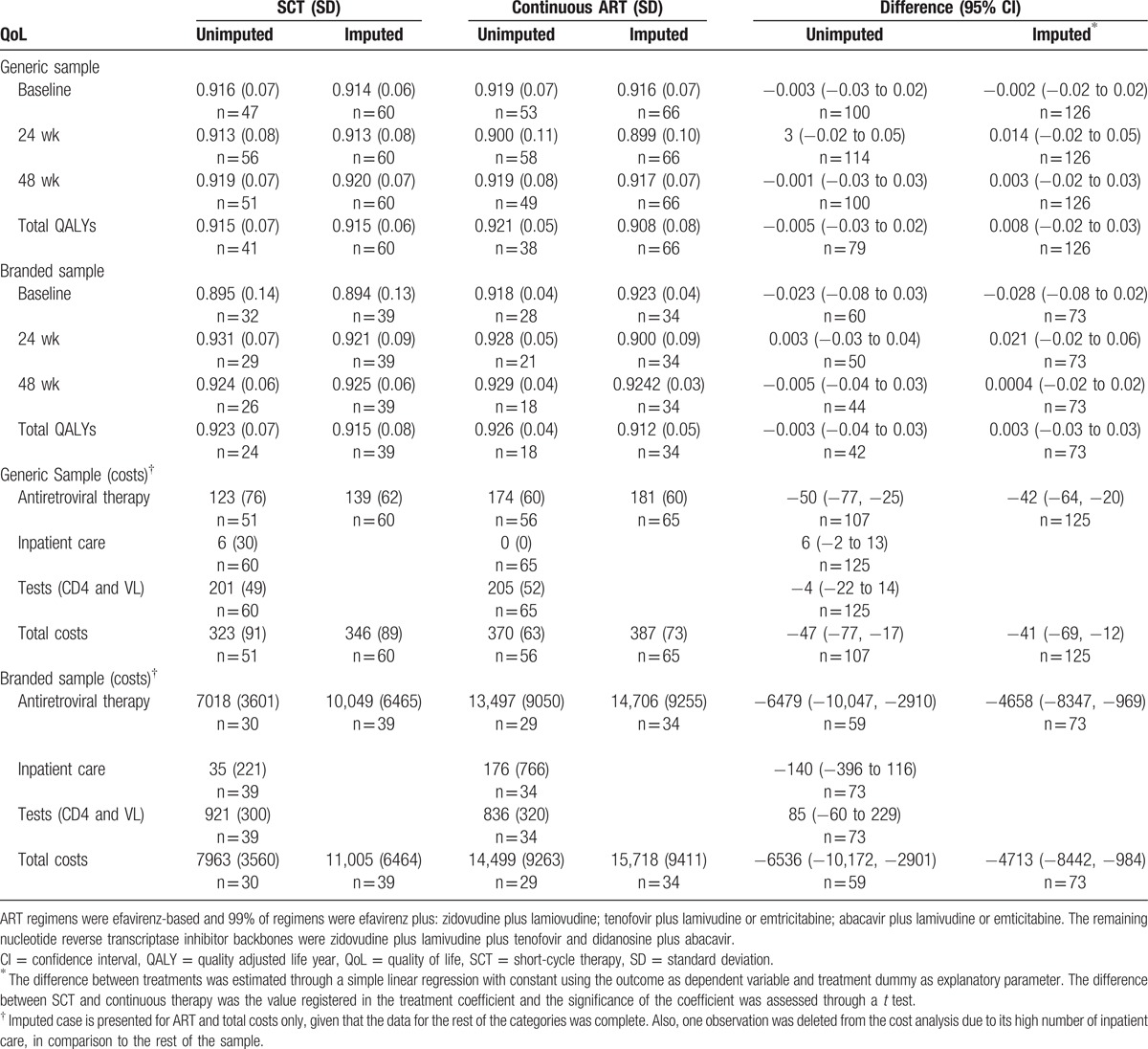
For the generic and branded groups, there was no significant difference between SCT and continuous therapy for PedsQL scores at week 0, 12, and 24, and total QALYs. Significant differences were identified for ARV drug and total costs in both groups (Table 1).
Table 1.
Pooled unimputed and imputed QoL and costs by trial arm.
At the country level, SCT significantly reduced total costs in most countries. In Germany and Ukraine, a decrease in total costs was nonsignificant (due to a very small sample size of 3 observations in Germany and an outlier in the SCT group in Ukraine who was treated with abacavir) (see Fig. 1).
