Supplemental Digital Content is available in the text
Keywords: dengue, hemorrhagic dengue fever, insulin pathway, plasma markers, protein array, severe dengue, TPM1
Abstract
Dengue virus is one of the most widespread flaviviruses that re-emerged throughout recent decades. The progression from mild dengue to severe dengue (SD) with the complications such as vascular leakage and hemorrhage increases the fatality rate of dengue. The pathophysiology of SD is not entirely clear. To investigate potential biomarkers that are suggestive of pathogenesis of SD, a small panel of serum samples selected from 1 healthy individual, 2 dengue patients without warning signs (DWS−), 2 dengue patients with warning signs (DWS+), and 5 patients with SD were subjected to a pilot analysis using Sengenics Immunome protein array. The overall fold changes of protein expressions and clustering heat map revealed that PFKFB4, TPM1, PDCL3, and PTPN20A were elevated among patients with SD. Differential expression analysis identified that 29 proteins were differentially elevated greater than 2-fold in SD groups than DWS− and DWS+. From the 29 candidate proteins, pathways enrichment analysis also identified insulin signaling and cytoskeleton pathways were involved in SD, suggesting that the insulin pathway may play a pivotal role in the pathogenesis of SD.
1. Introduction
Dengue virus (DENV) is one of the most widespread flaviviruses that re-emerged throughout recent decades, as it has made a global public health concern and is no longer related to tropical and subtropical countries only. Approximately 2.5 billion individuals from more than 110 countries are at danger of DENV infection each year due to the lack of an efficient vaccines and a specific treatment.[1] There are 4 serotypes of dengue viruses known as DENV-1, DENV-2, DENV-3, and DENV-4, where the infection of any serotype of DENV yields a wide range of symptoms ranging from mild dengue fever to severe dengue (SD) with complications such as plasma leakage and severe bleeding. Clinically, the features of acute DENV infection are rather nonspecific, as the symptoms such as high fever, arthralgia, headache, myalgia, and rash are also observed in other diseases such as chikungunya viral infection. However, 5% to 10% of the patients eventually develop into the life-threatening, severe form of DENV infection, characterized by thrombocytopenia and vascular leakage, leading to hypervolemic shock organ impairment and death if not promptly treated.[2,3]
Although the cause of severe form of dengue infection is not clear, it has been revealed that secondary infection with a different serotype of DENV,[4] or even a homotypic reinfection[5] is a major risk factor for SD probably due to antibody-dependent enhancement.[4] The phenomena of original antigenic sin,[6] immune evasion that inhibits interferon (IFN)-α and IFN-β signaling by suppressing Jak-Stat activation,[7] a cytokine storm,[8] and autoimmune responses[9] are thought to contribute to the pathogenesis of severe form of dengue disease. However, contradictory findings have been proposed by Hernández et al,[10] where no significant association was observed between the level of proinflammatory cytokines and disease severity, reflecting the complex nature of pathophysiology of dengue disease.
The present pilot study aims to identify immune-related proteins expressed in dengue patients with different disease severity through immunome profiling. Protein microarray platform allows analysis of more than 10 patient samples against many spatially isolated antigens coated on a single glass slide with a higher sensitivity than conventional enzyme-linked immunosorbent assay (ELISA). This study utilized an immunomics technique using a biotin carboxyl carrier protein (BCCP) domain affinity tag to promote fusion of post-translational biotinylated proteins to an array surface to include a total of 1636 proteins that play important functions in human immune response including kinases and transcription factors. The array also allows the detection of autoantibodies in patient serum samples, making them effective tools for biomarker discovery. The differential expressions of immune related proteins identified in patients with different dengue classifications could provide insights of the potential pathways of disease progression and hence, enable discovery of novel treatment strategies, and appropriate clinical managements.
2. Methodology
2.1. Sample collection
Blood samples from patient diagnosed with DENV infection were collected under the IRB reference number 926.4 with informed consent. The day of illness and clinical symptoms of the patients were remarked during blood collection and the disease severity was classified into 3 categories, including dengue without warning signs (DWS−), dengue with warning signs (DWS+), and SD determined by the signs and symptoms elaborated in Supplementary Table 1. Standard criteria as determined by WHO and MOH Malaysia were followed. The serum samples were subjected to DENV polymerase chain reaction (PCR), NS1 antigen ELISA, IgM ELISA, and hemagglutination inhibition (HI) test to confirm DENV infection.
