Abstract
Primary vascular dysregulation (PVD) is believed to be a main cause of local vasospasm and impaired autoregulation as a possible contributing factor in the pathogenesis of normal tension glaucoma (NTG). Subjects with PVD respond stronger to psychological stress. Autonomic dysfunction is another reason of instable ocular blood flow and may be studied by means of assessment of heart rate variability (HRV) especially during a hand-cold provocation test (CPT).
To compare the shift of HRV parameters in NTG and healthy subjects after a cold provocation test and to assess the relation between structural damage, circulatory parameters and autonomic dysfunction in NTG.
HRV was studied using CPT in 78 NTG patients and 60 health control matches. The ocular blood flow was measured using color Doppler imaging (CDI). The 24 hours blood pressure (BP) monitoring was carried out. The cardiovascular fitness assessment was made to all patients before and after CPT. Mean group difference of HRV parameters was compared between NTG and healthy subjects using the Euclidean metric. The mean ocular perfusion pressure (MOPP) was measured. Optic nerve head and retinal nerve fiber layer (RNFL) were evaluated using spectral-domain optic coherence tomography (SD-OCT). The relation between HRV, CDI, and SD-OCT-parameters was assessed.
In contrast to healthy subjects, a predominance of the sympathetic activity after the CPT was revealed in the NTG group. The end diastolic velocity (EDV) in central retinal artery (CRA) and short posterior ciliary artery (SPCA) was significantly reduced in NTG compared to healthy eyes. In NTG, the main HRV parameter characterizing the total effect of autonomic blood circulation regulation (SDNN) correlated with MOPP (0.75, P = .035), SPCA EDV (0.93, P < .001), and CRA EDV (0.9, P < .001). The average daily diastolic BP correlated with RNFL (0.67, P = .009).
The NTG patients have the disturbance of the autonomic nervous system, which increases in response to stress provocation and is related to ocular blood flow and structural damage.
Keywords: heart rate variability, normal tension glaucoma, ocular blood flow, ocular perfusion pressure
1. Introduction
One of the most recognized current cause of normal tension glaucoma (NTG) is an instable ocular blood flow of optic disc,[1] which may be the result of primary vascular dysregulation (PVD) and therefore the impaired autoregulation of retinal blood flow.[2] Excessive activity of the sympathetic link of the autonomic nervous system (ANS) is one of the possible causes leading both to disturbance of blood supply to the ONH and to a decrease in ocular perfusion pressure (OPP) in the vessels of the optic nerve and choroid.[3–5] Heart rate variability (HRV) is a well-known tool that allows studying the autonomic modulation of the heart sympathovagal balance.[4] Systemic parasympathetic and sympathetic neuropathies have been reported in patients with primary open-angle glaucoma (POAG) and NTG.[5–8] Furthermore, it has been revealed that the sympathovagal balance of the autonomic nervous system (ANS) in patients with NTG is shifted toward sympathetic activity,[9,10] and the sympathetic predominance is responsible for the faster rate of central visual field (VF) progression in NTG.[11] However, the relation between the autonomic dysfunction and structural damage in NTG is not clear.
It is believed that patients with an instable ocular blood flow respond stronger to psychological stress as it has been described in patients with primary vascular dysregulation (PVD).[12] Cold stimulation is a well-established provocation test used for detecting abnormal vascular reactivity in patients with autonomic failures.[13]
Pathological response to cold in patients with glaucoma has been previously demonstrated in our research[14] and we have described a significant reduction of retrobulbor blood flow in NTG.[15] Recently, some authors studied the peripheral blood flow in glaucoma patients after a cold provocation test (CPT)[1] and as a response to posture change.[16] Meanwhile, there is no literature data concerning the influence of CPT on HRV in NTG compared to healthy subjects.
The objective of this study is to compare the shift of HRV parameters in NTG and healthy subjects after CPT and to assess the relation between the structural damage, circulatory parameters, and autonomic dysfunction in NTG.
2. Materials and methods
2.1. Study design
The study was approved by the Ethical Committee (Institutional Review Board) at the Institution of the Federal Medical and Biological Agency of the Russian Federation and was conducted in accordance with Good Clinical Practice within the tenets of the Declaration of Helsinki. Each patient/healthy subject was required to sign an informed consent form before being enrolled in the study and prior to any measurements being taken.
2.2. Study subjects
Around 138 eyes of 138 subjects (78 patients with early and moderate NTG and 60 age-matched healthy subjects) were included into this study. The patients were recruited from April, 2015 up to January, 2016. All patients were Caucasian.
NTG was diagnosed on the basis of characteristic changes in the optic disc detected by ophthalmoscopy, which was performed by one glaucoma specialist (NK) and confirmed by 2 other glaucoma specialists. The inclusion criteria included pathological deviation from the normal neuroretinal rim, glaucomatous optic disc cupping, peripapillary atrophy, wedge-shaped defects of the retinal nerve fiber layer (RNFL) adjacent to the optic disc edge, hemorrhages at the optic disc boundary, glaucomatous VF loss on at least 2 consecutive tests, an open angle on gonioscopy (not less than 30°), ametropia ≤ 0.5 dpt, intraocular pressure (IOP) of 21 mm Hg or lower (without topical treatment) in repeated measurements on different days, follow-up at our clinic for at least 4 years with visits at 3- to 5-month intervals, and no ocular pathology other than glaucoma.