Figure 1.
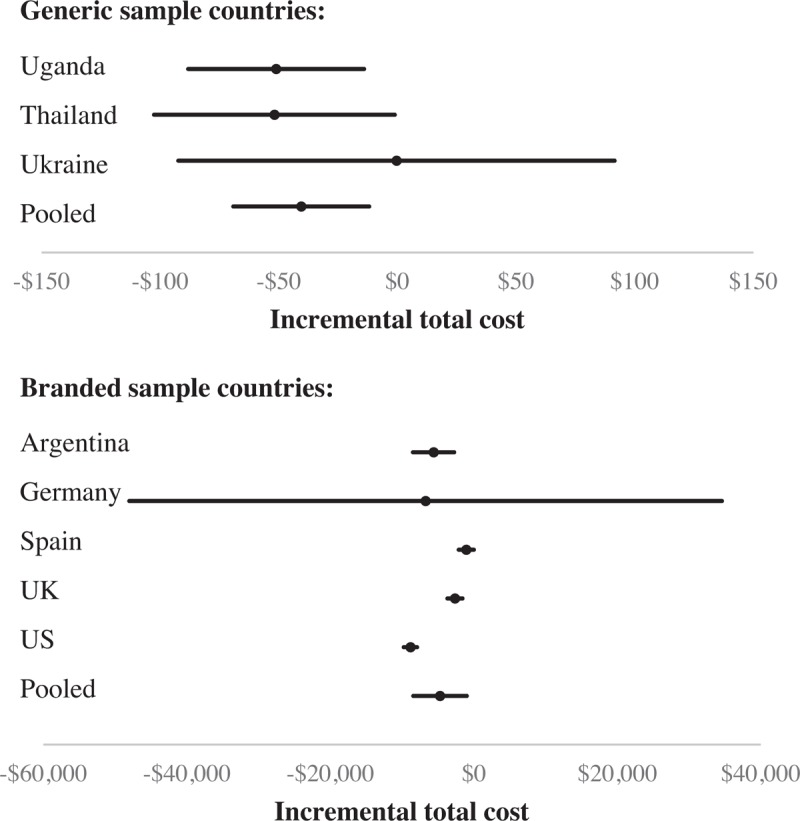
Incremental total cost of SCT compared with continuous therapy. A forest plot for incremental total cost of SCT versus continuous therapy is shown by country based on raw imputed data. Mean incremental costs (95% confidence intervals) are denoted by black circles (black lines). The pooled results include the values of all the patients inside a sample (generic/branded). SCT = short-cycle therapy.
3.2. Cost-effectiveness
For both groups, 3 modeling strategies were considered plausible. Differences between strategies result from different statistical approaches to the hierarchical data structure and the most appropriate strategy for each sample was determined by goodness-of-fit measures.
For the generic group, results show that countries differ minimally in measured QALYs and costs, and so a nonhierarchical specification is preferred. By contrast, for the branded group, estimation from the random-coefficients specification performs better than other strategies, implying that the pooled results may not apply to certain countries due to fundamental differences between clusters. See Supplementary Material: eMethods for the complete selection process.
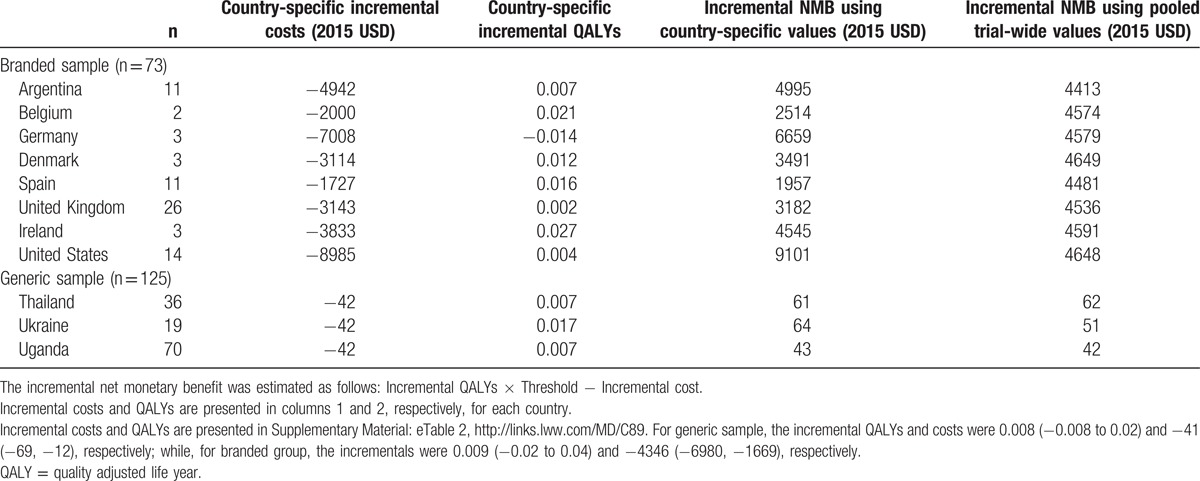
The pooled results (Table 2) indicate that SCT offers significant total cost savings of USD 41 per patient over continuous therapy over the 48-week time horizon in countries using generic drugs and USD 4346 per patient in countries using branded ARV drugs while accruing nonsignificant QoL benefits of 0.008 and 0.009 QALYs, for the generic and branded groups, respectively. Country-specific results for both groups are reported in Table 3. Although pooled results differ from country-specific estimates in some cases, whether using pooled or country-specific results, SCT is a cost-effective alternative to continuous therapy in every country.