2.2. Sample selection
A panel of serum samples comprised of 1 healthy individual as a healthy control (HC), 2 patients with DWS−, 2 patients with DWS+, and 5 patients with SD were selected for this pilot analysis. The samples were selected randomly to avoid any biases introduced by inappropriate selection. Other variables such as the history of previous therapy and medical care, or other medical conditions that might confound to the differences in between the experimental and control samples were also identified.
2.3. Immunome profiling
Serum samples were placed in a shaking incubator for thawing at 20°C for 30 minutes. About 22.5 μL of sample was diluted with 4.5 mL of serum assay buffer (SAB) containing 0.1% v/v Triton, 0.1% w/v BSA in PBS. An Immumome protein array (Sengenics, Malaysia) was removed from storage buffer and placed in a slide box containing 200 mL cold SAB, shaken on an orbital shaker at 50 rpm for 5 minutes. The slides were then placed in a slide hybridization chamber containing the diluted serum samples and incubated on a horizontal shaker at 50 rpm for 2 hours. After incubation, the array was rinsed with SAB buffer and incubated with a hybridization solution containing a mixture of Cy3-rabbit anti-human IgG solution (Dako Cytomation, Denmark) diluted 1:1000 in SAB buffer at 50 rpm for 2 hours. Finally, the slides were dipped in SAB buffer followed by pure water and dried at 240 g for 2 minutes. The hybridization signals were measured with a microarray laser scanner (Agilent Technologies, Petaling Jaya, Selangor, Malaysia) at 10 μm resolution. Fluorescence levels were detected and the data sorting and analysis were performed using Agilent Feature Extraction software on a Linux operating system with customized computer scripts. The list of 1636 proteins markers are listed in the Supplementary Table 2.
2.4. Quality control
Quality control based on raw and normalized data was done to verify the quality of protein array. The median of the raw signal intensities from the quadruplet protein spots on each slide was calculated and the median background signals were subtracted from the raw median signal intensities. The signal intensities of 2 positive controls including IgG and Cy3BSA were also inspected. The percentage of coefficient of variant (CV%) of intra-protein, intra-slide, and inter-array was calculated to determine the variations between the quadrupled signal intensity for each protein spot on the slide
2.5. Data analysis
The samples were matched in pairs between the experimental groups (DWS−, DWS+, and SD) with the control groups. Spot segmentation was performed to identify and detect spots automatically and accurately using GenePix Pro 7 software. A stringent filter was implemented to select only those protein markers that are present in 100% of samples of all study groups. The protein markers in DWS−, DWS+, and SD samples were further filtered to have > 2-fold expression change (FC) compared with the control. The transcripts identified were then clustered using a hierarchical algorithm with unsupervised Pearson clustering of the proteins was performed by using the normalized RFU from protein array analysis. Clustering for rows and columns were performed using Euclidean distance and complete linkage method. Differentially expressed proteins were plotted as a separate bar with yellow and red colors as up and downregulated genes, respectively. Complex Heatmap[11] package from Bioconductor was used for clustering and annotating the heat map. Data mining and statistical analysis for both quality control and identification of biomarkers were done using customized scripts created in R and Perl.
3. Results
3.1. Samples characteristics
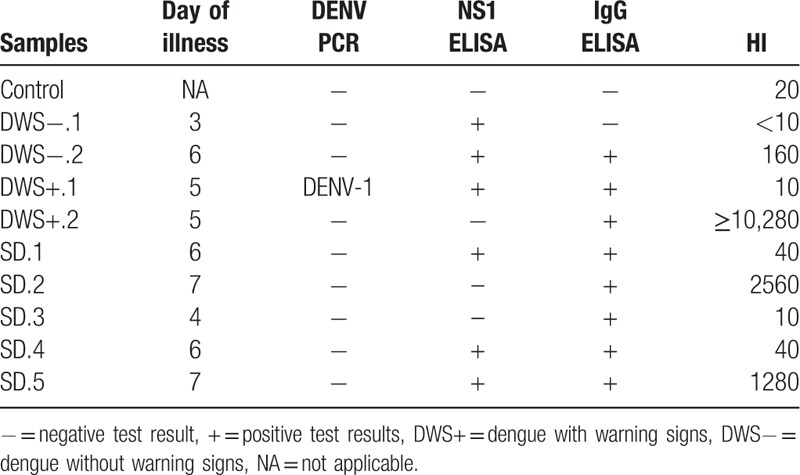
The clinical diagnosis of the serum samples selected in this study is reported in Table 1. The day of illness of the patients at the time of collection ranged from 3 to 7 days, and only 1 sample was found to be DENV PCR positive with serotype DENV-1. Six samples were NS1 positive, while 8 samples were IgM positive. The anti-DENV antibodies titration determined from HI revealed that most of the samples contained low concentration of neutralizing antibodies (<10, 10, 20, and 40), while 4 samples have a HI titer of 160, 1280, 2560, and ≥10,240.