The healthy participants were recruited from the people accompanying the patients and had IOP of <21 mm Hg for both eyes, a normal Humphrey Swedish Interactive Threshold Algorithm (SITA) 24-2 standard visual field with mean deviation (MD), and pattern standard deviation (PSD) within 95% limits of the normal reference. They also had a glaucoma hemifield test within 97% limits, a central corneal thickness ≥ 500 μm, a normal-appearing optic nerve head (ONH), a normal RNFL, an open anterior chamber angle as observed by gonioscopy, and no history of chronic ocular or systemic corticosteroid use. The age and race distribution of the healthy subjects matched that of the glaucoma patients.
Exclusion criteria were the following: large refractive errors (outside of ± 6.00 dpt sphere or 2.00 dpt cylinder), pupil diameter < 3 mm, systemic administration of beta-blockers and calcium-channel blockers, concomitant ocular disease (except for early cataract), chronic autoimmune diseases, diabetes mellitus, acute circulatory disorders in past medical history, and any concomitant diseases involving the administration of steroid drugs. A history of ocular arterial or venous obstruction (branch or central occlusion) and systemic conditions associated with venous congestion (e.g., heart failure) were also considered as exclusion criteria. The patients were instructed to avoid caffeine intake, smoking, and exercise for 5 hours prior to the study visit.
If both eyes of a patient were eligible, 1 eye was randomly chosen.
Those patients, who previously used antiglaucoma drops, were asked to discontinue the drug for a period of 21 days (drug washout period), while others were newly diagnosed glaucoma cases. The medical histories of all patients were carefully obtained with special attention paid to the signs of primary or secondary cardiovascular dysregulation (migraine, vasospasm, and neurocirculatory dystonia).[17]
All patients underwent Doppler ultrasound scanning to exclude pathology of the brachiocephalic vessels and were referred to the therapeutic outpatient clinic of the Department of A.I. Burnazyan Federal Medical and Biophysical Center of FMBA, Moscow, Russia, where they followed the physical examinations of their health status, including, clinical chemistry, the hematological and urine analysis, hepatitis, and human immunodeficiency virus serology.
2.3. Study examinations
All participants underwent complete ophthalmologic examinations including best corrected acuity, slit lamp examination, IOP measurement using analyzer of biomechanical properties of eyes (Ocular Response Analyzer, ORA, Reichert Ophthalmic Instruments Inc., Depew, NY), gonioscopy, anterior chamber angle measurement (Visante OCT, Carl Zeiss, Germany), pachymetry (SP-100, Tomey GmbH, Germany), dilated fundus biomicroscopy using 78-diopter lens, stereoscopic optic disc photography, and standard automated perimetry (SAP) using a Humphrey Field Analyzer (HFA, Carl Zeiss Meditec Inc., Dublin, CA) with SITA. Only reliable SAP results, which were defined as false-negative and false-positive responses of < 33% and fixation loss of < 20%, were eligible for the study. Glaucomatous VF defects were determined as having a cluster of 3 or more nonedge points with P < .05 and at least 1 point with P < .01 in the pattern deviation probability plot, PSD of less than 5%; or glaucoma hemifield test results outside normal limits. Both the glaucoma patients and the healthy subjects underwent SAP at least twice before this study.
The study included 24-hour blood pressure (BP) monitoring or automated measurement of BP for 24 hours at fixed intervals according to a preset program. Measurements were made on an outpatient basis in the conditions of normal patient activity. The device measured heart rate, systolic, and diastolic BP at set intervals using the oscillometric method, that is, by analyzing pulse phenomena in a blood pressure cuff.
Mean ocular perfusion pressure (MOPP) was calculated on the basis of IOP and arterial BP measurements immediately before the optical coherence tomography (OCT) scanning and investigation of retrobulbar blood flow, after a 10-minute resting period in the sitting position. Systemic BP was measured using the Riva Rocci technique. MOPP was calculated using the formula: MOPP = (2/3 diastolic BP + 1/3 systolic BP) × 2/ 3 – IOP.
BP was measured at rest in the sitting position. When measuring BP in the sitting position, a back had a support, and the middle shoulder point was at the heart level (the fourth intercostal space). The measurement was made using Adyutor mechanical tonometer.
2.4. OCT image acquisition and processing
All subjects also underwent optic disc area measurement at RTVue XR Avanti SD-OCT (Optovue, Inc., Fremont, CA) using the traditional ONH scan. This scan consists of 12 radial scans of 3.4 mm in length and 6 concentric ring scans ranging from 2.5 to 4.0 mm in diameter, all centered on the optic disc. The retinal pigment epithelium (RPE) tips are automatically detected by the software and are joined to delineate the optic disc margin and to calculate the disc area. All the examinations for a particular subject were performed on the same day. OCT was performed in the macular area as well. The tracking mode was used.
The ganglion cell complex (GCC) scan mode measured macular inner retinal layer thickness from the internal limiting membrane (ILM) to the inner nuclear layer—Avg.GCC. The GCC scan was centered on the fovea and covered a square grid on the central macula of 7.0×7.0 mm. The GCC thickness was determined with the GCC scanning protocol, which consists of 15 vertical line scans covering a 7.0×7.0 mm area centered about 1 mm temporal to the fovea. The GCC scanning protocol also included a central horizontal line scan for registration of vertical scans and fovea center searching. The characteristics of GCC (global loss volume—GLV, focal loss volume—FLV) were also measured.
2.5. HRV assessment
The cardiovascular fitness assessment was made to all patients before and after CPT using Rhythm-MET hardware–software complex developed by the Federal State Unitary Enterprise “Science Center of Radiation and Chemical Safety and Hygiene of the Medical and Biological Agency of the Russian Federation” according to the previously described method.