Table 2.
Model selection: Trial-wide results for both samples.
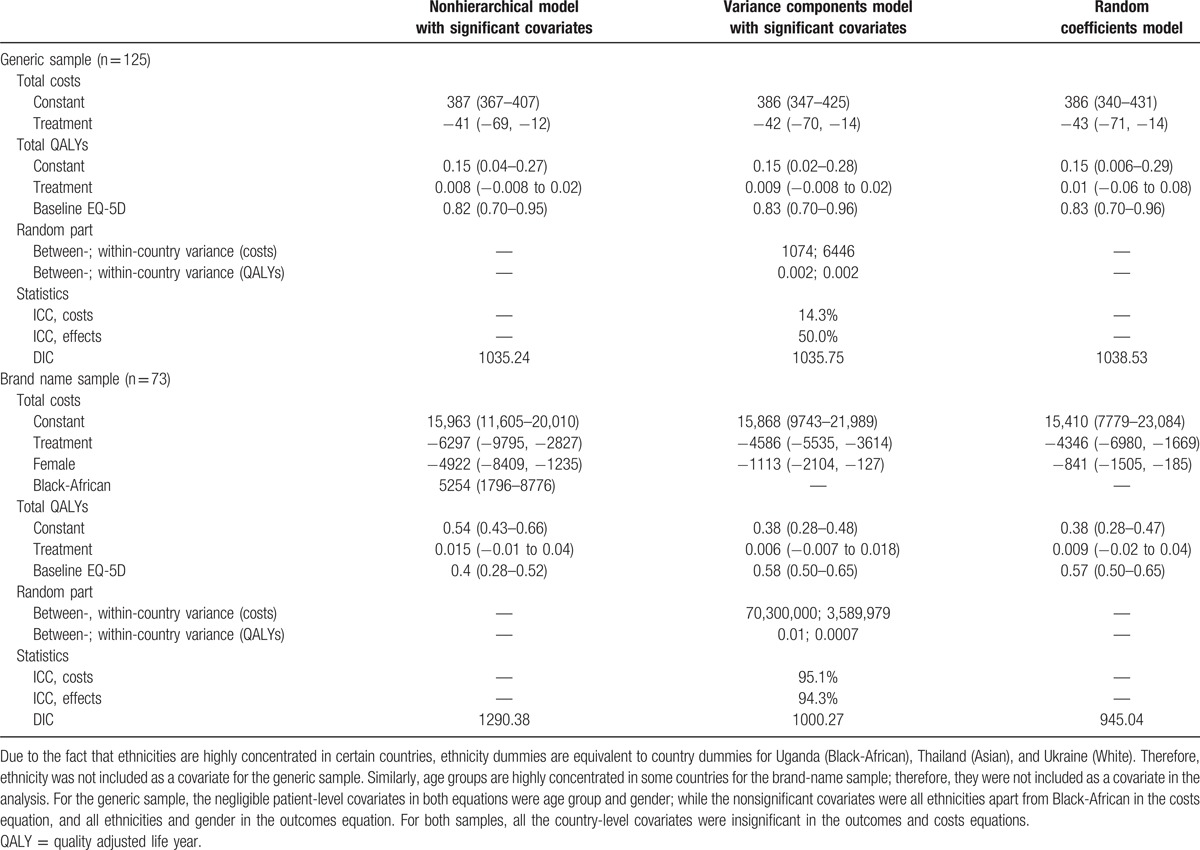
Table 3.
