Supplemental Digital Content is available in the text
Keywords: lidocaine, meta-analysis, nasogastric intubation, visual analog scale
Abstract
Background:
Nasogastric (NG) tube insertion is a common procedure in the clinical setting that causes much discomfort and pain for the patient. Pain control is often suboptimal, as many NG tube insertions are performed without any pain-relieving supplements. The aim of this study was to summarize and critically evaluate the evidence from randomized controlled trials (RCTs) on the effect and adverse effects of lidocaine agents in reducing pain and discomfort associated with NG tube insertion.
Methods:
Databases from the Cochrane Library, MEDLINE, EMBASE, Airiti Library, PerioPath Index to Taiwan Periodical Literature, and Cumulative Index of Nursing and Allied Health (CINAHL) were searched from inception to April 2017. RCTs focusing on lidocaine before NG tube insertion were appraised. The primary outcome was the visual analog scale (VAS) score. The modified Jadad scale was used for quality assessment. Mean difference (MD) with 95% confidence intervals (95% CIs) and odds ratio (OR) for binary outcomes were assessed by a random effects model. Heterogeneity was determined by using the Cochran Q test and I2 statistics. Publication bias was analyzed by using a funnel plot analysis.
Results:
Ten RCTs enrolling 734 patients were included in the meta-analysis. Eight of the 10 RCTs reporting VAS scores had sufficient quantitative data to be pooled through meta-analysis. Results revealed a significant reduction in VAS score, with a MD of −26.05 and a CI of −28.21 to −23.89 with moderate heterogeneity (P < .001, I2 = 56%). There were no significant changes in difficulty of NG tube insertions (MD = −0.30, 95% CI, −1.30 to 0.70, P = .55), number of NG tube insertion attempts (MD = −0.22, 95% CI, −0.98 to 0.53, P = .56), nasal bleeding (OR = 0.62, 95% CI, 0.11–3.41, P = .59), and vomiting (OR = 0.30, 95% CI, 0.07–1.27, P = .10).
Conclusion:
This meta-analysis suggests that applying lidocaine before NG tube insertion can alleviate pain and discomfort by 26% without increasing nasal bleeding or vomiting.
1. Introduction
Nasogastric (NG) tubes are flexible lumen tubes that are passed proximally from the nose into the stomach. NG tube insertions are common medical procedures performed in the clinical setting. They are used for both diagnostic and therapeutic purposes such as bowel obstruction, administration of medications, enteral nutrition, or stomach lavage.[1,2] Pain and discomfort associated with the procedure has long been recognized and reported and has even been described as the single most painful routine procedure in the emergency department.[3] Many patients experience oropharyngeal discomfort. However, pain control is often suboptimal, as many NG tube insertions are performed with ordinary lubricant jelly alone, without any additional pain-relieving supplements. Reasons for inadequate pain control measures include poor recognition of pain, inconvenience, unavailability, and insufficient research on alternative treatment options.[4–6]
A previous systematic review and meta-analysis in 2010 concluded that nebulized lidocaine introduced before NG tube insertion significantly reduced the associated pain and discomfort of the procedure.[7] Gallagher[8] reviewed 6 randomized clinical trials that have all demonstrated that the application of various forms of lidocaine helps alleviate some of the discomfort associated with NG tube placement. A randomized controlled trial (RCT) demonstrated that lidocaine gel significantly reduces pain and gagging sensations associated with the procedure but is associated with more difficult NG tube insertion than the use of lubricant gel.[9] However, adverse effects were not documented. To date, there are no agreed upon guidelines or international consensus in the provision of local anesthesia in patients undergoing NG tube insertion. The aim of this study was to perform an updated systematic review and meta-analysis to evaluate the benefits and adverse effects of lidocaine agents in reducing pain and discomfort associated with NG tube insertion.