Table 1.
The day of illness and the results for DENV PCR, NS1 ELISA, IgM ELISA, and HI for serum samples from 1 healthy control, 2 DWS−, 2 DWS+, and 5 SD.
3.2. Overall fold changes of protein expressions in each category
We investigated the transcriptional profile associated with the development of SD comparing to the DWS− and DWS+ using plasma protein microarray. Using unsupervised Pearson clustering of proteins, the pattern of protein expression was revealed in the heat map (Fig. 1). Among differentially expressed proteins, about 56% of the upregulated proteins formed the biggest cluster and similarly, 90% of the downregulated proteins are clustered together. Clustering of similar set of samples was observed in all the samples for the categories of control, DWS−, DWS+, and SD, with the exception of 1 patient with SD, probably due to an earlier day of illness (Day 4) when the sample was collected as compared with other patients in the same category (Day 6–7). High expression was observed uniquely in SD patients for PFKFB4, TPM1, PDCL3, and PTPN20A compared with other dengue samples. Of note, TPM1 where expression levels that as high as 50,000 RFU were observed in 3 out 5 SD patients indicting its importance SD. TP53 and ASNA1 were expressed highly in patients with DWS−, while TP53 constantly showed a high expression across all the dengue samples compared with control. AFF4, COPS6, and KRT19 also showed a notable high expression among DWS+ and DWS− patients. According to the clustering, DWS− patients showed more immune responses as compared with DWS+ patients probably due to the early infection stage in the patients.
Figure 1.
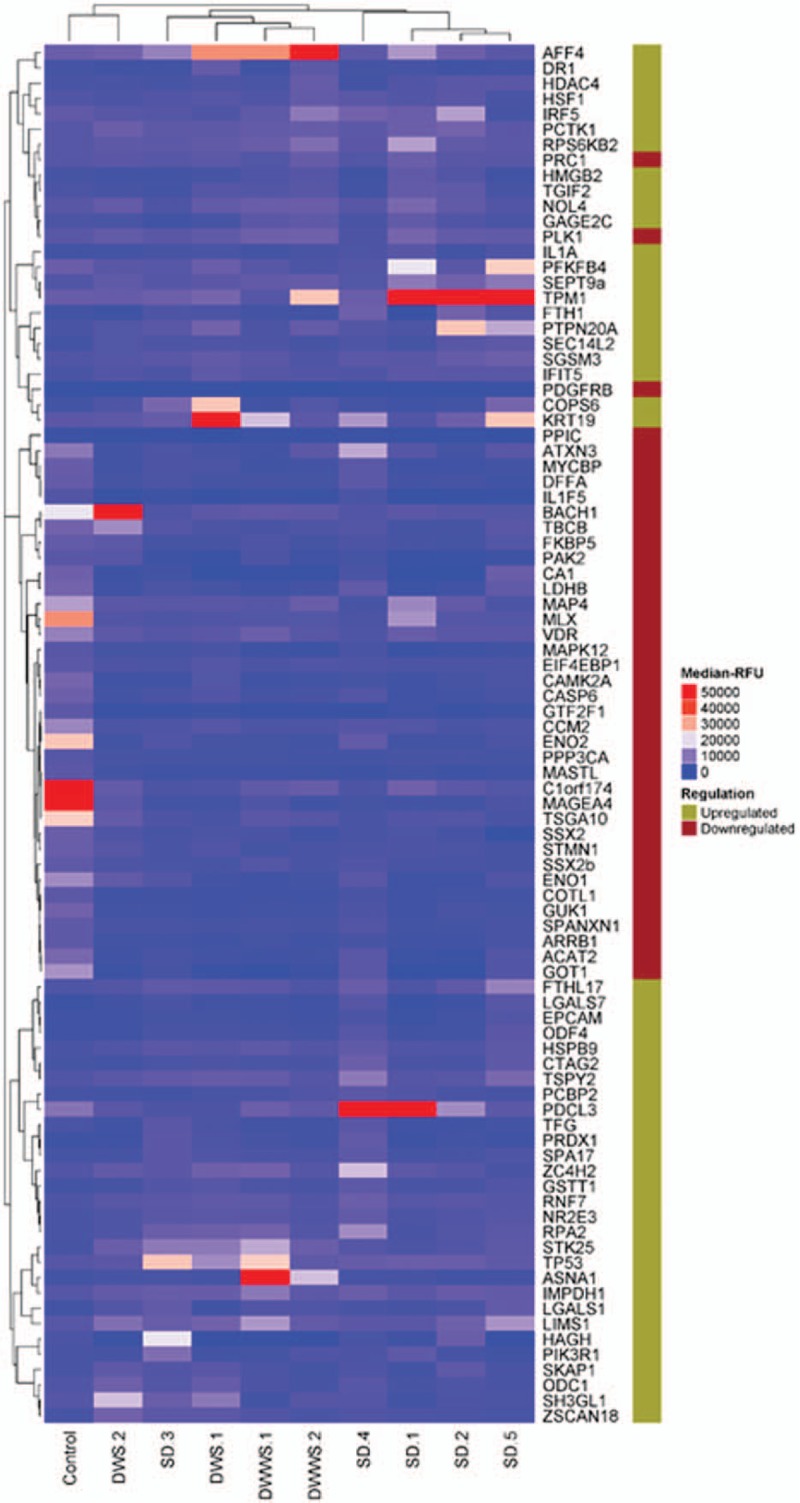