Rhythm-MET hardware–software complex was used in the present study. The method of its work is based on a comprehensive analysis of HRV, systemic hemodynamics, and vegetative regulation. Photoplethysmograms recorded from a phalanx with an infrared sensor,[18] located in the microprocessor module of data input and processing, were used as the source of data on HRV and peripheral blood flow. Cardiointervals obtained from photoplethysmograms were processed in accordance with the recommendations for assessment of HRV parameters and their subsequent generalization, including hemodynamics parameters, as well as for the assessment of functional status (FS) and functional reserves (FR) of the cardiovascular system according to the results of the examination at rest and after conducting CPT in order to form groups that are homogeneous in FS and FB. CPT also analyzed the parameters of time dependence of the amplitude of photoplethysmogram and recovery time after the test.
A generally accepted CPT was used as a provocation test. The testing procedure was the following: a patient's hand was dipped into cold water (+4°C) with small pieces of ice; moreover, the hand was covered with plastic ice bags for 30 seconds. The registration of RR-intervals was made at the end of the CPT.
The following characteristics were taken into account in accordance with the international standard:[19]
Standard deviation of NN-interval (SDNN): the parameter of HRV characterizing the total effect of autonomic blood circulation regulation. A reduction in SDNN reflects low HRV indicating a high tone of heart sympathetic activity. The decrease in SDNN reflects a decrease in HRV, which indicates an increase in the tone of heart sympathetic activity.
The parameter of parasympathetic autonomic regulation activity (RMSSD).
Total spectral power (TP): the parameter of the absolute activity level of regulatory systems.
Power in the high-frequency range (HF): the parameter of the spectral power of heart rate respiratory undulations reflecting the activity level of respiratory center. The high- frequency band reflects fast changes in beat-to-beat variability due to parasympathetic activity.
Power in the low-frequency range (LF): The low-frequency band is considered to be a fair approximation of sympathetic activity. The very low-frequency band reflects mostly sympathetic stimulation.
The low/high-frequency ratio is defined as a ratio of low-frequency to high- frequency power (LF/HF). A higher ratio indicates increased sympathetic activity or reduced parasympathetic activity.
The number of pairs of consecutive NN-intervals: the parameter of predominance degree of parasympathetic regulation over sympathetic one (pNN50).
Autonomic regulation index (ARI): the parameter used to evaluate the activity of ANS. The increased ARI shows the activation of sympathetic regulation, but the decreased ARI shows the activation of parasympathetic regulation.
Variation range characterizing the degree of HRV (TINN).
2.6. Color Doppler imaging
Ocular and retrobulbar blood flow was registered by means of color Doppler imaging (CDI) and impulse Doppler sonography (multifunctional VOLUSON 730 Pro System, General Electric Medical Systems, Germany) using a 10 to 15 MHz linear transducer. The methodology has been described earlier. Blood flow was studied in the ophthalmic artery (OA), central retinal artery (CRA), central retinal vein (CRV), lateral, and medial short posterior ciliary arteries (SPCA). We registered the Doppler frequency shift and obtained quantitative blood flow parameters: peak systolic blood flow velocity (PSV), end diastolic blood flow velocity (EDV), mean blood flow velocity (Vmean), and the resistivity index (RI).
3. Statistical analysis
Statistical processing and analysis of the results related to HRV parameters were aimed at identifying of those parameters of the NTG group that would have statistically significant differences from the mean group values at the comparison of the results related both to CPT (Аcold) and to a rest period (Аrest before the test). The same calculation was made for the control group. The mean group difference of the considered parameter was determined by averaging of the individual values of differences of this parameter determined for each person in the compared groups.
The Euclidean distance from the experimental point i with the coordinates 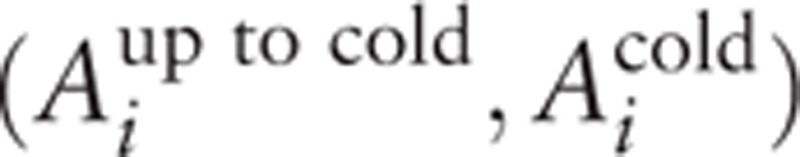 to the centre of the coordinates of plane (Aup to col, Acol) was used as a measure of difference. At the same time, the coordinates of “experimental points” relating to the rest conditions (before the test) have the following coordinates:
to the centre of the coordinates of plane (Aup to col, Acol) was used as a measure of difference. At the same time, the coordinates of “experimental points” relating to the rest conditions (before the test) have the following coordinates: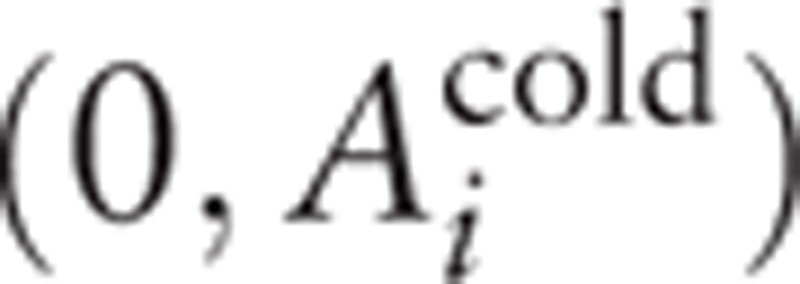 . Since the samples are dependent (the same composition of subjects), the Wilcoxon signed-ranks test for matched pairs was used. The essence of this method is that instead of estimating the difference of distributions at one-dimensional scale of the considered parameter we estimated the distance (the modulus of a vector) of each of the experimental points from the natural mathematical object—the center of coordinates—in the space of 2 variables. The resulting set of the moduli of vectors is one-dimensional and, therefore, standard statistical processing methods may be applied.