2. Methods
2.1. Database and search strategy
This systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) guidelines (Supplement 1).[10] We searched the following databases from inception to the end of April 2017: the Cochrane Library, MEDLINE, Cumulative Index of Nursing and Allied Health (CINAHL), EMBASE, Airiti Library, and PerioPath Index to Taiwan Periodical Literature. We searched for RCTs using the search terms: “nasogastric tube,” “NGT,” “lidocaine,” “lignocaine,” “lidocaine hydrochloride,” and “xylocaine.” For the Airiti Library and PerioPath Index to Taiwan Periodical Literature databases, we used the Chinese search terms: “liduokayin” (English translation: lidocaine), “weiguan” (English translation: gastric tube), “biweiguan” (English translation: nasogastric tube) (Supplement 2). The search was supplemented by reviews of reference lists of all relevant studies. Two reviewers (PCS and SJL) conducted the search independently, and disagreements were resolved through discussion with the third author (TLY). As this meta-analysis collected data from published articles, ethical approval was not necessary.
2.2. Inclusion and exclusion criteria
In our search for eligible studies, no restriction was placed on language. All authors are literate in both English and Chinese. HCC is also a native Korean speaker. Duplicate publications and those irrelevant to the topic of this study are excluded. Inclusion criteria for the studies were RCTs on human subjects, the effect of lidocaine on NG tube insertion, normal saline or K-Y lubricant gel or no intervention used for the control group, and the visual analog scale (VAS) score as the primary clinical outcome assessed. Studies were excluded if they were non-RCTs or crossover studies, lacked VAS scores, or if the control group used any substance other than the ones mentioned above.
2.3. Data extraction and statistical analysis
The authors independently used the modified Jadad scale to assess the methodological quality of each included study (Supplement 3). The modified Jadad scale includes 8 items to evaluate if randomization was done (score range 0–1), if randomization was appropriate (score range −1 to 1), if blinding was done (score range 0–1), if blinding was appropriate (score range −1 to 1), if withdrawals and dropouts were described (score range 0–1), if inclusion and exclusion criteria were described (score range 0–1), if adverse reactions were assessed (score range 0–1), and if the statistical analysis was described (score range 0–1).[11] The score of each study ranges from 0 (the lowest quality) to 8 (the highest quality). Studies were classified as moderate if they had a score of 4 or 5. If 2 authors had different opinions when assessing and selecting the included studies, agreement was reached by consensus with a third author.
The authors independently extracted the data from all included studies and the following data were collected: first author's name, year of publication, country, patient source, number of patients, age, type of intervention, control intervention type, clinical outcomes, and adverse effects. We have tried contacting the authors of certain studies to request for more in-depth study data that we felt would be beneficial to our research, but we were unable to obtain the raw data.