Heat map protein expression for 10 clinical samples comprised of 1 healthy individual (as control), 2 patients with DWWS, 2 patients with DWS, and 5 patients with SD. The expression level of the studied proteins was presented as relative fluorescence unit plotted with different colors.
3.3. The SD onset is associated with proteins involved in insulin signaling pathways
In patients who developed SD, we identified 29, 28, and 12 differentially abundant (fold change >2) plasma proteins associated with SD, DWS+, and DWS−, respectively (Fig. 2, left panel and Supplementary Table 3). We also identified 120 and 1 plasma proteins that were differentially downregulated (fold change > -2) associated with DWS− and DWS+, respectively (Fig. 2, right panel). Interestingly, there was no protein that was differentially downregulated identified in SD patients, suggesting that SD is likely due to overexpression of proteins that “exacerbate” the disease rather than under expression of proteins that “regulate” the disease. From the 29 proteins that differentially heighten among SD, the pathways enrichment analysis identified that these markers are associated with insulin signaling pathways and cytoskeleton pathways (Fig. 2B).
Figure 2.
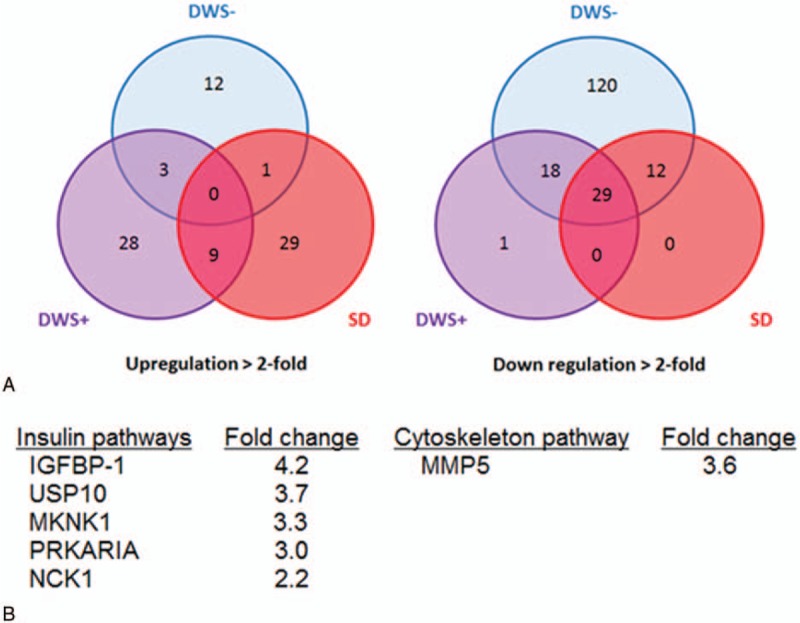
Differential expression of proteins and pathways involve in SD. (A) Venn diagrams depict plasma proteins that differentially upregulated and downregulated in a patient with DWS−, DWS+, and SD. (B) Pathways involved in the pathophysiology of severe dengue revealed by pathway enrichment analysis. DWS− = dengue without warning signs; DWS+ = dengue with warning signs; SD = severe dengue.