Cost effectiveness by group and country.
4. Discussion
The BREATHER multicountry trial showed that for HIV-infected young people, SCT with long-acting drugs was noninferior and maintained virological suppression compared with continuous therapy.[10] Study participants expected SCT to be easier than staying on continuous therapy (88% at trial baseline), and at the end of the trial this expectation was confirmed with 90% of those in the SCT group reporting that SCT made life easier (than continuous therapy) particularly as going out with friends was easier.[10] These findings were confirmed in a qualitative study using a subsample of BREATHER showed that participants described a positive SCT experience and a preference to SCT over continuous therapy.[14] This study assesses the cost-effectiveness of SCT as an option for young people in a wide range of countries. We find SCT offers significant cost savings and small, nonsignificant gains in health-related QoL compared with continuous therapy in all countries.
The magnitude of cost-savings with SCT and resulting cost-effectiveness estimates depend, however, upon whether a country has access to generic ARV drugs or faces the full costs of branded drugs. Countries inside the Global Fund procurement program show substantial homogeneity in outcomes and costs indicating results could generalize to other low- and middle-income countries where viral load monitoring is available. Although there is more heterogeneity across the countries purchasing branded drugs, SCT is cost-effective in all the countries evaluated.
During the model selection process, statistical tests demonstrated that a multilevel approach was required for the branded sample; however, for the group of countries purchasing generic drugs, a simpler cluster analysis performed well. Given that SCT is highly likely to be cost-effective in all cases, other LMICs acquiring ARV drugs through the Global Fund can reasonably rely on the pooled results, although countries purchasing branded ARV drugs may wish to undertake cost-effectiveness studies of SCT specific to their own jurisdiction.
This finding implies that where baseline and relative risks are similar across settings and where countries have access to commonly procured commodities, such as through Global Fund mechanism, it is unlikely to be necessary to repeat cost-effectiveness analyses in all jurisdictions. However, where countries negotiate their own prices with manufacturers, jurisdiction-specific analyses may be preferable.
This study is the first to assess the cost-effectiveness of SCT, and is one of few that explores the economics of youth-friendly forms of HIV treatment. Adolescents are highlighted as a particularly vulnerable population in HIV epidemics and it is recognized that existing evidence on youth-friendly approaches is limited and of generally poor quality. Young people are expected to have many years of taking ARV drugs ahead of them and the option of SCT has the potential to effectively reduce treatment fatigue and improve clinical results over the longer term.
This study applies a distinctive methodology by implementing a multilevel framework in all steps of the analysis. Despite employing robust methods, the evaluation has some limitations. First, analysis was restricted to 48 weeks as QOL and cost data were only collected up to 48 weeks (time point for the trial's primary analysis). Since HIV patients may live a near-normal lifespan with treatment; a 48-week time horizon is limited and represents a truncated time-horizon. Subsequent follow-up to 144 weeks of the BREATHER trial participants, maintaining original randomization, demonstrated that noninferior virological suppression on SCT versus continuous therapy was sustained; there were also no significant differences in grade 3/4 adverse events or ART-related adverse events between groups.[38] By 144 weeks 27/99 SCT participants had returned to continuous therapy (14 for viral rebound and 13 for other reasons; e.g., discontinuation of efavirenz, patient preference); most patients with viral rebound resuppressed on the same ART regimen. Although, the cost savings per year due to reduced ARV drug consumption on SCT are likely to diminish somewhat over time as some patients return to CT, BREATHER results suggest that SCT could offer substantial savings with ∼70% of SCT participants still taking weekends off out to 144 weeks.
Second, monitoring and clinic visits were more frequent and comprehensive than existing clinical practice in many countries. Where generic drugs are purchased the monitoring strategy is unlikely to include 3-monthly viral load monitoring as occurred in the trial. Further research may be warranted to assess if noninferiority and cost-effectiveness are maintained in real clinical settings even without enhanced monitoring.
Third, in some countries inside the branded sample, certain ARV drugs (i.e., efavirenz, tenofovir) will come off patent in the short run, which could have implications in the present analysis such as reducing the total cost gap between continuous therapy and SCT due to a considerable decrease in ARV drug acquisition costs.
A 4th potential limitation is that the health-related QoL mapping and resulting QALY estimates were based upon UK data and it is unclear whether there may be differences in values in other countries. However, there is no reason to believe use of UK health values in any way biased results one direction or another.