Data were analyzed using the mean difference (MD) with 95% confidence intervals (95% CIs) for continuous outcomes and odds ratio (OR) for binary outcomes. RevMan version 5.3.5 software (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark) was used for all data analyses. Meta-analysis was conducted when the trials had acceptable clinical homogeneity and statistical heterogeneity. Because of the significant heterogeneity expected among the studies, a random effects model was employed using the DerSimonian and Laird method.[12] Heterogeneity was quantified using the Cochran Q test and I2 statistics.[13] A P value < .10 for Chi-square testing of the Q statistic or an I2 > 50% was considered as a statistically significant heterogeneity. In accordance with the Cochrane methodology,[14] we performed a sensitivity analysis by individually removing each study to investigate whether choice of summary statistic is critical to the results of the meta-analysis. Subgroup analyses were also performed to determine sources of heterogeneity. Potential publication bias was assessed by observing the symmetry of funnel plots.[15]
3. Results
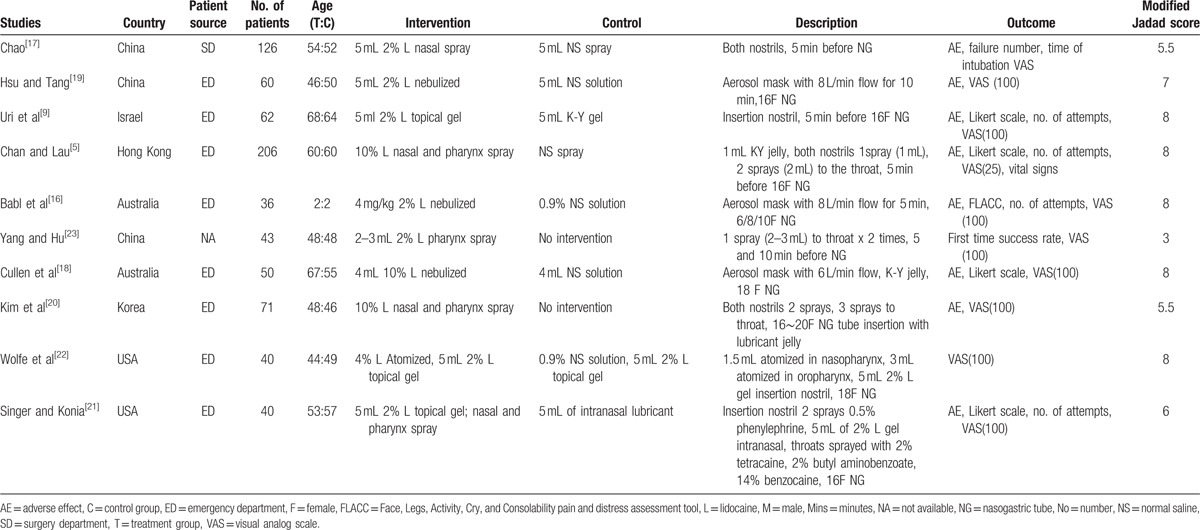
A total of 10 RCTs were included for quality assessment using the modified Jadad scale.[5,9,16–23] Seven of the 10 included RCTs achieved a full or nearly full score (modified Jadad score ≧6; Table 1).[5,9,16,18,19,21,22] Two RCTs had a moderate quality rating (modified Jadad score ≧4; Table 1).[17,20] One study had a Jadad score of 3 (Table 1).[23] The characteristics of the included trials are summarized in Table 1. A total of 734 participants were enrolled in the 10 studies and the mean age of the treatment and control group was 49 and 50 years, respectively. Only 1 of the 10 studies was conducted on children and had an age range of 1.0 to 3.8 years.[16] Five of the studies were conducted in Asia, 2 in Australia, 2 in the United States, and 1 in Israel. Six studies were written in English,[5,9,16,18,21,22] 3 studies in Chinese, [17,19,23] and 1 study in Korean.[20] Eight of the 10 studies recruited patients from the emergency department, 1 study allocated patients from the surgery department, and 1 study did not clarify their patient source. All patients required NG intubation with the most common indication being related to gastrointestinal diseases. The size of the NG tubes used was cited in all of the studies except for 2.[17,23] In adult patients, the sizes ranged from 14F to 20F, while in child patients, the sizes ranged from 6F to 10F.
Table 1.
Characteristics of the included studies.
We used the VAS scores as the main outcome. Eight of the 10 RCTs reporting VAS scores had sufficient quantitative data to be pooled through meta-analysis.[5,9,16,18–22] The other 2 RCTs were excluded, as 1 study did not clarify the measurement of the VAS score used,[17] while the other study did not provide sufficient information on the study design used.[23] The flowchart of the study selection process is summarized in Fig. 1. Thus, the analysis from the 8 included RCTs consisted of a total of 565 subjects. The primary outcome of most RCTs was to measure pain during NG insertion. Two studies[5,18] assessed discomfort that was considered as synonymous with pain. Seven studies used the VAS 100 mm measurement and 1 study[5] used the VAS 25 mm measurement, which for the purpose of our analysis was converted to the 100 mm scale by multiplying the result by 4. Overall, the meta-analysis showed a significant reduction in VAS score in the lidocaine group with a MD = −26.05, 95% CI, −28.21 to −23.89, P ≤ .001, I2 = 56%; forest plot is shown in Fig. 2.
Figure 1.