. Since the samples are dependent (the same composition of subjects), the Wilcoxon signed-ranks test for matched pairs was used. The essence of this method is that instead of estimating the difference of distributions at one-dimensional scale of the considered parameter we estimated the distance (the modulus of a vector) of each of the experimental points from the natural mathematical object—the center of coordinates—in the space of 2 variables. The resulting set of the moduli of vectors is one-dimensional and, therefore, standard statistical processing methods may be applied.
We used standard methods of descriptive statistics: calculation of mean, standard deviations, T-tests, methods of nonparametric statistics—Wilcoxon test, Mann–Whitney U test, implemented in the corresponding statistics packages (IBM SPSS Statistics version 21, StatPlus).
Parameters with the P < .05 were considered statistically significant. Since a number of parameters (GCC, GLV, systolic BP, and MOPP) are depended on the anterior–posterior axis and the age of the subjects, we carried out an adjustment for these parameters on the basis of the linear regression model.
4. Results
The patient characteristics are given in Table 1.
Table 1.
Characteristics of healthy subjects and patients with normal tension glaucoma.
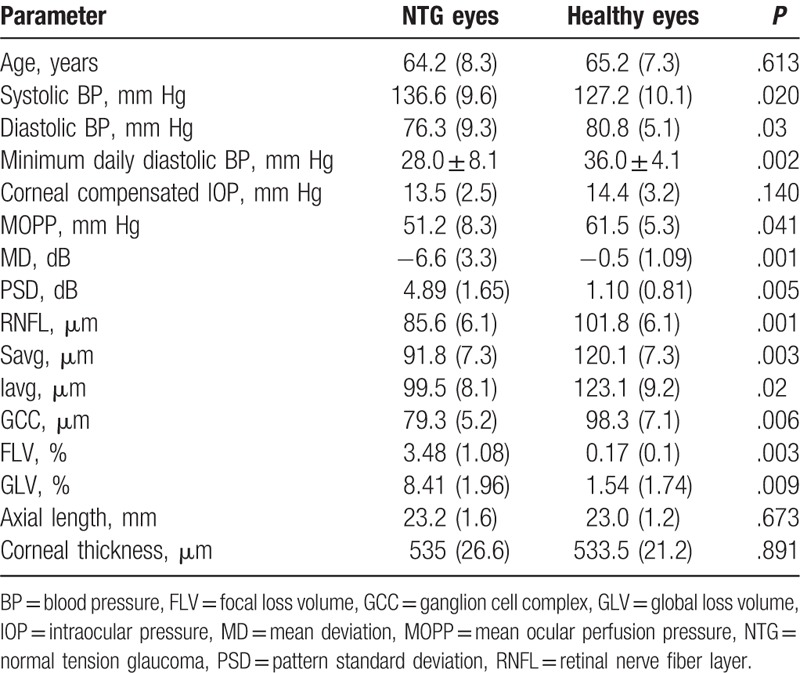
Table 1 shows the median of data and standard deviation (in parentheses), the exact Mann–Whitney U test (P∗) between the healthy eyes (control group) and NTG.
MOPP was significantly lower in the patients with NTG than in the control patients. The daily BP fluctuations in the study group ranged widely: from 75 to 145 mm Hg for systolic BP and from 38 to 95 mm Hg for diastolic BP. However, we used the values of BP at the time of HRV study and IOP measurement to calculate MOPP. According to the results of 24-hour BP monitoring, the average systolic and diastolic BP was significantly lower in the patients with NTG than in the control patients. The same result was obtained with respect to the minimum daily diastolic BP. A significant difference in night pulse pressure was also observed: it amounted to 37.0 ± 5.1 1 mm Hg in the NTG patients and 48.0 ± 9.1 mm Hg in the control group (P = .04).
HRV parameters in the studied groups and their shifts after the CPT are shown in Table 2. The Euclidean metric is applied. The exact Mann–Whitney U test (P∗) between the NTG and healthy eyes (control group) before a cold test: P = .003; P = .02.
Table 2.
The average heart-rate variability parameters in the patients with normal tension glaucoma and healthy subjects before and after the cold provocation test.
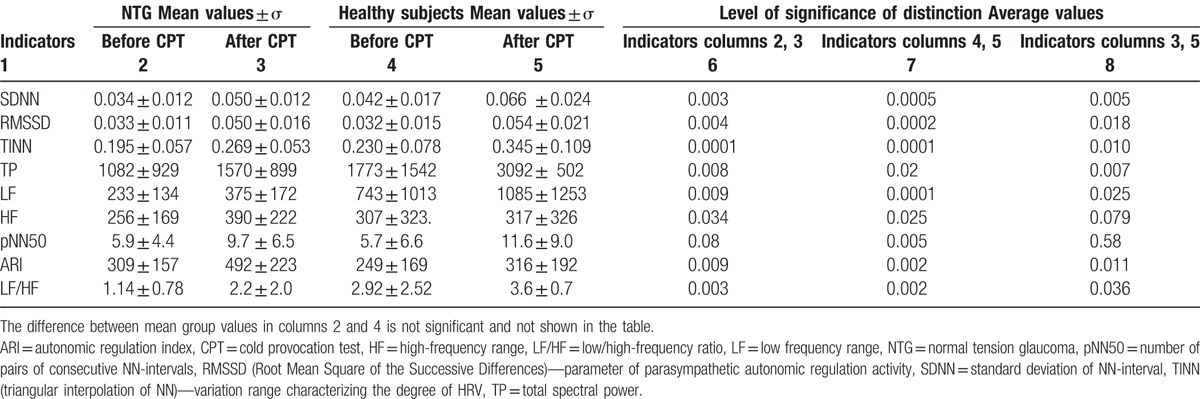
Table 2 shows that there are statistically significant differences between the studied parameters before and after the CPT both for the NTG group and healthy subjects. At the same time, when the CPT revealed the difference between the NTG group and healthy subjects, it was established that only 2 parameters as HF and pNN50 did not significantly differ, so they should be less important in case of their further use.