5. Conclusion
SCT, in which patients have weekends off from taking long-acting ARV drugs, is a cost-effective alternative to continuous therapy for young people. The cost effectiveness of SCT compared with continuous therapy was driven by lower ARV drug costs and differences in the other cost categories were negligible. Despite differences between countries, country-specific results reinforced the results of the pooled analysis; SCT did not have a significant impact on QoL but significantly reduced treatment costs.
Although countries differed in whether they had access to generic ARV drugs or purchased branded drugs, this study shows that SCT is cost-effective in all settings. As such, SCT can be considered as an adolescent-friendly alternative ART approach to current standard of care for young people.
Acknowledgments
The authors thank to Andrew Phillips for comments. The authors thank all of the young people, their families, and staff from the centers participating in the BREATHER trial.
Supplementary Material
Excluding missing data for existing treatment entries.
Abbreviations: AIDS = acquired immunodeficiency syndrome, ARV = antiretroviral, CHOICE = Choosing Interventions that are Cost-Effective, HIV = human immunodeficiency virus, MI = multiple imputation, PedsQL = Pediatric Quality of Life Inventory, QALY = quality adjusted life year, QoL = quality of life, SCT = short-cycle therapy, USD = US dollar.
Luis Enrique Tierrablanca and Jessica Ochalek contributed equally to the writing of this article and the implementation of the statistical analysis; Paul Revill made a substantial contribution to the writing of the article and made key recommendations for the development of the economic evaluation and Susan Griffin made key recommendations for the development of the economic evaluation. Deborah Ford and Ab Babiker provided comments on the statistical analyses. All authors provided input and comments on the manuscript, revised for important intellectual content, and approved the final version. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
The BREATHER trial was funded by the UK National Institute for Health Research Health Technology Assessment (projects: 08/53/25 and 11/136/108); European Commission through EuroCoord (FP7/2007/2015); PENTAFoundation; UK Medical Research Council; and INSERM SC10 to US19, France. The trial was sponsored by the Paediatric European Network for Treatment of AIDS (PENTA) Foundation. This work was supported by the Economic and Social Research Council and core support to the UK Medical Research Council (MC_UU_12023/26). Jamie Inshaw made the BREATHER trial data available for analysis and Wolf Rogowski provided the unit cost data for Germany.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
Contributor Information
Collaborators: the BREATHER (PENTA 16) Trial Group
References
- [1].Cohen MS, Hellmann N, Levy JA, et al. The spread, treatment, and prevention of HIV-1: evolution of a global pandemic. J Clin Invest 2008;118:1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].WHO; UNICEF; UNAIDS. Global Update on HIV Treatment 2013: Results, Impact and Opportunities. Geneva: World Health Organization; 2013. [Google Scholar]
- [3].Granich R, Gupta S, Hersh B, et al. Trends in AIDS deaths, new infections and ART coverage in the top 30 countries with the highest AIDS mortality burden; 1990–2013. PLoS ONE 2015;10:e0131353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].WHO. Global Update on the Health Sector Response to HIV, 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- [5].Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS 2014;28:128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kim S-H, Gerver SM, Fidler S, et al. Adherence to antiretroviral therapy in adolescents living with HIV. AIDS 2014;28:1945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lamb MR, Fayorsey R, Nuwagaba-Biribonwoha H, et al. High attrition before and after ART initiation among youth (15–24 years of age) enrolled in HIV care. AIDS 2014;28:559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].WHO. Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations. Geneva: World Health Organization; 2016. [PubMed] [Google Scholar]
- [9].WHO. What's new in adolescent treatment and care. WHO Fact Sheet, 2015. [Google Scholar]
- [10].BREATHER (PENTA 16) Trial Group. Weekends-off efavirenz-based antiretroviral therapy in HIV-infected children, adolescents, and young adults (BREATHER): a randomised, open-label, non-inferiority, phase 2/3 trial. Lancet HIV 2016;3:e421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].De Truchis P, Assoumou L, Landman R, et al. Efficacy of a maintenance four-days-a-week regimen, the ANRS162-4D trial. Paper presented at 21st International AIDS Conference, 2016. [Google Scholar]