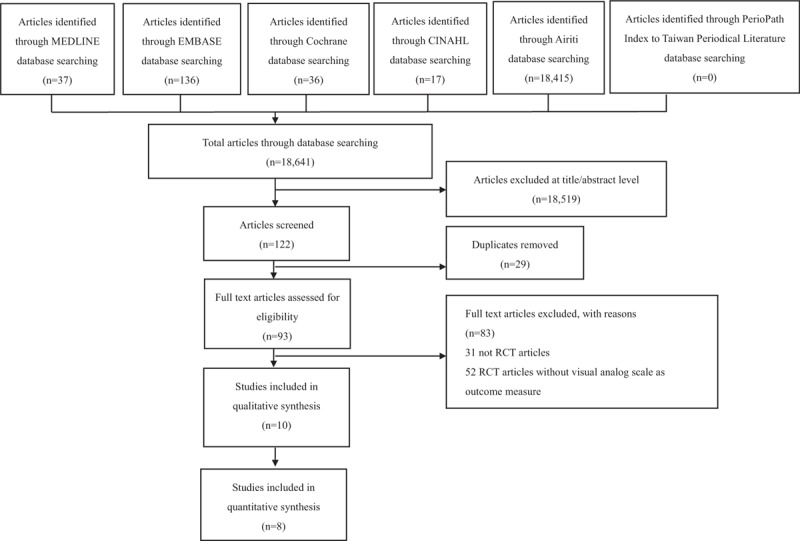
Flow diagram of the publication selection. CINHAL = Cumulative Index of Nursing and Allied Health, RCT = randomized controlled trial.
Figure 2.
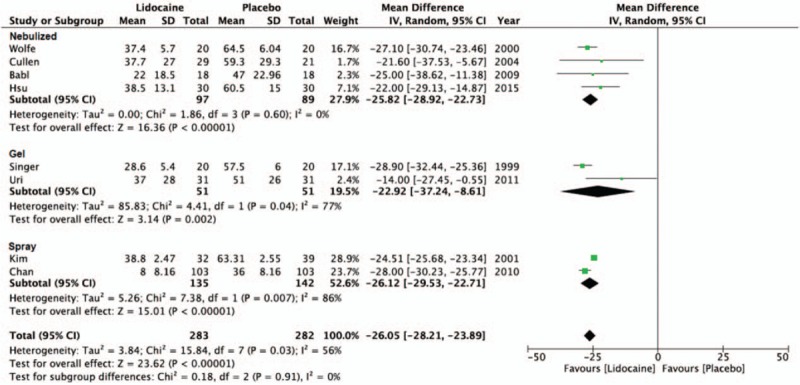
The forest plot for visual analog scale score outcomes between lidocaine and placebo groups by delivery method.
To explore the cause of heterogeneity, we performed a subgroup analysis according to the lidocaine concentration (Fig. 3) and method of delivery (Fig. 2). In 4 of the 8 studies, lidocaine used in 2% concentration as the intervention was effective in reducing discomfort (MD = −24.18, 95% CI, −30.25 to −18.12, P < .001, I2 = 55%).[9,16,19,21] Results from 1 study using 4% lidocaine were also shown to decrease pain (MD = −27.10, 95% CI, −30.75 to −23.45, P < .001)[22] and 3 studies using 10% lidocaine yielded a similar outcome (MD = −25.94, 95% CI, −29.07 to −22.81, P < .001, I2 = 74%).[5,18,20] In the subgroup analysis of the different delivery methods of lidocaine, 4 studies employing the use of nebulized/atomized lidocaine showed reduction in pain (MD = −25.82, 95% CI, −28.92 to −22.73, P < .001, I2 = 0%).[16,18,19,22] Two studies comparing lidocaine gel to K-Y and lubricant gel also found lidocaine to be more effective in decreasing discomfort (MD = −22.92, 95% CI, −37.24 to −8.61, P = .002, I2 = 77%).[9,21] The remaining 2 studies testing the effect of nasal and pharynx spray of lidocaine also had lower VAS scores than the control group (MD = −26.12, 95% CI, −29.53 to −22.71, P < .001, I2 = 86%).[5,20]
Figure 3.