Quantitative and qualitative representation of the preferred direction of the shift in vegetative regulation in the NTG group and healthy subjects after the CPT is given in Table 3, where the values of the shift in parameters (A) are presented in relative units with the ratioδA = (Aafter − Abefore)/Abefore × 100%, and the qualitative conclusion is built on the analysis of the following inequation Aafter < Abefore, or Aafter > Abefore taking into account the accepted physiological interpretation of the considered parameter.
Table 3.
The relative change in heart-rate variability parameter after the cold provocation test.
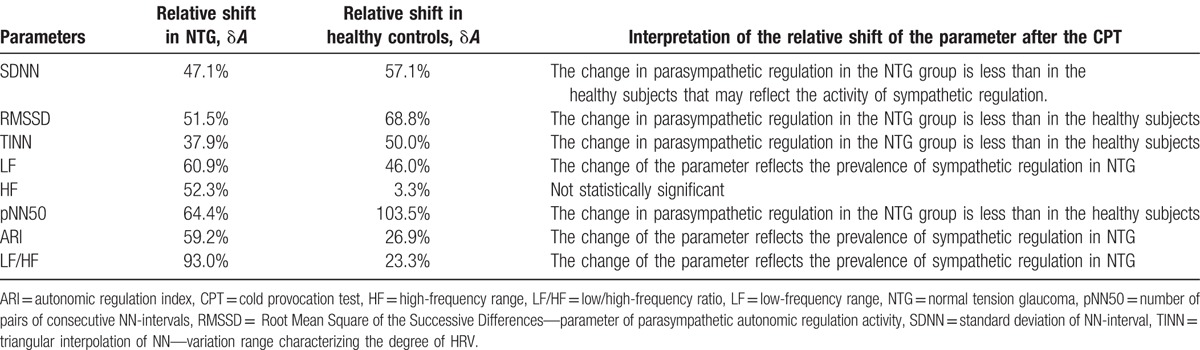
The analysis of relative shifts in the HRV parameters given in Table 3 and the weighted sum of the individual conclusions on these parameters provide the basis for the conclusion that CPT has a more pronounced sympathetic response of the autonomic nervous system in the NTG group compared to the control group. This difference is especially noticeable from the relative shifts in such parameters as LF: δLFNTG = 61% > δLFHeal = 46% and as LF/HF: δLF/HFNTG = 93% > δLF/HFHeal = 23%.
Thus, based on the results of the conducted analysis of the CPT effect on the HRV parameters, these parameters can be recommended as additional ones for the diagnosis of NTG. First of all, this refers to LF and LF/HF and then to SDNN, HF, and ARI.
The parameters of retrobulbar blood flow in NTG were also decreased in the NTG patients compared to the healthy subjects (Table 4).
Table 4.
Color Doppler imaging variables of the retrobulbar vessels in the studied groups.
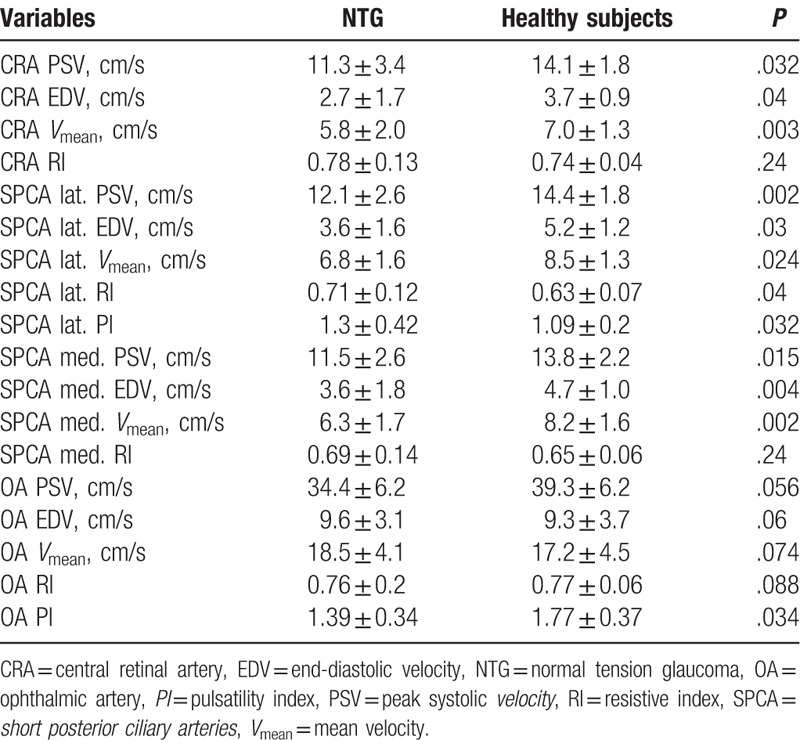
Table 4 shows the mean group values and the standard deviation, the exact Mann–Whitney U test (P∗) between the healthy eyes (control group) and early POAG.