4. Discussion
Compared with DWS− and DWS+, patients suffering from SD often have poorer outcomes and higher mortality rates.[12] To better understand the pathophysiology of this advanced form of dengue disease, we investigated the plasma biomarkers in SD patients using plasma protein microarray. We identified several plasma proteins such as PFKFB4, TPM1, PDCL3, and PTPN20A were highly expressed among SD patients comparing to other forms of dengue diseases, particularly TMP1 levels were unequivocally high in majority of SD patients. The pathways enrichment analysis also suggested that insulin pathways and cytoskeleton pathways were involved in the pathophysiology of SD.
The progression of mild dengue to SD is postulated to be due to overexaggerated host immune responses such as antibody-dependent enhancement[13] and cytokine storm,[14] leading to increased vascular permeability that results in a potentially fatal SD. Although most studies found a significant association of cytokines production with dengue severity,[15–17] contradictory results have also been reported.[18] Perhaps the SD is not just inflammatory responses induced by cytokines, but also damage-associated molecular patterns (DAMPs) generated during the inflammatory process.
Among 5 SD patients, 3 showed elevation of Tropomyosin (TPM) up to 50,000 RFU underlining the importance of TPM in the pathogenesis of SD. TPM is an actin-binding protein that exists in both muscle and nonmuscle cells.[19] In muscle cells, TPM plays a central role in muscle contraction through regulating the cooperative binding of actin to myosin in response to the calcium ion flux. However, the role of tropomyosin in nonmuscle cells remains unclear. It is thought to be involved in stabilization and modulation of the actin filaments during the intracellular transport in normal cells[19,20] and was known to be differentially expressed during viral infection.[21]
TMP has multiple isoforms. Approximately 40 isoforms of TPM are identified in mammals[22] and was reported to be elevated alongside with viral replication.[23] TPM is highly conserved among most eukaryotic organism[24] and has been implicated in allergic reactions caused by house dust mite, and food allergic including shrimp and snails.[25] Furthermore, this protein was also significantly upregulated and is correlated with pleural effusion and severe lung damage in influenza virus infected mice,[26] suggesting that pathogenesis through TPM1 might be related to vascular damage. It is plausible that the elevation of TPM-1 in plasma of SD patient as a consequence of excessive cell death, thereby releasing TPM into circulations as DAMPs, and hence leading to mast cell activation.
Mast cells activation plays an important role in dengue virus-induced vascular pathology. St John et al[27] showed that pro-inflammatory mediators produced by mast cell such as chymase are predicted in dengue hemorrhagic fever. Using an animal model, the author found that the systemic elevation of these mast-cell pro-inflammatory mediators promote vascular leakage and mast cell stabilizing drugs can restore vascular integrity.[27] However, how exactly dengue infection induced mast cell activation remains to be elucidated.
Other proteins that are highly elevated among SD include PDCL3, PFKFB4, and PTPN20A. PDCL3, also known as Viral IAP associated factor 1 (VIAF), modulates the caspases activation during apoptosis and assists in the folding of proteins essential in the regulation of cell cycle progression.[28] The upregulation of PDCL3 in patients with SD indicates a possible role in the induction of apoptosis,[29] probably in the vascular beds that contribute to hemorrhage and vascular leakage observed in these patients. The roles of PFKFB4, a kinase that regulates glycolytic by-products[30] and PTPN20A, a tyrosine phosphatase that regulates fundamental cellular processes[31] have not been discovered in DENV infection; hence, further studies can be performed to investigate the upregulation of these proteins in patients with SD.
In addition, pathways enrichment analysis showed that the insulin signaling and cytoskeleton pathways are differentially unregulated in patients with SD compared with patients with DWS− and DWS+. This is consistent with Htun et al[32] who reported, using meta-analysis that diabetes has been identified as a risk factor that increased severe clinical presentation of dengue disease. Insulin is known to be able to induce a dose-dependent increase in blood flow by reducing vascular resistance in skeletal muscle,[33] mainly achieved by increased vasodilation of the microcirculation[34] through activation of the insulin receptor.[35] As proteins involve in insulin signaling were elevated up to 4-fold among SD patients, it is plausible that such elevation may cause a sudden surge of vasodilation in capillaries leading to the plasma leakage and hypovolemia.