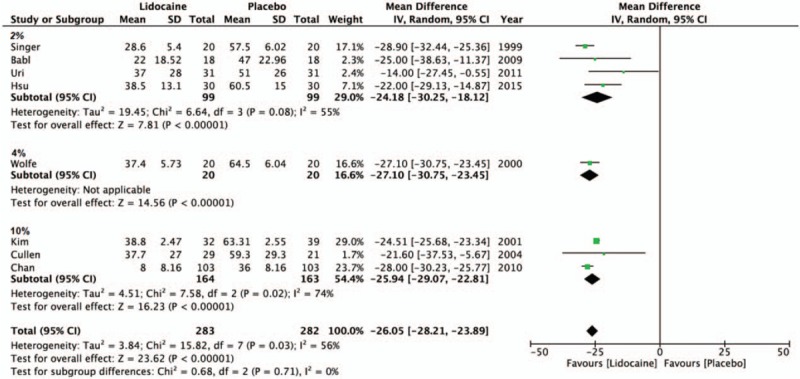
The forest plot for visual analog scale score outcomes between lidocaine and placebo groups by concentration.
Sensitivity analysis was also performed to determine whether the outcomes were altered after the removal of any of the studies. All results were stable and no significant difference was found. These results indicated that no single study carried enough weight to significantly influence the pooled results. Given the asymmetry of the funnel plot on visual inspection, publication bias is likely to be present (Supplement 4).
The difficulty of NG tube insertion was assessed using a 5-point Likert scale in 4 studies.[5,9,16,18] The scale marked “minimal,” “slight (less difficult than usual),” “moderate (usual amount of difficulty),” “substantial (more difficult than usual),” and “extreme.” The meta-analysis did not show a significant reduction in Likert scale (MD = −0.30, 95% CI, −1.30 to 0.70, P = .55) nor an increase in the number of NG tube insertion attempts (MD = −0.22, 95% CI, −0.98 to 0.53, P = .56).[5,9,16] For the adverse effect of nasal bleeding, no significant difference was found between the lidocaine group and the placebo group when all the patients were pooled into our meta-analysis, OR = 0.62; 95% CI, 0.11–3.41, P = .59.[5,16,18,19,21] There was also no detectable difference in vomiting between the 2 groups (OR = 0.30; 95% CI, 0.07–1.27, P = .10).[5,9,17–19,21]
4. Discussion
NG tube insertion is a common procedure in the clinical setting for all ages of patients. Although a useful tool, it is also a procedure that causes much discomfort and pain for the patient. The aim of this paper is to create a comprehensive up-to-date summary of the studies done on methods to minimize patient discomfort in NG tube insertions.
In our review, we did not limit our search on method of lidocaine delivery; rather, methods included nebulized, atomized, spray or topical gel, etc. All of the selected publications used a randomized placebo-controlled design, which reduced the risk of bias, and were critically appraised using the modified Jadad scale. We selected 10 articles of moderate and high-quality RCTs with 5 articles achieving a full score of 8.[5,9,16,18,22] We expanded our search to incorporate papers in all languages and all age groups. Our study demonstrated that the application of lidocaine before NG tube insertion was associated with 26% less pain and discomfort as evaluated by the VAS score and should be considered in daily medical practices.
Previously, Li et al[24] reviewed 6 RCTs involving 384 patients and reported that the administration of intranasal lidocaine spray reduced the discomfort of NG tube insertion. Kuo et al[7] also conducted a systemic review and meta-analysis of 5 RCTs with 212 subjects regarding the use of nebulized and atomized lidocaine to reduce the pain of NG tube insertion. In this study, they concluded that nebulized lidocaine decreased pain in patients by 57.7% as assessed by the VAS. However, they focused solely on the adult population and only included studies written in the English language. Their review did not discuss the adverse effects, difficulty of NG insertions, or number of NG tube insertion attempts.