High correlation between the average pulse BP and OCT parameters was found out: with cup volume: r = −0.65, P = .002, with rim volume: r = 0.574, P = .008 and with lin. cup/disc ratio: r = −0.756, P = .009. High correlation was also found out between the average daily diastolic BP and RNFL thickness (Avg. RNFL): r = 0.668 (P = .009).
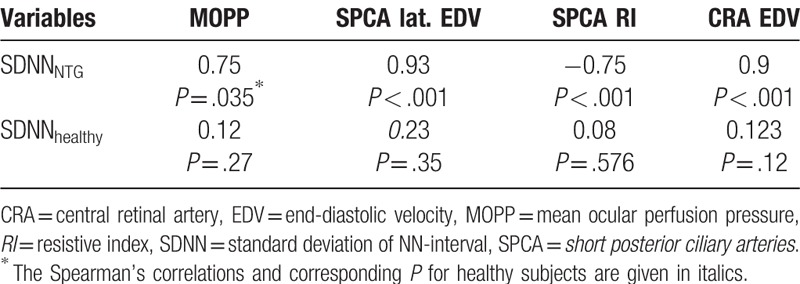
The pronounced correlation between the main parameter of HRV – SDNN – and MOPP and structural parameters are shown in Table 5.
Table 5.
Correlations between standard deviation of NN-interval, mean ocular perfusion pressure and circulatory parameters in studied groups.
5. Discussion
The present study demonstrates a dysfunction of ANS in NTG compared to healthy subjects and the shift of its balance towards predominance of sympathetic activity in response to CPT.
Sympathetic neural activity (SNA) causes an increase of heart rate, stroke volume, and vasoconstriction. SNA regulates the circadian variation of BP and is closely related to nocturnal dipping. The present study observes the significant dipping of diastolic BP in NTG compared to healthy subjects. This may be a consequence of the activation of the sympathetic innervation. Chronic increased SNA can lead to arterial and cardiac remodelling, endothelial dysfunction, increased tissue oxygen demand and subsequent decreasing of the ischamia threshold in all organs, including the eye.
It is known that the regulation of ocular hemodynamics is carried out at different levels: organ, tissue, and local (autoregulatory). Cell, membrane, and neurohumoral mechanisms play their own roles. An optic nerve and its vasculature have rich sympathetic (from cervical sympathetic system) and parasympathetic (from oculomotor nerve) innervations. There is the evidence of presence of the choroidal neuroplexus represented by numerous internal autonomic ganglia, forming the autonomous perivascular network around the choroidal vessels. It is believed that it plays vasodilatory function aimed at enhancing the ocular blood flow at light load.[20] Apparently, the autonomic dysfunction leading to the failure of optic nerve and retinal blood flow play a significant role in ocular physiology and pathophysiology in general and, particularly, in glaucoma.[5]
According to the results of the present study, the significant reduction in ocular blood flow in the major vessels supplying the optic nerve has been revealed in the NTG patients, and this reduction has correlated to HRV.
It has been recognized that the reduction of MOPP is closely connected with glaucoma progression.[21,22] High direct correlation of MOPP with SDNN, observed in the present study, logically explains the possibility of ocular perfusion reduction in the conditions of the increased activity of sympathetic blood flow regulation.
Our results are consistent with the literature data. According to Riccadonna et al, HRV, as well as the nocturnal diastolic BP variability, reduced in NTG compared to POAG patients. Furthermore, these differences were more prominent in more severe clinical forms of NTG. The authors suggested a relation between the extent of autonomic disorder and severity of glaucoma.[23] It is known that an increase of the sympathetic ANS in response to the provocation tests is typical for people with PVD. The development of NTG is associated with possible PVD.[2] The present study has pointed out to the role of PVD in the NTG pathogenesis. It is believed, that the main cause of PVD is a vascular endotheliopathy and PVD itself does not lead to the optic disc blood flow deficiency, but it is realized through autoregulation failures.[24] It can be assumed that the autoregulation failure has been the cause of reduced blood flow in the examined patients in the present study.
According to the literature, PVD leads to the significant imbalance of sympathetic and parasympathetic departments of ANS, namely, the predominance of sympathetic innervations.[8]
Phelps and Corbett[25] indicated for the first time PVD as a possible cause of NTG. They proved this thesis with the fact that the patients with NTG often suffer from migraine. Later it was confirmed by other authors.[8,21,22,26] Another important risk factor for NTG progression is fluctuations of perfusion pressure.[26] It is believed that these fluctuations are primarily determined by the presence of PVD. Although the role of PVD in the pathogenesis of glaucomatous optic neuropathy has been discussed for many years, only recent studies due to the use of modern technologies could prove that patients with NTG, but not healthy individuals, suffer from the retinal blood flow autoregulation failure in the conditions of provocation tests.[27] From this point of view, the dysfunction of the autonomic blood flow regulation seems to be of high importance and its study attracts attention of the researches. Wierzbowska et al[9] studied HRV in NTG and revealed the sympathovagal balance of ANS in NTG patients that shifted towards sympathetic activity with no change of 24-hour pattern of BP variability as compared to the healthy subjects. Na et al[10] also observed significantly decreased SDNN values in patients with NTG.
The matter is that ocular blood flow in PVD can be normal in normal conditions, but a failure occurs in the conditions of a provocation test, for example, a CPT. As retinal vessels in PVD are generally less prone to dilation, keeping tension of a vascular wall, then there is no sufficient expansion of arteries at stress tests.[24] This fact explains the instability of ocular perfusion in patients with PVD.