- [12].Cohen CJ, Colson AE, Sheble-Hall AG, et al. Pilot study of a novel short-cycle antiretroviral treatment interruption strategy: 48-week results of the five-days-on, two-days-off (FOTO) study. HIV Clin Trials 2007;8:19–23. [DOI] [PubMed] [Google Scholar]
- [13].Reynolds SJ, Kityo C, Hallahan CW, et al. A randomized, controlled, trial of short cycle intermittent compared to continuous antiretroviral therapy for the treatment of HIV infection in Uganda. PLoS ONE 2010;5:e10307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bernays S, Paparini S, Seeley J, et al. Qualitative study of the BREATHER trial (Short Cycle antiretroviral therapy): is it acceptable to young people living with HIV? BMJ Open 2017;7:e012934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].The Global Fund to Fight AIDS. Price & Quality Reporting—Sourcing & Management of Health Products, 2015. [Google Scholar]
- [16].MSF Access Campaign. Untangling the Web of Antiretroviral Price Reductions: 17th Edition, July 2014, 2014. [Google Scholar]
- [17].British Medical Association and Royal Pharmaceutical Society of Great Britain. British National Formulary, 2015. [Google Scholar]
- [18].Vademecum nacional de Medicamentos, 2015. [Google Scholar]
- [19].Danish Medicines Agency. Prices & Reimbursement: Medicine Prices, 2017. [Google Scholar]
- [20].Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents, 2017. [Google Scholar]
- [21].Blasco AJ, Pérez-Molina JA, Blasco AJ, et al. Costs and cost-efficacy analysis of the 2014 GESIDA/Spanish National AIDS Plan recommended guidelines for initial antiretroviral therapy in HIV-infected adults. Enferm Infecc Microbiol Clin 2015;33:156–65. [DOI] [PubMed] [Google Scholar]
- [22].Institut national d’assurance maladie-invalidité. Spécialités pharmaceutiques - Liste des prix/bases de remboursement - 01/11/2015 - Lijst van de prijzen en vergoedingsbases - Farmaceutische specialiteiten, 2015. [Google Scholar]
- [23].Rote Liste Service GmbH. Rote Liste, 2015. [Google Scholar]
- [24].Database of prescription and generic drugs, clinical guidelines. MIMS online, 2013. [Google Scholar]
- [25].WHO. Cost Effectiveness and Strategic Planning (WHO-CHOICE), 2015. [Google Scholar]
- [26].Phillips A, Shroufi A, et al. Working Group on Modelling of Antiretroviral Therapy Monitoring Strategies in Sub-Saharan Africa. Sustainable HIV treatment in Africa through viral-load-informed differentiated care. Nature 2015;528:S68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nakagawa F, Miners A, Smith CJ, et al. Projected lifetime healthcare costs associated with HIV infection. Paper presented at 18th Annual Conference of the British HIV Association, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Belaunzarán-Zamudio PF, Caro-Vega YN, Shepherd BE, et al. Monitoring of HIV treatment in seven countries in the WHO region of the Americas. Bull World Health Organ 2015;93:529–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Brennan A, Jackson A, Horgan M, et al. Resource utilisation and cost of ambulatory HIV care in a regional HIV centre in Ireland: a micro-costing study. BMC Health Serv Res 2015;15:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Centers for Medicare & Medicaid Services. Clinical Laboratory Fee Schedule, 2016. [Google Scholar]
- [31].Brazier JE, Rowen D, Mavranezouli I, et al. Developing and testing methods for deriving preference-based measures of health from condition-specific measures (and other patient-based measures of outcome). Health Technol Assess 2012;16:1–14. [DOI] [PubMed] [Google Scholar]
- [32].Stevens K, Ratcliffe J. Measuring and valuing health benefits for economic evaluation in adolescence: an assessment of the practicality and validity of the child health utility 9D in the Australian adolescent population. Value Health 2012;15:1092–9. [DOI] [PubMed] [Google Scholar]
- [33].Khan KA, Petrou S, Rivero-Arias O, et al. Mapping EQ-5D utility scores from the PedsQL™ generic core scales. Pharmacoeconomics 2014;32:693–706. [DOI] [PubMed] [Google Scholar]
- [34].Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2015. [Google Scholar]
- [35].Rabe-Hekseth S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. College Station, TX: Stata Press; 2012. [Google Scholar]
- [36].Woods B, Revill P, Sculpher M, et al. Country-level cost-effectiveness thresholds: initial estimates and the need for further research. Value Heal 2016;19:929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Faria R, Gomes M, Epstein D, et al. A guide to handling missing data in cost-effectiveness analysis conducted within randomised controlled trials. Pharmacoeconomics 2014;32:1157–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Turkova A. Long-term effects of weekends off ART in HIV-1-infected young people ON EFV+2NRTI. Paper presented at CROI Conference, 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