One limitation of this study was that the sample size was small. Although we showed that the levels of TMP1 were high in majority of the SD cases (3 out of 5 SD cases), however these specimens were selected randomly and hence they are representative samples. As the purpose of this pilot study was to gain new insight into the pathophysiology of SD, the study needs to be duplicated in a larger cohort.
In conclusion, we identified TPM-1 and insulin pathway may potentially play an important role in the pathogenesis of SD. Hence, regulating the insulin signaling pathway may be the key intervention to reduce plasma leakage in patients with SD. Future studies should verify these findings in a larger cohort.
Acknowledgment
The authors would like to acknowledge all the participants and staff at University Malaya Medical Centre (UMMC) for participant recruitment.
Supplementary Material
Footnotes
Abbreviations: AFF4 = AF4/FMR2 Family Member 4, BCCP = biotin carboxyl carrier protein, COPS6 = COP9 Signalosome Subunit 6, CV = coefficient of variant, Cy3BSA = Cy3 labelled bovine serum albumin, DAMPs = damage-associated molecular patterns, DENV = dengue virus, DWS− = dengue patients without warning signs, DWS− = dengue warning signs negative (dengue with warning signs), DWS+ = dengue patients with warning signs, DWS+ = dengue warning signs positive (dengue without warning signs), ELISA = enzyme-linked immunosorbent assay, FC = fold change, HC = healthy control, HI = hemagglutination inhibition, KRT19 = Keratin 19, NS1 = nonstructural protein 1, PCR = polymerase chain reaction, PDCL3 = phosducin like 3, PFKFB4 = 6-phosphofructo-2-kinase/ fructose-2 =6-biphosphatase 4, PTPN20A = protein tyrosine phosphatase; nonreceptor type 20A, RFU = relative fluorescence unit, SAB = serum assay buffer, SD = patients with severe dengue, SD = severe dengue, TP53 = tumor protein p53, TPM1 = tropomyosin-alpha 1, VIAF = viral IAP-associated factor 1.
Funding/support: This study is supported by University Malaya Research Grant RP042A-15HTM and RP042B-15HTM.
The study sponsor did not have involvement in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Centre for Disease Control. 2014. Dengue: Clinical Guidance. https://www.cdc.gov/dengue/clinicallab/clinical.html. [Google Scholar]
- [2].Halstead SB. Dengue. Curr Opin Infect Dis 2002;15:471–6. [DOI] [PubMed] [Google Scholar]
- [3].WHO. 2015. Dengue and Severe Dengue. http://www.who.int/mediacentre/factsheets/fs117/en/. [Google Scholar]
- [4].Sam S, Omar S, Teoh B, et al. Review of dengue hemorrhagic fever fatal cases seen among adults: a retrospective study. PLOS One 2013;7:e2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Waggoner J, Balmaseda A, Gresh L, et al. Homotypic dengue virus reinfections in Nicaraguan children. J Infect Dis 2016;214:986–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Rothman A. Immunitu to dengue virus: a tale of original antigenic sin and tropical cytokine stroms. Nat Rev Immuno 2011;11:532–43. [DOI] [PubMed] [Google Scholar]
- [7].Munoz-Jordan JL, Sanchez-Burgos GG, Laurent-Rolle M, et al. Inhibition of interferon signaling by dengue virus. Proc Natl Acad Sci U S A 2003;100:14333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pang T, Cardosa M, Guzman M. Of cascades and perfect storms: the immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome. Immunol and Cell Biol 2007;85:43–5. [DOI] [PubMed] [Google Scholar]
- [9].Wan S, Lin C, Yeh T, et al. Autoimmunity in dengue pathogenesis. J Formos Med Assoc 2013;112:3–11. [DOI] [PubMed] [Google Scholar]
- [10].Hernández S, Puerta-Guardo H, Aguilar H, et al. Primary dengue virus infections induce differential cytokine production in Mexican patients. Mem Inst Oswaldo Cruz 2016;111:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016;32:2847–9. [DOI] [PubMed] [Google Scholar]
- [12].Kuno G. Emergence of the severe syndrome and mortality associated with dengue and dengue-like illness: historical records (1890 to 1950) and their compatibility with current hypotheses on the shift of disease manifestation. Clin Microbiol Rev 2009;22:186–201. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sasaki T, Setthpramote C, Kurosu T, et al. Dengue virus neutralization and antibody-dependent enhancement activities of human monoclonal antibodies derived from dengue patients at acute phase of secondary infection. Antiviral Res 2013;98:423–31. [DOI] [PubMed] [Google Scholar]