The present study assessed the shift of HRV parameters in NTG as a response to CPT. This test along with some others (oxygen, isometric exercise, brachial arterial occlusion, or light flicker) was used to examine blood flow regulation in NTG.[9,28,29]
Gherghel et al[13] modified CPT involving immersion of a right hand in 40°C warm water followed by 4°C cold water exposure. Glaucoma patients demonstrated a significant decrease in finger and ocular blood flow, while the healthy subjects exhibited increases in systolic BP and pulse pressure and a decrease in finger blood flow during cold provocation, but the ocular blood flow was unchanged. According to the authors, these findings suggest a systemic autonomic failure and ocular vascular dysregulation in POAG patients. However, this group did not examine the NTG patients.
According to the results of our study, the NTG patients have demonstrated an increase of SNA in response to CPT and this shift was significantly different compared to the healthy subjects.
Park with co-authors studied the NTG patients with different types of HRV, and reported that VF progression in patients with sympathetic predominance is faster than in patients with higher HRV. The authors concluded that the autonomic dysfunction, especially the decrease of SDNNs, was a predictor of central VF progression in NTG.[11]
In our study, we described the high correlation between HRV parameters, namely SDNN, and structural parameters: the more pronounced SNA is associated with the more severe glaucoma damage of ONH and RNFL. These results may be explained on the basis of the reduction of the blood supply to ONH and peripapillary retina. Indeed, according to the CDI data, the parameters of retrobulbar blood flow in NTG were reduced significantly compared to healthy eyes that is in consistent with our previous data.[15]
To the best of our knowledge, this is the first study that demonstrates the high correlation between local blood flow and HRV parameters in NTG: the more pronounced SNA was associated with the decreased ocular blood flow in OA and SPCA. Based on the results of the conducted studies of the effect of CPT on the HRV parameters, they can be recommended as additional parameters for the diagnosis of NTG. First of all, this refers to LF and LF/HF and then to SDNN, HF, and ARI.
Our study has several limitations that must be acknowledged. First, we did not study the progression pattern of the disease and we did not analyze the relation between HRV and functional loss.
Second, we did not evaluate the changes of circulatory parameters in response to CPT. Third, we did not assess test–retest variability, though it is known that HRV is a moderately to fairly good reliable measurement.[30]
However, our study has an advantage over other studies on HRV in NTG as we did not include patients on antihypertensive medication or topical medications that could influence BP and heart rate values.
In summary, we found out that the NTG patients have the disturbance of autonomic nervous system that increases in response to stress provocation and is related with ocular blood flow and structural damage.
This finding refers to the NTG pathogenesis and suggests the use of HRV assessment in glaucoma diagnostics and monitoring.
Footnotes
Abbreviations: ANS = autonomic nervous system, ARI = autonomic regulation index, BP = blood pressure, CDI = color Doppler imaging, CPT = cold provocation test, CRA = central retinal artery, CRV = central retinal vein, EDV = end-diastolic velocity, FLV = focal loss volume, FR = functional reserves, FS = functional status, GCC = ganglion cell complex, GLV = global loss volume, HF = high-frequency range, HRV = heart-rate variability, ILM = internal limiting membrane, IOP = intraocular pressure, LF = low-frequency range, LF/HF = low/high-frequency ratio, MD = mean deviation, MOPP = mean ocular perfusion pressure, NTG = normal tension glaucoma, OA = ophthalmic artery, OCT = optical coherence tomography, ONH = optic nerve head, OPP = ocular perfusion pressure, PI = pulsatility index, pNN50 = number of pairs of consecutive NN-intervals, POAG = primary open-angle glaucoma, PSD = pattern standard deviation, PSV = peak systolic velocity, PVD = primary vascular dysregulation, RI = resistive index, RMSSD = root mean square of the successive differences, RNFL = retinal nerve fiber layer, RPE = retinal pigment epithelium, SAP = standard automated perimetry, SDNN = standard deviation of the NN intervals, SD-OCT = spectral-domain optical coherence tomography, SNA = sympathetic neural activity, SPCA = short posterior ciliary artery, TINN = triangular interpolation of NN, TP = total spectral power, TPCA = temporal short posterior ciliary artery, VF = visual field.
The authors did not receive funding for the research and writing of the article.
The authors have no conflicts of interest to disclose.