Moderate heterogeneity was noted in our meta-analysis. Different doses and delivery methods of anesthesia may explain some of the heterogeneity. We conducted a subgroup analysis in delivery methods of anesthesia to reduce heterogeneity. In the subgroup analysis, the nebulized, gel, and spray form of lidocaine were all shown to decrease VAS pain score (MD = 25.82 vs 22.92 vs 26.12). Likewise, a subgroup analysis of the different concentrations of lidocaine all had a significant reduction in pain during NG tube insertions (MD = 2%:24.18 vs 4%:27.10 vs 10%:25.94). Thus, the use of lidocaine, regardless of concentration or method of delivery, can decrease patient discomfort. Although it seems that lidocaine is a feasible method to alleviate the pain felt by NG intubation, we are unable to recommend the exact dose or preferred method of delivery. Ducharme and Matheson [25] studied 30 healthy volunteers, comparing the anesthetic effects of 1.5 mL 4% atomized lidocaine, 1.5 mL 4% atomized cocaine, and 5 mL 2% lidocaine gel in NG intubations. All 3 medications were effective in decreasing pain as shown by VAS scores. However, overall discomfort was noted less in lidocaine gel and the subjects seemed to prefer this method above the other 2 atomized agents. More head-to-head studies of the many concentrations and methods of lidocaine delivery may be needed to determine the best protocol. We also realize that the different methods of lidocaine delivery are dependent on the cost, availability, and preparation time in each clinic setting. Thus, the emphasis is on individualized therapy and health care providers should make a recommendation best regarding each patient.
Difficulty of NG tube insertion as measured by the 5-point Likert scale and number of NG tube insertion attempts did not show a significant difference between the treatment and control groups. These findings suggest that the application of lidocaine before NG intubation was not associated with more difficult tube insertions nor did it increase the number of insertion attempts. This was in contrast to the study done by Uri et al,[9] where topical lidocaine gel used was associated with an increase in difficulty of NG tube insertions. On the contrary, Chan and Lau[5] found that the application of lidocaine spray reduced NG intubation difficulty, duration, and number of attempts.
In our analysis, there was no detectable difference regarding the adverse effect of nasal bleeding or vomiting between the 2 groups. Thus, applying lidocaine before intubation can reduce pain and discomfort without increasing nasal bleeding or vomiting. In contrast, Cullen et al[18] found that although nebulized lidocaine was shown to be effective in reducing the discomfort of NG intubation, it was also associated with an increased incidence of epistaxis. However, studies done by Chan and Lau,[5] Chao,[17] and Hsu and Tang[19] all demonstrated that lidocaine use was associated with a decreased frequency of nausea and vomiting. Future studies should consider further investigation of the adverse effects of lidocaine on NG tube insertions.
There are some limitations to our study. Only 10 publications were selected for our study but most of them were moderate and high-quality RCTs. Due to the small number of data collected from the included RCTs, the following variables were not assessed: chest pain, cough, shortness of breath, and endotracheal intubation. Additional research is needed to determine the most efficient method with the least adverse effect. Furthermore, only 1 study out of the 10 assessed was a pediatric RCT, thus we are unable to make a strong recommendation for child patients.[16] Most of the RCTs included in this meta-analysis focused on the effects of lidocaine on an adult population. Thus, more studies are needed to make a final conclusion on the benefits of lidocaine in children during NG intubations.
5. Conclusion
Current evidence confirms the clinical benefit of lidocaine in reducing pain associated with NG tube insertions as assessed by the VAS and that every health care provider should consider using some form of anesthesia before attempting this procedure.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CINAHL = Cumulative Index of Nursing and Allied Health, MD = mean difference, NG = nasogastric, OR = odds ratio, PRISMA-P = Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols, RCT = randomized controlled trial, VAS = visual analog scale.