- [14].Espada-Murao L, Morita K. Dengue and soluble mediators of the innate immune system. Trop Med Health 2011;39:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Herdiman T, Pohan S, Rika Bur, et al. Interleukin-18 levels in adult dengue fever and dengue hemorrhagic fever. Med J Indones 2004;13:86. [Google Scholar]
- [16].Malavige GN, Huang LC, Salimi M, et al. Cellular and cytokine correlates of severe dengue infection. PLoS One 2012;7:e50387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mangione JN, Huy NT, Lan NT, et al. The association of cytokines with severe dengue in children. Trop Med Health 2014;42:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cruz Hernandez SI, Puerta-Guardo HN, Flores Aguilar H, et al. Primary dengue virus infections induce differential cytokine production in Mexican patients. Mem Inst Oswaldo Cruz 2016;111:161–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Perry SV. Vertebrate tropomyosin: distribution, properties and function. J Muscle Res Cell Motil 2001;22:5–49. [DOI] [PubMed] [Google Scholar]
- [20].Wang CL, Coluccio LM. New insights into the regulation of the actin cytoskeleton by tropomyosin. Int Rev Cell Mol Biol 2010;281:91–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Organ E, Sheng J, Ruley H, et al. Discovery of mammalian genes that participate in virus infection. BMC Cell Biol 2004;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mayer DC, Leinwand LA. Sarcomeric gene expression and contractility in myofibroblasts. J Cell Biol 1997;139:1477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Organ EL, Sheng J, Ruley HE, et al. Discovery of mammalian genes that participate in virus infection. BMC Cell Biol 2004;5:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bessot JC, Metz-Favre C, Rame JM, et al. Tropomyosin or not tropomyosin, what is the relevant allergen in house dust mite and snail cross allergies? Eur Ann Allergy Clin Immunol 2010;42:3–10. [PubMed] [Google Scholar]
- [25].Popescu FD. Cross-reactivity between aeroallergens and food allergens. World J Methodol 2015;5:31–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhao D, Liang L, Li Y, et al. Proteomic analysis of the lungs of mice infected with different pathotypes of H5N1 avian influenza viruses. Proteomics 2012;12:1970–82. [DOI] [PubMed] [Google Scholar]
- [27].St John AL, Rathore AP, Raghavan B, et al. Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus-induced vascular leakage. Elife 2013;2:e00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jagya N, Varma S, Thakral D, et al. RNA-seq based transcriptome analysis of hepatitis E virus (HEV) and hepatitis B virus (HBV) replicon transfected Huh-7 cells. PLOS One 2014;9:e87835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Thongtan T, Panyim S, Smith D. Apoptosis in dengue virus infected liver cell lines HepG2 and Hep3B. J Med Virol 2004;72:436–44. [DOI] [PubMed] [Google Scholar]
- [30].PFKFB4 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 [Homo sapiens (human)]. Bethesda, MD: NCBI; 2016. https://www.ncbi.nlm.nih.gov/gene/5210. [Google Scholar]
- [31].PTPN20 Protein Tyrosine Phosphatase , Non-receptor Type 20 [Homo sapiens (human)]. Bethesda, MD: NCBI; 2016. https://www.ncbi.nlm.nih.gov/gene/26095. [Google Scholar]
- [32].Htun NS, Odermatt P, Eze IC, et al. Is diabetes a risk factor for a severe clinical presentation of dengue? Review and meta-analysis. PLoS Negl Trop Dis 2015;9:e0003741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Calles-Escandon J, Cipolla M. Diabetes and endothelial dysfunction: a clinical perspective. Endocr Rev 2001;22:36–52. [DOI] [PubMed] [Google Scholar]
- [34].Baron AD, Brechtel G. Insulin differentially regulates systemic and skeletal muscle vascular resistance. Am J Physiol 1993;265:E61–7. [DOI] [PubMed] [Google Scholar]
- [35].Clerk LH, Vincent MA, Lindner JR, et al. The vasodilatory actions of insulin on resistance and terminal arterioles and their impact on muscle glucose uptake. Diabetes Metab Res Rev 2004;20:3–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