References
- [1].Nicolela MT. Clinical clues of vascular dysregulation and its association with glaucoma. Can J Ophthalmol 2008;43:337–41. [DOI] [PubMed] [Google Scholar]
- [2].Konieczka, Ritch R, Traverso C, et al. Flammer syndrome. EPMA J 2014;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nesher R, Kohen R, Shulman S, et al. Diastolic double-product: a new entity to consider in normal-tension glaucoma patients. Isr Med Assoc J 2012;14:240–3. [PubMed] [Google Scholar]
- [4].Narita K, Murata T, Hamada T, et al. Interactions among higher trait anxiety, sympathetic activity, and endothelial function in the elderly. J Psychiatr Res 2007;41:418–27. [DOI] [PubMed] [Google Scholar]
- [5].Clark CV, Mapstone R. Systemic autonomic neuropathy in open-angle glaucoma. Ophthalmol 1986;64:179–85. [DOI] [PubMed] [Google Scholar]
- [6].Kumar R, Ahuja VM. A study of changes in the status of autonomic nervous system in primary open angle glaucoma cases. Indian J Med Sci 1999;53:529–34. [PubMed] [Google Scholar]
- [7].Brown CM, Dutsch M, Michelson G, et al. Impaired cardiovascular responses to baroreflex stimulation in open angle and normal-pressure glaucoma. Clin Sci 2002;102:623–30. [DOI] [PubMed] [Google Scholar]
- [8].Anders D, Vollenweider S, Cann J, et al. Heart-rate variability in women during 40-hour prolonged wakefulness. Chronobiol Int 2010;27:1609–28. [DOI] [PubMed] [Google Scholar]
- [9].Wierzbowska J, Wierzbowski R, Stankiewicz A, et al. Cardiac autonomic dysfunction in patients with normal tension glaucoma: 24-h heart rate and blood pressure variability analysis. Br J Ophthalmol 2012;96:624–8. [DOI] [PubMed] [Google Scholar]
- [10].Na KS, Lee NY, Park SH, et al. Autonomic dysfunction in normal tension glaucoma: the short-term heart rate variability analysis. J Glaucoma 2010;19:377–81. [DOI] [PubMed] [Google Scholar]
- [11].Park H-YL, Park S-H, Park CK. Central visual field progression in normal tension glaucoma patients with autonomic dysfunction. Invest Ophthalmol Vis Sci 2014;55:2557–63. [DOI] [PubMed] [Google Scholar]
- [12].Neves VJ, Moura MJ, Almeida BS, et al. Chronic stress, but not hypercaloric diet, impairs vascular function in rats. Stress 2012;15:138–48. [DOI] [PubMed] [Google Scholar]
- [13].Gherghel D, Hosking SL, Cunliffe IA. Abnormal systemic and ocular vascular response to temperature provocation in primary open-angle glaucoma patients: a case for autonomic failure? Invest Ophthalmol Vis Sci 2004;45:3546–54. [DOI] [PubMed] [Google Scholar]
- [14].Kurysheva NI, Nagornova ND. Vasospasm in the pathogenesis of glaucomatous optic neuropathy (in Russian). Glaucoma 2004;2:18–24. [Google Scholar]
- [15].Kurysheva N, Kiseleva T, Irtegova E, et al. Venous ocular blood flow in primary open angle glaucoma. Int J Ophthalmol Eye Res 2015;3:1–7. [Google Scholar]
- [16].Feke GT, Pasquale LR. Retinal blood flow response to posture change in glaucoma patients compared with healthy subjects. Ophthalmology 2008;115:246–52. [DOI] [PubMed] [Google Scholar]
- [17].Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8:96. [PubMed] [Google Scholar]
- [18].Ryabova TY, Shlapak VN, Shkurenko SA. Methods for checking and self-checking an output of the cardiovascular system and a checking device. PCT Gazette - Section III. International application numbers and corresponding international publication numbers, Weekly Indexes 19357-19404, Publication Number: WO 01/78598, No. of the international application: PCT/RU2000/000320, International filing date: 02.08.2000, Date of publication: 25.10.2001, Priority Date:2000109508 04/18/2000 RU. https://patentscope.wipo.int/search/ru/detail.jsf?docId=WO2001078598&redirectedID=true [Google Scholar]
- [19].Heart rate variability. Standards of measurement, physiological interpretation, Сlinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. European Heart Journal 1996;17:354–81. [PubMed] [Google Scholar]
- [20].Flugel C, Tamm E, Mayer B, et al. Species differences in choroidal vasodilative innervation: evidence for specific intrinsic nitregic and VIP-positive neurons in the human eye. Invest Ophthalmol Vis Sci 1994;35:592–9. [PubMed] [Google Scholar]
- [21].Leske M, Heijl A, Hyman L. EMGT Predictors of long term progression in the early manifest glaucoma trial. Ophthalmology 2007;114:1965–72. [DOI] [PubMed] [Google Scholar]
- [22].Leske M. Ocular perfusion pressure and glaucoma: clinical trial and epidemiologic findings. Curr Opin Ophthalmol 2009;20:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Riccadonna M, Covi G, Pancera P, et al. Autonomic system activity and 24-hour blood pressure variations in subjects with normal and high tension glaucoma. J Glaucoma 2003;12:156–63. [DOI] [PubMed] [Google Scholar]
- [24].Flammer J, Mozaffarieh M. Autoregulation, a balancing act between supply and demand. Can J Ophthalmol 2008;43:317–21. [DOI] [PubMed] [Google Scholar]
- [25].Phelps CD, Corbett JJ. Migraine and low tension glaucoma. A case-control study. Invest Ophthalmol Vis Sci 1985;25:1105–8. [PubMed] [Google Scholar]
- [26].Choi J, Kim KH, Jeong J, et al. Circadian fluctuation of mean ocular perfusion pressure is a consistent risk factor for normal-tension glaucoma. Invest Ophthalmol Vis Sci 2007;48:104–11. [DOI] [PubMed] [Google Scholar]
- [27].Drance CM, Douglas DV, Schilzer M. Response of blood flow to warm and cold in normal and low tension glaucoma patients. Am J Ophthalmol 1988;105:35–9. [DOI] [PubMed] [Google Scholar]
- [28].Plange N, Kaup M, Daneljan L, et al. 24-h blood pressure monitoring in normal tension glaucoma: night-time blood pressure variability. J Hum Hypertens 2006;20:137–42. [DOI] [PubMed] [Google Scholar]
- [29].Venkataraman ST, Flanagan JG, Hudson C. Vascular reactivity of optic nerve head and retinal blood vessels in glaucoma—a review. Microcirculation 2010;17:568–81. [DOI] [PubMed] [Google Scholar]
- [30].Piepoli M, Radaelli A, Ponikowski P, et al. Reproducibility of heart rate variability indices during exercise stress testing and inotrope infusion in chronic heart failure patients. Clin Sci 1996;91:87–8. [DOI] [PubMed] [Google Scholar]