This research did not receive any funding. There are no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Thomsen TW, Shaffer RW, Setnik GS. Nasogastric intubation. N Engl J Med 2006;354:e16. [DOI] [PubMed] [Google Scholar]
- [2].Tucker A, Lewis J. Procedures in practice. Passing a nasogastric tube. Br Med J 1980;281:1128–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Singer AJ, Richman PB, Kowalska A, et al. Comparison of patient and practitioner assessments of pain from commonly performed emergency department procedures. Ann Emerg Med 1999;33:652–8. [PubMed] [Google Scholar]
- [4].Bonia J. Paris PM SR. Foreword. Appleton-Lange, Pain Management in Emergency Medicine. West Hartford, CT:1987. [Google Scholar]
- [5].Chan CP, Lau FL. Should lidocaine spray be used to ease nasogastric tube insertion? A double-blind, randomised controlled trial. Hong Kong Med J 2010;16:282–6. [PubMed] [Google Scholar]
- [6].Czarnecki ML, Turner HN, Collins PM, et al. Procedural pain management: a position statement with clinical practice recommendations. Pain Manag Nurs 2011;12:95–111. [DOI] [PubMed] [Google Scholar]
- [7].Kuo YW, Yen M, Fetzer S, et al. Reducing the pain of nasogastric tube intubation with nebulized and atomized lidocaine: a systematic review and meta-analysis. J Pain Symptom Manage 2010;40:613–20. [DOI] [PubMed] [Google Scholar]
- [8].Gallagher EJ. Nasogastric tubes: hard to swallow. Ann Emerg Med 2004;44:138–41. [DOI] [PubMed] [Google Scholar]
- [9].Uri O, Yosefov L, Haim A, et al. Lidocaine gel as an anesthetic protocol for nasogastric tube insertion in the ED. Am J Emerg Med 2011;29:386–90. [DOI] [PubMed] [Google Scholar]
- [10].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [12].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [13].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [14].The Cochrane Collaboration, JPT H. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. 2011. [Google Scholar]
- [15].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Babl FE, Goldfinch C, Mandrawa C, et al. Does nebulized lidocaine reduce the pain and distress of nasogastric tube insertion in young children? A randomized, double-blind, placebo-controlled trial. Pediatrics 2009;123:1548–55. [DOI] [PubMed] [Google Scholar]
- [17].Chao JX. The application of lidocaine in nasogastric tube insertion in general surgery ward patients. J Huaihai Med 2015;33:402.(in Chinese). [Google Scholar]
- [18].Cullen L, Taylor D, Taylor S, et al. Nebulized lidocaine decreases the discomfort of nasogastric tube insertion: a randomized, double-blind trial. Ann Emerg Med 2004;44:131–7. [DOI] [PubMed] [Google Scholar]
- [19].Hsu YH, Tang XP. Nebulized lidocaine in reducing pain of nasogastric tube insertion: a nursing study. J Nurses Training 2015;30:1030–4. (in Chinese). [Google Scholar]
- [20].Kim TK, Chung SM, Kim IB, et al. Effect of local anesthesia for nasogastric tube placement. J Korean Soc Emerg Med 2001;12:433–8. (in Korean). [Google Scholar]
- [21].Singer AJ, Konia N. Comparison of topical anesthetics and vasoconstrictors vs lubricants prior to nasogastric intubation: a randomized, controlled trial. Acad Emerg Med 1999;6:184–90. [DOI] [PubMed] [Google Scholar]
- [22].Wolfe TR, Fosnocht DE, Linscott MS. Atomized lidocaine as topical anesthesia for nasogastric tube placement: a randomized, double-blind, placebo-controlled trial. Ann Emerg Med 2000;35:421–5. [PubMed] [Google Scholar]
- [23].Yang XR, Hu YG. The application of low temperature lidocaine in preventing pain and discomfort of nasogastric tube insertions. J Nurses Training 2006;21:568.(in Chinese). [Google Scholar]
- [24].Li W, Sun XM. Effectiveness of intranasal lidocaine spray before nasogastric tube insertion: a meta-analysis. Chin J Evid-based Med 2015;15:342–5. (in Chinese). [Google Scholar]
- [25].Ducharme J, Matheson K. What is the best topical anesthetic for nasogastric insertion? A comparison of lidocaine gel, lidocaine spray, and atomized cocaine. J Emerg Nurs 2003;29:427–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


